Abstract
The circadian pacemaker within the suprachiasmatic nucleus (SCN) confers daily rhythms to bodily functions. In nature, the circadian clock will adopt a 24-h period by synchronizing to the solar light/dark cycle. This light entrainment process is mediated, in part, at glutamatergic synapses formed between retinal ganglion afferents and SCN neurons. N-methyl-d-aspartate receptors (NMDARs) located on SCN neurons gate light-induced phase resetting. Despite their importance in circadian physiology, little is known about their functional stoichiometry. We investigated the NR2-subunit composition with whole cell recordings of SCN neurons within the murine hypothalamic brain slice using a combination of subtype-selective NMDAR antagonists and voltage-clamp protocols. We found that extracellular magnesium ([Mg]o) strongly blocks SCN NMDARs exhibiting affinities and voltage sensitivities associated with NR2A and NR2B subunits. These NMDAR currents were inhibited strongly by NR2B-selective antagonists, Ro 25-6981 (3.5 μM, 55.0 ± 9.0% block; mean ± SE) and ifenprodil (10 μM, 55.8 ± 3.0% block). The current remaining showed decreased [Mg]o affinities reminiscent of NR2C and NR2D subunits but was highly sensitive to [Zn]o, a potent NR2A blocker, showing a ∼44.2 ± 1.1% maximal inhibition at saturating concentrations with an IC50 of 7.8 ± 1.1 nM. Considering the selectivity, efficacy, and potency of the drugs used in combination with [Mg]o-block characteristics of the NMDAR, our data show that both diheteromeric NR2B NMDARs and triheteromeric NR2A NMDARs (paired with an NR2C or NR2D subunits) account for the vast majority of the NMDAR current within the SCN.
INTRODUCTION
The suprachiasmatic nucleus (SCN) of the hypothalamus generates a daily rhythm that is reflected in behavioral and bodily functions. The properties of this rhythm are determined in large part by the molecular machinery of neurons that form an oscillating cellular network within the SCN (Herzog 2007). In mammals, this circadian rhythm synchronizes to the environmental light-dark cycles through a direct pathway from the retina to the SCN, the retinohypothalamic tract (Johnson et al. 1988). During this light entrainment process, light depolarizes retinal ganglion cells that express the photopigment, melanopsin, which, in turn, releases glutamate from their terminals at synapses formed with neurons of the SCN (Berson et al. 2002; Hattar et al. 2002).
l-glutamate is the primary neurotransmitter of the retinohypothalamic tract and is required for the entrainment of the circadian pacemaker to the solar light-dark cycle (Hannibal 2002). Although, ionotropic glutamate receptors of the SCN, N-methyl-d-aspartate receptors (NMDARs) and amino-methyl proprionic acid/kainate receptors, participate in this process (Colwell and Menaker 1992; Ebling 1996), NMDAR activation is necessary and sufficient for “light-like” circadian clock resetting and the intracellular cascades that coordinate this process, such as Ca2+/cAMP response element binding protein (CREB) phosphorylation (Ding et al. 1997; Ginty et al. 1993; Schurov et al. 1999) and immediate early gene (Kornhauser et al. 1990, 1992; Schurov et al. 1999) and “clock” gene expression (Moriya et al. 2000a; Paul et al. 2003).
The NMDAR is composed of four subunits, two from the NR1 family and two from the NR2 (NR2A-D) or NR3 (NR3A-B) families (Cull-Candy and Leszkiewicz 2004). In excitatory neurotransmission, the NR2 subunit confers distinct pharmacological profiles, gating properties, extracellular magnesium and zinc sensitivities, and distinct intracellular cascades linking the NMDA receptor complex to gene transcription (Ali and Salter 2001; Cull-Candy and Leszkiewicz 2004). Despite the great importance of the NMDAR as the cellular gate to light entrainment, NR2-subunits and their stoichiometry within the SCN remain unresolved (Gannon and Rea 1994; Mikkelsen et al. 1993; Moriya et al. 2000a,b; O'Hara et al. 1995; Petralia et al. 1994; Wang et al. 2008; Watanabe et al. 1993). While there are conflicting reports on the presence of NR2A and NR2B within the SCN (Gannon and Rea 1994; Mikkelsen et al. 1993; Moriya et al. 2000a,b; O'Hara et al. 1995; Petralia et al. 1994; Wang et al. 2008; Watanabe et al. 1993), the consensus from previous work suggests strong transcript levels for NR2C (Ishida et al. 1994; Mikkelsen et al. 1993; Moriya et al. 2000a,b; O'Hara et al. 1995) and weak or absent NR2D transcripts (Moriya et al. 2000a; O'Hara et al. 1995). Although the subunit composition circumscribes the function of the NMDAR in excitatory transmission and light-entrainment, a detailed description of the NR2 subunit and its stoichiometry within the SCN is currently lacking.
In the present study, we show the subtypes and stoichiometry of SCN NMDARs through a whole cell voltage-clamp study of SCN neurons using a diverse selection of subunit-selective antagonists with distinct mechanisms of inhibition. Our results support the presence of [Mg]o-sensitive NR2A and NR2B subunits within the SCN. Our advance is to show that diheteromeric NR2B NMDARs have a major presence within the SCN. NR2A also has an important functional role, as triheteromeric receptors formed with [Mg]o-hyposensitive subunits, NR2C and -D. Also from extensive recordings taken during the subjective day and night, our work argues against the hypothesis that a circadian rhythm in total triheteromeric NR2A or diheteromeric NR2B NMDAR currents exists as a global feature of neurons within the SCN.
METHODS
Electrophysiological recordings
All mice used in this study were cared for and handled according to the Institutional Animal Care and Use Committee and Research Animal Resources guidelines at the University of Minnesota. According to previously published methods, C57B/6 mice (Harlan, Indianapolis, IN), 14–21 days old, were entrained to a 12-h light:12-h dark cycle (LD) in colony chambers with unrestricted access to food and water (Clark et al. 2005). Mice were killed using a guillotine to avoid altering immediate early gene expression and circadian rhythms caused by standard anesthesia (pentobarbital, isoflurane, propofol, CO2, urethan, chloralose, and halothane) (Prosser et al. 1994; Sica et al. 1999; Takayama et al. 1994). The brains of mice were isolated during the subjective day, ≥2 h prior to the offset of light in the colony chamber (Zeitgeber time 10; ZT 10) for subjective night recordings and at ZT 3 for subjective day recordings, to prevent procedural resetting of the circadian clock (Gillette 1986). Coronal hypothalamic brain slices (250–350 μm) were sectioned with a vibratome in ice-cold, oxygenated (95% O2-5% CO2) modified artificial cerebral spinal fluid (mACSF) (in mM): 220 sucrose, 26 NaHCO3, 3 KCl, 5 MgCl2, 1.25 NaH2PO4, 1.0 CaCl2, and 10 glucose (pH 7.2–7.4). The slices were incubated for 20–30 min at ∼36°C and allowed to cool to room temperature (20–23°C) in ACSF holding solution (mACSF with 130 mM NaCl and 2 mM CaCl2, omitting sucrose and MgCl2) for ∼2 h from the time the animal was killed until the appropriate epoch during the subjective day (ZT 5–10) or subjective night (ZT 12–18). Neurons were visualized within brain slices transferred to a recording chamber mounted on the stage of an upright microscope (E600 FN, Nikon, Tokyo, Japan) equipped with differential interference contrast optics and infrared videoimaging system (CoolSnaps ES, Roper Scientific, Tucson, AZ). Whole cell recordings were made from the soma of ventral-lateral SCN (Fig. 1A) neurons at room temperature (23–25°C) using a Multi-clamp 700A amplifier (Axon Instruments, Union City, CA) with fire-polished borosilicate pipettes (3–7 MΩ, Sutter Instruments, Novato, CA). Pipette and cell capacitance and series resistance (0–50%) were compensated. All traces were sampled at 5–10 kHz and low-pass filtered at 2 kHz.
Fig. 1.
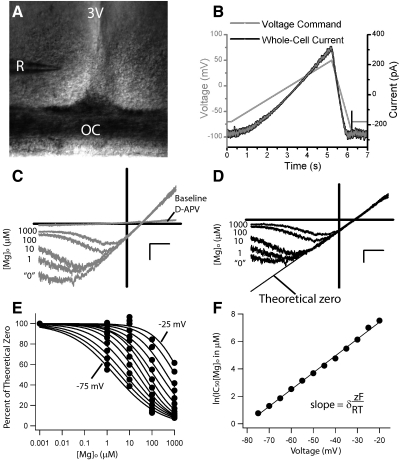
The experimental preparation, protocol and [Mg]o–block analysis of N-methyl-d-aspartate receptor (NMDAR) currents recorded from patch-clamped suprachiasmatic nucleus (SCN) neurons within the hypothalamic brain slice. A: this ×10 photograph was modified to show the 3rd ventricle (3V) and the optic chiasm (OC) in relation to the recording pipette (R) used during whole cell recordings of neurons within the SCN. B: voltage-clamped SCN neurons were given a voltage command protocol (mV, light gray trace), which served to inactivate voltage-gated conductances (ramp from −70 to 50 mV over 5 s) and to obtain an I-V record (ramp from 50 to −90 mV over 1 s). The black trace is an example of the current response (pA) to this voltage ramp protocol from an SCN neuron exposed to 100 μM NMDA and 100 μM d-Serine in nominally [Mg]o-free (“0” [Mg]o) physiological saline where voltage-gated sodium and L-type calcium channels (1 μM tetrodotoxin and10 μM nifedipine, respectively), GABAA (100 μM picrotoxin), and non-NMDAR ionotropic glutamate receptors (10 μM NBQX) were blocked. C: the NMDAR I-V curves are shown for baseline (without agonists), 0, 1, 10, 100, and 1,000 [Mg]o and d-APV (1,000 μM [Mg]o +100 μM d-APV) conditions recorded from a neuron within the SCN brain slice using 100 μM NMDA and 100 μM d-serine. The reversal potential was measured to be 12.5 ± 1.6 mV (n = 19). D: these currents are replotted for each condition with the d-APV curve deducted and normalized to the largest outward current of the set at 50 mV. The linear regression of the outward current, referred to as the “theoretical 0” condition, is also plotted (black line). E: the normalized current, with respect to the theoretical 0 line, is plotted as a function of [Mg]o for each voltage from −75 to −20 mV (in 5 mV increments). On this plot, the theoretical 0 points are set to 1 nM [Mg]o, and 0 [Mg]o condition is omitted. F: the natural log of the IC50 of [Mg]o (in μM) for each curve in C is plotted with respect to voltage (mV). The curve is fit with a linear regression to obtain the y intercept [IC50(0)] and slope (δzF/RT). The scale is set to C and D to 20 mV (horizontal bar) and 100 pA (vertical bar).
During recordings, a patch pipette was lowered in the recording solution (see following text), and positive pressure was applied to keep the electrode tip free of debris as it approached the soma. A gigaohm seal was achieved (>1 GΩ) with a brief suction pulse and, after 2 min recording in cell-attached mode, whole cell configuration was established after the cell membrane was ruptured by mouth suction. Neurons were distinguished from glia by their high-input resistances (>0.5–1 GΩ), initial sodium channel activation in the absence of tetrodotoxin (TTX) and spherical somas localized superior to the optic chiasm and inferior and lateral to the third ventricle.
