Abstract
During postnatal days (PD) 11–20, (+/−)3,4-methylenedioxy-methamphetamine (MDMA) treatment impairs egocentric and allocentric learning, and reduces spontaneous locomotor activity; however, it does not have these effects during PD 1–10. How the learning impairments relate to the stress hyporesponsive period (SHRP) is unknown. To test this association, the preweaning period was subdivided into 5-day periods from PD 1–20. Separate pups within each litter were injected subcutaneously with 0, 10, 15, 20, or 25 mg/kg MDMA ×4/day on PD 1–5, 6–10, 11–15, or 16–20, and tested as adults. The 3 highest MDMA dose groups showed reduced locomotor activity during the first 10 min (of 60 min), especially in the PD 1–5 and 6–10 dosing regimens. MDMA groups in all dosing regimens showed impaired allocentric learning in the Morris water maze (on acquisition and reversal, all MDMA groups were affected; on the small platform phase, the 2 high-dose groups were affected). No effects of MDMA were found on anxiety (elevated zero maze), novel object recognition, or egocentric learning (although a nonsignificant trend was observed). The Morris maze results did not support the idea that the SHRP is critical to the effects of MDMA on allocentric learning. However, since no effects on egocentric learning were found, but were apparent after PD 11–20 treatment, the results show that these 2 forms of learning have different exposure-duration sensitivities.
Keywords: MDMA, Ecstasy, Brain development, Learning and memory, Spatial learning, Morris water maze, Locomotor activity, Elevated zero maze, Novel object recognition
Introduction
(+/−)3,4-Methylenedioxymethamphetamine (MDMA) has become a widely abused drug, especially among adolescents and young adults. Prevalence of use rates at these ages are 4–12% over the last decade [Akaike et al., 1991; Johnston et al., 2007]. Knowledge of the effects of MDMA on the mature brain is extensive [Green et al., 2003], but little is known about its effects on the developing brain, despite the fact that use during pregnancy has been documented [McElhatton et al., 1999; Ho et al., 2001]. Regardless of this, no studies have yet been reported on the possible neurobehavioral effects of prenatal MDMA exposure in humans.
There are several studies in animals suggesting long-term CNS effects from exposure to MDMA during early brain development [Winslow and Insel, 1990; Meyer and Ali, 2002; Won et al., 2002; Kelly et al., 2002; Koprich et al., 2003; Meyer et al., 2004], and a few reporting no lasting effects [St. Omer et al., 1991; Broening et al., 1994; Broening et al., 1995; for a review, see Skelton et al., 2008]. In these experiments, rats were exposed to MDMA during stages of brain development analogous to the human first trimester and part of second trimester. There are even fewer models of second to third trimester-equivalent exposures.
Human second to third trimesters are analogous to rodent brain development during late gestation and into the neonatal period [West and Pierce, 1987; Bayer et al., 1993; Rice and Barone, 2000; Herlenius and Lagercrantz, 2004; Clancy et al., 2007a; Clancy et al., 2007b]. In developing an exposure model for MDMA comparable to third trimester human brain development, we focused on hippocampal development because of its involvement in learning and memory. In the dentate gyrus, incorporation of bromodeoxyuridine into proliferating hippocampal neurons shows peak labeling on postnatal day (PD) 9 followed by a gradual decline through PD 20 [Liu et al., 2003]. We previously found that rats exposed to MDMA exhibit long-term allocentric (spatial) learning and memory deficits in the Morris water maze (MWM) and egocentric (path integration) learning deficits in the Cincinnati water maze (CWM) when exposure was during PD 11–20, but not when exposure was during PD 1–10 [Broening et al., 2001]. We also demonstrated that the effects of PD 11–20 MDMA treatment on MWM performance were dose-related and selective for reference memory, whereas working memory [Vorhees et al., 2004] and cued learning [Williams et al., 2003b] in the MWM were unaffected. Novel object recognition was also impaired [Cohen et al., 2005]. We subsequently showed that the PD 11–20 MDMA-induced impairments were not due to growth retardation, injection, and/or litter effects [Williams et al., 2003b], and they were not attributable to maternal rearing factors [Broening et al., 2001; Williams et al., 2003b].
