Abstract
Myristoylation of the human immunodeficiency virus type 1 (HIV-1) proteins Gag and Nef by N-myristoyltransferase (NMT) is a key process in retroviral replication and virulence, yet remains incompletely characterized. Therefore, the roles of the two isozymes, NMT1 and NMT2, in myristoylating Gag and Nef were examined using biochemical and molecular approaches. Fluorescently labelled peptides corresponding to the N terminus of HIV-1 Gag or Nef were myristoylated by recombinant human NMT1 and NMT2. Kinetic analyses indicated that NMT1 and NMT2 had 30- and 130-fold lower Km values for Nef than Gag, respectively. Values for Kcat indicated that, once Gag or Nef binds to the enzyme, myristoylation by NMT1 and NMT2 proceeds at comparable rates. Furthermore, the catalytic efficiencies for the processing of Gag by NMT1 and NMT2 were equivalent. In contrast, NMT2 had approximately 5-fold higher catalytic efficiency for the myristoylation of Nef than NMT1. Competition experiments confirmed that the Nef peptide acts as a competitive inhibitor for the myristoylation of Gag. Experiments using full-length recombinant Nef protein also indicated a lower Km for Nef myristoylation by NMT2 than NMT1. Small interfering RNAs were used to selectively deplete NMT1 and/or NMT2 from HEK293T cells expressing a recombinant Nef–sgGFP fusion protein. Depletion of NMT1 had minimal effect on the intracellular distribution of Nef–sgGFP, whereas, depletion of NMT2 altered distribution to a diffuse, widespread pattern, mimicking that of a myristoylation-deficient mutant of Nef–sgGFP. Together, these findings indicate that Nef is preferentially myristoylated by NMT2, suggesting that selective inhibition of NMT2 may provide a novel means of blocking HIV virulence.
INTRODUCTION
AIDS is a growing problem worldwide, with UNAIDS estimating that 40 million individuals are currently infected by human immunodeficiency virus type 1 (HIV-1). No cure for AIDS exists, and new therapies are needed to combat the growing problem of HIV-1 drug resistance. Two important HIV-1 proteins, Gag and Nef, contain N-terminal myristic acid, which is added to the proteins during viral processing (Guy et al., 1987; Veronese et al., 1988). This protein lipidation occurs via transfer of a myristate moiety from myristoyl-CoA to the N terminus of the proteins through the action of the N-myristoyltransferase isozymes (NMT1 and NMT2). This involves recognition of an 8 aa target sequence at the N terminus of HIV-1 Gag and Nef proteins by NMT activity in the host cell.
Gag is a structural protein that binds tightly to the plasma membrane upon myristoylation (Bryant & Ratner, 1990; Zhou et al., 1994). Plasma membrane binding of Gag initiates recruitment of Gag molecules, RNA and various viral proteins, ultimately supporting the budding of newly formed viral particles (Bouamr et al., 2003; Gottlinger et al., 1989). Importantly, it has been demonstrated that nonmyristoylated Gag mutants fail to assemble into active viral particles and are unable to bud from the cell surface (Bryant & Ratner, 1990). Overall, there has been clear demonstration by multiple groups that disruption of Gag myristoylation effectively blocks the replication of HIV-1 and its release from infected cells.
Myristoylation of Nef also plays a critical role in HIV-1 pathogenesis. Nef is an HIV-1 accessory protein and enhances HIV-1 replication in the host cell (Shaheduzzaman et al., 2002). It is one of the first retroviral proteins to accumulate in an infected cell and plays a role in downregulating MHC class I molecules and cell-surface CD4 receptors (Garcia & Miller, 1992). Additionally, Nef greatly enhances viral infectivity, as wild-type viruses are six times more infectious than Nef− mutants (Miller et al., 1994), and it enhances reverse transcription in infected cells (Harris, 1999). Mutation of the N-terminal glycine residue of Nef blocks myristoylation, preventing its association with lipid rafts (Kaminchik et al., 1994), abolishing its incorporation into HIV-1 particles (Welker et al., 1998) and preventing it from downregulating the expression of MHC I and CD4 proteins (Peng & Robert-Guroff, 2001). As Nef is critical for high-titre virus replication in vivo, pharmacological inhibition of its myristoylation may be effective in blocking the progression of AIDS.
Human cells express two NMT isozymes, NMT1 and NMT2, with sequence similarity of approximately 77 % (Giang & Cravatt, 1998). Although the functional redundancy of these isozymes is a continuing central question, there have been few reports of differences in substrate preference for NMT1 and NMT2. Enhanced understanding of the roles of these isozymes is important, as they are potential drug targets regulating signalling processes important in cancer and retroviral pathogenesis (Bouamr et al., 2003; Harris, 1995; Pal et al., 1990). We have described differential roles for NMT1 and NMT2 in a cancer context (Ducker et al., 2005); however, their individual roles in the processing of viral proteins are incompletely characterized. Therefore, elucidation of the differences and/or overlap in NMT isozyme function is critical for the understanding of retroviral pathogenesis and the development of novel drug therapies.
METHODS
Materials
Synthetic peptides of 9 aa representing the N-terminal myristoylation sequence for HIV-1 Gag (GARASVLS) or Nef (GGKWSKLS), plus a C-terminal lysine conjugated to 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD) to enable fluorescence detection were prepared by AnaSpec. [3H]Myristoyl-CoA was obtained from American Radiolabelled Chemicals. Tissue culture reagents (medium, fetal bovine serum and antibiotics) were purchased from Gibco. The pQBI-nefGFP vector encoding Nef fused to SuperGlo green fluorescent protein (Nef-sgGFP) was purchased from Qbiogene. A myristoylation-deficient Nef-sgGFP mutant (with a G2A mutation) was constructed via site-directed mutagenesis using a QuikChangeII Site Directed Mutagenesis kit (Stratagene). DNA containing the GlyAAla mutation was isolated using a QIAprep Spin Mini-Prep kit and the construct was verified by DNA sequencing (Molecular Genetics Core Facility, Penn State College of Medicine, USA). The small interfering (si)RNAs NMT1-1 (5′-AATGAGGAGGACAACAGCTAC-3′) and NMT2-4 (5′-AAAAGGTTGGACTAGTACTAC-3′) were synthesized and purified by Ambion.
