Abstract
Integrins are a large family of heterodimeric transmembrane receptors that mediate cell-substratum adhesion. αvβ6 is an epithelial-specific integrin that is a receptor for the extracellular matrix (ECM) proteins fibronectin, vitronectin, tenascin and the latency associated peptide (LAP) of TGF-β. Integrin αvβ6 is not expressed in healthy adult epithelia but is upregulated during wound healing and in cancer. αvβ6 has been shown to modulate invasion, inhibit apoptosis, regulate the expression of matrix metalloproteases (MMPs) and activate TGF-β1. There is increasing evidence, primarily from in vitro studies, that suggest that αvβ6 may actually promote carcinoma progression. In this review we summarize what has been learnt in the past few years about the role of αvβ6 in cancer progression.
Keywords: Integrin αvβ6, cancer, MMPs, TGF-β1, EMT
INTRODUCTION
Integrins are heterodimeric trans-membrane receptors comprising alpha and beta subunits that function both as cell anchoring and signaling molecules [1]. There are 18 alpha and 8 beta subunits, each of which can bind to several partners giving rise to at least 24 distinct integrin heterodimers with different functions and ligand binding activities (Fig. (1)). Integrins serve as anchoring molecules by mediating the adhesion of the cellular cytoskeleton to the extracellular matrix (ECM). They also serve as bidirectional signaling molecules by mediating outside-in and inside-out signaling thereby controlling a variety of vital cell functions like adhesion, polarity, differentiation, migration and cell division [2]. These cell functions are critical during embryogenesis and help to maintain tissue homeostasis under normal physiological conditions. Under pathological states, defective integrin signaling can disrupt these functions resulting in abnormal cell division, migration and adhesion, which are hallmarks of cancer and metastasis [3, 4].
Fig. (1).
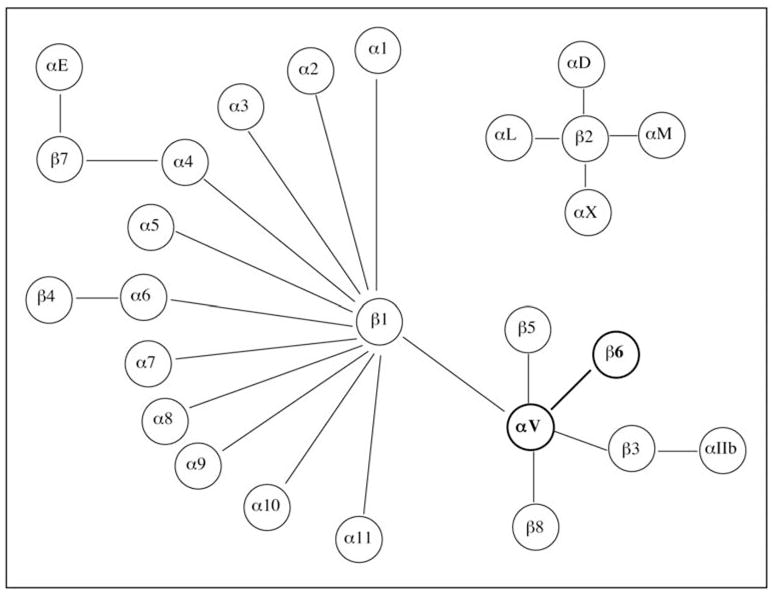
The 8α and 18β subunits form 24 distinct integrin heterodimers. Integrin β6 can only partner with αv.
The integrin αvβ6 is unique in that it is expressed exclusively in epithelial cells, and β6 partners only with αv forming a single heterodimer. During embryogenesis αvβ6 is expressed at high levels in the developing lung, skin and kidney epithelia and its expression is downregulated in healthy adult epithelia [5, 6]. However recent unpublished data from our laboratory suggest that αvβ6 is expressed at high levels in the adult hair follicles (C. MaGraw and S. Raghavan, unpublished observations).
The KO of β6 integrin has revealed an unexpected role for this epithelial integrin in downregulating local inflammation [7]. In the KO mice there is an increase in the number of macrophages in the skin that is associated with juvenile baldness. In the lungs there is a persistent accumulation of activated lymphocytes which results in an exaggerated response to acetylcholine (a bronchoconstrictor). These results suggest that β6 may have important anti-inflammatory effects and interventions targeting this integrin could affect the course of inflammatory disorders of the lungs and skin.