Treatment of developing animals with MDMA causes a significant release of corticosterone [Schaefer et al., 2006a; Schaefer et al., 2008]. Developmental exposure to stressors or high glucocorticoid levels result in deleterious long-term behavioral effects [Meaney et al., 1988]. The early postnatal period up to ~PD 14 is known as the stress hyporesponsive period (SHRP) when the adrenal response to stress is dampened [Sapolsky and Meaney, 1986]; however, when stressors are given, ACTH (adrenocorticotropic hormone) and corticosterone levels remain elevated for longer than they do in adults [Vazquez, 1998]. The SHRP is hypothesized to be the reflection of a neuroprotective mechanism that reduces the risk to developing neurons containing glucocorticoid receptors [Sapolsky and Meaney, 1986; Sapolsky, 1996]. While MDMA has multiple effects on the developing brain, including acute and long-term effects on 5-HT, PKA, and the 5-HT 1A receptors [Crawford et al., 2006; Schaefer et al., 2006a; Schaefer et al., 2008], an understanding of its effects during hypothalamic-pituitary-adrenal (HPA) axis development, and in particular in relationship to the SHRP, has not been explored. We have previously described a period of diminished corticosterone response to methamphetamine that indicates that the SHRP for this drug is from approximately PD 5 to 14 [Williams et al., 2006]. More recently, we have developed similar data for MDMA; these data show a drug-related diminished adrenal release of corticosterone from PD 5 to 13 [Schaefer et al., 2006b]. Therefore, we tested the effects of MDMA to determine if the period of vulnerability for learning deficits matched the drug-related SHRP. We predicted that exposures to MDMA within the SHRP would adversely affect later learning, whereas exposures outside the SHRP would be unaffected. This is based on findings that stressors such as maternal separation during the SHRP produce longer-lasting increases in ACTH and corticosterone than at other stages of development, and these long-lasting increases result in downregulation of hippocampal mineralocorticoid receptors and a permanent change in the mineralocorticoid receptor:glucocorticoid receptor ratio which has been suggested to determine the HPA axis set-point for stress responsiveness throughout life. This, in turn, is hypothesized to underlie the behavioral deficits that arise from exposure to stressors during the SHRP [Vazquez, 1998].
Accordingly, the present experiment was designed to test this idea by using 4 exposure intervals, 2 within and 2 outside the peak of the SHRP. Because the SHRP has imprecise boundaries, the exposure periods were set to be as close to what is known about the SHRP as possible from the drug-related data we developed previously [Williams et al., 2006; Schaefer et al., 2006b], but to be of the same length in order to equalize exposure duration and quantity for each treatment age (regimen). Accordingly, the following exposure intervals were tested: PD 1–5, 6–10, 11–15, and 16–20 with doses of 0, 10, 15, 20, and 25 mg/kg/dose for each regimen. Doses of MDMA were selected to compensate for the reduction in exposure interval compared to the previous 10-day exposure experiments [Broening et al., 2001; Williams et al., 2003b; Vorhees et al., 2004; Skelton et al., 2006; Vorhees et al., 2007].
Materials and Methods
Subjects
Nulliparous female Sprague-Dawley CD/IGS rats (Charles River, Raleigh, N.C., USA) were bred in-house to males of the same strain and supplier. Evidence of pregnancy was designated as embryonic day (ED) 0 and most females delivered on ED 22. Birth was designated PD 0 and litters were culled to 5 males and 5 females on PD 1. The animals were maintained in polycarbonate cages (46 × 24 × 20 cm) on a 14-hour light/10-hour dark cycle (lights on at 06: 00 h) with food and water available ad libitum in a vivarium at 21 ± 1° C with 50 ± 10% humidity, and accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The research protocol was approved by the Institutional Animal Care and Use Committee. Within each litter, 1 male and 1 female were uniquely identified from PD 1 to 5 with a permanent marker and by ear-punch thereafter. Each group received 4 doses given every 2 h each day. The groups received either 0, 10, 15, 20, or 25 mg/kg (+/−)3,4-methylenedioxymethamphetamine HCl (calculated as the free base and greater than 95% pure, obtained from the National Institute on Drug Abuse and dissolved in saline and given in a volume of 3 ml/kg).