Generation of recombinant NMTs
Recombinant NMT1 was constructed as a glutathione S-transferase (GST) fusion protein using the pGEX-5X-3 bacterial expression vector as described previously (French et al., 2004). cDNA encoding full-length NMT2 (Giang & Cravatt, 1998) was obtained from Dr Benjamin Cravatt (Scripps Research Institute, La Jolla, CA, USA) and cloned into the pGEX-5X-3 vector. Rosetta BL21(DE3) bacterial cells were transformed with the pGEX-NMT1 or pGEX-NMT2 vector according to the manufacturer's protocol. Colonies were selected and plasmid DNA was isolated followed by DNA sequence verification (Molecular Genetics Core Facility, Penn State College of Medicine, USA).
Preparation of NMT1 and NMT2 proteins was carried out as follows: bacterial stocks were grown in 25 ml Superbroth (111 mM glucose, 35 g tryptone l−1, 20 g yeast extract l−1, 85 mM NaCl, 100 mg ampicillin l−11, 34 mg chloramphenicol l−1) for 16 h at 30 °C. The culture was added to 500 ml Superbroth and grown to an OD600 of approximately 0.7-0.9 at 30 °C. IPTG was then added at a final concentration of 1 mM to induce the expression of recombinant NMT1 or NMT2, and cultures were incubated for an additional 3 h at 30 °C. At the end of induction, bacteria were harvested by centrifugation at 5000 g for 10 min at 4 °C and stored at −80 °C until lysis.
At the time of lysis, the cell paste was thawed on ice and added to lysis buffer (0.5 mg lysozyme ml−1 and bacterial protease inhibitor cocktail in cold PBS) at a concentration of 1 g paste per 3 ml buffer. The solution was stirred for 30 min at 4 °C and then cold PBS containing 10 μg DNase ml−1, 10 μg RNase ml−1 and 3 mM MgCl2 was added. The solution was stirred for 30 min at 4 °C and sonicated three times on ice (30 s, 40 % strength, 30 s between cycles). The solution was separated by centrifugation (15 000 g, 30 min, 4 °C) and the resulting supernatant removed and centrifuged for 1 h (118 000 g, 4 °C). The recombinant NMT1 or NMT2 was then purified by chromatography on glutathione-Sepharose B (Amersham Biosciences) using elution buffer containing 50 mM Tris/HCl (pH 8.0), 150 mM NaCl and 10 mM reduced glutathione. After the overnight batch elution, the sample was centrifuged and the supernatant mixed with 20 % glycerol in cold PBS. The supernatant was transferred to Amicon Ultra 4 centrifuge filter tubes (30 000 molecular mass cut-off) and centrifuged three times at 2200 g for 15 min to concentrate the protein to 1 mg ml−1. The concentrated protein was frozen in an ethanol/dry ice bath and stored at −80 °C until use.
In vitro NMT activity assays
The activities of NMT1 and NMT2 towards the Gag and Nef peptides were assessed as follows. Each reaction contained a total volume of 100 μl NMT assay buffer [30 mM Tris/HCl (pH 7.4), 0.5 mM EGTA, 0.455 mM EDTA, 0.05 % Tween-20]. β-Mercaptoethanol was added to a final concentration of 4.5 mM immediately before the experiment. Stock solutions of synthetic peptides were prepared in HPLC-grade water and stock solutions of myristoyl-CoA were dissolved in 100 % ethanol. During the reaction, appropriate amounts of NMT assay buffer, NMT1 or NMT2 and the synthetic peptide of interest were pre-incubated for 10 min at 37 °C with shaking. Myristoyl-CoA was added at the approximate Km of 9.3 μM (Rocque et al., 1993) to begin the reaction. The reactions were terminated after 30 min with 25 μl acetonitrile containing 2% trifluoroacetic acid (TFA). Each reaction was run in triplicate. Negative controls included the same reaction conditions without myristoyl-CoA.
The NMT assay samples were analysed using a Beckman-Coulter System Gold HPLC system interfaced with a Waters 470 Scanning Fluorescence Detector. The peptides were resolved on a reverse-phase, 18-carbon Chromolith Performance column (RP-18e, 10064.6 mm; Merck KGaA). The mobile phase consisted of a mixture of HPLC-grade acetonitrile containing 0.1% TFA and HPLC-grade water containing 0.1% TFA. Each run lasted 6 min beginning with an 85% acetonitrile/0.1% TFA mixture and progressing to a 15% acetonitrile/0.1% TFA mixture over the course of analysis. The NBD-labelled peptide in both its myristoylated and non-myristoylated forms was detected at its optimal excitation and emission wavelengths of 465 and 531 nm, respectively. The percentage of myristoylated peptide was calculated by dividing the peak area corresponding to the myristoylated peptide by total peak area and converting to μmoles of peptide myristoylated. The raw data were analysed using GraphPad Prism 3.0 and InStat 3.01.
The activities of NMT1 and NMT2 towards full-length Nef were assessed as follows. Each reaction took place in a total volume of 40 ml containing Nef, recombinant NMT1 or NMT2, [3H]myristoyl CoA, myristoyl-CoA and NMT assay buffer. β-Mercaptoethanol was added to the NMT assay buffer to a final concentration of 4.5 mM just prior to the experiment. Recombinant SF2-Nef (a kind gift from Dr Matthew Bentham, University of Leeds, UK) and NMT1 or NMT2 were pre-incubated for 10 min at 37 °C with shaking, and the reaction was initiated by the addition of myristoyl-CoA (50.5 μM, 1 μCi). Each reaction was run in duplicate. Negative controls consisted of samples under the same reaction conditions but without enzyme. The reactions were terminated after 25 min with 10 μl SDS loading buffer containing β-mercaptoethanol and samples were resolved by 10 % SDS-PAGE and electrotransferred overnight onto a PVDF membrane. Equivalent-sized sections were cut from each lane on the membrane corresponding to the molecular mass of the Nef protein, as determined by immunoblotting with an anti-Nef antibody (a gift from Dr Matthew Bentham). The samples were dissolved in Universol ES biodegradable liquid scintillation cocktail and 3H incorporation was determined by scintillation counting. Counts were adjusted for background and data were analysed using GraphPad Prism 3.0.
