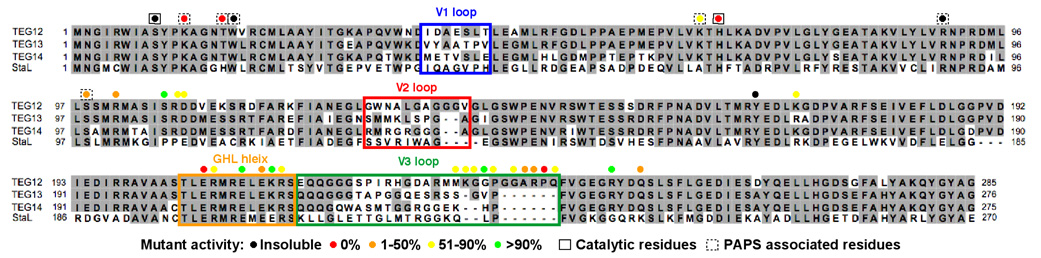
Figure 3.
ClustalW alignment of glycopeptide sulfotransferases. Highly variable sequences (V1, V2 and V3) and the flexible part of the GHL helix appear in colored boxes that match the coloration seen in Figures 2B and 2C. Results from alanine exchange mutagenesis experiments are color coded by percent activity. Results for catalytic and PAPS associated residues are shown in solid or dashed boxes, respectively. All other mutated residues are thought to interact with the glycopeptides. The H67A mutant was insoluble and therefore H67Q data is reported.