We have previously shown that the biological half-life of MDMA in PD-1 rats is 2.75 h and in PD-11 rats it is 4 h after a dose of 20 mg/kg given subcutaneously [Williams et al., 2004]. Hence, the 2-h interdose interval used herein should produce an internal dose higher than that induced by a single 20-mg/kg injection (depending on the exposure age), but the internal dose will be much less than what would occur if the daily dose were given all at once in a single dose.
All doses were given subcutaneously in the dorsum, and injection sites were varied to prevent irritation. Twenty litters were assigned to each of the 4 exposure regimens (ages): PD 1–5, 6–10, 11–15, and 16–20. Once the experiment began, an increase in off-spring mortality was immediately observed in the PD 1–5 regimen. Accordingly, 25 litters were assigned to this condition, hence a total of 85 litters were used in the experiment. Since not all litters were born with the full complement of 5 males and 5 females, up to 2 pups were cross-fostered from litters of the same age if necessary.
All litters were weighed on PD 1, 5, 6, 10, 11, 15, 16, and 20 and prior to each dose. Litters were separated on PD 28 and housed in same-sex pairs for the remainder of the experiment. Offspring were weighed weekly after PD 28. Testing began on PD 60.
Elevated Zero Maze
Animals were tested in an elevated zero maze as a test of anxiety [Shepherd et al., 1994]. Animals were placed in the center of one of the closed areas of the ring-shaped apparatus, and behavior was recorded for 5 min with an overhead camera connected to a DVD-R. The circular runway was 105 cm in diameter with a path width of 10 cm; 2 quadrants of the ring were enclosed by black acrylic sidewalls and 2 quadrants were open; the latter had a 1-cm clear acrylic curb to prevent slipping off the edge [Williams et al., 2003a]. The test was conducted under dim halogen lighting. The dependent measures were: number of open entries, time in the open (front paws and shoulders outside of the closed area), latency to first open entry, and number of head dips. The runway was cleaned with 70% ethanol between animals.
Locomotor Activity
Locomotor activity was measured for 1 h in a 41 × 41 × 30 cm Accuscan activity monitor equipped with 16 pairs of photo-detector-LED beams along the X and Y axes (Accuscan Electronics with VersaMax software, Columbus, Ohio, USA). The test was conducted 1–1.5 h following removal from the elevated zero maze. The apparatus was cleaned with a 70% ethanol solution between animals. Total horizontal, vertical, and regional (central vs. peripheral) distances moved were recorded in 5-min intervals.
Novel Object Recognition
One day following locomotor activity testing, animals began habituation to the testing arena used for novel object recognition. Circular polyethylene (91 cm in diameter) testing arenas were used with 51-cm high side walls. Each animal was given 10 min exposure to the arena per day for 3 days to habituate to the test environment. The test phase took place on the fourth day and was divided into 2 phases: familiarization and retention. The familiarization phase entailed placing 2 identical objects 25 cm from the sides of the arena and 41 cm apart. Rats were placed in the arena between the 2 objects and given a maximum of 10 min to accumulate 30 s of object exploration [Clark et al., 2000]; any animal not meeting this criterion was eliminated from further object-recognition testing. Object exploration was defined as the animal standing within 1 cm and oriented towards the object. Exploration of an object was defined as the animal sniffing or pawing the object; however, climbing on the object was not counted [Clark et al., 2000]. The retention phase began 1 h after familiarization. A new object was introduced along with a duplicate of 1 of the original objects. As in the familiarization phase, the animals had 10 min to complete 30 s of object exploration. A video camera connected to a monitor was placed over the testing arena and behavior was scored in real-time by an observer using ODLog (Macropod Software, www.macropodsoftware.com) scoring program. Time exploring the objects was analyzed.
Straight Channel
After 1 day of no testing, animals were then tested in a 15 × 244 cm straight swimming channel with a wire escape ladder mounted at one end. Each rat received 4 consecutive trials on the test day. On each trial, the rat was placed at one end facing the end wall and timed until it grasped the escape at the opposite end (maximum time = 2 min/trial).