NMT depletion by siRNAs
We have previously described the generation and validation of the siRNAs NMT1-1 and NMT2-4, which deplete human NMT1 and NMT2, respectively (Ducker et al., 2005). Fluorescently labelled scrambled BLOCK-iT siRNA (Invitrogen) or control (non-silencing) rhodamine-labelled siRNA (Qiagen) was used at a concentration of 50 nM as a negative control and to measure siRNA transfection efficiency, respectively. Human embryonic kidney 293T (HEK293T) cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 50 U penicillin/streptomycin ml−1 in an atmosphere of 5% CO2 at 37 °C. The cells were plated in poly-D-lysine-coated, 3.5 cm diameter, glass-bottomed dishes (MatTek) to obtain approximately 30-40% confluence within 24 h. On day 0, these cells were transfected with siRNA using Lipofectamine 2000 and antibiotic-free medium according to the manufacturer's protocol. Transfection groups comprised: 50 nM NMT1 siRNA, 50 nM NMT2 siRNA, 50 nM NMT1 plus 50 nM NMT2 siRNA, and 50 nM negative-control siRNA. These cells grew to approximately 90% confluence by day 2, at which time they were transfected with 1 μg Nef-sgGFP vector (see below).
Protein was isolated from HEK293T cells treated with siRNA at 24 or 48 h after Nef-sgGFP transfection (at the time of confocal visualization). Harvested cells were resuspended in hypotonic cell lysis buffer [50 mM Tris/HCl (pH 8.0), 140 mM NaCl, 1.5 mM MgCl2, 0.5% Igepal CA-630 and 5 mM EDTA]. Protein concentrations were determined using a fluorescamine assay (Bohlen et al., 1973) and samples were normalized for total protein content (50 μg per lane). Samples were separated by10% SDS-PAGE and electrotransferred overnight. Membranes were incubated in PBS-T (100 mM sodium phosphate, 100 mM NaCl, 0.1% Tween 20, pH 7.6) with 3% powdered milk (w/v) for 1 h at room temperature. Blots were incubated overnight at 4 °C with either NMT1 or NMT2 antibody (1:250 dilution; BD Transduction Laboratories). Following overnight incubation, blots were washed three times at room temperature in PBS-T and allowed to incubate for 1 h at room temperature with horseradish peroxidaseconjugated anti-mouse antibodies (1 : 40 000 dilution; Amersham Biosciences) diluted in PBS-T containing 3% powdered milk. Blots were washed three times at room temperature with PBS-T for 5 min and developed with an ECL Western Blot Detection System (Amersham Biosciences) according to the manufacturer's directions. Blots were imaged onto Hyperfilm MP (Amersham Biosciences) and appropriate bands were quantified using Quantity One software. β-Actin levels were determined as a loading control.
Nef localization studies
HEK293T cells were plated in poly-d-lysine-coated, 3.5 cm diameter, glass-bottomed dishes and transfected with NMT siRNA as described above. Transfection groups comprised: 50 nM NMT1 siRNA, 50 nM NMT2 siRNA, 50 nM NMT1 plus 50 nM NMT2 siRNA, and 50 nM negative-control siRNA. These cells grew to approximately 90 % confluence by day 2, at which time they were transfected with 1 μg Nef–sgGFP or the myristoylation-deficient Nef–sgGFP mutant using Lipofectamine 2000 at a concentration of 4 μg ml−1, with the medium being left on overnight. Cells were then visualized using confocal microscopy (Leica TCS SP2 AOBS system). Nuclei were visualized by washing cells once with antibiotic-free medium, incubating with Hoechst 33342 at a final concentration of 2 μg ml−1 for 10 min at 37 °C and washing again with medium. The plasma membrane was visualized using Alexa Fluor 594-labelled wheat germ agglutinin (Invitrogen) at a final concentration of 2 μg ml−1 according to the manufacturer's directions.
RESULTS
Kinetic parameters for Gag- and Nef-peptide myristoylation by NMT1 and NMT2
We and others have demonstrated that peptides of appropriate sequence can be myristoylated by recombinant human NMTs (French et al., 2004; Giang & Cravatt, 1998). We previously used an assay in which [3H]myristate was transferred from [3H]myristoyl-CoA to an immobilized peptide substrate to identify small-molecule inhibitors of human NMT1 (French et al., 2004). Here, we modified an assay that we developed to measure the activity of palmitoyl acyltransferases (Varner et al., 2002, 2003) for use in measuring NMT activity towards synthetic peptides. Each peptide was characterized for its ability to serve as a substrate for NMT1 and NMT2, using an HPLC-based separation technique. In this method, non-myristoylated Gag eluted at 2.9 min and myristoylated Gag at 4.0 min, providing sufficient resolution for quantification of the amounts of parental and myristoylated peptide. The native and processed Nef peptides exhibited similar resolution, with the non-myristoylated peak eluting at 2.7 min and the myristoylated peak eluting at 4.1 min.
Initial studies determined the time course and enzyme concentration dependence of the myristoylation reactions so that the kinetic studies could be conducted under initial velocity conditions (data not shown). The concentrations of the Gag and Nef peptides were systematically varied to allow the calculation of critical kinetic parameters, including Km, Kcat and catalytic efficiency, for the processing of each peptide by NMT1 and NMT2. The Michaelis–Menten constant, Km, was calculated for each peptide substrate using a least-squares regression model. Myristoylation of Gag by either NMT1 or NMT2 occurred with a fairly high Km compared with Km values for the Nef peptide, such that NMT1 and NMT2 had 30- and 130-fold higher Km values for Gag, respectively (Table 1). Whilst the Km values for Gag did not differ significantly between the two NMT isozymes, the Km of NMT2 for Nef was significantly lower than the Km for NMT1 (P=0.0001).
Table 1.