NMDAR currents were measured across a broad range of voltages with a slow voltage ramp, where the 5 s rising phase was used to inactivate voltage-gated conductances not pharmacologically inhibited (−70 to 50 mV) and the 1 s falling phase was used to generate current-voltage (I-V) relationships every 30 s (Fig. 1B). For [Mg]o experiments, outward currents at 50 mV were used to estimate NMDAR magnitude as inward currents rectified with increasing [Mg]o (Fig. 1C). To examine the NMDAR specifically, background currents where removed [minus the 100 μM 2-d-amino-5-phosphonovaleric acid (d-APV)] from the average trace representing each [Mg]o condition. For the Woodhull analysis of [Mg]o currents with and without ifenprodil, the resultant currents were then rescaled to outward currents to correct for receptor desensitization as this confound would exaggerate the degree of inhibition of [Mg]o (Fig. 1D). Because it has been shown that in the absence of [Mg]o, the NMDAR has a linear I-V relationship (Kuner and Schoepfer 1996), we fit the linear portion of the I-V with the largest outward current for each cell (Fig. 1D). This line, referred to as the “theoretical zero” [Mg]o condition, was used to normalize the “0”, 1, 10, 100, and 1,000 μM [Mg]o conditions after the background currents were removed (Kirson et al. 1999). This approach was taken because under nominally free [Mg]o conditions (“0”), the I-V was not linear, which was attributed to trace [Mg]o concentrations that could not be removed from our brain slice preparation. To quantify the affinity of [Mg]o for the NMDAR, we constructed [Mg]o concentration response curves from the set of whole cell currents normalized to the “theoretical zero” values (set to an inactive [Mg]o, 1 nM) for each voltage from −20 to −75 mV (Fig. 1E). The natural log of [Mg]o at 50% of the maximum normalized current (IC50) was plotted against voltage (Fig. 1F). This technique was used to quantify the voltage dependence of the NMDAR currents in relation to [Mg]o and [Zn]o, to identify the functional NMDAR subtypes present in the hypothalamic circadian pacemaker and, in the case of [Zn]o, to confirm the selectivity of the block.
During “puff” experiments, a pipette (1–2 MΩ) positioned ∼50 μm from the patch-clamped cell delivered 100 μM NMDA and d-serine dissolved in the recording solution with a pneumatic picopump (PV820, World Precision Instruments, Sarasota, FL). The peaks from the “puff” experiments were calculated as the difference between the mean peak amplitude during NMDA exposure and the baseline preceding the event and were normalized to the maximum amplitude obtained within each experiment (Fig. 4). In the experiments with puff and bath applications of NMDA, we estimated NMDAR rundown and desensitization, respectively, by comparing time-matched experiments with and without antagonists. This approach delineates the greatest extent of current decrement possible under these conditions as it is expected to overestimate the degree of rundown/desensitization under conditions where currents were reduced by antagonist co-application.
Fig. 4.
Ifenprodil significantly attenuates peak NMDAR current during acute agonist application. A: example current traces of a voltage-clamped SCN neuron responding to 100 μM NMDA and d-serine pipette applications (“puff”) alone and in the presence of 10 μM ifenprodil and 100 μM d-APV. The traces are scaled to 50 pA (vertical bar) and 2.5 s (horizontal bar). B: a time-series plot of mean normalized current from SCN neurons (±SE) recorded under ifenprodil (n = 3) and time-matched control (n = 3) conditions during the early subjective night [Zeitgeber time (ZT) 13–18]. The horizontal bar is 5 min. The puff pipette and bath solutions in these experiments contained antagonists of the voltage-gated sodium and L-type calcium channels (1 μM tetrodotoxin and10 μM nifedipine, respectively), GABAA (100 μM picrotoxin) and non-NMDAR ionotropic glutamate receptors (10 μM NBQX). Also, [Mg]o was omitted and 100 μM d-serine was supplemented to these solutions to optimize NMDAR current at −70 mV.
Solutions
All solutions were gravity fed at ∼3 ml/min to the brain slice. Solution was exchanged around the recording area with a 360 μm six-channel manifold tip (Perfusion Pencil, Automate Scientific, Berkeley, CA) positioned immediately above the slice and ∼1 mm away from the pipette tip. Additionally, a background solution fed the laminar perfusion chamber (Warner Scientific, Hamden, CT) to maintain the slice quality and to facilitate drug clearance. For experiments where NMDA receptor currents were studied with bath application and puff applications, recording pipettes were filled with (in mM) 125 Cs-methanesulfonate, 4 NaCl, 1 MgCl2, 5 MgATP, 9 Cs- bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA), 8 Na-HEPES, 1 Tris-GTP, 0.1 leupeptin, 10 Tris-phosphocreatine, 3 QX-314 (pH 7.2, osmolality: 290–295 mosM). The Mg2+-free recording solution contained (in mM) 0 MgCl2, 10 TEA-Cl, 120 NaCl, 26 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1.0 CaCl2, 10 glucose (pH 7.2–7.4, osmolality: 290–305 mOsm) supplemented with (in mM) 0.1 picrotoxin, 0.01 NBQX, 0.1 d-serine, 0.01 nifedipine, 0.001 tetrodotoxin and oxygenated (95% O2-5% CO2). Under these conditions, both extraneous voltage-gated (calcium-nifedipine, sodium-tetrodotoxin and QX-314 and potassium channels-cesium and/or TEA) and ligand-gated currents (GABAA-picrotoxin and non-NMDA ionotropic receptors—NBQX) were blocked. At the conclusion of NMDAR experiments, 100 μM 2-d-amino-5-phosphonovaleric acid (d-APV) conditions were used to block NMDAR currents. The contribution of glycine-activated NR3A and NR3B subunits, were excluded by recording from the SCN during a point of development where there is no detectable transcript (Bendova et al. 2009), by using a less effective agonist, d-serine (Chatterton et al. 2002) and defining NMDAR current based on d-APV block, which does not block NR3 subunits (Chatterton et al. 2002). Magnesium solutions, 1, 10, 100, and 1,000 μM, were prepared by adding appropriate volume from 1, 10, 100, and 1,000 mM stocks to 0 [Mg] recording solution without compensation for differences in ionic strength. Zinc solutions were buffered with EDTA (1 mM) and free [Zn]o (in M) were calculated with MaxChelator (Stanford, CA, http://maxchelator.stanford.edu/webmaxc/webmaxcS.htm) to be E-6 (1.70E-6), E-7 (1.03E-7), E-8 (1.05E-8), E-9 (1.75E-9), and E-10 (2.92E-10). Free calcium and magnesium were also examined, and although free [Mg]o did not change significantly from 1 μM, free [Ca]o was reduced in the E-10 [Zn]o condition so [Ca]o was supplemented to obtain 1.8 mM. For zinc experiments, slices were rinsed with 1 mM EDTA recording solutions prior to recording.
Reagents
Tetrodotoxin was purchased from Alomone Labs (Jerusalem, Israel). All other reagents were purchased from Sigma-Aldrich (St Louis, MO) or Tocris (Ellisville, MO). Many reagents were aliquoted and stored at −20 or −80°C according to manufacturer's instructions in either DMSO or H2O. Stock solutions of Nifedipine were protected from light with aluminum foil. At the time of the recording, stocks were diluted into recording solution.
Data analysis
Off-line waveform processing and analysis was conducted in Igor Pro 5.3 (Wavemetrics, Portland, OR) and/or Excel (Microsoft, Seattle, WA). Data were analyzed from 110 neurons that exhibited stable access resistances (<10% change and <40 MΩ) and stable recordings (leak currents within 100 pA of baseline at d-APV condition) and were responsive to 100 μM NMDA (>60 pA peak response). Waveforms recorded, in triplicate, from each cell within an experimental condition were averaged prior to further processing or statistical analyses. In all experiments, NMDAR currents were isolated by subtracting the averaged current of interest from the one blocked by the competitive antagonist, d-APV. These “raw” NMDAR currents were averaged between neurons within similar conditions. Statistical comparisons (see following text) were drawn between select inward, outward, or integrated currents. In the [Mg]o- and [Zn]o-titration experiments, currents were plotted, the outward currents normalized (in [Mg]o-titration experiments only) and the outward currents of the lowest cation concentration were fit with a linear regression (theoretical 0 line-Fig. 1D). These I-Vs were normalized with respect to the theoretical zero line to produce concentration response curves for voltages ranging from −20 to −75 mV (every 5 mV; Fig. 1E). For [Mg]o-titration experiments, these concentration response curves were fit to
and for [Zn]o-titration experiments, they were fit to
where A is the extent of inhibition, IC50 is the concentration for 50% inhibition, and n is the Hill coefficient. In addition to A and IC50, the Hill coefficient was a free parameter, which best described our data, as opposed to fits where the Hill coefficient was constrained to unity (data not shown). The linear regression relating the natural log-transformed IC50 to voltage (−20 to −75 mV) produced an estimate of the voltage dependence of block (δ) and the affinity of [Mg]o at 0 mV (IC50(0)), using a relation described by Woodhull (1973)
where z is valence, V is membrane potential, T is the temperature (293.35 K), and gas constant (R) and Faraday constant (F) have their standard meaning (Fig. 1F). Repeated-measure ANOVAs with Scheffé means comparison procedures or t-test were used to evaluate statistical significance of the bath applied and puff NMDA receptor experiments at an alpha level of 0.05 using SAS 9.1 (SAS Institute, Cary, NC), unless stated otherwise. Figures were produced in Igor Pro or Origin 7.0 (OriginLab, Northampton, MA) for publication.
RESULTS
SCN NMDARs show subtype-sensitivity to [Mg]o
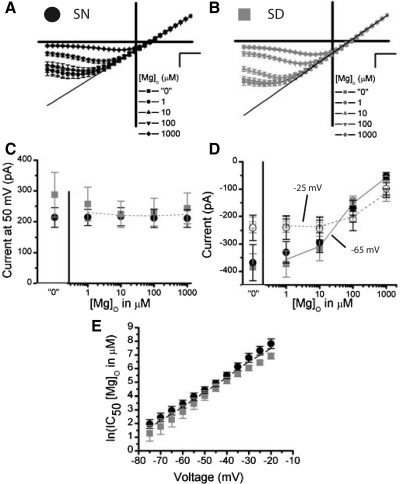
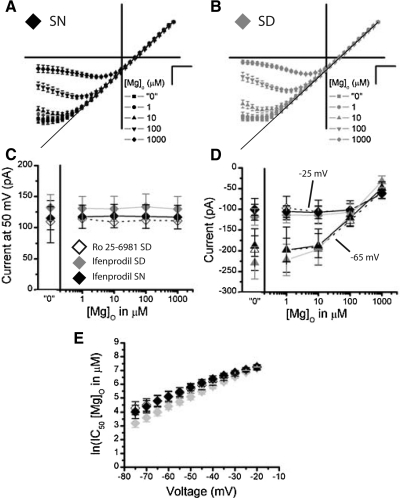
The extent of the [Mg]o block of inward currents through NMDARs depends critically on the subunit composition. Specifically, [Mg]o more strongly occludes NMDARs composed of NR2A or NR2B subunits than NMDARs containing NR2C or NR2D subunits (Cull-Candy and Leszkiewicz 2004). We examined the sensitivity of NMDAR currents over a range of [Mg]o (1-1,000 μM) where NR2A/B and N2C/D are differentially inhibited (Kuner and Schoepfer 1996). Figure 2, A and B, shows average I-V studies of NMDAR currents collected from SCN neurons during the subjective night (“SN”, ZT 13–16) and the subjective day (“SD”, ZT 6–10), respectively. These figures illustrate the cardinal voltage and concentration dependent inhibition of NMDAR current by [Mg]o; this departs from linearity as the membrane potential becomes more negative and decreases in NMDAR current magnitude as the [Mg]o increases. Quantitatively, the voltage required to elicit the largest inward current becomes more positive as the [Mg]o increases [F(4,88) = 62.64, P < 0.0001], where in solutions containing nominal “0” [Mg]o, the voltage eliciting maximum current is −66.7 ± 2.65 mV (−359.5 ± 37.4 pA) and decreases to −24.6 ± 4.4 mV (−108.1 ± 13.3 pA) at 1,000 μM [Mg]o. Figure 2C shows no effect of [Mg]o on mean outward currents, consistent with the established finding that [Mg]o does not inhibit outward NMDAR currents (Nowak et al. 1984) (P > 0.05). Because these experiments were conducted in series, from “0” to 1,000 μM [Mg]o, desensitization was also inappreciable (Fig. 2C). NMDAR currents do not vary between SD and SN measurements at 50 mV (P > 0.05, Fig. 2C). Figure 2D illustrates [Mg]o curves measured from the SN (black circles) and SD (gray squares) at two voltages, one voltage where [Mg]o strongly blocks the NMDAR (–65 mV) and one voltage where the [Mg]o block is relieved (–25 mV) at higher concentrations (Fig. 2, A and B). The impact of [Mg]o on NMDAR currents, although present at both voltages (P < 0.0001), is most salient at –65 mV, which elicits the largest current response at low [Mg]o but strongly attenuates current as [Mg]o approaches physiological concentrations [F(4,88) = 64.5, P < 0.0001]. Further analysis show no circadian difference between the SD and SN recordings for either −25 or −65 mV (P > 0.05; Fig. 2D). From this data, we constructed [Mg]o curves every 5 mV across a large range of voltages that elicited inward currents (−20 to −75 mV). To further explore the affinity of [Mg]o for NMDARs, the IC50's for [Mg]o were plotted as a function of voltage for the SD and SN means (± SE; Fig. 2E). Consistent with the preceding findings, the [Mg]o affinity for the NMDAR varied strongly with voltage [F(11,240) = 209.3, P < 0.0001] on the natural log-transformed [Mg]o IC50's. Although a tendency exists for NMDAR currents to be inhibited by less [Mg]o across voltages during the SN [4.2 ± 0.2 ln(μM), ∼66 μM, n = 9] than SD recordings [4.8 ± 0.2 ln(μM), ∼121 μM, n = 15], this main effect was not significant (P > 0.05). Analysis of the linear regression of these functions revealed that the proportionality constant, δ, interpreted here as an index of voltage-dependent [Mg]o affinity for the NMDAR, was overall indicative of a predominance of receptors containing NR2A and NR2B subunits (1.26 ± 0.07) (Kuner and Schoepfer 1996). In agreement with the data presented here, δ did not differ between the SD and SN recordings (1.20 ± 0.08 vs. 1.30 ± 0.15, P > 0.05). Although the circadian clock can be reset through NMDAR activation during the SN but not during the SD, these data demonstrate that the sensitivity of the NMDARs within the SCN to [Mg]o do not vary with this rhythmic phenomenon.