Cincinnati Water Maze
One day later, animals began testing in the CWM. The CWM was developed as previously described [Vorhees, 1987]. The CWM consists of 9 closed T-shaped cul-de-sacs that branch from a central channel extending from the starting point to the goal where an escape ladder is positioned. The arms of each T and the channels are 15 cm wide and the walls are 51 cm high. The water was 25 ± 1 cm deep and maintained at room temperature (22 ± 1° C). Testing was performed under infrared light using an infrared light emitter and closed circuit camera in order to eliminate extramaze (distal spatial) cues. Animals were acclimated to the dark for at least 5 min prior to being placed in the maze. To begin each trial, an animal was placed in the start position and allowed to find the goal (escape ladder). Two back-to-back trials per day were given with a 5-min limit per trial. Animals failing to escape within 5 min on their first daily trial were given at least a 5-min rest interval before their second trial. Errors, latency to escape, and number of returns to the start zone were recorded. An error was defined as head and shoulder entry into 1 of the arms of a T-section. Animals were tested for 15 consecutive days.
Morris Water Maze
The MWM tank was 210 cm in diameter with 51 cm high side walls, made of stainless steel, and painted flat black [Vorhees and Williams, 2006]. The maze was surrounded on all sides by white curtains that could be opened or closed to reveal or obscu re prominent room cues. In addition to the indigenous room cues, the 3 nearest walls (arbitrarily north, east, and west points) had large geometric figures prominently displayed. Animals were given 3 separate test phases. Each phase consisted of 4 trials per day (curtain open; 2-min limit per trial) for 5 successive days followed by 1 additional day on which a single probe trial was given (curtains open). The intertrial interval was 15 s spent on the platform. Animals not finding the platform within 2 min were removed from the water and placed on the platform. The platforms (either 10 × 10 cm or 5 × 5 cm) were made of clear acrylic with thin nylon screening attached to the surface. During testing, the platform was positioned 1.5–2 cm below the surface of the water, and was camouflaged by virtue of being transparent against a black back-ground. Water temperature was 21 ± 1 °C.
During phase 1 (acquisition phase), the 10 × 10 cm platform was located in the south-west quadrant halfway between the center and the sidewall. Rats were started at 1 of 4 positions located distal to the quadrant containing the platform in a random order, with the restraint that they received 1 trial from each of the 4 starting positions per day. The start positions used were: north-west, north, east, and south-east. These positions were used to eliminate short-path solutions such as when south or west start positions are used. The day after the last acquisition trial, each rat was given a single 30-second probe trial. For the probe trial, rats were started from a position they had never been started from before (north-east) with the platform removed.
The next day after the acquisition probe trial, rats were tested in the MWM in phase 2 (reversal) with the same 10 × 10 cm platform. For this phase, the platform was moved to the north-east quadrant. The same procedure used for acquisition was used for reversal (5 days, 4 trials/day). Start positions were south-east, south, west, and north-west. On the sixth day, the platform was removed and a 30-second reversal probe trial was given, with the start position being south-west.
The day after completion of the reversal phase, rats were tested in the MWM in phase 3 (shifted-reduced phase). For this phase, the platform was moved to the north-west quadrant, and the platform was changed to one 75% smaller than the original (i.e. 5 × 5 cm). All other aspects of the test were the same as for acquisition and reversal, including a 30-second probe trial the day after the last shifted-reduced trial.
Following completion of the 3 hidden platform phases of testing in the MWM, the animals were tested for cued learning. During this test, curtains were closed around the pool to obscure distal cues, and the platform had a plastic ball mounted above it on a steel rod in order to mark its location. During this test, both the start and platform positions were changed on every trial. Animals were given 4 trials on 2 successive days with a maximum of 2 min per trial and an intertrial interval of approximately 15 s (5 s on the platform and 10 s in a transfer cage).
Performance was recorded using Smart® video-tracking software (San Diego Instruments, San Diego, Calif., USA). For the learning trials, path length, cumulative distance from the platform, latency, and speed were analyzed. For the probe trials, dependent measures were time and distance in the target quadrant, number of site crossovers, average distance from the platform site, and mean search distance (the time in the target quadrant minus time in each of the other quadrants divided by 3). The tracker recorded the animal’s position every 0.2 s.
