Abstract
Despite ongoing debate about the nature of gender differences in mathematics achievement, little is known about gender similarities and differences in mathematical cognition at the neural level. We used fMRI to compare brain responses in 25 females and 24 males during a mental arithmetic task involving 3-operand addition and subtraction. We used voxel-based morphometry (VBM) to examine gender differences in brain structure. Although females and males did not differ in accuracy or response times (effect size d < 0.3), significant gender differences in functional brain activation were observed in the right dorsal and ventral visuo-spatial information processing streams (d > 1.1). Males showed greater dorsal stream activation in right intra-parietal sulcus areas important for numerical cognition, and angular gyrus regions of the default mode network that are typically deactivated during complex cognitive tasks, as well as greater ventral stream activation in the right lingual and parahippocampal gyri. VBM revealed an opposite pattern of gender differences – compared to males, females had greater regional density and greater regional volume in dorsal and ventral stream regions where males showed greater fMRI activation. There were no brain areas where females showed greater functional activation than males, and no brain areas where males showed greater structural density or volume than females. Our findings provide evidence for gender differences in the functional and structural organization of right hemisphere brain areas involved in mathematical cognition. Together with the lack of behavioral differences, our results point to more efficient use of neural processing resources in females.
Keywords: Gender, sex differences, mental arithmetic, fMRI, frontal lobe, parietal cortex, intraparietal sulcus, lingual gyrus, parahippocampal gyrus, dorsal visual stream, ventral visual stream, default mode network
In the past few decades, scientists and the public alike have debated the existence of gender differences in mathematical skills (Armstrong, 1981; Geary, 1996; Halpern, et al., 2007; Hyde, 2005; Kegel-Flom & Didion, 1995). The question of gender differences in mathematics has been particularly contentious because of its widespread implications for academic achievement, career choice, and professional success in engineering and the sciences (Jacobs, 2005). Despite the stereotypic view that males are “better” at mathematics, evidence for gender differences in mathematics has been mixed; some studies suggest differences favoring males, and others suggest differences favoring females (Gallagher & Kaufman, 2005). Cognitive and educational psychologists have advanced a number of reasons for potential gender differences in mathematical cognition; however, the neurobiological bases of these differences, if any, remain completely unknown. We used a cognitive neuroscience approach to investigate whether brain and cognitive processes underlying basic mathematical problem solving differ between females and males.
The literature on gender differences in mathematical abilities and achievement has been complex and inconsistent (Gallagher & Kaufman, 2005; Halpern, et al., 2007). A detailed meta-analysis of gender differences using over 100 studies found that performance on a range of mathematical tasks was similar in females and males (Hyde, Fennema, & Lamon, 1990). Several other studies, however, have suggested that males outperform females in standardized tests of mathematical achievement (Gallagher & Kaufman, 2005). Although the nature and origin of gender differences in mathematics remains unclear, variability in performance tends to be higher in males (Feingold, 1992; Geary, 1998; Willingham & Cole, 1997). Consequently, males tend to outperform females in select groups of high achieving and gifted children and college students (Benbow, Lubinski, Shea, & Eftekhari-Sanjani, 2000; Hyde, et al., 1990; Stanley, Colangelo, Assouline, & Ambroson, 1994), whereas females tend to outperform males at the lower end of the distribution (Barbaresi, Katusic, Colligan, Weaver, & Jacobsen, 2005). Recent studies, however, suggest that females’ underperformance in math relative to males is eliminated in more gender-equal cultures (Guiso, Monte, Sapienza, & Zingales, 2008), and in the United States, where the math gender gap has been closing over time (Goldin, Katz, & Kuziemko, 2006).
Recent brain imaging studies have contributed tremendously to our understanding of the cognitive processes involved in mathematical cognition (Dehaene, Molko, Cohen, & Wilson, 2004; Delazer, Girelli, Grana, & Domahs, 2003; Menon, Rivera, White, Glover, & Reiss, 2000). Both lesion and functional imaging studies have identified a network of brain regions that are important for mathematical cognition. These regions include the posterior parietal cortex (PPC), inferior frontal cortex, premotor cortex and basal ganglia. Collectively, these studies suggest that PPC areas, notably cortical areas in and around the intraparietal sulcus (IPS), play an important role in mathematical calculation and fact retrieval (Dehaene, et al., 2004; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999; Menon, Rivera, et al., 2000; Rivera, Reiss, Eckert, & Menon, 2005). Previous studies in mixed groups of college-aged females and males have suggested that PPC and prefrontal cortex contributions to MA tasks can be dissociated, and that PPC regions, notably the IPS, play an important role in calculation independent of other processing demands (Menon, Rivera, et al., 2000). All of these studies have used mixed groups of males and females with relatively small sample sizes that preclude determination of whether female and male brains differ in the way they process basic mathematical information.
In this study, we used functional magnetic resonance imaging (fMRI) to examine gender similarities and differences during a mathematical cognition task in young adults. In order to maximize our ability to examine differences in functional brain organization independent of behavioral differences, we chose a population of well-educated college-age males and females for whom the math cognition tasks we used would be similar in difficulty (Guiso, et al., 2008). We used a 3-operand mental arithmetic (MA) task involving simple addition and subtraction operations similar to those used in Menon et al. (2000), where participants were presented with equations of Arabic (e.g. 4+3−1=5) numerals, and asked to judge whether the equation was correct or incorrect. We used three-operand equations because this task is known to generate reliable and robust responses in PPC, prefrontal and subcortical regions involved in mathematical cognition, while at the same time providing variability in performance to facilitate examination of gender differences in brain-behavior relations. Finally, we examined whether any observed gender differences in functional activations were related to the underlying anatomical structure using voxel-based morphometry (VBM; Ashburner & Friston, 2000; Good, et al., 2001). Our analysis focused on gray matter density and volume after nonlinear corrections for brain volume so that local differences in brain structure and function could be compared more directly (Ilg, et al., 2008).
Accordingly, our aims were to (1) examine whether males and females engage the same brain areas during MA, (2) quantify the extent of similarities in brain response across males and females, (3) investigate whether the two groups engage similar brain processes in relation to performance, and (4) examine whether gender differences in function were related to underlying anatomical differences. Based on other related research on gender differences in cognitive tasks involving mental rotation and working memory (Bell, Willson, Wilman, Dave, & Silverstone, 2006; Frings, et al., 2006; Goldstein, et al., 2005; Thomsen, et al., 2000; Weiss, et al., 2003), we predicted that (1) behavioral performance would be similar in males and females, (2) gender differences in brain responses during mathematical cognition would exist even in the absence of overt gender differences in behavior, (3) males and females would show extensive overlap in activation of left and right IPS and angular gyrus, regions important for arithmetic calculation and fact retrieval, and (4) males would show greater activation in the PPC whereas females would show greater responses in the left and right frontal cortex. Understanding whether males and females differ in the brain systems they use for processing mathematical information may inform us about potential differences in strategy use and underlying vulnerability to stereotyping. In order to make appropriate links with prior behavioral and meta-analysis studies, we report effect sizes for gender differences in brain response. To our knowledge, few, if any, brain imaging studies of gender differences in cognitive function have reported effect sizes.
Method
Participants
Forty-nine healthy, right-handed, adults (25 females and 24 males) participated in this study. They ranged from 18 to 36 years of age, with a mean age of 23.99 (SD = 4.66). Mean ages of males (M = 23.53, SD = 4.92) and females (M = 24.36, SD = 4.51) were not significantly different, t(36) = 0.542, ns. Participants were recruited from the Stanford University community after giving written informed consent. All protocols were approved by the Human Subjects Committee at Stanford University School of Medicine, and participants were treated in accordance with the APA “Code of Conduct”.