Kinetic parameters for Gag and Nef peptide myristoylation by human NMT1 and NMT2 Values represent the mean value±SEM for three or four experiments. P values were calculated using an unpaired t-test; significant values (P<0.05) are indicated in bold.
| Kinetic parameter | Peptide | NMT1 | NMT2 | P value (NMT1 vs NMT2) |
|---|---|---|---|---|
| Km (μM) | Gag | 330±84 | 196±96 | 0.25 |
| Nef | 11±1 | 1.5±0.3 | 0.0001 | |
| P value (Gag vs Nef) | 0.024 | 0.0004 | ||
| Kcat (min−1) | Gag | 0.265±0.057 | 0.107±0.053 | 0.25 |
| Nef | 0.052±0.010 | 0.032±0.004 | 0.10 | |
| P value (Gag vs Nef) | 0.026 | 0.200 | ||
| Catalytic efficiency (min−1 μM−1) | Gag | 1±0.3 | 0.98±0.2 | 0.70 |
| Nef | 4.9±1.5 | 23.6±4.4 | 0.008 | |
| P value (Gag vs Nef) | 0.027 | 0.003 |
The catalytic constant, Kcat, was determined as a measure of the amount of substrate converted per unit enzyme (Kcat=Vmax* [E]) (Table 1). The amount of enzyme present in each reaction was measured by quantification of the band intensity of the purified GST–NMT fusion proteins via Coomassie staining (data not shown). The myristoylation of the Nef peptide by NMT1 occurred with a lower Kcat than the corresponding Kcat for the Gag peptide (Table 1). In contrast, the Kcat values for the Gag and Nef substrates were not significantly different for processing by NMT2. Interestingly, the Kcat values for either Gag or Nef did not differ significantly between the NMT isozymes. This indicated that, once the peptide is bound, myristoylation by NMT1 or NMT2 proceeds at comparable rates.
A comparison was also made of the catalytic efficiencies of NMT1 and NMT2 for the myristoylation of the Gag and Nef peptides. The catalytic efficiency measures the amount of substrate converted per unit time (Kcat/Km), and is considered to be the best indicator of the biological activity of the enzyme. NMT1 and NMT2 were 5- and 24-fold more efficient at myristoylating the Nef peptide compared with the Gag peptide (P=0.027 and 0.003), respectively (Table 1). Interestingly, NMT1 and NMT2 were equivalently efficient at myristoylating the Gag peptide, whilst NMT2 was approximately 5-fold more efficient at myristoylating the Nef peptide than NMT1 (P=0.008).
Kinetic experiments were carried out using SF2-Nef to determine whether the observed NMT isozyme selectivity extended to full-length Nef protein. In these experiments, purified SF2-Nef was incubated with [3H]myristoyl-CoA and NMT1 or NMT2, followed by purification of the Nef protein by SDS-PAGE, localization by immunoblotting and quantification of myristoylation by scintillation counting. The Nef protein concentration was varied from 0.1 to 5 μM (the maximum amount that could be added to the assays). As with the Nef peptide, both NMT1 and NMT2 myristoylated the SF2-Nef protein; however, the Km for NMT1 was approximately 2- to 5-fold that for NMT2. Overall, the kinetic studies indicated that Nef is a higher-affinity substrate than Gag for both NMT1 and NMT2, and that NMT2 is considerably more efficient at processing Nef than NMT1.
Competition between Gag and Nef peptide myristoylation
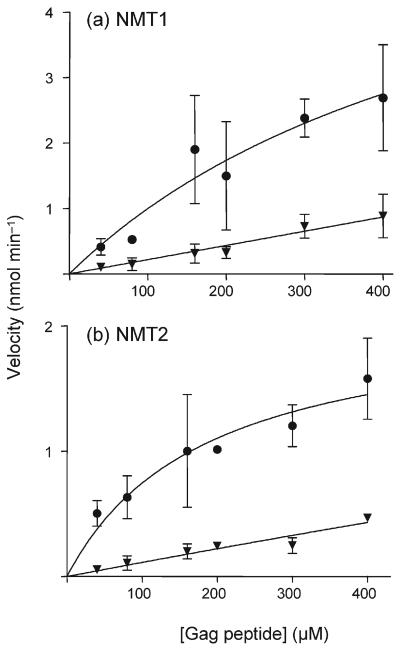
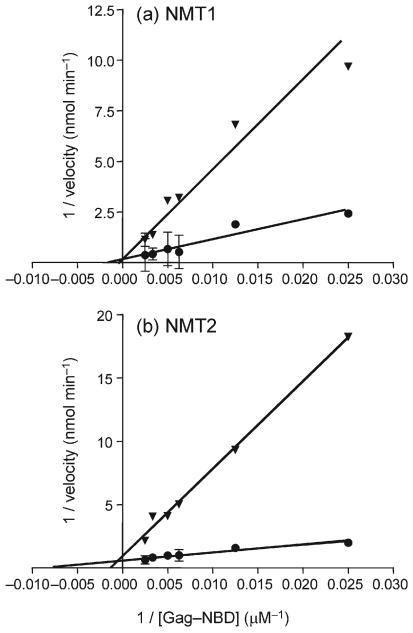
As Nef was found to be a preferred substrate for both NMT1 and NMT2, we determined its ability to act as a competitor for Gag peptide myristoylation in an in vitro system. Myristoylation assays were performed as indicated above with the addition of 16 μM Nef peptide (1.5 and 10.7 times the Km for NMT1 and NMT2, respectively) and adjustment of the HPLC gradient to allow resolution of Gag peptide and Nef peptide fluorescence. Myristoylation of the Gag peptide by either NMT1 or NMT2 was strongly suppressed by the presence of the Nef peptide (Fig. 1). In these assays, both peptides were simultaneously myristoylated, indicating that they may act as alternative substrates for the NMT isozymes. Lineweaver–Burk analyses indicated that the Nef peptide acted as a competitive inhibitor of Gag peptide myristoylation for NMT1 and NMT2 (Fig. 2). The Nef peptide demonstrated inhibition constant (Ki) values of 86 and 2 μM for NMT1 and NMT2, respectively. These data confirmed that Nef behaves as an alternative substrate for NMT1 and NMT2, and is preferentially myristoylated if present in the same system as Gag.
Fig. 1.
Inhibition of Gag peptide myristoylation by Nef peptide. Gag–NBD was myristoylated by recombinant NMT1 (a) or NMT2 (b) in the absence (●) or presence (▼) of 16 μM Nef–NBD. Reactions were initiated by the addition of 10 μM myristoyl-CoA and incubated at 37 °C for 30 min. Reactions were quenched with acetonitrile and analysed by HPLC to quantify myristoylation of the Gag peptide. Values represent the mean±SD and each graph represents four to five experiments.
Fig. 2.
Lineweaver–Burk plots of Gag peptide myristoylation in the presence or absence of Nef peptide. Gag–NBD was myristoylated by recombinant NMT1 (a) or NMT2 (b) in the absence (●) or presence (▼) of 16 μM Nef–NBD as detailed in Fig. 1. Values represent the mean±SD in a representative experiment, and lines were fitted by least-squares analyses.