The expression of αvβ6 is highly upregulated during wounding with an elevated expression observed at the wound edge within a few days of wounding [5, 8, 9]. This expression is maintained until wound closure is complete. Interestingly, Huang et al., [10] showed that the β6 KO keratinocytes could not migrate as efficiently on fibronectin or vitronectin compared to the WT keratinocytes suggesting an important role for this integrin in cell migration. Additionally integrin αvβ6 is highly upregulated in carcinomas of the breast, lung, oral and skin squamous cell carcinomas (SCC), colon, stomach and endometrium among others (see Table 1). Table 1 lists the different types of cancers that have been associated with αvβ6 and the expression of αvβ6 in them. In summary, αvβ6 functions to regulate epithelial remodeling during development, tissue repair, and neoplasia [5].
Table 1. Expression of β6 in Various Cancers.
Method of detection: immunohistochemistry/immunoprecipitation
| Cancer | Population Size | % αvβ6 | Conclusion/Reference |
|---|---|---|---|
| Endometrial | 126 | 42 | Promotes invasion like in OSCCs. [25] |
| Basal Cell | 15 nodular | 7 | Promotes invasion by myofibroblasts. [14] |
| 13 morphoeic | 77 | ||
| Liver | 63 | 71.4 | Higher % of immunoreactivity than primary colon cancer cells. [17] |
| Colon | 358 | 34 | Promotes colonic cell metastasis in the liver. [17] |
| Colon | 488 | 37 | Poor prognosis. [42] |
| Gastric | 300 | 36.7 | Prognostic marker in early stage tumors. [26] |
| Cervical squamous | 85 | 59 | Unfavorable Prognostic marker. [49] |
| Oral SCC | 17 | 100 | No expression of beta6 in normal buccal mucosa. [50] |
| 5 | 80 | αvβ6 present in oral leukoplakia. [51] | |
| 11 | 100 | αvβ6 is present at invasive front of the cancer. [31] | |
| Pancreas | 34 | 100 | Enhanced αvβ6 expression in well differentiated tumors compared to poorly differentiated ones [24]. |
| Breast | 90 | 18 | The grade 1 tumors were not positive for αvβ6 expression. [52] |
| Ovary | 45 | 100 | Staining intensity proportional to extent of malignancy. [20] |
In this review we focus on the signaling pathways that have most often been associated with an increased expression of αvβ6 in different cancers. We anticipate that a better understanding of the signaling cascade downstream of αvβ6 will help us determine how elevated levels of αvβ6 lead to the spread and development of cancer.
Expression of αvβ6 in Different Cancers
It is a well-established fact now that there is a wide prevalence of αvβ6-integrin expression in different kinds of cancer (table 1). The expression of αvβ6 is often associated with a poor prognosis [11]. Integrin αvβ6 seems to promote cell invasion and migration both of which are involved in metastasis. The signaling mechanisms that are operative in the presence of an increased expression of αvβ6 in these cancers still remain to be elucidated. There are many lines of evidence that suggest that the activation of TGF-β pathway is an important signaling pathway that is involved in the process [12]. Nevertheless more detailed studies remain to be performed as each cancer type represents a unique cellular context.
Basal Cell Carcinomas (BCCs) are one of the most prevalent forms of skin cancer. BCCs arise due to the deregulation of the Sonic hedgehog (Shh) pathway and the activation of its downstream transcriptional effectors, Gli 1 and 2 [13]. BCCs can be nodular or morphoeic, the former being slow growing and low risk, and the latter having an infiltrative growth pattern and being aggressive. Recently Marsh et al., showed that while αvβ6 was expressed in the low-risk BCCs, its expression was markedly upregulated in the morphoeic variants [14]. Using a BCC model (generated by transfecting Gli1& Gli2 into NTERT human keratinocytes) the authors showed that the increased expression of αvβ6 promoted trans-differentiation of stromal fibroblasts into myofibroblasts that was accompanied by a paracrine secretion of hepatocyte growth factor/scatter factor (HGF/SC) by the myofibroblasts. This aided the invasion of the BCC cells. Thus increased expression of αvβ6 promoted BCC invasion by modulation of the stroma. The TGF-β1 pathway was also activated by αvβ6 during the process.
In colon cancer (the second highest cancer causing mortality), high αvβ6 expression is associated with a more aggressive disease outcome [15]. In colon cancer models, at the leading edge of tumors, EMT accompanied by a loss of E-Cadherin was seen [16]. The TGF-β1 pathway was reported to be activated, which in turn promoted increased invasion of cancer cells through the stroma.
The ability of colon cancer cells to metastasize in the liver has long posed a problem in the cure and control of colon cancer itself. Investigations into the cause of such a phenomenon have shown that the expression of αvβ6 in colon cancer cells was accompanied by the elevated MMP9 expression at the invading edge of the tumor [17]. MMP9 can degrade collagen, one of the chief components of the basement membrane and thus chew through the barrier that would otherwise restrain migratory cells. Also in the liver, in the sites of metastasis, tenascin C and fibronectin which are the ligands of αvβ6 are expressed [17]. Integrin αvβ6 could probably bind to these ligands and trigger many downstream signals, which lead to carcinogenesis.