Experimental design
All participants performed an fMRI experiment involving two conditions – mathematical calculation (Calculation) and number identification (Identification). Participants were presented with 16 alternating blocks of Calculation and Identification trials, each block lasting 32 seconds. Each Calculation block consisted of eight 3-operand equations of the form a+b−c=d (e.g. 5+4−2=7), where a, b, c and d were all single-digit numerals ranging from 1 to 9. Each equation was presented for 3.5 seconds followed by a blank screen for 0.5 seconds. Participants were instructed to indicate whether the equation was correct (e.g., 4+5-2=7) or incorrect (e.g., 4+5-2=8). Half of the equations presented were correct, and the other half incorrect; the order of correct and incorrect equations was randomized. Each Identification block consisted of eight 7-symbol strings (e.g., 4@3&2#5), with numbers and symbols alternating in the same way as stimuli used in the Calculation task. None of the symbols used in these stimuli were related to mathematical calculation symbols. Each string was presented for 3.5 seconds followed by a blank screen for 0.5 seconds. Participants were instructed to indicate whether the string contained the numeral 5. Half of the strings presented contained the numeral 5; the placement of “5” within the string and the order of presentation of these strings were randomized. The Identification task was designed to control for basic number and visual processing, motor response and low-level attention (Menon, Rivera, et al., 2000). Our experimental design therefore allowed us to examine gender differences during MA more precisely than would have been possible with a purely perceptual baseline condition.
Behavioral data analysis
Mean reaction time (RT) and percentage of correct responses for the Calculation and Identification trials were computed for each participant. RT and accuracy were analyzed using a two-way analysis of variance (ANOVA) with factors: gender (Males, Females) and task condition (Calculation, Identification).
Brain Imaging
fMRI data acquisition
Images were acquired on a 3T GE Signa scanner using a standard GE whole head coil (software Lx 8.3). Head movement was minimized during scanning by a comfortable custom-built restraint. A total of 28 axial slices (4.5mm thickness) parallel to the AC-PC line and covering the whole brain were imaged using a T2* weighted gradient echo spiral pulse sequence (TR = 2000 msec, TE = 30 msec, flip angle = 70°, 1 interleave; Glover & Lai, 1998). The field of view was 20 cm, and the matrix size was 64 × 64, providing an in-plane spatial resolution of 3.125 mm. To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquiring functional MRI scans (Kim, Adalsteinsson, Glover, & Spielman, 2002). To aid in localization of functional data, a high resolution T1 weighted fast spoiled grass gradient recalled (FSPGR) inversion recovery 3D MRI sequence was used with the following parameters: TI = 300 msec, TR = 8 msec; TE = 3.6 msec; flip angle = 15°; 22 cm field of view; 124 slices in coronal plane; 256 × 192 matrix; 2 NEX, acquired resolution = 1.5 × 0.9 × 1.1 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9 mm spatial resolution. Structural and functional images were acquired in the same scan session.
fMRI data analysis
Preprocessing
The first 5 volumes were not analyzed to allow for signal equilibration effects. A linear shim correction was applied separately for each slice during reconstruction using a magnetic field map acquired automatically by the pulse sequence at the beginning of the scan (Glover & Lai, 1998). Functional MRI data were then analyzed using SPM5 analysis software (http://www.fil.ion.ucl.ac.uk/spm). Images were realigned to correct for motion, corrected for errors in slice-timing, spatially transformed to standard MNI space using the echo-planar imaging template provided with SPM5, resampled every 2 mm using sinc interpolation and smoothed with a 4mm full-width half-maximum Gaussian kernel to decrease spatial noise prior to statistical analysis. Translational movement in millimeters (x, y, z) and rotational motion in degrees (pitch, roll, yaw) was calculated based on the SPM5 parameters for motion correction of the functional images in each subject. None of the participants had movement greater than 3mm translation or 3 degrees of rotation.
Individual subject analyses
Task-related brain activation was identified using a general linear model. Individual subject analyses were first performed by modeling task-related conditions. For the mathematical cognition task, brain activity related to the two task conditions (Calculation and Identification) was modeled using boxcar functions convolved with a canonical hemodynamic response function and a temporal dispersion derivative to account for voxel-wise latency differences in hemodynamic response. Low-frequency drifts at each voxel were removed using a high-pass filter (0.5 cycles/min) and serial correlations were accounted for by modeling the fMRI time series as a first degree autoregressive process (Friston, et al., 1997). Voxel-wise t-statistics maps for each condition were generated for each participant using analysis of variance, along with the respective contrast images.
Group analyses
Group analysis was performed using the contrast images and a second-level analysis of variance. Voxel-wise t-statistics maps were then used to determine group-level activation for each task condition. Significant clusters of activation were determined using a voxel-wise statistical height threshold of (p < 0.01), with corrections for multiple spatial comparisons at the cluster level (p < 0.05). The following two within-group comparisons were performed: (1) Males (Calculation minus Identification); and (2) Females (Calculation minus Identification); two between-group comparisons were then performed to examine gender differences in activation and deactivation: (3) Males (Calculation minus Identification) – Females (Calculation minus Identification); and (4) Females (Calculation minus Identification) – Males (Calculation minus Identification). Conjunction analysis (Friston, Penny, & Glaser, 2005) was subsequently performed to determine brain regions with the most consistent overlap in activation across males and females. Activation foci were superimposed on high-resolution T1-weighted images and their locations were interpreted using known neuroanatomical landmarks (Duvernoy, Bourgouin, Cabanis, & Cattin, 1999). MNI coordinates were transformed to Talairach coordinates using a non-linear transformation (Brett, 2000).
ROI analyses
Region of Interest (ROI) analyses were used to further quantify brain responses. In the between-group comparisons, which involve double subtractions, additional analyses were conducted to distinguish between differences arising from Calculation task-related increases in activation, as opposed to Identification task-related decreases in activation (or “deactivation”). For each significant cluster that was detected in these comparisons, mean Z-scores were first computed for males (comparison #1 above) and females (comparison #2). Clusters in comparisons 3 and 4 were then categorized as arising from deactivation if (a) the mean Z score was significantly negative in comparisons #2 and #1, respectively; and (b) the mean Z score was not significantly different from 0 in comparisons #1 and #2, respectively. Both the magnitude and sign of the activations were examined in order to ascertain whether any observed brain-behavior relations were explained by task-related activation or deactivation. ROI analyses were conducted using the MaRsbar toolbox (http://marsbar.sourceforge.net). ROIs were based on clusters that showed significant activation and deactivation differences between males and females (Table 1). In order to elucidate the nature of gender differences arising from deactivation, we examined brain responses in regions that overlapped with the default mode network (DMN), as defined in a previous study in our lab (Greicius, Krasnow, Reiss, & Menon, 2003). We also examined gender differences in 10mm spheres around the activation peaks shown in Table 1. Finally, in order to further examine gender differences in activation, functional ROIs based on activation clusters from our previous study (Menon, Rivera, et al., 2000), were also used. We used these functional ROIs because they have been found to be consistently activated in several studies of mathematical cognition.
Table 1.