Statistical Procedures
Because the experiment used a split-litter design, offspring were matched on multiple factors by virtue of being littermates. In order to ensure that litter effects were controlled in the analyses, litter was treated as a random factor (block) in a completely randomized block model analysis of variance (ANOVA). In this model, group (i.e. doses) and sex were between factors within each block and litter was the block factor. Measures taken repetitively on the same animal, such as time interval for locomotor activity testing or day for maze testing, were treated as repeated-measure factors. Treatment age (regimen) was included as a between-subject factor since separate litters were used for each treatment regimen. Data were analyzed using general mixed-model ANOVA (Proc Mixed, SAS Institute, Cary, N.C., USA). Each data set was checked for best fit to different covariance models. In most cases, best-fit parameters indicated that the autoregressive-1 covariance structure was best. Significant interactions were analyzed using simple-effect slice ANOVA at each level of the repeated-measure factor. Kenward-Rogers degrees of freedom were used and can be fractional. Pairwise group comparison used the step-down Hochberg test (Proc Multtest, SAS Institute). Pairwise group comparisons were restricted to those between the saline and each MDMA treatment group within each treatment regimen (age). Based on multiple previous experiments with MDMA showing learning deficits, we predicted and only tested for MDMA-induced impaired performance on the learning tests (one-tailed). No predictions were made for the elevated zero maze or locomotor activity, any treatment effects on these were tested nondirectionally (two-tailed).
Results
General Characteristics
An analysis of mortality showed that for the PD 1–5 regimen, MDMA caused a significant increase in mortality (table 1). No significant MDMA-induced increase in mortality was found for the other 3 treatment regimens. All mortality in the experiment occurred either during the drug exposure period or shortly thereafter with one exception. The exception was 1 animal that died after weaning. The increased mortality observed in the PD 1–5 regimen was dose-dependent: increasing mortality with increasing MDMA dose. With the exception of a single animal in the PD 1–5 regimen, the saline controls were unaffected. Accordingly, the PD 1–5 mortality was the result of drug treatment and not early handling, maternal separation during dosing, or the stress associated with subcutaneous injections. Despite the increased mortality in the PD 1–5 regimen, the assignment of extra litters to the PD 1–5 regimen compensated for the losses sufficiently such that there were enough survivors to test behaviorally.
Table 1.
Offspring mortality from neonatal MDMA treatment
| Group | Sex | PD (regimen) |
|||
|---|---|---|---|---|---|
| 1–5 | 6–10 | 11–15 | 16–20 | ||
| Saline | male | 1/25 | 0/20 | 0/20 | 0/20 |
| female | 0/25 | 0/20 | 0/20 | 0/20 | |
| MDMA (mg/kg) | |||||
| 10 | male | 5/25 | 0/20 | 0/20 | 0/20 |
| female | 6/25** | 0/20 | 0/20 | 0/19 | |
| 15 | male | 7/25* | 1/20 | 0/20 | 0/20 |
| female | 7/25** | 0/20 | 0/19 | 0/19 | |
| 20 | male | 13/25** | 0/20 | 1/20 | 0/20 |
| female | 9/25** | 2/19 | 1/19 | 0/20 | |
| 25 | male | 17/25** | 1/19 | 2/20 | 0/20 |
| female | 20/25** | 1/20 | 2/19 | 0/20 | |
| Number of litters | 25 | 20 | 20 | 20 | |
Data show number of offspring that died during or shortly after treatment/total number of offspring. There was one death after weaning.
p < 0.05,
p < 0.01 compared to saline (same sex).
Analyses of body weights during treatment for the 4 different dosing regimens (10, 15, 20, and 25 mg/kg = groups MDMA-10, 15, 20, and 25, respectively) showed effects of drug treatment, sex, day, and drug treatment × day (all p < 0.0001). Slice effect ANOVA for the PD 1–5 regimen showed no treatment differences on PD 1 or 2; differences were significant on PD 3–5. On PD 3, the MDMA-25 and MDMA-20 groups were significantly lighter than saline controls, whereas on PD 4–5, all MDMA groups were lighter than controls.
Similarly for PD 6–10 body weights, slice ANOVA showed no drug treatment effects on PD 6, but all MDMA groups were lighter than saline controls on PD 7–10.
For PD 11–16 body weights, slice ANOVA showed no drug treatment effects on PD 11 and significant differences on PD 12–15 with all MDMA-treated groups lighter than saline controls.