The nature of the inhibition of Gag peptide myristoylation by NMT1 and NMT2 was examined further. Additional peptides containing 4 aa (GGKW), 6 aa (GGKWSK) or 8 aa (GGKWSKLS) corresponding to the N terminus of HIV-1 Nef, with or without myristate attached to the N-terminal glycine residue, were synthesized. These peptides were designated N4, N6 and N8, with the corresponding N-myristoylated peptides designated myr-N4, myr-N6 and myr-N8, respectively. Myristoylation assays with NMT2 were completed as described above, using varying concentrations of Gag and N4, N6, N8, myr-N4, myr-N6 or myr-N8 peptide at a concentration of 16 μM. Results indicated that N8 and myr-N8 both decreased Gag myristoylation by 90–95 % (data not shown). Furthermore, both the myristoylated and non-myristoylated forms of N6 also decreased Gag myristoylation, although to a lesser extent than N8. This further demonstrated that the Nef myristoylation sequence exhibits a stronger affinity for the NMT2 isozyme than the Gag myristoylation sequence in vitro.
Differential effects of depletion of NMT1 and NMT2 on Nef–sgGFP localization in intact cells
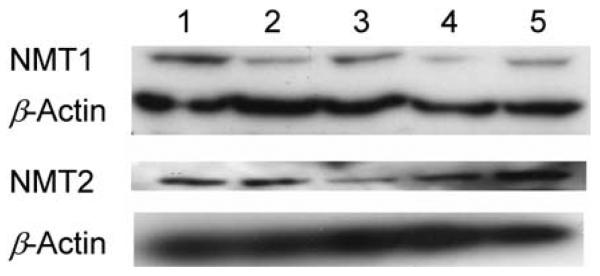
We have previously described siRNAs that are effective in selectively depleting NMT1 or NMT2 from intact cells (Ducker et al., 2005). These reagents were used to examine the roles of endogenous human NMT isozymes in myristoylation of Nef–sgGFP, whose distribution was monitored by confocal microscopy. As demonstrated in Fig. 3, treatment of the cells with the NMT siRNAs decreased NMT protein levels, typically by 60–80 % in six experiments.
Fig. 3.
Depletion of NMTs by siRNA treatments. HEK293T cells were left untreated (lane 1) or transfected with 50 nM NMT1 siRNA (lane 2), NMT2 siRNA (lane 3), NMT1 and NMT2 siRNA (lane 4) or negative-control siRNA (lane 5). Protein was isolated 48 h later, resolved by SDS-PAGE and probed with anti-NMT1, anti-NMT2 or β-actin antibodies as indicated. The Western blot is representative of five to six separate experiments.
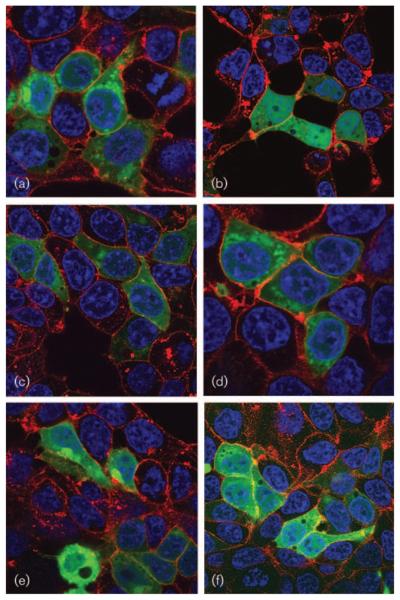
Confocal microscopy experiments demonstrated that cells that were untreated with siRNA exhibited mainly perinuclear and cytosolic fluorescence with a distinct punctate staining pattern (Fig. 4a), suggesting that Nef–sgGFP localizes mainly to the Golgi apparatus in HEK293T cells. Cells treated with a negative-control siRNA exhibited localization of Nef–sgGFP similar to untreated cells (Fig. 4c). In contrast, the non-myristoylated Nef–sgGFP mutant exhibited diffuse localization throughout the cell, including nuclear fluorescence and without distinct perinuclear localization or a punctuate expression pattern (Fig. 4b). Cells treated with NMT1 siRNA (Fig. 4d) exhibited a similar pattern to untreated cells, with Nef–sgGFP localizing mainly to the perinuclear and cytosolic regions, excluding the nucleus. However, cells treated with NMT2 siRNA exhibited non-punctate expression of Nef–sgGFP, with diffuse localization throughout the cell, including the nucleus (Fig. 4e). Additionally, cells treated with both NMT1 and NMT2 siRNA exhibited localization of Nef–sgGFP similar to that of treated NMT2 cells (Fig. 4f). Therefore, depletion of NMT2 from these cells caused marked alterations in the subcellular localization of the Nef fusion protein, producing a pattern similar to that of the non-myristoylated Nef protein. In contrast, depletion of NMT1 did not cause such a redistribution. These studies were consistent with the kinetic analyses in indicating that NMT2 preferentially myristoylates Nef in the intact cell.
Fig. 4.
Effect of NMT depletion on the subcellular localization of Nef–sgGFP. HEK293T cells were plated and treated 24 h later with 50 nM NMT1 siRNA, NMT2 siRNA, NMT1 and NMT2 siRNA, or negative-control siRNA. After 48 h, cells were transfected with 1 μg Nef–sgGFP. After an additional 24 h, cells were stained with Hoechst 33342 (blue) and wheat germ agglutinin (red) and subsequently imaged by confocal microscopy. (a) Cells transfected with Nef–sgGFP alone (green). (b) Cells transfected with a non-myristoylated Nef–sgGFP mutant. (c–f) Cells treated with scrambled siRNA (c), NMT1 siRNA (d), NMT2 siRNA (e) or NMT1 and NMT2 siRNA (f). Images are representative of three to four separate experiments.