Conjunction of brain activation in males and females during mental arithmetic
| Condition | Region | BA | Corrected p Value | # of Voxels | Peak Z Score | Peak MNI Coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Conjunction (Males & Females) (Calculation - Identification) |
||||||||
| L Intra-Parietal Sulcus | 7 | < 0.001 | 12342 | 7.52 | -22 | -70 | 42 | |
| L Superior Parietal Lobule | 7 | 7.36 | -26 | -64 | 46 | |||
| L Supramarginal Gyrus | 40 | 7.17 | -30 | -54 | 44 | |||
| L Inferior Frontal Gyrus | 44 | < 0.001 | 15151 | 7.07 | -46 | 12 | 28 | |
| L Inferior Frontal Gyrus/Anterior Insula | 47, 48 | 6.68 | -32 | 24 | 0 | |||
| R Inferior Frontal Gyrus/Anterior Insula | 47, 48 | 6.42 | 32 | 24 | -2 | |||
| R Supramarginal Gyrus | 40 | < 0.001 | 4287 | 6.37 | 36 | -50 | 44 | |
| R Intra-Parietal Sulcus | 7 | 6.26 | 30 | -70 | 46 | |||
| R Intra-Parietal Sulcus | 7 | 6.19 | 30 | -66 | 38 | |||
Overlap in brain activation across males and females during Calculation, compared to the Identification, condition (p < 0.01), as assessed using a conjunction analysis. The three major activation peaks in each cluster are shown with their corresponding Brodmann Area (BA), significance level of the cluster, the number of voxels in the cluster, the Z-score of the peak activated voxel, and the MNI coordinates.
Average signal strength, as measured by average t-score, and the spatial extent of activation, as measured by the percentage of voxels activated above a threshold of Z > 2.33 or Z < -2.33, were calculated for each ROI and compared across genders using an ANOVA.
Brain-behavior relationships
Finally, brain-behavior relationships were also examined using ROI analysis. Signal levels in each ROI were regressed against accuracy and RT separately in males and females and the slopes compared using a difference of slopes test. Regression analysis was used to examine whether the relation between performance (either accuracy or RT) and brain activation during Calculation versus Identification differed between males and females. Contrast images from each participant were entered into the regression analysis along with the behavioral covariates of interest. Positive and negative correlations in brain activation with increased accuracy or reaction time were compared in males and females. Voxel-wise t-statistics maps were generated and the clusters with significant correlations were determined using a voxel-wise statistical height threshold of (p < 0.01), with corrections for multiple spatial comparisons at the cluster level (p < 0.05).
VBM analysis
Thirty-four participants (17 males, 17 females) had T1-weighted images that could be used in VBM analysis of gender differences in brain anatomy. The remaining 15 participants either did not have structural MRI data or the data was of inadequate quality for VBM analysis. We performed optimized VBM using the VBM5 toolbox in SPM5 (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/). T1-weighted images from each participant were first segmented into gray, white and CSF images (Ashburner & Friston, 2005). Segmented gray matter images were “modulated” using non-linear warping procedures that correct for global brain differences and align homologous brain regions into a common space (Ashburner & Friston, 2000; Ilg, et al., 2008). The resulting images were then smoothed using a Gaussian kernel with 10mm FWHM (Ashburner & Friston, 2000). Between-group analysis was performed using SPM5 on the these smoothed images to determine brain voxels where local gray matter density and volume differed between males and females, after controlling for differences in brain size.
Results
Behavior
Accuracy
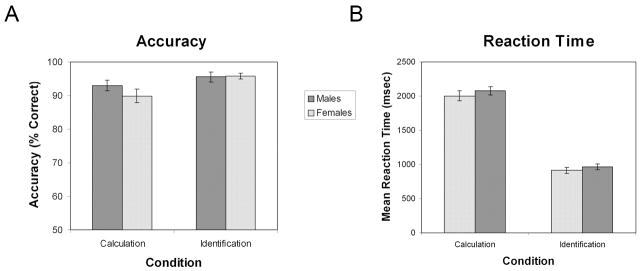
An Analysis of Variance (ANOVA) was performed on accuracy with factors task condition (Calculation and Identification) and gender (Males and Females; Figure 1a). There was a significant main effect of task condition, such that participants performed more accurately on the Identification task (M = 0.96, SE = .01) than on the Calculation task (M = 0.91, SE = .01), F(1,47) = 13.36,p < .01, partial η2 = 0.22. There was, however, no significant interaction between task condition and gender, F(1,47) = 2.16, p = .15, partial η2 = 0.04. Importantly, there was no main effect of gender; both males (M = 0.94, SE = 0.01) and females (M = 0.93, SE = 0.01) were equally accurate on both the Calculation and Identification conditions, F(1,47) = 0.61, p = .44, partial η2 = 0.01, Cohen’s d = 0.23. Post-hoc t-tests were performed to confirm that there was no difference in accuracy between males and females on either of the Calculation and Identification tasks individually. There was no significant difference in accuracy between males (M = 0.93, SE = .02) and females (M = 0.90, SE = .02) on Calculation trials, t(47) = 1.22, p = .23. There was also no significant difference in accuracy between males (M = 0.96, SE = .07) and females (M = 0.96, SE = .05) on Identification trials, t(47) = -0.14, p = .89.
Figure 1. Mental arithmetic task performance in males and females.
(A) Accuracy as a function of gender (male, female) and condition (Calculation, Identification). No significant difference between genders was found in either the Calculation or Identification conditions, and there was no significant interaction between gender and condition. In both males and females, accuracy was significantly greater in the Identification, compared to the Calculation, condition. (B) Reaction time as a function of gender and condition. No significant difference between genders was found in either the Calculation or Identification conditions, and there was no significant interaction between gender and condition. In both males and females, reaction time was significantly greater in the Calculation, compared to the Identification, condition.
Reaction Time
An ANOVA was performed on RT with factors task condition (Calculation and Identification) and gender (Males and Females; Figure 1b). As expected, there was a significant effect of task condition such that participants performed more quickly on the Identification task (M = 938.23 msec, SE = 31.13) than on the Calculation task (M = 2041.65 msec, SE = 48.31), F(1,47) = 728.22, p < .01, partial η2 = 0.94. There was, however, no significant interaction between task condition and gender, F(1,47) = 0.09, p = .76, partial η2 = 0.00. Importantly, there was no main effect of gender, F(1,47) = 0.86, p = .36, partial η2 = 0.02, Cohen’s d = 0.28; males (M = 1522 msec, SE = 50.17) and females (M = 1457 msec, SE = 49.16) performed equally quickly. Post-hoc t-tests were performed to confirm that there was no difference in RT between males and females on either of the Calculation and Identification tasks individually. There was no significant difference in RT between males (M = 2080.37, SE = 60.55) and females (M = 2002.94, SE = 74.71) on Calculation trials, t(47) = 0.80, p = .43. There was also no significant difference in RT between males (M = 964.47, SE = 44.86) and females (M = 911.99, SE = 43.19) on Identification trials, t(47) = 0.84, p = .40.