Metalloproteases play a pivotal role in maintaining tumor microenvironment by actively remodeling the ECM, which is required for invasion and migration. In OSCC (oral squamous cell carcinoma) it has been shown that increased αvβ6 expression leads to the activation of MMP3 which is responsible for enhanced cell migration and invasion on fibronectin. A series of signaling events are orchestrated after the binding of αvβ6 to its ligand, resulting in the activation of MMPs. While much remains unknown as to what those events might be, there have been some studies in this area. Li et al., [18] have shown that the association of Fyn kinase with αvβ6 results in its activation that in turn leads to the recruitment of FAK. The Raf-ERK-MAPK pathway is then activated that results in the MMP3 gene being transcriptionally activated. These series of events are responsible for the increased migration of OSCC cells with increased levels of αvβ6 that then leads to the spread and development of the cancer. Recently Ramos el al., [19] have also shown that by increasing expression of αvβ6 in OSCC cells they could detect an EMT like event, which involved the expression of vimentin and down regulation of E-Cadherin.
Ahmed et al., [20] reported the expression of αvβ6 in ovarian cancer cell lines and ovarian cancer tissues. Integrin αvβ6 was present in the cell lines in varying amounts ranging from high to moderate and low. Similarly the presence of αvβ6 was also detected in ovarian cancer tissues. Integrin αvβ6 was strongly expressed in Grade III tumors in comparison to Grade I tumors. Subsequently Ahmed et al., [21] also showed that Grade III ovarian tumors had a strong expression of αvβ6 throughout the tumor whereas in benign tumors the expression of αvβ6 was restricted only to the epithelial cells at the edge of the tumor. Mucinous tumors had a stronger expression of αvβ6 in comparison to the endometrioid or serous kind. In the ovarian cancer cells it was also shown that the increased expression of αvβ6 was accompanied by the secretion of HMW-uPA (High molecular weight-urokinase plasminogen activator), MMP2 and MMP9 into the tumor conditioned media. In the absence of increased expression of αvβ6 in the cells, there was marked reduction in the level of matrix degradation by uPA and MMPs. Thus αvβ6 probably plays a role in the degradation of the ECM in concert with uPA and MMPs. Interaction of αvβ6 with uPAR (urokinase plasminogen activator receptor) in ovarian cancer cells also delineates a hitherto unreported pathway that can promote cancer development. It has been shown in immunoprecipitation studies that αvβ6 interacts with uPAR [22]. This interaction is responsible for the increased cell migration and ERK activation in ovarian cancer cells. It can be speculated that this interaction may have a role in localized degradation of the ECM that can promote cancer cell migration and invasion. Increased levels of TGF-β1 have also been recorded in ovarian cancer cells and αvβ6 is known to play a role in TGF-β1 activation [23]. There is evidence to suggest that if αvβ6 integrin blocking antibodies are used that block TGF-β1 activation in these cells, then αvβ6-uPAR induced ERK activation is also abolished (as discussed by Saldhana et al.,) [22]. Thus it is possible that the αvβ6-uPAR interactions may have some role to play in the activation of TGF-β1. The αvβ6-uPAR interaction is found only in ovarian cancer cells and not in normal ovarian cells, and thus depicts the specific protein interactions found only in the cancer cells as opposed to normal cells. Thus it is more likely that such interactions will demarcate specific signalosomes that lead the cell towards metastasis.
Sipos et al., [24] showed that in gastroenteropancreatic adenocarcinoma, the expression of αvβ6 was restricted to the tumor cells, with staining being mainly confined to the cytoplasm and very weak membrane staining. The highest expression of αvβ6 was seen in the pancreatic ductal adenocarcinomas, followed by gastric carcinomas of the intestinal type and other intestinal adenocarcinomas. Additionally there was no correlation between the expression of αvβ6 and its various ligands like fibronectin and tenascin C. Unlike other carcinomas like OSCC where the expression of αvβ6 is at the leading edge of the tumor, in the case of gastroenteropancreatic adenocarcinoma the presence of αvβ6 was poor and inconsistent at the leading edge of the tumors. Integrin αvβ6 was strongly expressed in the well or moderately differentiated tumors in comparison to the poorly differentiated tumors. Thus, in contrast to its invasive role in OSCCs, in the context of the gastroenteropancreatic adenocarcinoma αvβ6 seems to be involved in carcinoma differentiation.