Fig. 2.
NMDAR currents are strongly inhibited by [Mg]o within the mouse SCN. The NMDAR I-Vs are plotted as an average (±SE) where each cell was scaled to its outward current at 50 mV, for all [Mg]o conditions in experiments conducted during the subjective night (SN; A, black, n = 9) and subjective day (SD; B, gray, n = 15). In the 0 μM [Mg]o condition, no [Mg]o was added to extracellular solutions. C: outward NMDAR currents at 50 mV are plotted as a function of [Mg]o for the SN (black circle) and SD (gray square). D: inward NMDAR currents at −25 mV (empty) and −65 mV (filled) are plotted as a function of [Mg]o for the SN (black circle, n = 9) and SD (gray square, n = 15). E: the IC50 (natural log) of [Mg]o (in μM) is plotted as a function of voltage (mV) for the SN (black circle, n = 9) and SD (gray square, n = 15). The scale is set to A and B is 20 mV (horizontal bar) and 0.5 arbitrary units (vertical bar).
NR2B subunits contribute significantly to whole cell NMDAR currents within the SCN
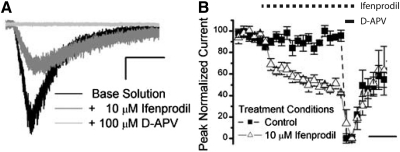
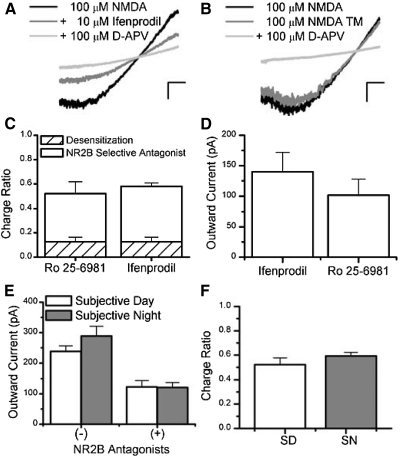
In the present study, we show no circadian differences in NMDAR currents in either the voltage-dependent or -independent measures under conditions where the receptor is activated with 100 μM NMDA and d-serine. From this assay, we have noted a pronounced degree of inhibition by [Mg]o consistent with the presence of NR2A or NR2B containing NMDARs. To dissect the subunit composition of NMDARs, we selected established compounds that could preferentially antagonize the NMDAR in a subtype-specific manner. Ifenprodil is a noncompetitive blocker that has a 180.6- to 429.4-fold preference for NMDARs containing the NR2B-subunit over channels composed of other subunits (Mott et al. 1998; Perin-Dureau et al. 2002; Whittemore et al. 1997; Williams 1993, 1995). We selected 10 μM ifenprodil, which is a 29.4- to 90-fold higher concentration than its IC50 (0.11–0.34 μM) and has been shown to be optimally selective and efficacious (Priestley et al. 1995; Williams 1993, 1995). To measure the contribution of NR2B containing subunits in whole cell NMDAR currents within SCN neurons, we co-administered ifenprodil (10 μM) with NMDA (100 μM) and d-serine (100 μM) in between conditions where total NMDAR currents and those blocked by d-APV (ifenprodil included) were measured. Figure 3A illustrates the NR2B component of the whole cell SCN NMDAR current by the significant decrement in current magnitude as a function of voltage in the presence of ifenprodil. Indeed this finding is reflected in the average outward current at 50 mV pooled across the SD and SN, which decreases from 281.6 ± 27.1 to 124.4 ± 14.5 pA [55.8 ± 3.2% inhibition, paired t-test, t(32) = 9.1, P < 0.0001]. Additionally, we used an ifenprodil-related compound, Ro 25-6981, in similar voltage ramp experiments, which has been reported to be both more potent than ifenprodil (23.5- to 26.7-fold, IC50 0.009–0.017 μM) and to exhibit greater subtype selectivity (3,058- to 5,777-fold for NR2B- over NR2A-containing subunits) (Fischer et al. 1997). Ro 25-6981 (3.5 μM) also significantly reduced the peak outward NMDAR current (at 50 mV) in SCN neurons from 224.6 ± 26.8 to 101.6 ± 26.4 [data not shown, 55.7 ± 9.4% inhibition, paired t-test, t(4) = 5.9, P = 0.0041]. Due to the slow onset of blockade and recovery from inhibition for ifenprodil and Ro 25-6981, we were concerned that the decrement in whole cell NMDA current could be attributed to time-dependent changes in current resulting from prolonged agonist application (Williams 1993). Thus we conducted identical whole cell voltage-clamp experiments but omitted the NR2B-selective antagonists to obtain conservative estimates of the NMDAR desensitization, contemporaneously. Figure 3B illustrates that modest steady-state NMDAR desensitization occurs with peak outward currents reducing from 145.7 ± 22.9 to 119.1 ± 17.3 pA [17.1 ± 3.1% inhibition, paired t-test, t(11) = 3.9, P = 0.0026]. This minor reduction in NMDAR current under control conditions excludes the possibility that the decrement in NMDAR current occurring during the application of NR2B-selective antagonists could be exclusively attributed to desensitization [55.8 (treated) vs. 17.1% (control) reduction]. In Fig. 3C, the contribution of NR2B subunits to the NMDAR complement is summarized as the fraction of d-APV-sensitive current in ifenprodil, Ro 25-6981 or time-matched conditions integrated across voltage (a voltage-independent measure). Specifically, the ifenprodil- and Ro 25-6981-sensitive components account for 0.58 ± 0.03 and 0.52 ± 0.10 of the NMDAR charge ratios in mouse SCN neurons, respectively, which are both significantly greater than the charge ratio decrement attributed to desensitization (control), 0.13 ± 0.04 [1-way ANOVA, F(2,47) = 35.11, P < 0.0001]. These data support a significant presence of NMDAR containing NR2B subunits within the SCN.
Fig. 3.
NMDARs containing NR2B subunits contribute to NMDAR current within the mouse SCN. A: a representative I-V plot derived from a patch-clamped ventral-lateral SCN neuron that was subjected to voltage ramps in the presence of 100 μM NMDA (black), supplemented with 10 μM ifenprodil (charcol) and 100 μM d-2-amino-5-phosphonovaleric acid (d-APV, gray). B: a representative I-V plot from a similar experiment as in A but with ifenprodil omitted, as a time-matched control (TM). C: this bar graph shows the average proportion of the charge (±SE) blocked by NR2B-selective antagonists, Ro 25-6981 (3.5 μM, n = 5) and ifenprodil (10 μM, n = 33), and the degree of desensitization that occurred in SCN neurons in similar “0” [Mg]o solutions omitting these antagonists (hatched bars, n = 12). D: this bar graph shows the mean outward NMDAR currents at 50 mV (±SE) for cells treated with ifenprodil (10 μM, n = 6) or Ro 25-6981 (3.5 μM, n = 5) recorded during the SD. E: the average outward NMDAR currents (±SE) treated with NMDA alone (minus) and with NR2B antagonists (plus) are compared during the SD (n = 11) and SN (n = 27) recordings. F: the charge ratio of the integrated NMDAR current sensitive to NR2B antagonists relative to the total integrated NMDAR current is plotted for SD (n = 11) and SN (n = 27) recordings. The scale is set to A and B is 20 mV (horizontal bar) and 100 pA (vertical bar).
To further reduce the impact of NMDAR desensitization on our recordings, we examined the degree of ifenprodil blockade on NMDAR currents during brief exposures of NMDA. In these experiments, we administered NMDA (100 μM) and d-serine (100 μM) on the cell from a pipette driven by a pneumatic pump, in [Mg]o -free recording solutions with or without ifenprodil. Figure 4A exemplifies the marked reduction in NMDAR current that occurs in the presence of ifenprodil in SCN neurons voltage-clamped to –70 mV. As shown in Fig. 4B, this reduction in normalized mean currents occurs slowly overtime, reaching its maximal effect in 9 min (49.7 ± 6.4% of baseline current). In contrast to our ramp experiments, acute time-matched controls do not significantly desensitize [92.8 ± 4.7% of baseline current, 1-sample t-test, t(4) = −1.20, P = 0.30]. The average integral of the normalized peak NMDAR current overtime (0–20 min) is significantly greater in control conditions (1,651.0 ± 50.1 min-1) than in ifenprodil-treated experiments [1,140.9 ± 100.0 min-1; 2-sample independent t-test, t(4) = 4.56, P = 0.01]. These data suggest that ifenprodil-sensitive currents account for a significant portion of the NMDAR current within the SCN.
To further characterize the NMDAR currents sensitive to NR2B-selective antagonists, we compared measurements taken during the SD (ZT 6–10) and SN (ZT 13–18). Because there were no significant differences between ifenprodil and Ro 25-6981 during the SD recordings in either charge ratio [ifenprodil = 0.52 ± 0.07 Ro 25-6981 = 0.52 ± 0.10; 2-sample t-test, t(9) = −0.04, P = 0.97] or outward current at 50 mV [ifenprodil: 157.1 ± 17.2 pA, Ro 25-6981: 123.1 ± 20.9 pA, t(36) = 0.75, P > 0.05)], we pooled these data (Fig. 3D). Although NR2B antagonists reduced NMDAR currents significantly [group means, NMDA = 274.1 ± 23.9 pA, + NR2B antagonist = 121.4 ± 13.0, F(1,36) = 73.99, P < 0.001, Scheffé, P < 0.001], no circadian differences were evident [groups means: SD = 180.5 ± 18.5 pA, SN = 204.5 ± 21.5 pA, F(1,36) = 0.38, P > 0.05, 2-way (1-factor) repeated measures ANOVA, Fig. 3E]. Consistent with these findings, NMDARs composed of NR2B subunits contribute >50% of the NMDAR charge transferred in the SCN, during both the SD (0.52 ± 0.05) and SN [0.59 ± 0.03, 2-sample independent t-test, t(36) = −1.19, P = 0.24, Fig. 3F]. From this data we conclude that NR2B subunits contribute substantially to NMDAR currents in the SCN but do not exhibit a circadian rhythm.
