Since the pioneering work of Everett and Sawyer, the idea that pituitary gonadotrophins provide the critical timing cue for ovulation has remained unquestioned [1]. It is widely accepted that the timing of ovulation depends entirely on the timing of luteinizing hormone (LH) secretion, itself driven by neuroendocrine releasing factors controlled by the circadian clock in the suprachiasmatic nucleus (SCN) [2,3]. As a consequence, there has been little investigation of a role for the ovary in this process. However, we and others have demonstrated the presence of endogenous circadian clocks in the rat ovary [4–6]. Here we describe a circadian rhythm of ovarian sensitivity to LH that determines the ovulatory response to gonadotrophins. It is plausible that the circadian clock in the ovary may set the responsiveness of the ovarian follicle to the LH surge. Our results significantly alter the classic view that gonadotrophins provide the only timing cue for ovulation. They suggest that the ovary itself plays a major role in the process and provide a new perspective that will inform future research on infertility and ovarian physiology.
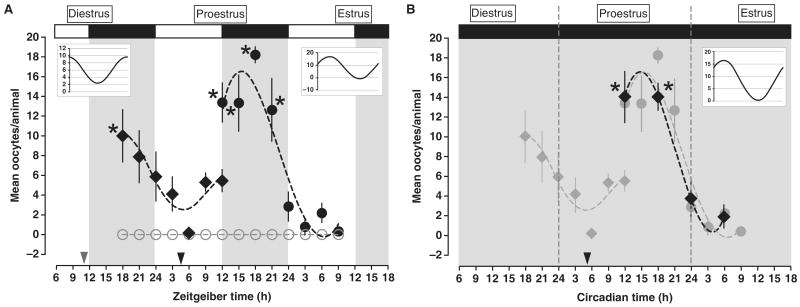
We blocked endogenous gonadotrophin secretion and assessed ovulation in response to timed exogenous LH treatments as a measure of phasic ovarian sensitivity. We suppressed endogenous gonadotrophin secretion with cetrorelix pamoate depot (CET), a highly selective and long-lasting GnRH receptor antagonist [7] (see Figure S1A in supplemental data, published with this article online). We first analyzed the pattern of ovarian sensitivity between the evening of diestrus and the afternoon of proestrus. Cycling rats maintained under a 12:12 L:D cycle (lights on 05:00h) were injected at ZT11 (Zeitgeber Time; ZT0 = lights on) on diestrus with CET (1 mg/0.1 m; i.m.). Beginning 7h later, groups of rats were treated with equine LH (eLH; 600 IU; see Figure S1B) at 3h intervals during the subsequent 18h (ZT18 and 21 on diestrus; ZT0, 3, 6, 9 and 12 on proestrus). Rats injected with eLH during the middle of the dark portion of the L:D cycle on diestrus ovulated more frequently and produced significantly more oocytes than did animals injected during the middle of the day (Figure 1A). The number of oocytes released between ZT6 and ZT9 increased and remained elevated through the end of the light phase on proestrus (ZT12; Figure 1A). Separate groups of cycling rats maintained under the same light–dark cycle were injected with CET at ZT5 on proestrus. Beginning 7h after CET treatment, groups of rats were treated with equine LH at 3h intervals during the subsequent 21h. Rats injected with eLH during the dark portion of the L:D cycle on proestrus ovulated more frequently and produced significantly more oocytes compared with animals injected during the light portion of estrus (ZT12–21 vs. ZT24–9; p < 0.001). The most robust response to eLH was seen during the middle of the night on proestrus; the smallest response was seen 9h into the light portion of the L:D cycle on estrus (Figure 1A). A multiple harmonic regression analysis (see supplemental methods) verified the significance of the diurnal rhythms of ovarian responsiveness on diestrus (F = 6.23, p < 0.01; Figure 1A inset on the left) and proestrus (F = 36.48, p < 0.001; Figure 1A inset on the right). Regardless of treatment time, animals receiving CET treatment on either day failed to ovulate in response to saline. Serum LH level was significantly reduced in all of the CET-treated animals when compared with serum from animals treated with saline vehicle (p < 0.001; see Figure S1A).
Figure 1.
Injections of eLH after cetrorelix-induced suppression of LH secretion reveal a circadian rhythm of ovarian sensitivity.
(A) Groups of rats housed under a 12:12 L:D cycle were injected at ZT11 on diestrus or ZT5 on proestrus with Cetrorelix pamoate depot (1 mg/0.1 ml; i.m.) followed by either eLH (600 IU in 0.2 ml sterile saline i.p.; black diamonds for diestrus; black circles for proestrus) or saline vehicle (0.2 ml; open gray circles for both diestrus and proestrus) every 3h beginning at ZT18 on diestrus and ZT12 on proestrus. Regardless of estrous cycle day, animals injected with eLH during the night ovulated more frequently and produced significantly more oocytes/ovulation. The discontinuity at ZT12 on proestrus is a consequence of a decline in the number of mature and responsive follicles in the animals injected at ZT11 on diestrus following 25h without LH/FSH support. Treatment with sterile saline failed to produce ovulation regardless of injection time. Asterisks indicate a significant increase in mean oocyte number above basal level (ZT6 on proestrus; ZT9 on estrus) within the eLH treated group as a function of time. The open and solid bars at the top of the figure indicate the light and dark portions of the L:D cycle. The dashed black line represents a non-linear regression generated with a fourth-order polynomial. The arrowheads on the abscissa indicate the time of CET treatment. Inset graphs: Curves generated by a CircWave multiple harmonic regression analysis (left; diestrus, right; proestrus; see supplementary experimental methods). Horizontal grid lines are included to emphasize the amplitude of the harmonic regressions. (B) Animals were injected at CT5 on proestrus with Cetrorelix pamoate depot (1 mg/0.1 ml; i.m.) followed by either eLH (600 IU i.p.; solid black circles) or sterile saline (data not shown) at one of 4 timepoints beginning at CT12 on proestrus. Treatment with sterile saline failed to produce ovulation regardless of injection time. Asterisks indicate a significant increase in mean oocyte number above basal level (ZT6) within the eLH treated group and between treatments as a function of time. The solid gray background indicates that animals were maintained under constant dim light. The light-gray data points and dotted curve are replicated from Figure 1A (ZT12 ∼ CT12) to emphasize the similarity of the results in dimLL to those in L:D. The dashed black line represents a non-linear regression generated with a fourth-order polynomial. The black arrowhead indicates the time of CET-treatment. In Figures 1A and B, data were considered significant at p < 0.05 and are presented as mean ± SEM. See Table S1 for the percentage and absolute numbers of rats that ovulated at each time point, as well as the mean number of eggs produced by each animal. Inset graph: Curve generated by same method as in (A).