Recently, Hetch et al., [25] reported the expression of αvβ6 in endometrial carcinoma. They showed that αvβ6 was expressed in 42% of the endometrial carcinomas. Tumors with an infiltrative pattern of invasion expressed αvβ6 at the leading edge. αvβ6 expression was highly concurrent with metastasis. Thus in endometrial carcinomas, like in OSCCs, αvβ6 seems to promote invasion which maybe correlated to the activation of matrix metalloproteinases (MMPs) and degradation of the ECM. Zhang et al., [26] reported the expression of αvβ6 in 36.7% gastric carcinoma samples and showed that expression was correlated with various stages of the cancer. More importantly they showed that the expression of αvβ6 in gastric carcinoma predicted reduced survival rates. The accuracy of such predictions was very high especially with respect to early stage tumors. In studying the relationship between the expression of αvβ6 and the progression of various cancers it seems that certain pathways, such as the MMP and TGF-β1 are reiteratively activated. In this next section we discuss the activation of these pathways and others by integrin αvβ6.
The Role of MMPs in αvβ6 Mediated Cancer Progression
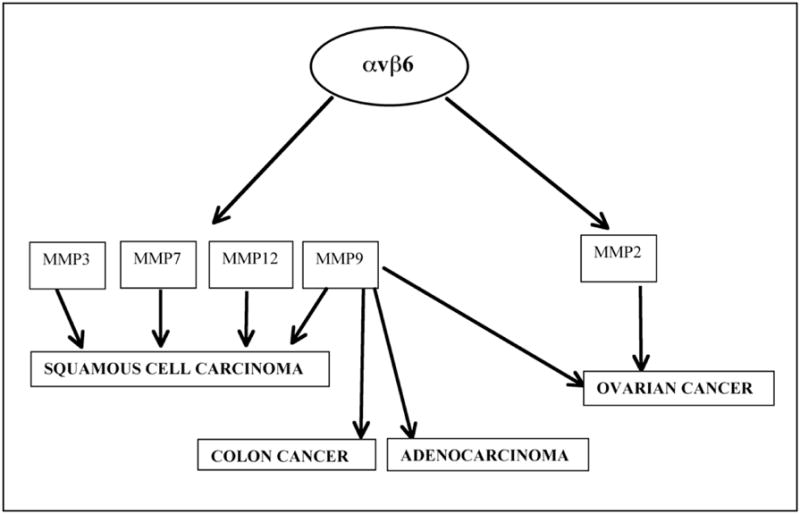
MMPs are a family of zinc-dependent metallo-endopeptidases that aid in the degradation of the components of the ECM [27]. Abnormal degradation rates often make cells more anchorage independent and lead to cancer. There have been numerous reports describing the role of αvβ6 in the activation/inhibition of the MMP pathway in concert with its various ligands like fibronectin, tenascin-C, vitronectin and the latency associated peptide (LAP) of TGFβ1 or TGFβ3. Such signaling pathways have also been responsible under various conditions to enhance the progression of a malignant phenotype (Fig. (2)). Additionally abnormal degradation products often bind to the integrins and activate them resulting in altered signaling in the cells leading to cancer.
Fig. (2).

Activation of different matrix metalloproteinases by αvβ6 in various cancers. Activation of MMP9 has the highest prevalence.
Al-Hazmi et al. [28] have reported a positive feedback loop for the activation of the matrix metalloproteinases, MMP2 and MMP9. MMP9 was shown to be responsible for the cleavage of fibronectin to produce the 120kDa cleaved fragment. Epithelial cells over expressing αvβ6 showed enhanced migration on the 120kDa cleaved fibronectin fragment but not on the full-length fibronectin. This was accompanied by an increased expression of both MMP9 and MMP2 creating a positive feedback loop, which could play an important role in the development of cancer.
Fouchier et al., [29] described the role of αvβ6 in bone metastasis of cancer cells. Their studies showed the involvement of αvβ6 in the adhesion of HT29-D4 adenocarcinoma cells to fibrinogen that had been processed by MMP9 in the presence of high concentration of Mn2+. This process occurred via the activation of the Extracellular Signal Regulated Kinase (ERK)/Mitogen Activated Protein Kinase (MAPK) pathway and MMP9.
Morgan et al., [30] demonstrated that the last 11 amino acids from the β6 cytoplasmic tail (EKQKVDLSTDC) was able to activate MMP9 which could increase the invasiveness of OSCC cells. When this fragment of the β6 cytoplasmic tail (EKQKVDLSTDC) was expressed in context of β3 integrin, MMP2 was activated. Thus the 11 amino acid region of the cytoplasmic tail of β6 integrin was able to activate different MMPs in the context of the different β subunits that it was associated with. Studies by Impola et al., [31] identified MMPs 7, 9 and 12 as prognostic markers in the invasive fronts of squamous cell carcinomas along with the presence of a high levels of αvβ6 integrin.