Brain imaging
Brain areas that showed overlap in activation across males and females during MA
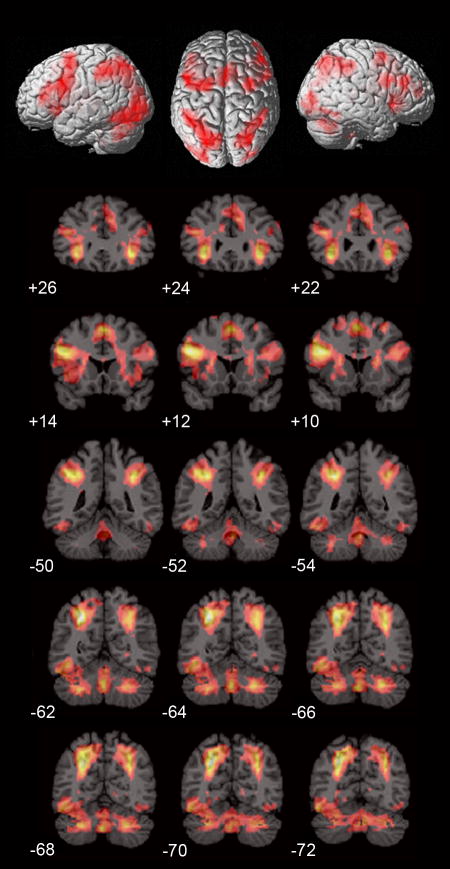
Conjunction analysis indicated that both males and females had significant overlap in the Calculation compared to Identification tasks bilaterally in the inferior frontal cortex (BA 44, 47) and adjoining anterior insula (BA 48), anterior cingulate cortex (BA 24), pre-supplementary motor area (BA 6), intraparietal sulcus (IPS) and adjoining superior parietal lobule (BA 7) and supramarginal gyrus (BA 40), lateral occipital cortex (BA 19), caudate nucleus and cerebellum (Figure 2, Table 1).
Figure 2. Conjunction of brain activation in males and females during mental arithmetic.
Similarities in brain activation during the Calculation, compared to the Identifaction, task in males and females (p < .01), as determined using conjunction analysis. Consistent activation across the two groups was detected bilaterally in the inferior frontal gyrus and adjoining anterior insula, anterior cingulate cortex, pre-supplementary motor area, intraparietal sulcus and adjoining supramarginal gyrus, lateral occipital cortex, caudate nucleus and the cerebellum.
Gender differences in brain activation during MA
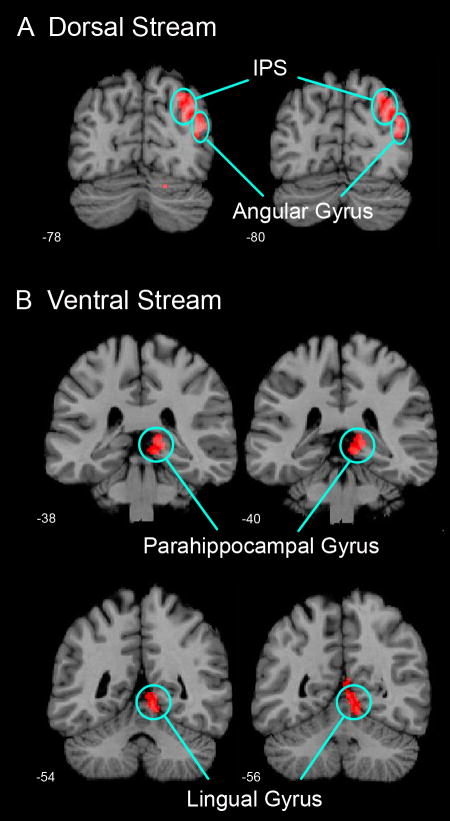
A between-group comparison revealed significantly greater activation in males, compared to females, in two right hemisphere clusters, encompassing the dorsal (Cohen’s d = 1.12) and ventral (Cohen’s d = 1.19) visual streams, respectively (Figure 3, Table 2). The dorsal stream cluster consisted of the right IPS (BA 7) and the right angular gyrus (BA 39). The ventral stream cluster included the right lingual gyrus (BA 18) and the right parahippocampal gyrus (PHG; BA 37). No brain regions showed greater activation in females compared to males.
Figure 3. Gender differences in brain activation during mental arithmetic.
Significant gender differences in activation were observed in two clusters encompassing (A) the dorsal visual stream and (B) the ventral visual stream during the Calculation, compared to the Identification, condition (p < .01, corrected for multiple spatial comparisons). In the dorsal stream, males showed greater activation in the right intra-parietal sulcus (IPS) and the right angular gyrus. In the ventral stream, males showed greater activation in the right parahippocampal gyrus and right lingual gyrus. All gender differences were lateralized to the right hemisphere. No brain areas showed greater activation in females, compared to males.
Table 2.
Gender differences in brain activation during mental arithmetic
| Condition | Region | BA | Cluster p-Value | Effect size | # of Voxels | Peak Z Score | Peak MNI Coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Males - Females (Calculation - Identification) |
|||||||||
| Dorsal Stream | R Intra-parietal Sulcus (IPS) | 7 | 0.049 | 1.12 | 306 | 3.78 | 32 | -78 | 48 |
| R Angular Gyrus | 39 | 0.049 | 2.88 | 40 | -78 | 42 | |||
| Ventral Stream | R Lingual Gyrus | 18 | 0.022 | 1.19 | 355 | 3.58 | 8 | -54 | 0 |
| R Parahippocampal Gyrus (PHG) | 37 | 0.022 | 3.05 | 8 | -40 | 0 | |||
| Females - Males (Calculation - Identification) |
|||||||||
| No significant clusters | |||||||||
Gender differences were observed in two clusters, one encompassing the dorsal visual stream and the other the ventral visual stream during the Calculation, compared to the Identification task (p < .01, corrected for multiple spatial comparisons at p < 0.05; cluster extent = 306 voxels). Localization of brain activation in the two clusters are shown, along with the corresponding Brodmann Area (BA), significance level of the cluster, effect size indicated by Cohen’s d value, the number of voxels in the cluster, the Z-score of the peak activated voxel, and the MNI coordinates.
ROI analysis of gender differences in activation and deactivation
An ROI analysis was conducted to further examine the profile of gender differences noted above in the between-group comparison. We examined differences in both activation (Calculation > Identification) and deactivation (Identification > Calculation) in the dorsal and ventral stream clusters identified in Figure 3.
ROI analysis of the dorsal visual stream cluster revealed positive task-related activation in males, whereas females did not activate significantly above the baseline (Figure 4A). To further assess the robustness of these gender differences, we then examined the spatial extent of activation within these regions. To accomplish this, we computed the number of voxels in the cluster that were activated above a threshold of Z = |2.33|, (voxel-wise p < 0.01). Males showed greater activation than females, while females showed greater deactivation than males.
Figure 4. Gender differences in dorsal and ventral visual stream activation during mental arithmetic.
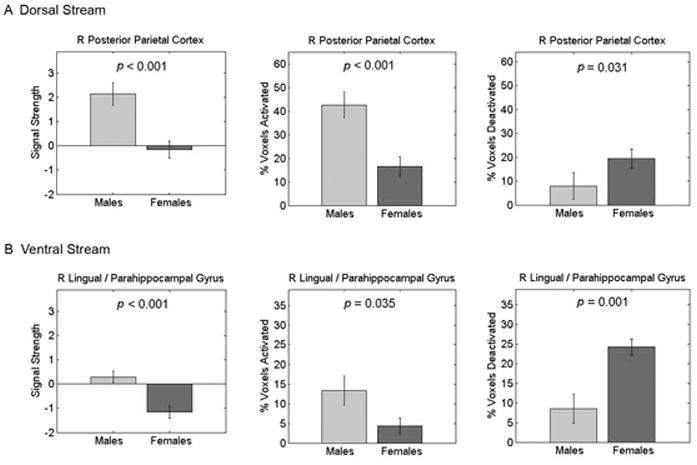
(Left) Average signal strength, (Center) percentage of voxels activated, and (Right) percentage of voxels deactivated in the (A) dorsal and (B) ventral visual streams. Gender differences in both task-related activation (Calculation > Identification) and deactivation (Identification > Calculation) were observed. Height thresholds of Z = 2.33 and Z = -2.33 (p < 0.01) were used to assess activation and deactivation. In the dorsal visual stream (A), males showed significant (positive) activation whereas females did not activate significantly above baseline. Consequently, activation was greater in males and deactivation was greater in females. In the ventral visual stream (B), females showed significant deactivation, whereas males did not activate significantly above baseline. Accordingly, activation was greater in males and deactivation was greater in females.