The same pattern was seen for PD 16–20 body weights, with slice ANOVA showing no differences on PD 16 and significant differences on PD 17–20 with all MDMA-treated groups weighing less than saline controls.
In order to determine if body weight reductions seen during dosing recovered, offspring were weighed weekly from the end of treatment to the end of behavioral testing (PD 21–98; table 2). A repeated-measure ANOVA on weekly weights showed a significant drug treatment effect [F(4,757) = 16.39, p < 0.0001], but no drug treatment × week interaction. All MDMA-treated groups had lower body weights than the saline-treated group. Sex (males weighed more than females) and week effects were significant as was the sex × week interaction (all p < 0.0001). Regimen was not a significant factor alone, but did interact with week (p < 0.0005). However, slice effect ANOVA performed each week failed to show any week as having a significant regimen effect. The week that came closest was week 12 (PD 98; p = 0.057).
Table 2.
Offspring body weights (in grams)
| Regimen | Group |
||||
|---|---|---|---|---|---|
| Saline | MDMA-10 | MDMA-15 | MDMA-20 | MDMA-25 | |
| Males | |||||
| PD 1–5 | |||||
| 21 | 58.2 ± 6.4 | 53.8 ± 6.6 | 53.1 ± 6.9 | 50.8 ± 9.1 | 56.1 ± 11.0 |
| 56 | 337.0 ± 6.4 | 343.4 ± 6.6 | 342.8 ± 6.9 | 333.2 ± 9.1 | 325.1 ± 11.0 |
| 98 | 503.2 ± 6.4 | 511.1 ± 6.6 | 495.6 ± 6.9 | 487.2 ± 9.1 | 478.5 ± 11.0 |
| n | 20 | 19 | 17 | 9 | 6 |
| PD 6–10 | |||||
| 21 | 61.0 ± 6.4 | 53.2 ± 6.4 | 52.2 ± 6.6 | 49.5 ± 6.4 | 48.1 ± 6.7 |
| 56 | 360.9 ± 6.4 | 345.5 ± 6.4 | 343.2 ± 6.6 | 330.9 ± 6.4 | 340.7 ± 6.7 |
| 98 | 525.1 ± 6.4 | 506.2 ± 6.4 | 511.0 ± 6.6 | 480.6 ± 6.4 | 493.6 ± 6.7 |
| n | 20 | 20 | 19 | 20 | 18 |
| PD 11–15 | |||||
| 21 | 59.1 ± 6.6 | 50.9 ± 6.4 | 48.4 ± 6.6 | 46.7 ± 6.6 | 45.7 ± 6.7 |
| 56 | 360.9 ± 6.6 | 350.2 ± 6.4 | 345.6 ± 6.6 | 338.6 ± 6.6 | 338.5 ± 6.7 |
| 98 | 533.4 ± 6.6 | 515.3 ± 6.4 | 498.4 ± 6.6 | 508.3 ± 6.6 | 509.4 ± 6.7 |
| n | 19 | 20 | 19 | 19 | 18 |
| PD 16–20 | |||||
| 21 | 56.7 ± 6.3 | 49.0 ± 6.4 | 47.7 ± 6.4 | 47.7 ± 6.4 | 46.8 ± 6.4 |
| 56 | 354.3 ± 6.3 | 350.0 ± 6.4 | 351.3 ± 6.4 | 346.6 ± 6.4 | 343.7 ± 6.4 |
| 98 | 513.3 ± 6.3 | 515.3 ± 6.4 | 510.7 ± 6.4 | 507.4 ± 6.4 | 508.7 ± 6.4 |
| n | 20 | 20 | 20 | 20 | 20 |
|
| |||||
| Females | |||||
| PD 1–5 | |||||
| 21 | 55.9 ± 6.6 | 52.8 ± 6.9 | 52.4 ± 7.5 | 50.8 ± 7.5 | 54.5 ± 15.3 |
| 56 | 226.7 ± 6.6 | 222.6 ± 6.9 | 221.6 ± 7.5 | 221.0 ± 7.5 | 217.8 ± 15.3 |
| 98 | 302.2 ± 6.6 | 290.7 ± 6.9 | 292.8 ± 7.5 | 290.4 ± 7.5 | 290.2 ± 15.3 |
| n | 19 | 17 | 14 | 14 | 3 |
| PD 6–10 | |||||
| 21 | 58.5 ± 6.4 | 52.2 ± 6.4 | 49.8 ± 6.4 | 48.8 ± 6.7 | 48.4 ± 6.6 |
| 56 | 238.0 ± 6.4 | 227.9 ± 6.4 | 223.4 ± 6.4 | 222.1 ± 6.7 | 222.1 ± 6.6 |