Thomas et al., [32] showed for the first time that when αvβ6 bound LAP, there was increased migration of OSCC cells via activation of MMP9. Interestingly, in contrast to bound (insoluble) LAP (which is a component of the ECM), soluble LAP downregulate αvβ6 induced adhesion and migration of OSSC cells. Ramos et al., [33] have shown that by over-expressing αvβ6 in SCC9 lines they could make SCC9 lines more invasive and migrate faster on fibronectin. Fibronectin matrix assembly was down regulated and there was up regulation of MMP3. This highlighted the role of αvβ6 in cancer progression by enhancing the degradation of the ECM by activating MMP3, thereby increasing the migration of cells and making tumors more invasive. Thomas et al., [34] created a panel of SCC lines expressing different levels of αvβ6 and showed an increased invasiveness of those cells due to the up regulation of MMP9.
Two independent groups have demonstrated that the ERK/MAPK pathway plays a role in the activation of MMPs by αvβ6, either independently or in concert with the plasminogen receptor (uPAR) [20, 35]. Li et al., [18] demonstrated that upon binding to fibronectin the Fyn kinase was recruited to β6 integrin which in turn recruited FAK to the focal adhesion complex activating the Raf-ERK-MAPK pathway. This leads to the transcriptional activation of MMP3 and provides a putative molecular mechanism, whereby increased expression of αvβ6 enhances the metastasis in OSCC cells. Thus αvβ6 seems to play a role in the activation of MMPs in various cancers. Even though detailed studies are still needed to highlight the exact molecular mechanism of such a phenomenon, to date the ERK/MAPK and TGF-β1 pathways seems to be playing a role in the activation of MMPs by αvβ6 in various cancers.
Activation of TGF-β by αvβ6 Integrin
TGF-β family has 3 isoforms TGF-β1, TGF-β2 and TGF-β3. Each of the TGF-βs is synthesized as homodimeric pro-TGF-β, which is comprised of TGF-β covalently linked to the latency associated protein (LAP). Pro-TGF-β is cleaved by furin-like enzymes inside the cell and remains attached to LAP in a non-covalent linkage forming the small latent complex (SLC). The TGF-β in this form is inactive and cannot bind to its receptor. It has to be released from this complex in order to be active. SLCs are found and secreted in very few instances. Mostly TGF-β is secreted as a large latent complex (LLC). The LLCs are formed by the association of latent TGF-β binding proteins (LTBPs) to the SLCs (TGFβ/LAP) by disulphide covalent bonds [36]. LTBPs have 4 isoforms; LTBP1, LTBP2, LTBP3 and LTBP4. Other than LTBP3 all other LTBPs can bind to all isoforms of TGF-β. The LTBPs belong to the superfamily of fibrillin-like ECM proteins and bind other ECM proteins like fibronectin and vitronectin. Thus the LLCs provide a large pool of latent TGF-β in the ECM that is available when needed and can be activated accordingly. The activation of TGF-β1 by αvβ6 has been studied by various groups [32, 37, 38]. A common mechanism that has emerged from such studies is that after the binding of αvβ6 to the RGD peptide present in the latency associated peptide (LAP) [that is associated with TGF-β1], αvβ6 binds to the actin cytoskeleton. This in turn triggers a conformational change in TGF-β1-LAP-LTBP1 complex (LLC). This releases TGF-β1 from the LLC, which then binds to its receptor, thus activating the pathway (Fig. (3)) [36]. Integrin αvβ6 cannot activate TGF-β bound to any other LTBP such as LTBP3. The different isoforms of the LTBPs have significant differences in the sequence of the hinge region, suggesting that this region has a significant role in the specific association with and activation of TGF-β by various integrins.
Fig. (3).
The TGF-β1 LLC. (A) TGF-β1 is found at the cell membrane as a large latent complex (LLC). The LLC comprises of TGF-β1, the latency-associated protein (LAP), and LTBP1 which belongs to the fibrillin superfamily. LTBP1 anchors the LLC to the ECM. TGF-β1 is inactive in this form. (B) Activation of TGF-β by αvβ6. Integrin αvβ6 binds to the RGD sequence in LAP inducing a conformational change and the binding of αvβ6 to the actin cytoskeleton. The binding to αvβ6 to the actin cytoskeleton further induces a conformational change that releases TGF-β1 from LAP. The unbound TGF-β1 is now active and can go and bind its receptor and activate the TGF-β1 signaling cascade.