Ifenprodil-insensitive current exhibits reduced [Mg]o affinity
Based on the work presented so far, we have shown a steep voltage-dependent [Mg]o-affinity of whole cell NMDAR current; a pattern anticipated from neurons composed of NR2A and/or NR2B subtypes (Cull-Candy and Leszkiewicz 2004). Consistent with this notion, we have shown that ifenprodil and Ro 25-6981 inhibit NMDAR currents, supporting a prevalence of NR2B subtypes. Thus one would predict that a strongly voltage-dependent profile with respect to [Mg]o would remain after eliminating current carried through NMDARs containing NR2B, if NR2A containing receptors contribute to the remaining current. To test this conjecture, NMDAR currents were recorded during [Mg]o-titration experiment with “0”, 1, 10, 100, 1,000 μM [Mg]o in the presence of 10 μM ifenprodil or 0.5 μM Ro 25-6981. Current-voltage averages of SCN neurons collected during the SN (Fig. 5A) and SD (B) at various [Mg]o concentrations demonstrate that ifenprodil-insensitive and Ro 25-6981-insensitive (data not shown) inward currents rectify in a voltage- and [Mg]o-dependent fashion, exhibiting little [Mg]o blockade at low concentrations and considerable blockade at 1,000 μM. The voltage required to elicit the largest inward current becomes more positive as [Mg]o increases, exhibiting a similar linear relationship from 10 to 1,000 μM between antagonists (data not shown). In fact, similar current magnitudes remain after 10 μM ifenprodil or 0.5 μM Ro 25-6981 was used (Fig. 5, C and D). The outward currents measured at 50 mV show, as expected, no [Mg]o dependence, suggesting inappreciable desensitization during this titration series for each blocker. Additionally, the outward currents recorded in the presence of NR2B antagonists were about half of those measured in their absence (compare Figs. 2C and 5C). The inward currents showed significant [Mg]o-dependence at −25 mV [F(4,64) = 38.2, P < 0.0001] and −65 mV [F(4,64) = 63.97, P < 0.0001], which maintained maximum current amplitudes until 100 and 10 μM, respectively (Fig. 5D). Consistent with the preceding findings, the affinity of [Mg]o for the NR2B antagonist-insensitive NMDAR current varied with voltage [F(11,176) = 136.76, P < 0.0001] on the natural log-transformed [Mg]o IC50s. As suggested by the overlapping curves for Ro 25-6981 SD, ifenprodil SD, and ifenprodil SN, the [Mg]o affinities did not differ across treatment groups (P > 0.05, Fig. 5E). Further, the proportionality constant, δ, did not differ significantly between the ifenprodil (0.81 ± 0.05) and Ro 25-6981 (0.66 ± 0.08) recordings [2-sample t-test t(17) = 1.10, P > 0.05]. These data show that Ro 25-6981 and ifenprodil blockade reveals NMDAR currents of similar magnitude and [Mg]o sensitivity.
Fig. 5.
NMDAR currents insensitive to NR2B blockers are weakly inhibited by [Mg]o within the mouse SCN. The NMDAR I-Vs are plotted as a mean (±SE) where each cell was scaled to its outward current at 50 mV, for all [Mg]o conditions treated with 10 μM ifenprodil in experiments conducted during the SN (A, black, n = 9) and SD (B, gray, n = 7). In the “0” μM [Mg]o condition, no [Mg]o was added to extracellular solutions. C: outward NMDAR currents at 50 mV are plotted as a function of [Mg]o for the 10 μM ifenprodil-treated, SN (black, n = 7) and SD (gray, n = 9) and 0.5 μM Ro 25-6981-treated (SD, white, n = 3) conditions. D: inward NMDAR currents at −25 mV (diamond) and −65 mV (triangle) are plotted as a function of [Mg]o for the 10 μM ifenprodil-treated, SD (gray, n = 9) and SN (black, n = 7), and 0.5 μM Ro 25-6981-treated (SD, white, n = 3) conditions. E: the mean IC50 (natural log) of [Mg]o (±SE) is plotted as a function of voltage (mV) for the 10 μM ifenprodil-treated, SD (gray) and SN (black), and 0.5 μM Ro 25-6981-treated (SD, white) conditions. The scale is set to A and B is 20 mV (horizontal bar) and 0.5 arbitrary units (vertical bar).
Consistent with our previous ifenprodil and Ro 25-6981 experiments (Fig. 3), there were no differences between the SD and SN revealed in these [Mg]o-titration experiments (Fig. 5). There was no circadian difference in outward or inward currents (P > 0.05, Fig. 5, C and D) or [Mg]o-affinity (P > 0.05, E). Although a tendency exists for ifenprodil-insensitive NMDAR currents to be inhibited by more [Mg]o across voltages during the SN recordings [5.81 ± 0.13 ln(μM), ∼334 μM, n = 7], as shown by the upward shift in IC50 curves, when compared with the SD [5.26 ± 0.15 ln(μM), ∼193 μM, n = 9], this main effect was not significant (P > 0.05). During the SD, Ro 25-6981-treated cells exhibited voltage-dependent [Mg]o affinities that were similar to those recorded in the presence of ifenprodil, regardless of the circadian time of their recordings [P > 0.05, 5.78 ± 0.18 ln(μM), ∼324 μM, n = 3]. Further, the proportionality constant did not differ significantly among the ifenprodil SD (0.87 ± 0.06), ifenprodil SN (0.76 ± 0.08), or Ro 25-6981 SD (0.66 ± 0.08) recordings (P > 0.05). These data show a quantitatively consistent pattern of [Mg]o inhibition in currents remaining when NR2B-selective antagonists are used; this occurs independent of the light-induced phase-resetting rhythm within the SCN.
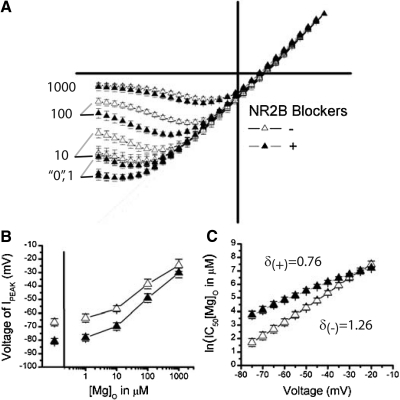
When considering these experiments in comparison to the work presented earlier, several striking differences exist. The I-V relationships show less [Mg]o rectification in the experiments where NR2B antagonists were used (Fig. 5, A and B) when compared with those where they were not (Fig. 2, A and B). This effect is revealed when mean I-Vs are normalized to the maximum outward current and displayed together (Fig. 6A). One can see that NR2B-antagonist-treated SCN neurons approach linearity under low Mg (“0”, 1, and 10 μM) conditions, deviating more at hyperpolarized voltages, when compared with nontreated conditions. Further, a blunted, less parabolic shape is evident in the ifenprodil-treated I-Vs when compared with the untreated conditions, also indicating a decreased affinity of NMDARs for [Mg]o. This is illustrated in Fig. 6B where the voltage required to elicit the peak inward current reached a plateau at approximately −81 mV in NR2B-treated NMDARs, which is close to the maximum voltage of −90 mV that would always elicit the largest inward current if no rectification occurred (linear I-V). This finding is in stark contrast with NMDAR currents recorded without NR2B-selective blockers, showing more extensive rectification at more hyperpolarized voltages (approximately −67 mV at 0 [Mg]o, Fig. 6B). When compared between groups at each [Mg]o, NR2B-antagonist-treated cells pass a larger fraction of current compared with their untreated cohort, most salient when comparing “0”, 1, and 10 μM [Mg]o conditions (Fig. 6A). In Fig. 6C, the ln(IC50) quantifies the extent of [Mg]o inhibition across voltage, showing significant main effects for ifenprodil treatment [F(1,41) = 14.95, P = 0.0004], voltage [F(11,451) = 353.50, P < 0.0001] and for their interaction [F(11,451) = 22.67, P < 0.0001]. Thus NMDAR currents require more [Mg]o on average to achieve a similar level of inhibition with ifenprodil [5.61 ± 0.09 ln(μM), ∼273 μM, n = 19], as shown by the upward shift in ln(IC50) curves, when compared with current recorded without ifenprodil [Fig. 6C, 4.58 ± 0.13 ln(μM), ∼98 μM, n = 24]. Additionally, the amount of [Mg]o needed depends on voltage, requiring larger incremental increases in [Mg]o as voltages approach the reversal potential; an effect more pronounced for antagonist-treated (δ = 0.79 ± 0.05, n = 19) than untreated neurons (δ = 1.25 ± 0.07, n = 24). These data reveal a distinct profile of [Mg]o inhibition between NMDAR currents with and without NR2B contribution, suggesting the presence of a receptor species less sensitive to [Mg]o.
Fig. 6.
NMDAR currents exhibit less [Mg]o block in the presence of NR2B blockers than in their absence. A: the scaled mean I-V curves (±SE) are superimposed to contrast recordings in the presence (+) and absence (−) of NR2B antagonists for 0, 1, 10, 100, 1,000 μM [Mg]o. B: the average voltage that elicited the peak inward current is plotted as a function of [Mg]o for cells treated with (+, n = 19) or without (−, n = 24) NR2B antagonists. C: the mean IC50 (natural log) of [Mg]o (±SE) is plotted as a function of voltage (mV) for conditions with (+, n = 16) or without (−, n = 24) NR2B antagonists. The proportionality constants are displayed for their respective conditions.
Triheteromeric NMDARs, containing NR2A subunits, significantly contribute to whole cell NMDAR currents in the SCN
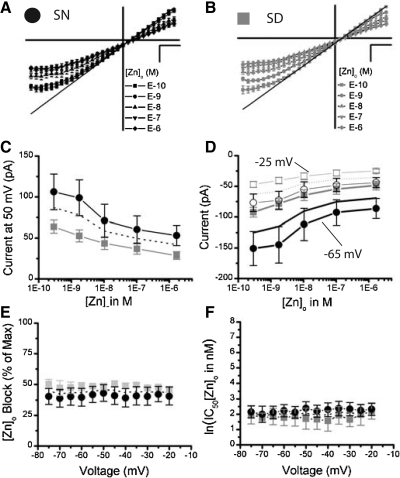
To examine if the SCN contains NMDARs with NR2A subunits, we performed zinc titration experiments as [Zn]o selectively inhibits current passed through NR2A NMDAR subtypes with >100-fold affinity over other NMDARs (Paoletti et al. 1997). To do this, each SCN neuron was subjected to voltage ramps in a series of E-10, E-9, E-8, E-7, E-6 M [Zn]o (see methods) as well as one condition intended to remove background currents (E-6 [Zn]o minus the 100 μM d-APV). All conditions were conducted in 10 μM ifenprodil to remove NR2B containing receptors. Using ifenprodil and [Zn]o jointly improves the selectively of [Zn]o for NR2A from ∼100- to 1,000-fold with respect to other subtypes because [Zn]o is a more potent inhibitor of NR2B (IC50 ∼0.7 μM) than NR2C (IC50 ∼15–18 μM) or NR2D (IC50 ∼9.2 μM) (Paoletti et al. 1997; Rachline et al. 2005). Nanomolar [Zn]o blocks NMDAR current and does not exhibit a voltage-dependent block until low affinity binding sites are sufficiently occupied, which occurs in the micromolar range (Paoletti et al. 1997). Consistent with high-affinity occupancy, the normalized average I-Vs show a uniform concentration-dependent reduction in both inward and outward currents (Fig. 7, A and B). These experiments included 1 μM [Mg]o that conferred the minor inward rectification across all [Zn]o conditions at hyperpolarized voltages (Fig. 7, A and B). The average outward currents at 50 mV showed significant [Zn]o-dependence [F(4,36) = 23.65, P < 0.0001, Fig. 7C, dotted line], which precluded this parameter as an internal control for desensitization. For inward current, there was also a significant effect of [Zn]o at both −25 mV [F(4,36) = 28.69, P < 0.0001, dotted line] and −65 mV [F(4,36) = 24.08, P < 0.0001, solid line Fig. 7D]. In contrast to [Mg]o, [Zn]o does not completely block diheteromeric NR2A NMDARs at the nanomolar concentration used here (Paoletti et al. 1997) (Fig. 7, A and B). Thus an additional free parameter that accounted for the magnitude of inhibition was used to fit the concentration response curves for [Zn]o at voltages between −75 to −20 mV (see methods). Neither the magnitude of block (Fig. 7E, dotted line) nor the affinity of [Zn]o block (Fig. 7F, dotted line), varied with voltage (P > 0.05). However, [Zn]o decreased the ifenprodil-insensitive currents by 44.2 ± 1.12% (Fig. 7E) and the ln(IC50) for [Zn]o was 2.05 ± 0.09, equating to 7.77 nM (Fig. 7F). We show here that NR2A subunits contribute significantly to NMDAR current in SCN neurons through a pharmacologically distinct method using a noncompetitive NR2A-subtype selective antagonist.