ROI analysis in the ventral stream cluster revealed a different profile: females showed significant deactivation, whereas males did not activate significantly above baseline (Figure 4B). This was confirmed in a parallel analysis of the spatial extent of activation, with females deactivating significantly more voxels than males.
Within the dorsal stream cluster, we further examined localized responses in the right IPS and the right angular gyrus using 10mm spheres around the corresponding peaks of activation from the between group comparison shown in Table 2. Both males and females showed positive activation in the IPS, but males showed significantly greater activation than females, p = .007 (Figure 5). In the right angular gyrus, however, males showed positive activation and females showed deactivation. As a result, brain responses in the right angular gyrus were significantly greater in males (p = .003).
Figure 5. Gender differences in right posterior parietal cortex during mental arithmetic.
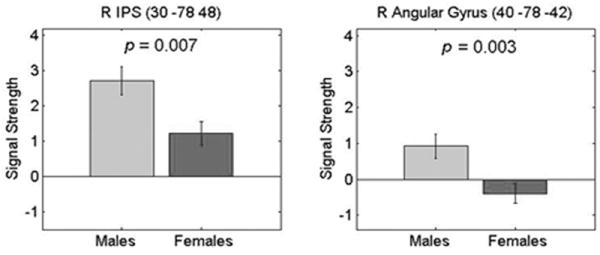
Signal strength in the right intra-parietal sulcus (IPS) (Left), and the right Angular Gyrus (Right) in males and females. Significant gender differences were observed in both the IPS (p = 0.007) and the angular gyrus (p = 0.003). These gender differences were the result of both task-related activation (Calculation > Identification) and deactivation (Identification > Calculation). The right IPS showed activation in both males and females, while the right angular gyrus showed positive activation in males and deactivation in females. 10mm sphere ROIs about the peak activated voxels in the posterior parietal cortex within the dorsal stream (see Figure 3) are shown here. The MNI coordinates (x y and z in mm) of the peaks are shown above each graph.
We repeated the above analysis using 10mm spheres around the activation peaks within the ventral visual stream clusters (Table 2) encompassing the right lingual gyrus and the right PHG. Females showed significant deactivation in both regions, while activation did not significantly differ from baseline in males (Figure 6). As a result, brain responses were significantly greater in males, compared to females, in the right lingual gyrus and the right PHG, p = 0.013 and p = 0.016, respectively.
Figure 6. Gender differences in right lingual and parahippocampal gyri during mental arithmetic.
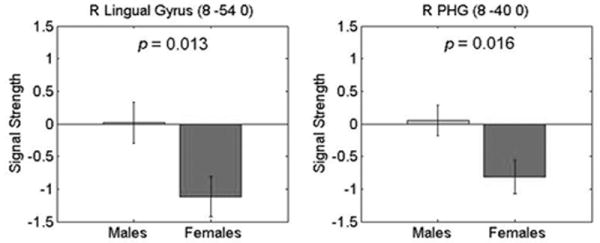
Signal strength in the right lingual gyrus (Left) and in the right parahippocampal gyrus (PHG) (Right) in males and females. Significant gender differences were observed in the lingual gyrus (p = 0.013) and the PHG (p = 0.016). In both regions, gender differences were the result of significant task-related deactivation (Identification > Calculation) in females. Signal strength in males was not significantly different from baseline. 10mm sphere ROIs about the peak activated voxels within the ventral visual stream (see Figure 3) are shown here. Other details as explained in Figure 5.
Gender differences within the default mode network
Both the dorsal and ventral stream clusters that differed in functional responses between males and females overlapped with regions comprising the default mode network (DMN). The DMN is a network of brain regions that is consistently deactivated across a wide range of cognitive tasks (Greicius, et al., 2003; Raichle, et al., 2001). As shown in Supplementary Figure S1, overlap with the DMN was observed in the right angular and lingual gyri as well as the PHG, but not the right IPS. To further quantify brain responses in males and females in relation to the DMN, we examined brain responses in the dorsal and ventral stream clusters that overlapped with the DMN. This analysis revealed that within the DMN, females tend to more strongly deactivate both the dorsal and ventral streams (Supplementary Figure S2, left hand column).
Outside the DMN, within the PPC regions of the dorsal stream, males showed significant positive activation, while females did not show significant activation above baseline (Supplementary Figure S2A, right hand column). Within the ventral stream, deactivation extended outside the DMN in females, whereas males showed positive task-related activation (Supplementary Figure S2B, right hand column).
Gender differences in frontal lobe and subcortical structures
Frontal lobe and subcortical structures were extensively activated in both males and females during the Calculation task, as seen in Figure 2. To confirm the lack of gender differences observed in these regions, we used functional ROIs based on significant clusters of activation detected in a previously published study of MA (Menon, Rivera, et al., 2000). There was no overlap in participants of the present and prior studies. The left inferior and bilateral middle frontal gyri, right thalamus and left cerebellum showed activation in males and females as measured both by mean signal strength (Supplementary Figure S3) and percentage of voxels activated (Supplementary Figure S4). No gender differences were seen in any of the frontal or subcortical regions for either average signal strength or spatial extent of activation.
Gender differences in brain-behavior relations
We used a general linear model to examine gender differences in the relationship between brain responses and behavioral measures (accuracy and RT). The analysis was conducted using both voxel-wise whole brain analysis, as well as ROI analyses consisting of both the dorsal and ventral clusters, as well as the 10mm sphere ROIs around the peaks of activation in the IPS, angular gyrus, lingual gyrus, and PHG. In each case, there were no gender differences in the profile of brain-behavior relations as assessed using a difference of slopes test.
Gender differences in anatomy
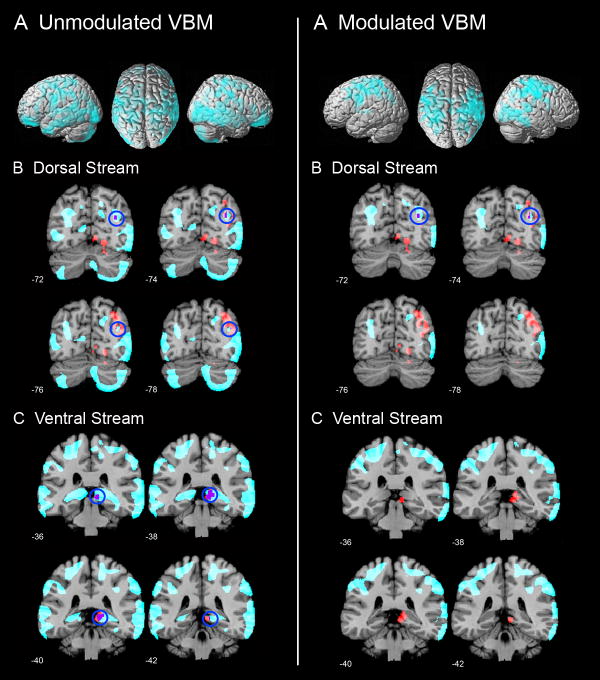
The aim of this analysis was primarily to examine whether the observed functional differences between males and females arose from underlying anatomical differences. VBM showed that females had greater regional density and volume in a number of posterior brain areas where we had detected functional activation differences between males and females (Figure 7). Specifically, females showed greater density bilaterally in the IPS, PHG, extrastriate cortex (BA 17), supramarginal gyrus (BA 40), angular gyrus (BA 39), superior parietal lobule (BA 7), and inferior temporal cortex (BA 37), p < 0.05, cluster-level corrected for multiple comparisons at p < 0.05; Figure 7, left column. Males, on the other hand, did not show greater density in any brain region. Females also showed greater regional volume bilaterally in the IPS, supramarginal gyrus (BA 40), angular gyrus (BA 39), superior parietal lobule (BA 7), and in the right extrastriate cortex (BA 17), p < 0.05, cluster-level corrected for multiple comparisons at p < 0.05; Figure 7, right column. Again, males did not show greater regional volume in any brain region.