TGF-β1-LAP can bind other integrins like αvβ1 but cannot be activated by it [39]. Although both αvβ6 and αvβ1 can bind the TGF-β1-LAP-LTBP1 complex, only αvβ6 is able to trigger the release of active TGF-β1 from the LLC. One possible explanation for this could be that the actin cytoskeleton binding properties of αvβ6 versus αvβ1 maybe very different. It would be interesting to explore the exact mechanism of such a phenomenon and investigate the possibilities of different “signalosomes” at the cytoplasmic interface of the integrins, especially in context to ABPs (actin binding proteins), which influence the differential binding of these integrins to actin. It has also been shown that the ERK/MAPK pathways are involved in the upregulation of MMPs through the activation of the TGF-β1 by αvβ6. Thus it is interesting that there is significant evidence in literature that actin cytoskeletal reorganization involving the ERK/MAPK pathways are implicated in the development of a malignant phenotype [40, 41]. The role of αvβ6 with respect to the development of a malignant phenotype through such means remains to be further investigated.
Role of αvβ6 Integrin in Epithelial to Mesenchymal Transition
Epithelial to mesenchymal transition (EMT) has been known to play a role in important physiological processes like embryonic development and wound healing. EMT is characterized by the loss of adherence of cells and increased motility. The downregulation of E-cadherin at the adherens junctions (AJs) is the hallmark of such a phenomenon and numerous molecular pathways involving various candidate genes have been implicated in the process. The role of EMT in the progression of cancer and in the development of metastasis by TGF-β has been explored, implicating the role of integrin αvβ6 in it [42]. There have been extensive studies conducted on the colon carcinoma model by Bates et al., [15, 16, 42], and they have demonstrated that αvβ6 is up regulated in their EMT model. They found that β6 mRNA was upregulated under the influence of the Ets1 transcription factor. Additionally TGF-β1 was activated by the upregulation of αvβ6 in the EMT model and there was an increased release of soluble TGF-β1 from the inactive TGFB1-LAP complex. They found that the increased levels of αvβ6 in this EMT model actually increased the migration of the cells on fibronectin. This demonstrated that αvβ6 was accelerating the processes that enhance metastasis. By analyzing around 500 patient samples the authors could correlate the poor prognosis of colon carcinoma patients with high expression of αvβ6. Interestingly, recently Ramos et al., [19] have shown that increased expression of αvβ6 in OSCC cells triggered EMT accompanied by loss of E-cadherin and gain in vimentin expression. Importantly, the 11 amino acid cytoplasmic tail of β6 integrin was shown to be responsible for this effect. However, the precise function of increased αvβ6 expression in the development of EMT in the various forms of OSCCs remains to be explored.
Role of Endocytosis in Cancer Progression
Recently there has been a report of the interaction of HAX-1 (HS1 associated protein) with αvβ6 [43]. In the study, the authors show that this interaction was responsible for the clathrin-mediated endocytosis of the receptor which was responsible for the promotion of the invasive behavior of the OSCCs. While there have been many reports showing the increased expression of αvβ6 in different cancers, there have been comparatively few insights into the exact mechanism by which this overexpression of αvβ6 results in progression of cancer. Recent data seem to implicate a role for endocytosis in cancer progression [44]. Evidence is emerging that endocytosis may play a pivotal role in the various forms of cancer by controlling the net signaling output of a cell at various stages. Receptors can undergo clathrin or non-clathrin mediated endocytosis whereby they may either be recycled to the membrane or sent to the lysosome for degradation. These kinds of fate determinations are often the defining points in the cell-signaling pathways that control the development of cancers. The interaction between HAX-1 and αvβ6 is perhaps the first evidence for endocytosis playing an important function in the genesis of cancer by a specific integrin.
Although there is increasing evidence that the overexpression of αvβ6 in various forms of cancer is associated with a more aggressive phenotype, the signaling mechanisms underlying this phenomenon are only just starting to be elucidated. This area of research should be explored intensively as it will reveal interesting pathways causing cancer in different cells by αvβ6. Such studies will pave the pathway towards the development of therapeutics to control and cure these cancers at an early stage.
Until recently, ERK was the only known protein directly interacting with αvβ6 integrin, but now more candidates, like Fyn Kinase and HAX-1, have emerged. However, keeping in mind the repertoire of cancers where αvβ6 is upregulated and the different pathways which may be linked to the cause of metastasis in each cancer, there appears to exist a goldmine of information still to be retrieved about the interacting partners of αvβ6 integrin and their role in the cause and development of cancer.