Fig. 7.
NMDAR currents insensitive to NR2B blockers are significantly reduced by the NR2A antagonist, [Zn]o, within the mouse SCN. The NMDAR I-Vs are plotted as a mean (±SE) where each cell was scaled to its largest outward current at 50 mV, for all [Zn]o conditions (E-6, E-7, E-8, E-9, E-10 M) treated with 10 μM ifenprodil and 1 μM [Mg]o in experiments conducted during the SN (A, n = 6) and SD (B, n = 5). C: the mean outward NMDAR currents (±SE) at 50 mV are plotted as a function of [Zn]o for the SN (black circles, n = 6) and SD (gray squares, n = 5) conditions. D: the mean inward NMDAR currents (±SE) at −25 mV (empty) and −65 mV (filled) are plotted as a function of [Zn]o for the SN (circle, n = 6) and SD (square, n = 5). E: the mean percent of the total NMDAR current blocked by [Zn]o (±SE) is plotted as a function of voltage (mV). F: the mean IC50 (natural log) of [Zn]o (±SE) is plotted as a function of voltage (mV) for the SN (black circles) and SD (gray squares) conditions. The mean IC50 for [Zn]o across voltage is 9.3 and 6.2 nM for the SN and SD recordings, respectively.
This study shows a trend of larger NMDAR currents recorded during the SN than the SD, which is both consistent (Figs. 3 and 7) and inconsistent (Figs. 2 and 5) with our previous studies. Although this may appear the case, there was no significant difference in outward or inward current magnitude between the SD (gray squares) and SN (black circles; P > 0.05, Fig. 7, C and D). The proportion of [Zn]o blockade or affinity also do not differ significantly with circadian time (P > 0.05, Fig. 7, E and F). Thus it appears that although the NMDARs may vary in numbers between neurons within the SCN and between studies, their subtype compositions are remarkably similar.
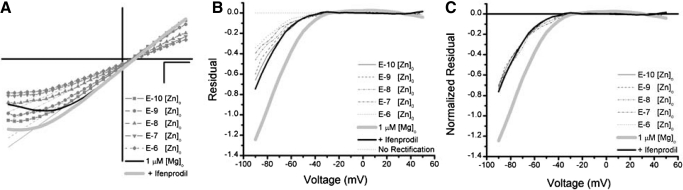
Diheteromeric NMDARs composed of NR2A, like NR2B, are strongly inhibited by [Mg]o. We demonstrated a significant difference between currents recorded in the presence and absence of NR2B blockers (Fig. 6), where in the former case the [Mg]o rectification was reduced to the profile anticipated by NR2C and/or NR2D containing subunits. Paradoxically, these same ifenprodil-insensitive currents, showed significant sensitivity to nanomolar concentrations of [Zn]o (∼44% block; Fig. 7) suggestive of a population receptors bearing NR2A subunits. To test these competing hypotheses, we examined the difference in [Mg]o sensitivity in the presence of [Zn]o and ifenprodil at 1 μM [Mg]o. Given the reduced sensitivity of ifenprodil-insensitive NMDAR currents to [Mg]o, one would predict a further decrease in the degree of [Mg]o block in the current remaining once [Zn]o is applied due to the strong sensitivity of NR2A subunits to both divalents (Hatton and Paoletti 2005). In contrast, if [Zn]o blocked triheteromers of NR2A complexed with NR2C or NR2D, although it would retain its potency, no significant change in [Mg]o affinity would be expected in the current recorded with both divalents. At 1 μM [Mg]o, inward currents recorded with or without NR2B antagonists diverged at voltages hyperpolarized beyond approximately −40 mV (Fig. 6A). For comparison, these curves are superimposed on the averaged currents recorded during the [Zn]o-titration experiments (Fig. 8A, light gray and black lines), all normalized to the peak outward current at 50 mV (E-10 [Zn]o). To quantify the sensitivity to 1 μM [Mg]o, linear fits were taken for each [Zn]o curve to represent the theoretical zero [Mg]o condition. The difference between each curve and their theoretical zero line is plotted as a function of voltage in Fig. 8B. As expected, the more pronounced the [Mg]o block, the larger the difference between the curves, producing functions highly nonlinear with voltage. Consistent with the data presented in Fig. 6, the differences were significantly smaller in the presence of ifenprodil when compared with experiments were this antagonist was omitted. The difference between the curves diminished when recorded in [Zn]o, appearing to have a concentration dependent decrease in [Mg]o sensitivity. However, when these differences where normalized to outward current at 50 mV, as [Zn]o blocks inward and outward currents uniformly (Fig. 7), this trend vanished (Fig. 8C). Further, the degree of [Mg]o rectification recorded in ifenprodil did not change with [Zn]o but were markedly different from data where ifenprodil was omitted (Fig. 8C). Because the [Mg]o-sensitivity did not decrease once NR2A subunits were blocked, these data strongly support the hypothesis that NR2A subunits are incorporated in NMDARs containing [Mg]o-hyposensitive NR2 subunits, NR2C and/or NR2D.
Fig. 8.
The NMDARs remaining after ifenprodil and [Zn]o are weakly inhibited by [Mg]o within the mouse SCN. A: the scaled mean I-V represents each [Zn]o condition, plotted with respective linear regressions of the outward currents, of all SN and SD experiments (n = 11). For comparison, the mean I-Vs of previous 1 μM [Mg]o experiments recorded with (charcoal line) and without (gray line) NR2B blockers are also displayed. B: the difference between the linear regression and the respective curve fit (Residual) is plotted as a function of voltage for each [Zn]o condition and previous 1 μM [Mg]o recorded with (charcoal line) and without (gray line) NR2B blockers. The “no rectification” curve represents the case were no difference between the linear regression and the experimental data exists. C: the residuals plotted in B are normalized to their outward current at 50 mV (see A) and plotted as a function of voltage for each [Zn]o condition. The previous 1 μM [Mg]o recorded with (charcoal line) and without (gray line) NR2B blockers are also plotted.
DISCUSSION
Using a combination of pharmacological agents and voltage-clamp protocols, we demonstrate the predominance of NR2A and NR2B subunits arranged in tri- and diheteromeric complexes, respectively, within the SCN. Magnesium strongly blocks SCN NMDARs showing affinities correlating with receptors composed of NR2A or NR2B subunits. A key finding here is the observation that when ifenprodil, a NR2B selective antagonist, is applied the affinity of [Mg]o for the NMDAR is significantly reduced. The great selectively of ifenprodil and its pronounced effect (54.8–59.0% block), suggests a significant portion of NMDARs within the SCN contain NR2B subunits. Further, it unmasked a population that is more insensitive to [Mg]o, such as NMDARs containing NR2C or NR2D subunits. Interestingly, when this remaining current was treated with [Zn]o, which has a strong selectivity for the NR2A containing subunits, the ifenprodil-insensitive current was reduced by 44%, and it did not impart a further decrease in [Mg]o affinity expected if diheteromeric NR2A subunits were blocked. Rather no change in [Mg]o affinity was observed. Considering this and the fact that the block of [Zn]o is incomplete for diheteromeric NR2A subtypes and even less efficacious for triheteromeric ones (Hatton and Paoletti 2005), this evidence supports triheteromeric receptors containing NR2A and excludes significant contributions from alternative combinations. The discussion in the following text will elaborate on these experiments and the stoichiometry for NMDAR subtypes within the SCN.
Magnesium block distinguishes NMDAR subtypes within the SCN
Extracellular magnesium confers a voltage- and concentration-dependent block to NMDARs that reduces inward current (Cull-Candy and Leszkiewicz 2004). Previous work has categorized NMDAR receptors into strongly rectifying subtypes, such as diheteromeric NR2A or NR2B channels, and weakly rectifying subtypes, such as NR2C or NR2D channels (Kuner and Schoepfer 1996). We collected data exclusively at subphysiologic concentrations, spanning 3 log units, which confer a broad range of inhibition where these NMDAR classes can be distinguished in voltage-clamp experiments. From these recordings, the classic inhibition by [Mg]o was observed that decreased inward current with concentration and imparted no inhibition on outward currents (Figs. 2, C and D, and 5, C and D). We analyzed the interaction between voltage and the affinity of [Mg]o for the NMDAR, showing that it decreases significantly as voltage increases; denoting the depolarization-dependent relief of [Mg]o blockade essential for its activity under physiologic [Mg]o conditions (Figs. 2E and 5E).
We used the parameter δ in our analyses that describes how steeply the extent of block depends on voltage (Woodhull 1973) as an aid to distinguish NMDAR subtypes on the basis of [Mg]o block characteristics. Previous work has estimated δ values for diheteromeric NMDARs derived from NR2A or -B diheteromers to be ∼1.0–1.1, and for NR2C and D diheteromers to be ∼0.7–0.8 (Kuner and Schoepfer 1996). We found that δ changed with ifenprodil application from 1.26 before to 0.76 after. The magnitude of our estimates is higher than expected, which we ascribe to our experimental conditions. For one, we may have underestimated our actual [Mg]o levels, such that the percent of block that we achieved may reflect higher [Mg]o levels resulting from basal [Mg]o that could not be removed from our slice preparations. Higher background levels of [Mg]o could disproportionately impact the lower concentrations (0, 1, and 10 μM) and inflate the affinity of [Mg]o for the NMDAR. The later possibility is likely as our nominal “0” [Mg]o condition exhibited more rectification, where a linear relationship is observed when no [Mg]o is present (Kirson et al. 1999). Another reason could be the high concentration of intracellular cesium (>125 mM) included in our pipette. High [Cs]i levels increase the [Mg]o IC50 and the voltage dependence compared with conditions were [Cs]i is lower (Qian and Johnson 2006). Despite the impact of these issues on our values, our findings are in the range of published estimates and, more importantly, show a change in the steepness of the [Mg]o-voltage relation once ifenprodil is applied. The voltage-dependent study of the [Mg]o block in combination with other techniques and inhibitors was useful in reducing the subtype possibilities and may serve future investigators addressing similar issues.