Figure 7. Gender differences in gray matter and its overlap with functional differences.
Left Panel (A) Unmodulated VBM results showing parietal, prefrontal and temporal cortical regions where females showed greater gray matter density than males. No brain areas showed greater gray matter density in males, compared to females. (B, C) Significant gender differences in gray matter density were observed in the two clusters encompassing the dorsal visual stream and ventral visual stream regions, respectively, where males showed greater functional responses than females. Brain regions that showed structural differences are highlighted in cyan, functional differences are shown in red and the voxels that show overlap in structural and functional differences are shown in purple.
Right Panel (A) Modulated VBM results showing parietal and prefrontal cortical regions where females showed greater gray matter volume than males. No brain areas showed greater gray matter volume in males, compared to females. (B, C) Significant gender differences in gray matter volume were observed in the dorsal visual stream regions where males showed greater functional responses than females.
Discussion
Our study provides new insights into the organization of brain systems involved in mathematical cognition in females and males. Although brain responses showed considerable overlap in many regions that are known to be involved in MA, significant gender differences were also observed, despite the lack of overt behavioral differences. Gender differences were all localized to the right posterior regions of the brain, where number and space interact (Ansari, 2008; Hubbard, Piazza, Pinel, & Dehaene, 2005). Gender differences in activation were localized to two clusters: one within the dorsal visuospatial stream, and the other within the ventral visuospatial processing stream (Goodale & Westwood, 2004; Milner & Goodale, 2007; Ungerleider & Mishkin, 1982). Within the dorsal stream, males showed greater activation than females in the right IPS and the right angular gyrus regions of the PPC; within the ventral visual stream, males showed greater activation than females in the right lingual and the right PHG. Interestingly, there were no brain areas where females showed greater functional activation than males. In addition, significant differences were found in the structural density and volume of areas overlapping with those activated by the mathematical tasks such that females had greater density and volume than males.
Similar levels of performance in males and females
To our knowledge, only two behavioral studies have used three-operand arithmetic problems similar to the ones used in our study. Our results are consistent with the findings from these studies in that there were no differences between males and females in either accuracy or RT (Geary, Saults, Liu, & Hoard, 2000; Royer, Tronsky, Chan, Jackson, & Marchant, 1999). Geary et al. (2000) gave college-age participants two minutes to solve as many three-operand addition problems as possible, and observed no gender differences in the number of problems accurately solved during the task (d = 0.04). Although Royer et al. (1999) did not directly compare performance between genders on their three-operand addition task, the reported mean accuracies of 94.07 (SD = 8.04) for males and 94.04 (SD = 8.04) for females are not significantly different. Similarly, reaction times did not appear to differ between the genders, with a mean reaction time of 1.94 sec (SD = 0.783) for males, and 2.19 sec (SD = 0.801) for females. Our study extends these findings in two ways: (1) we used both addition and subtraction operations in three-operand problems, and (2) we presented each problem in a time-limited format, giving participants only four seconds to answer each problem. Our findings contribute to growing evidence that gender differences in mathematics performance are small, even in high achieving young adults. Taken together, these results provide further support for the gender similarities hypothesis (Hyde, 2005), and argue against the notion of innate gender differences in mathematical calculation. Methodologically, these results are important here because the lack of gender differences in behavior allows us to examine differences in the functional organization of brain areas independent of performance.
Gender differences in the dorsal visual stream
Within the dorsal stream, differences were observed in the right IPS and the right posterior angular gyrus. The right IPS showed greater activation in the MA, compared to the Identification task, in both males and females (Figure 5). However, the magnitude of this response was significantly different – males showed greater activation than females. The right IPS region where gender differences were observed is part of the set of canonical parietal lobe regions that are activated during MA tasks. Functional imaging studies have shown that the IPS region plays a crucial role in arithmetic calculations, independent of other processing demands such as working memory (Delazer, et al., 2003; Gruber, Indefrey, Steinmetz, & Kleinschmidt, 2001; L. Zago & Tzourio-Mazoyer, 2002). Behavioral studies in patients with damage to the PPC demonstrate significant deficits in performance of mental addition and subtraction tasks (Levin, Goldstein, Spiers, Heilman, & Valenstein, 1993). In addition, the PPC has been classically thought to underlie the acalculia component of Gerstmann’s syndrome (Levin, et al., 1993), although the precise overlap with the IPS regions showing differences in our study remains unclear. No consistent view of the differential role of the left and right IPS in MA has yet emerged; but our findings suggest that males may engage the right IPS more heavily to perform MA tasks. It is noteworthy that sex differences in similar right PPC regions have also been reported in three-dimensional mental rotation tasks, with males showing greater responses (Hugdahl, Thomsen, & Ersland, 2006; Thomsen, et al., 2000; Weiss, et al., 2003). The precise foci of these gender differences differ across studies, and the extent to which the two cognitive processes overlap neurally is at present unclear. Nevertheless, these findings are consistent with the view that numerical and visuo-spatial information processing overlap, at least partially, in the right IPS (L Zago, et al., 2008).
A different pattern of gender differences was detected in the right inferior PPC, in a region that includes the angular gyrus, extending posteriorly to the transition zone between the parietal and occipital cortex (Duvernoy, et al., 1999). In this region, males showed slight activation, while females showed deactivation (Figure 5). One potential issue in interpreting these findings is that it leaves unclear whether the observed gender differences in deactivation arose from activation of these regions during the Identification condition. In order to address this issue, we analyzed a different fMRI dataset acquired in a separate group of 11 female and 10 male participants with both number identification and passive fixation “rest” baseline conditions. We found no gender differences in the number identification, compared to the passive fixation, condition in the angular gyrus (Supplementary Figure S5). Further, no gender differences were detected in either the dorsal or ventral stream clusters when we compared brain responses during the number identification condition with the passive fixation condition (Supplementary Figure S6). These analyses indicate that gender differences reported here arise from differences in deactivation during the MA Calculation task as opposed to activation differences during the Identification task.
Angular gyrus deactivation has been reported in several studies in response to greater cognitive load, and this region is also known to overlap with the default mode network (DMN) (Greicius, et al., 2003; Raichle, et al., 2001). The DMN is a set of tightly coupled brain regions that consistently shows significant deactivation during demanding cognitive tasks. We found that angular gyrus regions that overlapped with the canonical DMN (Greicius, et al., 2003) showed significant deactivation in females, with baseline activity in the males (Figure S4). In contrast, angular gyrus regions outside the DMN showed significant positive activation in males, with baseline activity in females. Although the functions of the lateral parietal lobe component of the DMN are not yet well understood, emerging evidence suggests that angular gyrus regions noted above may play an important role in memory retrieval (Vincent, et al., 2006; Wagner, Shannon, Kahn, & Buckner, 2005). Taken together, these findings suggest that the functional organization of inferior parietal lobule systems that contribute to computational and retrieval processes during mathematical information processing is different in males and females.