To determine if the diurnal rhythm of eLH-induced ovulation is endogenous and circadian we replicated a portion of our initial experiment with rats maintained in constant dim light (dimLL). Rats in dimLL displayed clear free-running circadian rhythms of activity and a strong rhythm of ovarian sensitivity to eLH treatment (Figure 1B). Six of seven rats ovulated in response to eLH treatment during the subjective night at CT12 (CT12 = activity onset); 14 ± 2.58 oocytes/ovulation) and 5/5 rats ovulated following treatment at CT18 (14 ± 1.34 oocytes/ovulation). Rats treated during the subjective night ovulated more frequently and produced significantly more oocytes/ovulation than animals treated during the subjective day (CT0, 3/6 rats ovulated, 3.7 ± 1.94 oocytes/ovulation; CT6, 3/7 rats ovulated, 1.9 ± 1.12 oocytes/ovulation; p < 0.01 vs. CT12 and CT18). Multiple harmonic regression analysis validated the robustness of this free-running circadian rhythm of ovarian sensitivity (inset Figure 1B; F = 18.14, p < 0.001). Animals treated with CET and injected with saline failed to ovulate, regardless of treatment phase.
Our data suggest that a circadian clock drives the sensitivity of the ovary to LH and thus participates in the timing of ovulation. The rhythm of ovarian sensitivity may be driven from outside the ovary by, for example, rhythms of circulating melatonin, glucocorticoids, thyroid hormones or other endocrine or neural factors. Alternatively, the oscillator may be located in the ovary. This is a strong possibility since we know that the ovary has its own circadian clock [6]. If the ovarian clock plays a role in the timing of ovulation, what mechanism might underlie its influence? One (of several) possibilities involves the regulation of prostanoid levels. A significant step in the response of the ovarian granulosa cell to LH is the increase in the level of prostaglandin E2 and prostaglandin F2α, which together mediate the inflammatory response preceding follicular rupture [8]. The rate-limiting step in prostaglandin (PG) synthesis is the activity of cyclooxygenase-2 (COX2). COX2 expression is regulated by E-box promoter elements which are targets of the CLOCK–BMAL1 transactivator complex [9,10]. Further, the timing of COX2 gene expression is highly conserved. In several species COX2 mRNA begins to increase approximately 10h before ovulation [8]. CLOCK–BMAL1 binding to the COX2 promoter may regulate the timing of COX2 expression on the day of ovulation. Thus an increase in COX2 and PG activity in the ovary, in anticipation of the LH surge, might establish a critical period for follicular rupture.
Supplementary Material
Acknowledgments
The authors acknowledge the technical assistance of excellent undergraduates at the University of Virginia including Susan Cha, Jordan Davis, Neel D. Trivedi and Gwendolyn Yao. We thank Jennifer Mohawk and Pinar Pezuk for helpful discussion and comments on the manuscript. We gratefully acknowledge the technical support of Jeff Hager, Denise T. Holmes and Naomi Ihara. The Cetrorelix Pamoate depot formulation was a gift from Dr. Sabine Engel at AEterna Zentaris GmBH (Frankfurt, DE). This work was supported in part by NIH grant MH56647 and NSBRI grant NCC 9-58-HPF 00406 (to M.M.). M.T.S. was supported by a fellowship from the Center for Reproduction Research at the University of Virginia. T.Y. was supported by a fellowship from the Japan Society for the Promotion of Science for Young Scientists. The authors have no conflicts of interest to disclose.
Footnotes
References
- 1.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- 2.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- 3.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 4.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- 5.He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302:111–118. doi: 10.1007/s11010-007-9432-7. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotrophins. Endocrinology. 2009;150:4338–4347. doi: 10.1210/en.2008-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs M, Schally AV, Csernus B, Rekasi Z. Luteinizing hormone-releasing hormone (LH-RH) antagonist Cetrorelix down-regulates the mRNA expression of pituitary receptors for LH-RH by counteracting the stimulatory effect of endogenous LH-RH. Proc Natl Acad Sci USA. 2001;98:1829–1834. doi: 10.1073/pnas.031582398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 9.Morris JK, Richards JS. An E-box region within the prostaglandin endoperoxide synthase-2 (PGS-2) promoter is required for transcription in rat ovarian granulosa cells. J Biol Chem. 1996;271:16633–16643. doi: 10.1074/jbc.271.28.16633. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Antaya M, Boerboom D, Lussier JG, Silversides DW, Sirois J. The delayed activation of the prostaglandin G/H synthase-2 promoter in bovine granulosa cells is associated with down-regulation of truncated upstream stimulatory factor-2. J Biol Chem. 1999;274:35037–35045. doi: 10.1074/jbc.274.49.35037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.