Diheteromeric NR2B receptors are a major NMDAR subtype within the SCN
Phenylethanolamines (ifenprodil and Ro-25 6981) bind the N-terminal domain (NTD) of the NR2B subunit (Perin-Dureau et al. 2002); this results in the inhibition of the receptor, presumably by inducing a shift in its proton sensitivity (Mott et al. 1998). When comparing diheteromeric NMDARs, ifenprodil preferentially inhibit NMDARs composed of NR2B subunits than NR2A, NR2C, or NR2D by >180-fold while Ro 25-6981 exhibits a >3058-fold NR2B-selectivity (Fischer et al. 1997; Hatton and Paoletti 2005; Priestley et al. 1995; Williams 1993, 1995). Hatton and Paoletti showed that the potency of ifenprodil is retained if at least one NR2B-NTD is present, such that diheteromers (NR2B/B) and triheteromers (NR2A/B) show submicromolar affinities (0.14 and 0.80 μM IC50's, respectively) (Hatton and Paoletti 2005). In contrast, the efficacy of ifenprodil exhibits a superadditive relation where binding of both NR2B-NTDs in a diheteromeric receptor is significantly more than doubling the inhibition conferred to a triheteromeric receptor with one NR2B-NTD (Hatton and Paoletti 2005). For example, the blockade conferred by 10 μM ifenprodil reaches a plateau at ∼94% for diheteromeric (NR2B/B) and ∼20% for triheteromeric (NR2A/B) receptors (Hatton and Paoletti 2005). Additionally, the quality of the block differs between receptors with and without NR2B-NTDs. Ifenprodil blockade is voltage independent for NR2B receptors at low micromolar-submicromolar concentrations (Mott et al. 1998; Williams 1993, 1995). The blockade of non-NR2B NMDARs has significantly reduced affinity with a voltage-dependent block similar to [Mg]ο, bearing strong inward and minimal outward rectification (Williams 1993).
We demonstrate that the NMDAR currents within SCN neurons are highly sensitive to NR2B phenylethanolamine antagonists, ifenprodil and Ro 25-6981. In fact, throughout this study, we found that both ifenprodil and Ro 25-6981 reduced outward NMDAR current by ∼54.8–59.0% or charge ratio, a voltage-independent measure, by 52–61% (see Figs., 3C, 4, B and E, and 8B). The concentrations chosen for this study were based on previous reports that suggested maximum efficacy and selectivity of NR2B-subtypes from receptors composed of other subtypes (Fischer et al. 1997; Priestley et al. 1995; Williams 1993, 1995). To avoid concern that 3.5 μM Ro 25-6981 could block significant non-NR2B receptors, we also conducted some experiments at 0.5 μM. Examination of these currents revealed that this change in concentration was not reflected in the current remaining (+50 mV; 3.5 μmml: 101.6 ± 26.4 pA vs. 0.5 μmml: 109.7 ± 33.8 pA; compare Ro 25-6981, Fig. 3D to 5C “0” [Mg]o condition). In addition, there was no increase in the voltage dependence between concentrations that would be expected if receptors composed of non-NR2B were blocked (Fischer et al. 1997). On the contrary, the current insensitive to these drugs exhibited a voltage-dependent decrease (Fig. 6, “0” μM [Mg]o). Because the profile expected to be present if other NR2 subtypes were inhibited was not evident at the concentrations of phenylethanolamines used here, these concentrations were believed to be specific.
Our data support a population of NR2B diheteromers within the SCN. The phenylethanolamine concentrations used here do not block NR2B diheteromers completely (∼94% block). Thus if ifenprodil blocked 54.8–59.0% of the NMDAR current, then 58.3–62.8% of total NMDAR current is expected to be attributed by these receptor subtypes. However, if NR2B form triheteromers with another NR2 subunit, then these concentrations block significantly less. Hatton and Paoletti (2005) show with triheteromeric receptors composed of NR2B and NR2A (mutant N614KT690I) that ifenprodil at 10 μM block just over 20% as compared with 94% for diheteromeric NR2B receptors (Hatton and Paoletti 2005). If this efficacy holds for nonmutated triheteromers, a predominance of this organization is not feasible. In fact, any efficacy less than the percent blocked would implicate for a homogenous triheteromeric NR2B receptor population a total current exceeding 100% of the NMDAR current (∼20% efficacy would require a total current of 274–295%). Also, if the triheteromeric receptors exist within the SCN, they are unlikely to be composed with NR2A subunits. Primarily, the [Mg]o sensitivity of the current remaining after NR2B blockade exhibits significantly reduced affinity for [Mg]o (Figs. 5 and 6); this is consistent with NR2C and NR2D pharmacology not with NR2B and/or NR2A diheteromeric or NR2A/B triheteromeric NMDARs (Kuner and Schoepfer 1996). Further, when [Zn]o and ifenprodil were used together in 1 μM [Mg]o solutions, no change in [Mg]o rectification occurred (Fig. 8). It has been suggested that when [Mg]o- and [Zn]o-sensitive subunits (NR2A) are coexpressed with subunits less sensitive to [Mg]o and [Zn]o (an NR2A mutant akin to NR2C and NR2D), the reduced [Mg]o dependence in the current remained after [Zn]o block (Hatton and Paoletti 2005), supporting a dominant effect of [Mg]o-hyposensitive subunits within triheteromeric complexes. Although we cannot exclude the possibility that a small fraction of the current blocked with these drugs affected triheteromeric receptors, including NR2B subunits that were too small to detect with our [Mg] o assay, we believe this contribution is insignificant. Due to the profound block of the phenylethanolamines and the change in [Mg]o in the current remaining, we believe that a significant portion of the NMDAR within the SCN are NR2B diheteromers and are not triheteromeric NR2B receptors complexed with NR2A, -C, or -D.
Our data suggest a predominance of diheteromeric NR2B NMDARs within the SCN. Previous work has given conflicting accounts of NR2B levels within the SCN. Numerous experiments examining NR2B transcript have been conducted with levels varying from strong (O'Hara et al. 1995; Wang et al. 2008) to weak (Mikkelsen et al. 1993; Moriya et al. 2000a,b) to absent (Gannon and Rea 1994; Watanabe et al. 1993). Reports on NR2B protein products have varied similarly from strong (Wang et al. 2008) to light (Petralia et al. 1994). Although reports have varied among immunohistochemical, in situ hybridization, Northern and Western blot assays, phenylethanolamines have consistently shown significant NR2B contribution to SCN NMDAR currents. In addition to our study, recent work has shown with 3 μM ifenprodil a 47 ± 5% reduction in whole cell currents (Wang et al. 2008) and a 40 ± 7% in evoked excitatory postsynaptic currents (Kim et al. 2006). Although it was not discussed in these studies, the substantial blockade observed precludes triheteromeric NR2B receptors as the dominant species within the SCN. Our studies using phenylethanolamines in combination with [Zn]o and [Mg]o pharmacology more strongly support a dominant diheteromeric assembly of NR2B over a triheteromeric one. In summary, both our work in combination with recent functional work (Kim et al. 2006; Wang et al. 2008) clarifies the early assertion that NR2B is expressed and has significant functional contribution within the SCN as diheteromeric receptor (Mikkelsen et al. 1993; Moriya et al. 2000b; O'Hara et al. 1995).
Triheteromeric receptors with NR2A are present within the SCN
Our studies support the presence of NR2A subtypes in the SCN with the use of the potent and selective blocker [Zn]o. Analogous to blockade of NR2B subunits by phenylethanolamines, [Zn]o confers a high affinity voltage-independent block by binding to the N-terminal domain (NTD) of NR2A subunits (Choi and Lipton 1999; Low et al. 2000; Paoletti et al. 2000). Using a similar analysis employed in our [Mg]o-titration experiments, the [Zn]o-titration experiments blocked ∼44% of the ifenprodil-insensitive NMDAR current in a concentration-dependent manner (Fig. 7E). In combination with ifenprodil, the selectively of [Zn]o for NR2A subtypes increases as [Zn]o preferentially blocks NR2A containing subunits by ∼100-fold for NR2B and ∼1,000-fold for NR2C/D subtypes (Rachline et al. 2005). Further, our estimates of the affinity of [Zn]o for the NMDAR (IC50 ∼7 nM) are in very close accordance to previously published values, 5.2–17.6 nM (Choi and Lipton 1999; Paoletti et al. 1997; Rachline et al. 2005; Williams 1996). Last, our study was conducted at concentrations below those reported to inhibit other NR2 subtypes, and we did not observe the concentration and voltage-dependent inward [Zn]o block characteristic of these subtypes (Paoletti et al. 1997; Rachline et al. 2005) (Figs. 7 and 8). Therefore our work is consistent with the presence of NR2A receptor assemblies within the SCN as determined by the selective and potent blocker [Zn]o.
At first glance, the contribution of the NR2A subunit to NMDAR currents within the SCN may appear modest, based on the limited blockade observed (Fig. 7). However, interpreting these data in the context [Zn]o- and [Mg]o-blockade characteristics, our work suggests that this subunit may govern the function of a larger portion of NMDARs. The blockade of NR2A diheteromeric channels by [Zn]o is incomplete (∼70–80% maximal block) over the submicromolar concentrations (Paoletti et al. 1997; Rachline et al. 2005). Although we have reason to believe differently (see following text), if we assume that [Zn]o blocked a homogenous population of NR2A subtypes with a 70–80% efficacy, then 24.2–28.4% of the total current can be ascribed to NR2A subtypes considering the fraction of ifenprodil-insensitive current remaining (∼44% block of 44.0–45.2% ifenprodil-insensitive current remaining based on Fig. 3). From our [Mg]o-titration studies of the ifenprodil-insensitive current within SCN neurons, we observed a pronounced decrease in [Mg]o affinity consistent with NR2C and NR2D subtypes (Kirson et al. 1999; Kuner and Schoepfer 1996). In our [Zn]o-titration studies, we included [Mg]o, which could distinguish NMDAR subtypes. Although [Zn]o blocked a significant portion of the remaining current, we did not observe an apparent reduction in [Mg]o block that would be expected if the contribution of highly [Mg]o-sensitive subtypes, such as diheteromeric NR2A or triheteromeric NR2A/B, were removed (Fig. 8C). In fact, we did not observe any shift at all, suggesting [Zn]o blocked a homogenous population that was relatively insensitive to [Mg]o. For this reason, we believe [Zn]o blocked triheteromeric NR2A NMDARs in a manner that retained a high [Zn]o affinity (∼7 nM) and voltage independence expected from previous work with this receptor population (Hatton and Paoletti 2005).
As with the efficacy of ifenprodil on NR2B triheteromeric receptors, [Zn]o also exhibits reduced efficacy for NR2A-containing triheteromers. Previous work has estimated the extent of the high-affinity block of triheteromeric NR2A receptors between 17 and 38% with [Zn]o, being 28.7% for NR2A/2C triheteromers(Hatton and Paoletti 2005). Assuming that [Zn]o blocked a homogenous NR2A-containing triheteromeric receptor population, our estimate of 44% is higher than the values previously published. Because the [Zn]o increased in concentration from lowest to highest, we believe that desensitization could have exaggerated the degree of block. Although ∼16% difference appears large, it would account for only 6–8% of the total NMDAR current. Desensitization of this magnitude is reasonable within the conditions of our experiments (Figs. 3C and 5C). As suggested in the preceding text, we discount the possibility that triheteromeric NR2A/B receptors contribute significantly to the [Zn]o-sensitive current. In combination, ifenprodil and [Zn]o are poorly efficacious and, in the order administered here, would block ∼40% of the NR2A/B triheteromeric current (Hatton and Paoletti 2005). The remaining current would exhibit strong [Mg]o-block qualities, which contrasts with our findings (Fig. 8). Because no ligands have been developed that can specifically target triheteromeric subtypes, we did not have the advantage of testing this hypothesis directly. Despite this limitation, we believe that based on the pharmacologic profiles of ifenprodil, [Zn]o, and [Mg]o and their combined impact on the NMDAR currents recorded, that most of the NMDARs remaining after phenylethanolamine blockade incorporate NR2A with NR2C or NR2D subunits.