Gender differences in the ventral visual stream
Gender differences were also observed in the ventral visual stream. Like in the parietal cortex, these differences were restricted to the right hemisphere. Differences were observed in both the lingual gyrus and PHG, which form part of a hierarchically organized processing stream for encoding visual stimuli (Menon, Eliez, Glover, & Reiss, 2000) that converges into the hippocampus. Interestingly, no gender differences were observed in the hippocampus. Based on previous studies, the hippocampus does not play a critical role in retrieval of MA facts in adults, since these facts are thought to be consolidated into long-term memory, primarily in the PPC (Dehaene, Piazza, Pinel, & Cohen, 2003; Delazer, et al., 2004; Rivera, et al., 2005). Similar to the findings regarding the right angular gyrus regions noted above, gender differences in the ventral visual stream regions were also related to greater deactivation in females (Figure 6). The lingual gyrus and PHG regions are not generally thought of as being part of the canonical DMN, but these regions variably overlap with the posterior cingulate cortex regions which are typically deactivated during high-level cognitive tasks (Greicius, et al., 2003). Unlike the IPS and angular gyrus regions noted above, however, the specific role of these ventral visual regions in mathematical information processing is less clear.
A similar pattern of gender differences in the ventral visual stream has been reported during a mental rotation task in which the right medial lingual gyrus showed greater activation in males (Seurinck, Vingerhoets, de Lange, & Achten, 2004). Interestingly, gender differences in the lingual gyrus have also been observed during passive viewing of simple visual stimuli. For example, in one study, men activated the right lingual gyrus (BA 18) 30% more than women in response to flickering checkerboard stimuli (Kaufmann, Elbel, Gossl, Putz, & Auer, 2001). Unfortunately, these studies did not determine whether the gender difference in lingual activation was primarily due to activation by the males, or deactivation by the females, as was seen in the current study. Nevertheless, these studies provide converging evidence for gender differences in the functional organization of early visual pathways. The manner in which these differences contribute to, or bias processing of, complex numerical and symbolic strings, such as those used in our MA task remains to be investigated.
Interpreting gender differences in brain activation
Our findings clearly indicate that although there was considerable overlap in the brain areas activated by males and females during our mathematical cognition task, there were significant differences in brain responses as well. Our study includes one of the largest sample sizes for which gender differences have been examined in any functional brain imaging study to date. The large sample size (N = 49) and large effect sizes (d > 1.1) suggest that the differences observed in the dorsal and ventral visual streams are likely to be robust and replicable. To our knowledge, this is the first study to report effect sizes for gender differences in functional brain imaging studies. When compared with small effect sizes reported in behavioral studies, our results suggest that functional brain imaging may provide more sensitive measures of gender differences in information processing than purely behavioral measures.
Our findings raise an intriguing question - how do we interpret differences in brain response in the absence of behavioral differences? One possible interpretation is that males and females might differ in the cognitive strategy used, even though their performance levels are similar. In children, differences in visualization of multiple solution paths has been suggested as a possible mechanism of gender differences in strategy during math cognition tasks (Geary, et al., 2000). To our knowledge, no such differences have been reported in adults. Given that our study contains well-educated participants, from a relatively gender-equal society (Guiso, et al., 2008), it is unlikely that males and females applied different strategies to solve the fairly simple MA tasks that we used here. In agreement with this observation, we did not find any gender differences between brain-behavioral relations in any brain region.
A more likely reason for the observed gender differences in functional responses is that there are significant structural differences between male and female brains in these regions. Our VBM analysis provides strong evidence that this may indeed be the case. We found that females had greater gray matter density in both dorsal and ventral stream regions where functional task-related gender differences were observed. Specifically, within the dorsal stream, females showed greater gray matter density in and around the right IPS and angular gyrus regions where males showed greater functional responses. Within the ventral stream, a similar pattern was observed, with females showing significantly greater gray matter density in and around the right parahippocampal cortex regions where males showed greater functional activation. The lingual gyrus was the only region where prominent functional differences were observed without accompanying structural differences. In addition, females showed greater gray matter volume in dorsal stream regions where males showed greater functional responses.
Interestingly, the structural differences observed in our study are consistent with findings from several recent MRI and cytoarchitectonic studies. These studies found that females have thicker gray matter in temporal and parietal cortices after controlling for brain size differences (Amunts, et al., 2007; Im, et al., 2006; Sowell, et al., 2006). For example, Sowell et al. found that cortical thickness in the right inferior parietal and posterior temporal regions was up to 0.45 mm thicker, on average, in females, even without correcting for the normally smaller total brain volume in females (Sowell, et al., 2006). These areas overlap with the right IPS region where we found gender differences in structure and function in our study. Furthermore, consistent with our finding of right lateralized gender differences in parietal lobe activation, structural imaging studies have revealed gender differences in hemispheric asymmetry within the parietal cortex and the parieto-occipital cortex gray matter, with males having greater asymmetry than females, especially in the right hemisphere (Amunts, et al., 2007; Goldstein, et al., 2001; Gur, et al., 1999; Kovalev, Kruggel, & von Cramon, 2003; Shah, et al., 2004). Our study converges on the above findings and points to important gender differences in the functional and structural organization of the right PPC. Exactly how these structural differences could lead to functional differences in the extent of activation, and more surprisingly, in the magnitude of activation found in the right IPS and angular gyrus is presently unclear. We hypothesize that increased regional volume and density of gray matter in females may result in more efficient information processing modules.
In agreement with this suggestion, a study of motor mental rotation, where females and males performed equally well, found that the slope of the fMRI signal per degree of mental rotation was more than two times higher in men than in women (Christova, Lewis, Tagaris, Uğurbil, & Georgopoulos, 2008). In that study, such processing efficiency was primarily restricted to the prefrontal cortex; weaker effects were also found in the right IPS, perhaps because of the small sample size used – four males and four females. Our study, conducted with a much greater sample size suggests that similar processing efficiency principles may apply in the PPC in the context of mathematical cognition tasks. Taken together, these findings highlight the need for further studies to examine how functional modules are organized in males and females and how they differentially influence information processing.
Supplementary Material
Acknowledgments
We thank Sonia Crottaz-Herbette, Jason Hom and Caitlin Costello for assistance with data acquisition and analysis, and Elena Rykhlevskaia for assistance with the VBM analysis. This research was supported by NIH grants HD047520 and HD059205, and NSF grant BCS/DRL 0750340.
Abbreviations used
- DMN
Default Mode Network
- fMRI
Functional Magnetic Resonance Imaging
- IPS
IntraParietal Sulcus
- MA
Mental Arithmetic
- PHG
ParaHippocampal Gyrus
- PPC
Posterior Parietal Cortex
- ROI
Region Of Interest
- RT
Reaction Time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Armstrong E, Malikovic A, Homke L, Mohlberg H, Schleicher A, et al. Gender-specific left-right asymmetries in human visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(6):1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci. 2008;9(4):278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Armstrong JM. Achievement and Participation of Women in Mathematics: Results of Two National Surveys. Journal for Research in Mathematics Education. 1981;12:356–372. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barbaresi W, Katusic S, Colligan R, Weaver A, Jacobsen S. Math learning disorder: incidence in a population-based birth cohort, 1976-82, Rochester, Minn. Ambul Pediatr. 2005;5(5):281–289. doi: 10.1367/A04-209R.1. [DOI] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. NeuroImage. 2006;30(2):529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Benbow CP, Lubinski D, Shea DL, Eftekhari-Sanjani H. Sex differences in mathematical reasoning ability at age 13: their status 20 years later. Psychological science : a journal of the American Psychological Society / APS. 2000;11(6):474–480. doi: 10.1111/1467-9280.00291. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 2000 http://www.mrccbu.cam.ac.uk/Imaging/mnispace.html.