DISCUSSION
The importance of protein myristoylation in retroviral virulence, budding and assembly has been demonstrated extensively (Bouamr et al., 2003; Harris & Neil, 1994; Hill & Skowronski, 2005; Jacobs et al., 1989; Pal et al., 1990; Yu & Felsted, 1992). On this basis, NMT has previously been considered as a target for anti-HIV-1 drugs. Several studies have examined the effects of myristic acid analogues on protein myristoylation and function (Heuckeroth & Gordon, 1989). These analogues act as alternative substrates for NMT and are transferred to substrate proteins, resulting in a less hydrophobic modification of the protein, disrupting its targeting to lipid rafts in the plasma membrane. Studies of the effects of myristate analogues on the replication of HIV-1 have consistently validated NMT as a target for the development of anti-AIDS drugs (Bryant et al., 1989, 1991; Devadas et al., 1992; Lindwasser & Resh, 2002). Importantly, studies by Bryant et al. (1991) demonstrated synergistic inhibition of HIV-1 replication using combinations of a myristate analogue and the reverse transcriptase inhibitor azidothymidine (AZT). Additionally, the inhibitory effects of the myristate analogues were more prolonged than the effects of AZT. There are few studies of the effects of NMT inhibitors (distinguished from alternative substrates) on HIV-1 replication. In one case, N-myristoyl glycinal diethylacetal was shown to inhibit Gag myristoylation in MT-2 and CEM/LAV cells, and to inhibit HIV-1 replication substantially in MT-4 cells (Tashiro et al., 1989). Similarly, several serinal-based NMT inhibitors have been shown to suppress HIV-1 replication in infected CEM cells (Takamune et al., 1999).
Complementary to studies with pharmacological inhibitors of NMT, work from the Shoji laboratory has demonstrated that infection of CEM cells with HIV-1 results in a progressive decrease in the expression of NMT by these cells (Takamune et al., 2001). This is associated with markedly increased cytotoxicity of the serinal NMT inhibitors towards HIV-1-infected CEM cells compared with uninfected CEM cells (Takamune et al., 2002). This indicates that NMT inhibitors can act as selective cytotoxins for HIV-1-infected lymphocytes and may be effective in destroying HIV-1 reservoirs in latently infected cells.
A major limitation in previous studies has been the lack of consideration of the potential differential roles of NMT1 and NMT2 in the processing of HIV-1 proteins. Although it is known that two NMT isozymes exist in humans, there have been few demonstrations of differences or redundancies in enzymic function between NMT1 and NMT2 (Ducker et al., 2005). Additionally, recent studies have indicated that NMT1-knockout mice experience embryonic lethality, whereas knockout of NMT2 appears to have little effect on early embryonic development (Yang et al., 2005), supporting the hypothesis that these isozymes play unique roles in cell biology.
Our studies utilized complementary biochemical and molecular approaches to demonstrate that the two NMT isozymes exhibit differences with regard to Gag and Nef myristoylation. The in vitro kinetic data clearly indicated marked differences between Gag and Nef myristoylation by both isozymes, with Nef being a higher-affinity substrate than Gag for both isozymes. In addition, the values for Km and catalytic efficiency showed significant differences for Nef myristoylation between the isozymes, with NMT2 exhibiting a stronger preference for both full-length Nef and its representative N-terminal octapeptide than NMT1. As Nef has lower Km values than Gag, it follows that Nef peptides would be myristoylated preferentially by either isozyme. Our data indicate that Nef decreases Gag myristoylation by both NMT1 and NMT2 in vitro, with Ki for NMT2 being substantially lower than that for NMT1. Additionally, peptides corresponding to the N-terminal first 6 or 8 aa in the Nef sequence decreased Gag myristoylation by NMT2. The exact nature of inhibition in vivo remains unclear due to potential spatial and temporal restrictions, and thus merits further study. However, these results support the kinetic data that the Nef myristoylation sequence is a preferred substrate for NMT2 compared with NMT1, and that the relative affinity of these enzymes for HIV-1 Nef is greater than HIV-1 Gag. Subcellular localization studies using a GFP-tagged Nef protein have confirmed the isozyme selectivity in an intact cell system. In this assay, wild-type Nef–sgGFP exhibited perinuclear localization with a punctate staining pattern, consistent with targeting to the Golgi apparatus as has been observed previously (Greenberg et al., 1997). In contrast, a non-myristoylated Nef–sgGFP mutant was diffusely localized including nuclear localization, consistent with a recent report in which a G2A Nef mutant was shown to exhibit diffuse subcellular localization via indirect immunofluorescence (Fackler et al., 2006). Knockdown of NMT2 with siRNA also caused the Nef–sgGFP reporter to exhibit diffuse, non-punctate cytosolic and nuclear localization similar to that of the G2A mutant. Knockdown of NMT1, however, did not have a dramatic effect on the perinuclear localization of Nef–sgGFP or the punctate expression pattern. Overall, this molecular approach supports the biochemical findings indicating that NMT1 and NMT2 play differential roles in the myristoylation and subsequent biological activity of Nef.
It has been suggested that myristoylation may not be essential for membrane localization of Nef (Bentham et al., 2006). However, the same report also stated that a complex interplay of signals at the N terminus of Nef directs its subcellular localization, making it highly likely that myristoylation is in fact critical for proper Nef function, such as interaction with downstream effectors at the membrane. In fact, recent studies have confirmed the importance of myristoylation for both membrane localization and functionality of Nef (Fackler et al., 2006), particularly when expressed in the context of the lentiviral genome. The fact that Nef interacts with a large number of effector proteins may underlie the seeming differences in conclusion from the present studies and a report from Hill & Skowronski (2005) suggesting that NMT1 binds to Nef more tightly than NMT2. In those studies, Nef and either NMT1 or NMT2 were ectopically expressed in Cos7 or Jurkat T cells, the tagged Nef protein was immunoprecipitated and co-precipitated NMT isozymes were quantified by immunoblotting. The authors indicated that the spectrum of Nef-binding proteins was altered somewhat by the selection of the tag (Hill & Skowronski, 2005) and may be further complicated by the differential expression levels of exogenous NMT1 and NMT2 in these experiments. Nevertheless, this report primarily focused on the binding of NMT1 and NMT2 to Nef, which is consistent with our data demonstrating that both isozymes can myristoylate the Nef protein. The present studies, however, focus on the catalytic processing of HIV-1 Gag and Nef, where we believe the kinetic studies as well as the RNA interference experiments clearly indicate that NMT2 is the predominant isozyme involved in processing Nef at levels that may be expressed in HIV-1-infected cells.