αvβ6 in Therapeutics of Cancer
Since αvβ6 is not expressed in normal adult epithelia but only under special wound healing conditions and in cancers, it lends itself to be a very good molecular target for therapy. Targeting αvβ6 should not affect adjoining normal tissues, a common problem in targeting specific molecules in the treatment of cancer. Integrin αvβ6 is highly expressed in greater than 95% of OSCCs and thus imaging αvβ6 in such tissues would serve as a very efficient means of imaging of the cancer tissue for the purposes of diagnosis and treatment. Hausner et al., [45] have successfully designed a heavy lead peptide mimicking the normal αvβ6 ligand. This peptide binds to αvβ6 and could help identify the αvβ6 tumors specifically in mice. Such peptides can be successfully used to treat OSCC during imaging in surgeries or diagnosis or chemotherapy for identifying distinct regions of treatment. US patent 7150871 claims that there was successful reduction of metastasis in lung carcinoma cells over expressing αvβ6 when they were rendered the monoclonal antibody 10D5 that binds specifically to β6 integrin. An earlier finding showed the direct binding of ERK to the β6 subunit in colon cancer cells was responsible for the progression of the cancer [46]. The report hinted at this pathway being used in cancer therapeutics. It may be possible that the binding of 10D5 directly to β6 disrupts its binding to ERK and thus helps in the reduction of metastasis. This needs further probing by analyzing the cytosolic MAPK activity in such cells exposed to the 10D5 antibody. In fact a recent study in colon cancer cells by Zhou-Yang et al., [47] revealed that apoptosis was enhanced when αvβ6 function was blocked by the 10D5 antibody. The block in cell death was also accompanied by decreased phosphorylation of ERK. This could be an important pathway regulated by αvβ6 integrin and could be targeted for therapy and small molecule inhibition studies. Kogelberg et al., [48] have successfully designed a single chain humanized form of an antibody scFv B63 which can bind to αvβ6 integrin and inhibit αvβ6 mediated cell adhesion. This antibody has been designed from the viral coat protein-1, VP1 of the O1 strain of the foot and mouth disease virus. VP1 is known to be a high affinity ligand for αvβ6. A peptide derived from VP1 was seen to exhibit αvβ6 specific binding both in vitro and in vivo. A 17-mer peptide derived from VP1 was used to create the single chain antibody successfully which can be used to treat cancer invasion mediated by the αvβ6 integrin.
In summary, there is very convincing evidence in literature correlating the severity of various forms of cancer with the expression of αvβ6. These observations have been made in many cancers, including those of the lung, breast, colon, stomach and the ovary. We predict that detailed studies on the correlation of αvβ6 expression with cancer progression and understanding its molecular mechanism(s) will help generate molecular tools that could be used to identify specific stages of cancer in patients and in directing proper treatment.
Acknowledgments
We thank Dr. R. DasGupta for critical reading of the manuscript. SR and AB are funded by the NIH grant 5-R03AR054022. SR was supported by a Dermatology Foundation Career Award, and DF Small Research Grant.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti FG, Ruoslahti E. Integrin signaling. Science (New York, NY) 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 3.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 5.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108 (Pt 6):2241–51. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 6.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–7. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 7.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–8. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark RA, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MW. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. British J Dermatol. 1996;135:46–51. [PubMed] [Google Scholar]
- 9.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, et al. Keratinocytes in human wounds express alpha v beta 6 integrin. J Investig Dermatol. 1996;106:42–8. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Wu J, Spong S, Sheppard D. The integrin alphavbeta6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111 (Pt 15):2189–95. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 12.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 13.Lupi O. Correlations between the Sonic Hedgehog pathway and basal cell carcinoma. Int J Dermatol. 2007;46:1113–7. doi: 10.1111/j.1365-4632.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 14.Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ. alpha vbeta 6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res. 2008;68:3295–303. doi: 10.1158/0008-5472.CAN-08-0174. [DOI] [PubMed] [Google Scholar]
- 15.Bates RC. Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT) Cell cycle Georgetown, Tex. 2005;4:1350–2. doi: 10.4161/cc.4.10.2053. [DOI] [PubMed] [Google Scholar]
- 16.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–70. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 17.Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, et al. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879–87. doi: 10.1111/j.1349-7006.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Yang Y, Hu Y, Dang D, Regezi J, Schmidt BL, et al. Alphavbeta6-Fyn signaling promotes oral cancer progression. The J Biol Chem. 2003;278:41646–53. doi: 10.1074/jbc.M306274200. [DOI] [PubMed] [Google Scholar]
- 19.Ramos DM, Dang D, Sadler S. The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res. 2009;29:125–30. [PubMed] [Google Scholar]