- Christova P, Lewis S, Tagaris G, Uğurbil K, Geogopoulos A. A voxel-by-voxel parametric fMRI study of motor mental rotation: hemispheric specialization and gender differences in neural processing efficiency. Exp Brain Res. 2008 doi: 10.1007/s00221-008-1405-x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Molko N, Cohen L, Wilson AJ. Arithmetic and the Brain. Curr Opin Neurobiol. 2004;14:218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three Parietal Circuits for Number Processing. Cognitive Neuropsychology. 2003;20(3/4/5/6):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;7(284):970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Lochy A, Bartha L, Brenneis C, Trieb T. The acquisition of arithmetic knowledge - an FMRI study. Cortex; a journal devoted to the study of the nervous system and behavior. 2004;40(1):166–167. doi: 10.1016/s0010-9452(08)70936-5. [DOI] [PubMed] [Google Scholar]
- Delazer M, Girelli L, Grana A, Domahs F. Number processing and calculation--normative data from healthy adults. The Clinical neuropsychologist. 2003;17(3):331–350. doi: 10.1076/clin.17.3.331.18092. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F. The Human Brain: Functional Anatomy, Vascularization and Serial Sections with MRI. New York: Springer; 1999. [Google Scholar]
- Feingold A. Sex Difference in Variability in Intellectual Abilities: A New Look at an Old Controversy. Review of Educational Research. 1992;62:61–84. [Google Scholar]
- Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A. Gender-related differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport. 2006;17(4):417–421. doi: 10.1097/01.wnr.0000203623.02082.e3. [DOI] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston K, Penny W, Glaser D. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, Kaufman JC. Gender Differences in Mathematics: An Integrative Psychological Approach. Cambridge University Press; 2005. [Google Scholar]
- Geary DC. Sexual Selection and Sex Differences in Mathematical Abilities. Behavioral and Brain Sciences. 1996;19:229–284. [Google Scholar]
- Geary DC. Male, Female: The Evolution of Human Sex Differences. Washington DC: American Psychological Association; 1998. [Google Scholar]
- Geary DC, Saults SJ, Liu F, Hoard MK. Sex differences in spatial cognition, computational fluency, and arithmetical reasoning. Journal of experimental child psychology. 2000;77(4):337–353. doi: 10.1006/jecp.2000.2594. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1998;39(3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Goldin C, Katz LF, Kuziemko I. The Homecoming of American College Women: The Reversal of the College Gender Gap. Journal of Economic Perspectives. 2006;20(4):133–156. [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, et al. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19(4):509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral cortex (New York, NY: 1991) 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Goodale M, Westwood D. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 2004;14(2):203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculation. Cerebral cortex (New York, NY: 1991) 2001;11(4):350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- Guiso L, Monte F, Sapienza P, Zingales L. Diversity. Culture, gender, and math. Science. 2008;320(5880):1164–1165. doi: 10.1126/science.1154094. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The Science of Sex Differences in Science and Mathematics. Psychological Science in the Public Interest. 2007;8(1):1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard E, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nat Rev Neurosci. 2005;6(6):435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Thomsen T, Ersland L. Sex differences in visuo-spatial processing: an fMRI study of mental rotation. Neuropsychologia. 2006;44(9):1575–1583. doi: 10.1016/j.neuropsychologia.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The gender similarities hypothesis. The American Psychologist. 2005;60(6):581–592. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Fennema E, Lamon SJ. Gender differences in mathematics performance: a meta-analysis. Psychological bulletin. 1990;107(2):139–155. doi: 10.1037/0033-2909.107.2.139. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger A, Gaser C, Liebau Y, Dauner R, Wöller A, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28(16):4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 2006;31(1):31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Jacobs JE. Twenty-five years of research on gender and ethnic differences in math and science career choices: what have we learned? New directions for child and adolescent development. 2005;110(110):85–94. doi: 10.1002/cd.151. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Elbel GK, Gossl C, Putz B, Auer DP. Frequency dependence and gender effects in visual cortical regions involved in temporal frequency dependent pattern processing. Human brain mapping. 2001;14(1):28–38. doi: 10.1002/hbm.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel-Flom P, Didion CJ. Women, math, and test scores. Science. 1995;270(5235):364–365. [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;48(4):715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. NeuroImage. 2003;19(3):895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- Levin HS, Goldstein FC, Spiers PA, Heilman KM, Valenstein E. Anonymous. Clinical Neuropsychology. Vol. 3. New York: Oxford University Press; 1993. Acalculia; pp. 91–22. [Google Scholar]
- Menon V, Eliez S, Glover GH, Reiss AL. Analysis of distributed neural system involved in spatial, novelty and memory processing. Human Brain Mapping. 2000;11(2):117–129. doi: 10.1002/1097-0193(200010)11:2<117::AID-HBM50>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. NeuroImage. 2000;12(4):357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Milner A, Goodale M. Two visual systems re-viewed. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005;15(11):1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Royer JM, Tronsky LN, Chan Y, Jackson SJ, Marchant H., 3rd Math-Fact Retrieval as the Cognitive Mechanism Underlying Gender Differences in Math Test Performance. Contemporary educational psychology. 1999;24(3):181–266. doi: 10.1006/ceps.1999.1004. [DOI] [PubMed] [Google Scholar]
- Seurinck R, Vingerhoets G, de Lange FP, Achten E. Does egocentric mental rotation elicit sex differences? NeuroImage. 2004;23(4):1440–1449. doi: 10.1016/j.neuroimage.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age. Cerebral cortex (New York, NY: 1991) 2006 doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JC, Colangelo N, Assouline SG, Ambroson DL. Anonymous. Talent Development: Vol 2 Proceedings from the 1993 Henry B and Jocelyn Wallace National Research Symposium on Talent Development. Vol. 2. Vandalia, OH: Ohio Psychology Press; 1994. Gender Differences for Able Elementary School Students on Above-grade-level Ability and Achievement Tests; pp. 141–148. [Google Scholar]
- Thomsen T, Hugdahl K, Ersland L, Barndon R, Lundervold A, Smievoll AI, et al. Functional magnetic resonance imaging (fMRI) study of sex differences in a mental rotation task. Medical science monitor : international medical journal of experimental and clinical research. 2000;6(6):1186–1196. [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two Cortical Visual Systems. In: Ingle DJ, Mansfield RJW, Goodale MS, editors. The Analysis of Visual Behaviour. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of neurophysiology. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in cognitive sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weiss E, Siedentopf CM, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, et al. Sex differences in brain activation pattern during a visuospatial cognitive task: a functional magnetic resonance imaging study in healthy volunteers. Neuroscience letters. 2003;344(3):169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Willingham WW, Cole NS. Gender and Fair Assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Zago L, Petit L, Turbelin M, Andersson F, Vigneau M, Tzourio-Mazoyer N. How verbal and spatial manipulation networks contribute to calculation: An fMRI study. Neuropsychologia. 2008;46(9):2403–2414. doi: 10.1016/j.neuropsychologia.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Zago L, Tzourio-Mazoyer N. Distinguishing visuospatial working memory and complex mental calculation areas within the parietal lobes. Neuroscience letters. 2002;331(1):45–49. doi: 10.1016/s0304-3940(02)00833-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.