The studies described herein suggest that blocking Nef myristoylation by NMT2 may be an additional approach for the development of new anti-AIDS drugs. Nef plays a critical role in the early stages of viral replication and enhances viral infectivity (Harris, 1999; Miller et al., 1994), as well as retroviral evasion of the immune system through downregulation of CD4 receptors and MHC I molecules (Garcia & Miller, 1992; Greenway et al., 2003; Harris, 1999). As N-terminal myristoylation of Nef plays a critical role in creating infectious HIV-1 virions, blocking Nef myristoylation may prevent the release of newly synthesized infectious viral particles. Nef is a key early gene in HIV-1 replication, and blockade of an essential step in its cellular signalling pathway will disrupt downstream synthesis of late-stage viral proteins, greatly affecting viral replication. A recent study indicated that disruption of interactions of Nef with host-cell proteins might be one means of inhibiting HIV-1 infectivity (Olszewski et al., 2004). Our findings expand this idea by suggesting that targeting Nef myristoylation by NMT2 may be a novel strategy in preventing the spread of infectious virions from host cells.
Nef is an attractive target for therapeutic intervention as it is produced in much lower quantities than other retroviral proteins. Although Gag has a lower affinity than Nef for the NMT isozymes, the cell compensates by producing a large quantity of Gag (approx. 2000 copies per virion) (Scarlata & Carter, 2003) compared with Nef (approx. 1 % of Gag levels) (Coffin, 2002). Furthermore, plasma membrane binding of non-myristoylated Gag particles can be rescued by oligomerization with myristoylated Gag proteins (Bouamr et al., 2003). Thus, whilst Gag is a continuing antiretroviral target, it is likely to be difficult to completely eliminate Gag myristoylation and processing into virions using pharmacological agents. Targeting the NMT2-specific myristoylation of Nef may be more feasible, as Nef is produced in lower quantities than Gag and is produced earlier in the viral replication cycle.
One of the greatest challenges facing antiviral therapies is the process of acquired drug resistance, in which viral enzymes rapidly mutate to evade virus-targeting drugs. It is important to recognize that myristoylation of HIV-1 Gag and Nef proteins is accomplished by the host, i.e. human NMT, as the virus does not encode an enzyme with this activity. Therefore, attempts to manipulate HIV-1 replication pharmacologically must focus on the development of human NMT inhibitors. We believe that targeting the human NMT2 enzyme will provide a more reliable target and circumvent this problem of virus drug resistance. Pharmacological inhibition of NMT2 will probably not impair normal function in host cells, as NMT2 knockout mice are phenotypically unchanged. Therefore, development of selective NMT2 inhibitors represents a novel approach for the identification of new anti-AIDS agents.
ACKNOWLEDGEMENTS
We thank Dr Kevin J. French of Apogee Biotechnology Corporation, USA, for cloning NMT1–GST and NMT2–GST, Dr Benjamin Cravatt of the Scripps Research Institute, USA, for full-length NMT cDNAs, and Dr Matthew Bentham of the University of Leeds, UK, for the recombinant SF2-Nef and anti-Nef antibody.
REFERENCES
- Bentham M, Mazaleyrat S, Harris M. Role of myristoylation and N-terminal basic residues in membrane association of the human immunodeficiency virus type 1 Nef protein. J Gen Virol. 2006;87:563–571. doi: 10.1099/vir.0.81200-0. [DOI] [PubMed] [Google Scholar]
- Bohlen P, Stein S, Dairman W, Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973;155:213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Bouamr F, Scarlata S, Carter C. Role of myristylation in HIV-1 Gag assembly. Biochemistry. 2003;42:6408–6417. doi: 10.1021/bi020692z. [DOI] [PubMed] [Google Scholar]
- Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant ML, Heuckeroth RO, Kimata JT, Ratner L, Gordon JI. Replication of human immunodeficiency virus 1 and Moloney murine leukemia virus is inhibited by different heteroatom-containing analogs of myristic acid. Proc Natl Acad Sci U S A. 1989;86:8655–8659. doi: 10.1073/pnas.86.22.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant ML, Ratner L, Duronio RJ, Kishore NS, Devadas B, Adams SP, Gordon JI. Incorporation of 12-methoxydodecanoate into the human immunodeficiency virus 1 Gag polyprotein precursor inhibits its proteolytic processing and virus production in a chronically infected human lymphoid cell line. Proc Natl Acad Sci U S A. 1991;88:2055–2059. doi: 10.1073/pnas.88.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JM, Hughes SH, Varmus HE. Retroviruses (electronic version) Cold Spring Harbor Press; Woodbury, NY: 2002. [Google Scholar]
- Devadas B, Lu T, Katoh A, Kishore NS, Wade AC, Mehta PP, Rudnick DA, Bryant ML, Adams SP. Substrate specificity of Saccharomyces cerevisiae myristoyl-CoA: protein N-myristoyltransferase. Analysis of fatty acid analogs containing carbonyl groups, nitrogen heteroatoms, and nitrogen heterocycles in an in vitro enzyme assay and subsequent identification of inhibitors of human immunodeficiency virus I replication. J Biol Chem. 1992;267:7224–7239. other authors. [PubMed] [Google Scholar]
- Ducker CE, Upson JJ, French KJ, Smith CD. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol Cancer Res. 2005;3:463–476. doi: 10.1158/1541-7786.MCR-05-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Moris A, Tibroni N, Giese SI, Glass B, Schwartz O, Krausslich HG. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology. 2006;351:322–339. doi: 10.1016/j.virol.2006.03.044. [DOI] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Schrecengost RS, Copper JE, Xia Z, Smith CD. Cyclohexyl-octahydro-pyrrolo[1,2-α]pyrazine-based inhibitors of human N-myristoyltransferase-1. J Pharmacol Exp Ther. 2004;309:340–347. doi: 10.1124/jpet.103.061572. [DOI] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Downregulation of cell surface CD4 by Nef. Res Virol. 1992;143:52–55. doi: 10.1016/s0923-2516(06)80080-4. [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. A second mammalian N-myristoyltransferase. J Biol Chem. 1998;273:6595–6598. doi: 10.1074/jbc.273.12.6595. [DOI] [PubMed] [Google Scholar]
- Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Bronson S, Lock M, Neumann M, Pavlakis GN, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M. HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. J Biosci. 2003;28:323–335. doi: 10.1007/BF02970151. [DOI] [PubMed] [Google Scholar]
- Guy B, Kieny MP, Riviere Y, Le Peuch C, Dott K, Girard M, Montagnier L, Lecocq JP. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Harris M. The role of myristoylation in the interactions between human immunodeficiency virus type I Nef and cellular proteins. Biochem Soc Trans. 1995;23:557–561. doi: 10.1042/bst0230557. [DOI] [PubMed] [Google Scholar]
- Harris M. HIV: a new role for Nef in the spread of HIV. Curr Biol. 1999;9:R459–R461. doi: 10.1016/s0960-9822(99)80282-6. [DOI] [PubMed] [Google Scholar]
- Harris MP, Neil JC. Myristoylation-dependent binding of HIV-1 Nef to CD4. J Mol Biol. 1994;241:136–142. doi: 10.1006/jmbi.1994.1483. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Gordon JI. Altered membrane association of p60v-src and a murine 63-kDa N-myristoyl protein after incorporation of an oxygen-substituted analog of myristic acid. Proc Natl Acad Sci U S A. 1989;86:5262–5266. doi: 10.1073/pnas.86.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BT, Skowronski J. Human N-myristoyltransferases form stable complexes with lentiviral Nef and other viral and cellular substrate proteins. J Virol. 2005;79:1133–1141. doi: 10.1128/JVI.79.2.1133-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, Gheysen D, Thines D, Francotte M, de Wilde M. The HIV-1 Gag precursor Pr55gag synthesized in yeast is myristoylated and targeted to the plasma membrane. Gene. 1989;79:71–81. doi: 10.1016/0378-1119(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Kaminchik J, Margalit R, Yaish S, Drummer H, Amit B, Sarver N, Gorecki M, Panet A. Cellular distribution of HIV type 1 Nef protein: identification of domains in Nef required for association with membrane and detergent-insoluble cellular matrix. AIDS Res Hum Retroviruses. 1994;10:1003–1010. doi: 10.1089/aid.1994.10.1003. [DOI] [PubMed] [Google Scholar]
- Lindwasser OW, Resh MD. Myristoylation as a target for inhibiting HIV assembly: unsaturated fatty acids block viral budding. Proc Natl Acad Sci U S A. 2002;99:13037–13042. doi: 10.1073/pnas.212409999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski A, Sato K, Aron ZD, Cohen F, Harris A, McDougall BR, Robinson WE, Jr, Overman LE, Weiss GA. Guanidine alkaloid analogs as inhibitors of HIV-1 Nef interactions with p53, actin, and p56lck. Proc Natl Acad Sci U S A. 2004;101:14079–14084. doi: 10.1073/pnas.0406040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Reitz MS, Jr, Tschachler E, Gallo RC, Sarngadharan MG, Veronese FD. Myristoylation of Gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res Hum Retroviruses. 1990;6:721–730. doi: 10.1089/aid.1990.6.721. [DOI] [PubMed] [Google Scholar]
- Peng B, Robert-Guroff M. Deletion of N-terminal myristoylation site of HIV Nef abrogates both MHC-1 and CD4 down-regulation. Immunol Lett. 2001;78:195–200. doi: 10.1016/s0165-2478(01)00250-4. [DOI] [PubMed] [Google Scholar]
- Rocque WJ, McWherter CA, Wood DC, Gordon JI. A comparative analysis of the kinetic mechanism and peptide substrate specificity of human and Saccharomyces cerevisiae myristoyl-CoA : protein N-myristoyltransferase. J Biol Chem. 1993;268:9964–9971. [PubMed] [Google Scholar]
- Scarlata S, Carter C. Role of HIV-1 Gag domains in viral assembly. Biochim Biophys Acta. 2003;1614:62–72. doi: 10.1016/s0005-2736(03)00163-9. [DOI] [PubMed] [Google Scholar]
- Shaheduzzaman S, Krishnan V, Petrovic A, Bittner M, Meltzer P, Trent J, Venkatesan S, Zeichner S. Effects of HIV-1 Nef on cellular gene expression profiles. J Biomed Sci. 2002;9:82–96. doi: 10.1007/BF02256581. [DOI] [PubMed] [Google Scholar]
- Takamune N, Misumi S, Furuishi K, Shoji S. Blockage of HIV-1 production through inhibition of proviral DNA synthesis by N,O-didecanoyl serinal dimethylacetal. IUBMB Life. 1999;48:311–315. doi: 10.1080/713803526. [DOI] [PubMed] [Google Scholar]
- Takamune N, Tanaka T, Takeuchi H, Misumi S, Shoji S. Down-regulation of N-myristoyl transferase expression in human T-cell line CEM by human immunodeficiency virus type-1 infection. FEBS Lett. 2001;506:81–84. doi: 10.1016/s0014-5793(01)02892-7. [DOI] [PubMed] [Google Scholar]
- Takamune N, Hamada H, Misumi S, Shoji S. Novel strategy for anti-HIV-1 action: selective cytotoxic effect of N-myristoyltransferase inhibitor on HIV-1-infected cells. FEBS Lett. 2002;527:138–142. doi: 10.1016/s0014-5793(02)03199-x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Shoji S, Kubota Y. Antimyristoylation of the gag proteins in the human immunodeficiency virus-infected cells with N-myristoyl glycinal diethylacetal resulted in inhibition of virus production. Biochem Biophys Res Commun. 1989;165:1145–1154. doi: 10.1016/0006-291x(89)92722-8. [DOI] [PubMed] [Google Scholar]
- Varner AS, De Vos ML, Creaser SP, Peterson BR, Smith CD. A fluorescence-based high performance liquid chromatographic method for the characterization of palmitoyl acyl transferase activity. Anal Biochem. 2002;308:160–167. doi: 10.1016/s0003-2697(02)00212-9. [DOI] [PubMed] [Google Scholar]
- Varner AS, Ducker CE, Xia Z, Zhuang Y, De Vos ML, Smith CD. Characterization of human palmitoyl-acyl transferase activity using peptides that mimic distinct palmitoylation motifs. Biochem J. 2003;373:91–99. doi: 10.1042/BJ20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese FD, Copeland TD, Oroszlan S, Gallo RC, Sarngadharan MG. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988;62:795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker R, Harris M, Cardel B, Krausslich HG. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Shrivastav A, Kosinski C, Sharma RK, Chen MH, Berthiaume LG, Peters LL, Chuang PT, Young SG, Bergo MO. N-myristoyltransferase 1 is essential in early mouse development. J Biol Chem. 2005;280:18990–18995. doi: 10.1074/jbc.M412917200. [DOI] [PubMed] [Google Scholar]
- Yu G, Felsted RL. Effect of myristoylation on p27nef subcellular distribution and suppression of HIV-LTR transcription. Virology. 1992;187:46–55. doi: 10.1016/0042-6822(92)90293-x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Parent LJ, Wills JW, Resh MD. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]