- 20.Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, et al. Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–44. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N, Riley C, Rice GE, Quinn MA, Baker MS. Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J Histochem Cytochem. 2002;50:1371–80. doi: 10.1177/002215540205001010. [DOI] [PubMed] [Google Scholar]
- 22.Saldanha RG, Molloy MP, Bdeir K, Cines DB, Song X, Uitto PM, et al. Proteomic identification of lynchpin urokinase plasminogen activator receptor protein interactions associated with epithelial cancer malignancy. J Proteome Res. 2007;6:1016–28. doi: 10.1021/pr060518n. [DOI] [PubMed] [Google Scholar]
- 23.Bristow RE, Baldwin RL, Yamada SD, Korc M, Karlan BY. Altered expression of transforming growth factor-beta ligands and receptors in primary and recurrent ovarian carcinoma. Cancer. 1999;85:658–68. doi: 10.1002/(sici)1097-0142(19990201)85:3<658::aid-cncr16>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, Kalthoff H, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathol. 2004;45:226–36. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 25.Hecht JL, Dolinski BM, Gardner HA, Violette SM, Weinreb PH. Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16:543–7. doi: 10.1097/PAI.0b013e31816bc5ee. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W, Wang JY, et al. Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol (Royal College of Radiologists (Great Britain)) 2008;20:61–6. doi: 10.1016/j.clon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 28.Al-Hazmi N, Thomas GJ, Speight PM, Whawell SA. The 120 kDa cell-binding fragment of fibronectin up-regulates migration of alphavbeta6-expressing cells by increasing matrix metallopro-teinase-2 and -9 secretion. Eur J Oral Sci. 2007;115:454–8. doi: 10.1111/j.1600-0722.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 29.Fouchier F, Penel C, Pierre Montero M, Bremond P, Champion S. Integrin alphavbeta6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn2+ Eur J Cell Biol. 2007;86:143–60. doi: 10.1016/j.ejcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Morgan MR, Thomas GJ, Russell A, Hart IR, Marshall JF. The integrin cytoplasmic-tail motif EKQKVDLSTDC is sufficient to promote tumor cell invasion mediated by matrix metalloproteinase (MMP)-2 or MMP-9. J Biol Chem. 2004;279:26533–9. doi: 10.1074/jbc.M401736200. [DOI] [PubMed] [Google Scholar]
- 31.Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, et al. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol. 2004;202:14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GJ, Hart IR, Speight PM, Marshall JF. Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells. Br J Cancer. 2002;87:859–67. doi: 10.1038/sj.bjc.6600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos DM, But M, Regezi J, Schmidt BL, Atakilit A, Dang D, et al. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 2002;21:297–307. doi: 10.1016/s0945-053x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 34.Thomas GJ, Lewis MP, Hart IR, Marshall JF, Speight PM. AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer. 2001;92:641–50. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Niu J, Dorahy DJ, Gu X, Scott RJ, Draganic B, Ahmed N, et al. Integrin expression in colon cancer cells is regulated by the cytoplasmic domain of the beta6 integrin subunit. Int J Cancer. 2002;99:529–37. doi: 10.1002/ijc.10397. [DOI] [PubMed] [Google Scholar]
- 36.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 39.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell. 1998;9:2627–38. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldner JC, Brandt BH. Cancer cell motility-on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Investig. 2005;115:339–47. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsay AG, Keppler MD, Jazayeri M, Thomas GJ, Parsons M, Violette S, et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 2007;67:5275–84. doi: 10.1158/0008-5472.CAN-07-0318. [DOI] [PubMed] [Google Scholar]
- 44.Lanzetti L, Di Fiore PP. Endocytosis and cancer: an ‘insider’ network with dangerous liaisons. Traffic (Copenhagen, Denmark) 2008;9:2011–21. doi: 10.1111/j.1600-0854.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 45.Hausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer Res. 2007;67:7833–40. doi: 10.1158/0008-5472.CAN-07-1026. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed N, Niu J, Dorahy DJ, Gu X, Andrews S, Meldrum CJ, et al. Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene. 2002;21:1370–80. doi: 10.1038/sj.onc.1205286. [DOI] [PubMed] [Google Scholar]
- 47.Zhao-Yang Z, Ke-Sen X, Qing-Si H, Wei-Bo N, Jia-Yong W, Yue-Tang M, et al. Signaling and regulatory mechanisms of integrin alphavbeta6 on the apoptosis of colon cancer cells. Cancer Lett. 2008;266:209–15. doi: 10.1016/j.canlet.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 48.Kogelberg H, Tolner B, Thomas GJ, Di Cara D, Minogue S, Ramesh B, et al. Engineering a single-chain Fv antibody to alpha v beta 6 integrin using the specificity-determining loop of a foot-and-mouth disease virus. J Mol Biol. 2008;382:385–401. doi: 10.1016/j.jmb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, et al. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212:316–24. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 50.Jones J, Watt FM, Speight PM. Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med. 1997;26:63–8. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 51.Hamidi S, Salo T, Kainulainen T, Epstein J, Lerner K, Larjava H. Expression of alpha(v)beta6 integrin in oral leukoplakia. Br J Cancer. 2000;82:1433–40. doi: 10.1054/bjoc.1999.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer (Tokyo, Japan) 2000;7:19–26. doi: 10.1007/BF02967183. [DOI] [PubMed] [Google Scholar]