An important consideration is the concentration of agonists used in this study. Although 100 μM d-serine is saturating for the glycine site at almost 2 log units larger than its EC50 (Preistley et al. 1995), it is equivocal whether 100 μM NMDA is saturating for the glutamate site. The issue is that although 100 μM NMDA is greater than the three times the EC50 for NR2B, -C, and -D diheteromers, reports suggest that NMDA is less potent for NR2A diheteromers, where 100 μM NMDA would equate to 1.06–4.27 times the EC50 (23.4–94.1 μM) (Erreger et al. 2007; Ishii et al. 1993; Mott et al. 1998; Ren et al. 2003). A NMDA concentration that is sub-saturating for NR2A subtypes with respect to other receptor compositions could result in an underestimation of the NR2A component and a relative overestimation of the non-NR2A subtypes. An additional issue is that if the antagonists used to estimate the receptor populations alter the affinity of the agonist, the population of receptors could further be underestimated. Although both of these issues may have influenced our results, we believe there effect is minimal. First, steady-state NMDAR currents, like those measured here, show lower EC50 for NMDA compared with peak NMDAR current measured immediately following NMDAR application (Ren et al. 2003; Zheng et al. 2001). Ren et al. (2003) demonstrate that steady-state currents recorded from NR2A diheteromers exhibit EC50 values for NMDA ∼23 μM that saturate close to 100 μM as compared with peak currents ∼40 μM NMDA that saturated close to 300 μM (Ren et al. 2003). The second issue is the allosteric interaction between N-terminal domain and the glutamate-binding domain of NR2A and -B diheteromeric receptors that results in an apparent increase in glutamate-site agonist potency with NTD-site antagonists, like [Zn] and ifenprodil. Kew et al. (1996) illustrate that the mKd for NMDA is 7.1 μM in the absence of ifenprodil, but when ifenprodil is increased to 1 μM, the mKd for NMDA decreases to 2.1 μM, where <20% of the steady-state current remains (Kew et al. 1996). The difference between the amount of current blocked with and without ifenprodil was decreased by a small fraction for 100 μM NMDA when compared with the hypothetical case where no shift in the apparent affinity would have occurred by the NTD antagonist. The reason is the main effect of the magnitude of block is determined by the extent of activation of the NMDAR (i.e., how much current there is to block). In our studies, we are achieving close to saturating conditions for all receptor subtypes. The shift in potency induced by NTD-antagonists, ifenprodil and Zn, although significant in terms of EC50 for NMDA, would have little impact on our receptor contribution estimates because of the extent of block achieved with the concentration of NTD ligands used.
Previous efforts to demonstrate the presence of NR2A are limited and, as with the literature on NR2B subunits within the SCN, have produced conflicting data. Early experiments have reported NR2A transcript to be relatively small (Moriya et al. 2000b; O'Hara et al. 1995) or absent (Gannon and Rea 1994; Mikkelsen et al. 1993; Watanabe et al. 1993). In contrast, functional experiments have yielded more significant results. Moriya et al. (2000b) demonstrated that the rate of entrainment of the NR2A knockout mice was slowed during low-intensity light. Further, intracellular calcium levels of SCN neurons were reduced in response to saturating concentrations of NMDA (100–300 μM) in comparison to wild-type littermates (Moriya et al. 2000b). One recent study found a concentration of [Zn]o (10 μM) expected to saturate the NTD of the NR2A decreased the NMDAR response of SCN by 19% (Wang et al. 2008). In our study, [Zn]o would have blocked similar proportion of the total NMDAR current (19.3–19.9%) and charge ratio (18.0–21.1%) assuming a similar percentage of current remained after 10 μM ifenprodil as in our earlier studies (Fig. 3). When considered as a whole, this literature conveys a message made explicit in our work that NR2A subunits have a significant functional role in the SCN both in vivo and in vitro despite low transcript levels. When this notion is considered with the fact that the low [Mg]o affinity did not change with [Zn]o, it supports the hypothesis that a limited NR2A subunit expression could account for its more pronounced functional role if expressed as triheteromeric receptor with a [Mg]o-hyposensitive subtype. Additionally, because the extent of blockade in the presence of ifenprodil (Fig. 7) and absence (Wang et al. 2008) are comparable and the relative [Mg]o affinity decreases for ifenprodil-insensitive currents (Fig. 5), it is unlikely that NR2A and NR2B form triheteromers. The experiment performed here and the work of others within field support our hypothesis that NR2A form triheteromeric receptors with NR2C and/or NR2D subunits.
Do NR2C and NR2D subunits contribute to the SCN NMDAR currents?
As described in the preceding text, we believe that NR2B diheteromeric receptors and NR2A triheteromeric receptors account for the vast majority of the NMDARs within the SCN. If this is the case, then what NMDAR subtype(s) could complex with NR2A and account for the reduced [Mg]o affinity? There is a strong consensus among previous studies that NR2C subtypes are localized to the SCN (Ebling 1996). Studies of NR2C transcripts have shown strong signals on Northern blots (O'Hara et al. 1995) and have localized NR2C transcript to the SCN using in situ hybridization (Ishida et al. 1994; Mikkelsen et al. 1993; Moriya et al. 2000a,b). Moriya et al. examined NR2C knockout animals and found that intracellular calcium increases in SCN neurons (100–300 μM NMDA) were blunted in these animals with respect to wild-type, supporting their functional presence in the SCN (Moriya et al. 2000b). Although there is previous support for NR2C, there is very little evidence of NR2D transcripts within the SCN. In two previous studies that reported strong NR2C transcript, NR2D was miniscule (O'Hara et al. 1995) or absent (Moriya et al. 2000a). In conclusion, although our work suggests a population of [Mg]o-hyposensitive NMDARs populate the murine SCN, we did not identify the subtype directly. Previous work supports NR2C subunits as an important subunit within the SCN. Based on this evidence collectively, we believe that NR2A and NR2C form triheteromers within the SCN.
Are NR3 subtypes involved in NMDAR currents within the SCN?
The NR3 NMDAR family is composed of NR3A and NR3B subunits that can form diheteromers (NR1/NR3) or triheteromers (NR1/NR2/NR3). In heterologous expression systems, NR1/NR3 receptor currents rapidly activate (and desensitize) in response to glycine rather than glutamate-site agonists (Chatterton et al. 2002; Smothers and Woodward 2007). In contrast, when NR3 subtypes are expressed with NR1 and NR2, NMDAR currents require both NMDA and glycine to activate but have reduced currents, an additional small single-channel conductance and reduced [Mg]o-block and [Ca]o permeability when compared with NR1/NR2 diheteromeric NMDAR currents (Chatterton et al. 2002; Ciabarra et al. 1995; Das et al. 1998; Perez-Otano et al. 2001; Sasaki et al. 2002; Sucher et al. 1995). Due to the lack of nonspecific ligands and restricted developmental expression, the functional presence of these channels has been difficult to demonstrate in native systems. Although there is some evidence to support glycine-activated NMDAR in neurons (Chatterton et al. 2002), recent advances show that when NR3A expression is induced with tetracycline in transgenic neurons, glycine-activated currents were not recorded (Tong et al. 2008). Rather NR3 subunits appear to form triheteromers with NR1/NR2 subunits that retain NMDA and glycine affinity but decreased [Mg]o block and [Ca]o permeability (Tong et al. 2008). Therefore although NR3 can form functional diheteromeric channels in heterologous expression systems, the triheteromeric NR3 channels appear to be the predominant form in native neurons.
In the context of our study, the contribution of NR1/NR2A/NR3 triheteromeric currents are an attractive explanation because they are activated with glutamate- and glycine-site agonists but exhibit reduced [Mg]o affinity. However, we believe that if NR3 subtypes responded to NMDA and d-serine in our recordings, their contributions were small. First, NMDAR currents were recorded at a developmental period when NR3 transcripts disappear from SCN neurons (P14-21). NR3A transcript levels are low during early development peaking at P10 and disappearing after P14 (Bendova et al. 2009). In contrast, NR3B transcripts levels are highest at E20, dropping to low levels during the early postnatal period and disappearing after P14 (Bendova et al. 2009). Second, when NR3 subunits are at peak levels during development, only 5–8.7% of the NR3 protein immunoprecitate with NR2 subunits (Al-Hallaq et al. 2002). The contribution of glycine-activated NR1/NR3 is more unlikely because we used d-serine rather than glycine, which is a poor agonist of NR1/NR3 diheteromeric receptors (Chatterton et al. 2002), and we defined NMDAR current by d-APV sensitivity, which does not antagonize NR1/NR3 diheteromers. Thus any contribution of NR1/NR3 diheteromers that d-serine activated was subtracted as background. These lines of evidence make it unlikely that current from either NR1/NR3 diheteromers or NR1/NR2/NR3 triheteromers contributed to our findings. Further studies will define the role of NR3 in circadian physiology and its involvement in light entrainment during a development period when newborn pups shift from synchronizing to maternal cues to the solar light-dark cycle.
No significant circadian differences in NMDAR currents
Because light-induced phase resetting is prevented by NMDAR antagonists (Colwell and Menaker 1992) and NMDA can produce a phase-response curve, in vivo and in vitro, that shares striking resemblance to phase-response curves produced by light, in vivo (Ding et al. 1994; Mintz and Albers 1997; Mintz et al. 1999; Shibata et al. 1994), several investigators have examined if these observations are, in part, produced by circadian regulation of the NMDAR. Although several studies have investigated SD and SN differences in NMDAR with electrophysiologic and calcium imaging techniques, the results have been diverse: some have reported SN increases (Colwell 2001; Pennartz et al. 2001) and some SD increases (Ikeda et al. 2003) and other experiments have reported no differences (Ikeda et al. 2003; Pennartz et al. 2001). Similar variation is seen in the circadian expression of NMDAR transcripts, which have been reported to peak during the SD (pan-NR1, NR2C) (Bendova et al. 2009; Ishida et al. 1994) or SN (NR2B) (Wang et al. 2008) or with no circadian variation (NR1-a, NR1-1, NR1-2, NR1-4) (Bendova et al. 2009). Although fewer publications have reported on circadian variation in NMDAR protein, no clear theme has been shown for protein levels, where NMDAR protein levels either peak during the SN (immunohistochemistry NR1) (Bendova et al. 2009) or shown no variation total protein levels (Western blot, NR2B) (Wang et al. 2008). In summary, although circadian rhythms in NMDARs have been shown, a clear, reproducible phenomenon has not been duplicated between investigators.
In our study we found no significant differences in the total NMDAR current recorded during the SD (ZT 5–10) and early SN (ZT 12–16; Fig. 3E). Further, no significant difference was found in the treated or untreated components of [Mg]o (Fig. 2, C and D), [Zn]o (Fig. 7, C and D), or phenylethanolamines (Figs. 3E and 5, C and D) during the SD and SN. We found no daily rhythm to [Mg]o block as reflected in our measurements of inward currents, outward currents, or affinity estimates (Figs 2, C–E). We also found no circadian rhythm in the ifenprodil-sensitive NMDAR current in average outward current, the current proportion or charge ratio with or without [Mg]o (Figs. 3, E and F, and 5, C and D). By implication, we found no circadian rhythm in the portion of the NMDAR current insensitive to NR2B antagonists during the SD and SN (Figs. 3E, 5, C, and D, and 7, C and D [E-10 [Zn]o point]). We also did not observe a consistent, albeit insignificant, trend in our data that may be resolved with greater statistical power. Within our experiments we show a trend in global NMDAR current that is larger during the SN (Fig. 3E), the SD (Fig. 2C), or equivalent (Fig. 2D) for “0” Mg condition. In some phenylethanolamine experiments, the antagonist appeared to block more during the SN (Figs. 3, E and F, and 5, C and D) and other examples blocked more during the SD (Fig. 7, C and D). In summary, we showed no SD/SN rhythms indicative of circadian regulation of the NMDAR.
In the circadian rhythm field, a parsimonious explanation to account for the diverse reports on the circadian patterning of NMDARs is lacking. Several physiological and methodological explanations have been suggested. In our own study, we did not assess the circadian rhythms in our slices with a method independent of our NMDAR current measurements. As a result, although we took standard precautions (see methods), we cannot exclude the possibility that the lack of rhythmicity in our data could have resulted from a change to the circadian clock from a factor that we were unaware of. Previous reports show that the circadian rhythm of the SCN can be altered or abolished with the use of anesthetics (Prosser et al. 1994), blocking calcium influx (Nahm et al. 2005), or following tissue preparation (Gillette 1991). Another possibility is that physiologic factors could account for the differences. One notion is that the neurons involved in phase resetting are distinct from the oscillating neurons that produce output rhythms (Silver et al. 1996). Another possibility is that the phosphorylation state of the NMDAR may be responsible for its circadian activities rather than total NMDAR levels (Wang et al. 2008). Although we cannot exclude the possibility that an important factor will account for the variability in our data, it is clear that a global increase in NMDAR numbers on SCN neurons during the early SN is not evident in total NMDAR, NR2B, non-NR2B or NR2A components under our experimental conditions.
Conclusion
The work presented here offers an explanation to the existing gap in knowledge in the function of SCN neurons. Numerous reports have documented NMDAR modulation by protein factors, such as brain-derived neurotropic factor (Kim et al. 2006; Michel et al. 2006a) and PACAP (Harrington et al. 1999; Kopp et al. 2001; Michel et al. 2006b; Minami et al. 2002), through tyrosine-kinase and protein kinase A modulation. For many years, the prevailing belief is that the NR2C subunits dominated in glutamate-mediated phase resetting (Ebling 1996). Unlike NMDAR composed of NR2A and -B subunits, NR2C subtypes are resistant to intracellular modulation by kinase phosphorylation (Kutsuwada et al. 1992). This study explains the disparity, namely that NR2B are a major NMDAR subtype within the murine SCN and are known to be exquisitely sensitive to modulation. NR2C are likely to be coupled with NR2A, which may also confer a degree of modulation to neurons within the SCN. Future work will examine the role of these receptor subtypes in cellular entrainment and how protein modulators regulate this process.
GRANTS
This work was supported in part by National Eye Institute Grants R01EY-012949 and R21EY-018885 and a McKnight Foundation grant.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Jian Ding and Christopher Colwell for technical and scientific training of J. P. Clark.
Present address of J. P. Clark: Department of Surgical Neurophysiology, University of California, 505 Parnassus Ave, Box 0112, San Francisco, CA. 94143.
REFERENCES
- Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP. Association of NR3A with the N-methyl-d-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol 62: 1119–1127, 2002 [DOI] [PubMed] [Google Scholar]
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol 11: 336–342, 2001 [DOI] [PubMed] [Google Scholar]
- Bendova Z, Sumova A, Mikkelsen JD. Circadian and developmental regulation of N-methyl-d-aspartate-receptor 1 mRNA splice variants and N-methyl-d-aspartate-receptor 3 subunit expression within the rat suprachiasmatic nucleus. Neuroscience 159: 599–609, 2009 [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073, 2002 [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415: 793–798, 2002 [DOI] [PubMed] [Google Scholar]
- Choi YB, Lipton SA. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 23: 171–180, 1999 [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci 15: 6498–6508, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JP, 3rd, Sampair CS, Kofuji P, Nath A, Ding JM. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol 289: R656–662, 2005 [DOI] [PubMed] [Google Scholar]
- Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci 13: 1420–1428, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J Biol Rhythms 7: 125–136, 1992 [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004: re16 2004 [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393: 377–381, 1998 [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266: 1713–1717, 1994 [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci 17: 667–675, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol 50: 109–132, 1996 [DOI] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-d-aspartate glutamate receptors. Mol Pharmacol 72: 907–920, 2007 [DOI] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283: 1285–1292, 1997 [PubMed] [Google Scholar]
- Gannon RL, Rea MA. In situ hybridization of antisense mRNA oligonucleotides for AMPA, NMDA and metabotropic glutamate receptor subtypes in the rat suprachiasmatic nucleus at different phases of the circadian cycle. Brain Res Mol Brain Res 23: 338–344, 1994 [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res 379: 176–181, 1986 [DOI] [PubMed] [Google Scholar]
- Gillette MU. Suprachiasmatic nucleus electrophysiology in vitro: rhythmic activity and endogenous clock properties. In: The Mind's Clock, edited by Klein DC, Moore R, Reppert S. New York: Oxford Univ. Press, 1991, p. 125–143 [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260: 238–241, 1993 [DOI] [PubMed] [Google Scholar]
- Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res 309: 73–88, 2002 [DOI] [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci 19: 6637–6642, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295: 1065–1070, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46: 261–274, 2005 [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci 8: 790–802, 2007 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur J Neurosci 17: 58–70, 2003 [DOI] [PubMed] [Google Scholar]
- Ishida N, Matsui M, Mitsui Y, Mishina M. Circadian expression of NMDA receptor mRNAs, epsilon 3 and zeta 1, in the suprachiasmatic nucleus of rat brain. Neurosci Lett 166: 211–215, 1994 [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem 268: 2836–2843, 1993. [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res 460: 297–313, 1988 [DOI] [PubMed] [Google Scholar]
- Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol 497: 761–772, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Choi HJ, Colwell CS. Brain-derived neurotrophic factor regulation of N-methyl-d-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons. J Neurosci Res 84: 1512–1520, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Schirra C, Konnerth A, Yaari Y. Early postnatal switch in magnesium sensitivity of NMDA receptors in rat CA1 pyramidal cells. J Physiol 521: 99–111, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MD, Meissl H, Dehghani F, Korf HW. The pituitary adenylate cyclase-activating polypeptide modulates glutamatergic calcium signalling: investigations on rat suprachiasmatic nucleus neurons. J Neurochem 79: 161–171, 2001 [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron 5: 127–134, 1990 [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science 255: 1581–1584, 1992 [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci 16: 3549–3558, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature 358: 36–41, 1992. [DOI] [PubMed] [Google Scholar]
- Low CM, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular determinants of coordinated proton and zinc inhibition of N-methyl-d-aspartate NR1/NR2A receptors. Proc Natl Acad Sci USA 97: 11062–11067, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Clark JP, Ding JM, Colwell CS. Brain-derived neurotrophic factor and neurotrophin receptors modulate glutamate-induced phase shifts of the suprachiasmatic nucleus. Eur J Neurosci 24: 1109–1116, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci 7: 15, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen PJ, Ebling FJ. Distribution of N-methyl d-aspartate (NMDA) receptor mRNAs in the rat suprachiasmatic nucleus. Brain Res 632: 329–333, 1993 [DOI] [PubMed] [Google Scholar]
- Minami Y, Furuno K, Akiyama M, Moriya T, Shibata S. Pituitary adenylate cyclase-activating polypeptide produces a phase shift associated with induction of mPer expression in the mouse suprachiasmatic nucleus. Neuroscience 113: 37–45, 2002 [DOI] [PubMed] [Google Scholar]
- Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res 758: 245–249, 1997 [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19: 5124–5130, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Horikawa K, Akiyama M, Shibata S. Correlative association between N-methyl-d-aspartate receptor-mediated expression of period genes in the suprachiasmatic nucleus and phase shifts in behavior with photic entrainment of clock in hamsters. Mol Pharmacol 58: 1554–1562, 2000a [DOI] [PubMed] [Google Scholar]
- Moriya T, Takahashi S, Ikeda M, Suzuki-Yamashita K, Asai M, Kadotani H, Okamura H, Yoshioka T, Shibata S. N-methyl-d-aspartate receptor subtype 2C is not involved in circadian oscillation or photoic entrainment of the biological clock in mice. J Neurosci Res 61: 663–673, 2000b [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1: 659–667, 1998 [DOI] [PubMed] [Google Scholar]
- Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci 25: 9304–9308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature 307: 462–465, 1984 [DOI] [PubMed] [Google Scholar]
- O'Hara BF, Andretic R, Heller HC, Carter DB, Kilduff TS. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res Mol Brain Res 28: 239–250, 1995 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci 17: 5711–5725, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron 28: 911–925, 2000 [DOI] [PubMed] [Google Scholar]
- Paul KN, Fukuhara C, Tosini G, Albers HE. Transduction of light in the suprachiasmatic nucleus: evidence for two different neurochemical cascades regulating the levels of Per1 mRNA and pineal melatonin. Neuroscience 119: 137–144, 2003 [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Hamstra R, Geurtsen AM. Enhanced NMDA receptor activity in retinal inputs to the rat suprachiasmatic nucleus during the subjective night. J Physiol 532: 181–194, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci 21: 1228–1237, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci 22: 5955–5965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci 14: 6102–6120, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol 48: 841–848, 1995 [PubMed] [Google Scholar]
- Prosser RA, Edgar DM, Heller HC, Miller JD. A possible glial role in the mammalian circadian clock. Brain Res 643: 296–301, 1994 [DOI] [PubMed] [Google Scholar]
- Qian A, Johnson JW. Permeant ion effects on external Mg2+ block of NR1/2D NMDA receptors. J Neurosci 26: 10899–10910, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci 25: 308–317, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Honse Y, Karp BJ, Lipsky RH, Peoples RW. A site in the fourth membrane-associated domain of the N-methyl-d-aspartate receptor regulates desensitization and ion channel gating. J Biol Chem 278: 276–283, 2003 [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, Nakanishi N, Sucher NJ, Lipton SA. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol 87: 2052–2063, 2002 [DOI] [PubMed] [Google Scholar]
- Schurov IL, McNulty S, Best JD, Sloper PJ, Hastings MH. Glutamatergic induction of CREB phosphorylation and Fos expression in primary cultures of the suprachiasmatic hypothalamus in vitro is mediated by coordinate activity of NMDA and non-NMDA receptors. J Neuroendocrinol 11: 43–51, 1999 [DOI] [PubMed] [Google Scholar]
- Shibata S, Watanabe A, Hamada T, Ono M, Watanabe S. N-methyl-d-aspartate induces phase shifts in circadian rhythm of neuronal activity of rat SCN in vitro. Am J Physiol Regulatory Integrative Comp Physiol 267: R360–364, 1994 [DOI] [PubMed] [Google Scholar]
- Sica AL, Gootman PM, Ruggiero DA. CO(2)-induced expression of c-fos in the nucleus of the solitary tract and the area postrema of developing swine. Brain Res 837: 106–116, 1999 [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport 7: 1224–1228, 1996 [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-d-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther 322: 739–748, 2007 [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci 15: 6509–6520, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci Lett 176: 59–62, 1994 [DOI] [PubMed] [Google Scholar]
- Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, Lipton SA, Nakanishi N. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol 99: 122–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Schroeder A, Loh D, Smith D, Lin K, Han JH, Michel S, Hummer DL, Ehlen JC, Albers HE, Colwell CS. Role for the NR2B subunit of the N-methyl-d-aspartate receptor in mediating light input to the circadian system. Eur J Neurosci 27: 1771–1779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-d-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol 338: 377–390, 1993 [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Ilyin VI, Woodward RM. Antagonism of N-methyl-d-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition. J Pharmacol Exp Ther 282: 326–338, 1997 [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44: 851–859, 1993 [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-d-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neurosci Lett 184: 181–184, 1995 [DOI] [PubMed] [Google Scholar]
- Williams K. Separating dual effects of zinc at recombinant N-methyl-d-aspartate receptors. Neurosci Lett 215: 9–12, 1996 [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol 61: 687–708, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Erreger K, Low CM, Banke T, Lee CJ, Conn PJ, Traynelis SF. Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci 4: 894–901, 2001 [DOI] [PubMed] [Google Scholar]