| 98 | 316.4 ± 6.4 | 299.3 ± 6.4 | 288.1 ± 6.4 | 298.3 ± 6.7 | 298.5 ± 6.6 |
| n | 20 | 20 | 20 | 18 | 19 |
| PD 11–15 | |||||
| 21 | 57.5 ± 6.4 | 47.6 ± 6.4 | 45.3 ± 6.6 | 44.9 ± 6.7 | 43.1 ± 6.9 |
| 56 | 236.3 ± 6.4 | 218.1 ± 6.4 | 216.6 ± 6.6 | 221.7 ± 6.7 | 213.9 ± 6.9 |
| 98 | 316.9 ± 6.4 | 291.7 ± 6.4 | 291.5 ± 6.6 | 297.8 ± 6.7 | 288.8 ± 6.9 |
| n | 20 | 20 | 19 | 18 | 19 |
| PD 16–20 | |||||
| 21 | 56.4 ± 6.4 | 48.5 ± 6.6 | 47.0 ± 6.6 | 44.7 ± 6.4 | 44.3 ± 6.4 |
| 56 | 235.5 ± 6.4 | 229.4 ± 6.6 | 223.2 ± 6.6 | 230.0 ± 6.4 | 228.1 ± 6.4 |
| 98 | 315.1± 6.4 | 306.0 ± 6.6 | 296.9 ± 6.6 | 305.0 ± 6.4 | 305.8 ± 6.4 |
| n | 20 | 19 | 19 | 20 | 20 |
|
| |||||
| Mean across age and sex |
274.9 ± 2.1 | 265.8 ± 2.1* | 262.1 ± 2.1* | 260.3 ± 2.2* | 259.6 ± 2.6* |
Data are presented as least-squares means ± 8 SEM; n = the number of animals per group. PD 56 was chosen to show weight shortly before the start of behavioral testing on PD 60. PD 98 was chosen to show weight near the end of behavioral testing on PD 101.
p < 0.0001 compared to saline.
Elevated Zero Maze
No significant effects of drug treatment (either main effects or interactions) were found on time in open, number of open entries, latency to first open entry, or number of head dips. There were no effects of sex on time in open, latency to first open entry, or head dips. There was a significant effect of sex on number of open entries (p < 0.0001) reflecting that females were more active than males. There was a regimen effect on all 4 measures. Across all treatment groups, animals in the PD 11–15 regimen spent more time in the open, made more open entries, had shorter latencies to first open entry, and had more head dips than animals in the other regimens. This was followed closely by the PD 6–10 regimen, suggesting that handling/injection stress during these ages made the animals less anxious as adults. At the other end of the continuum, the PD 1–5 regimen had the least time in open, fewest open entries, and fewest head dips, suggesting they were more anxious. Data for time in open and number of open entries are shown in table 3.
Table 3.
Elevated zero maze: principal measures of performance are shown (time in open and number of open zone entries)
| Regimen | Group |
||||
|---|---|---|---|---|---|
| saline | MDMA-10 | MDMA-15 | MDMA-20 | MDMA-25 | |
| PD 1–5 | |||||
| Time in open, s | 82.1 ± 7.9 | 82.7 ± 7.9 | 84.3 ± 8.3 | 66.2 ± 9.5 | 81.5 ± 13.1 |
| Open entries, n | 8.1 ± 0.7 | 7.4 ± 0.7 | 8.4 ± 0.8 | 6.3 ± 0.9 | 8.2 ± 1.2 |
| Number of animals | 37 | 36 | 32 | 23 | 11 |
| PD 6–10 | |||||
| Time in open, s | 95.6 ± 7.7 | 100.1 ± 7.7 | 95.5 ± 7.8 | 94.0 ± 7.8 | 103.9 ± 7.9 |
| Open entries, n | 9.1 ± 0.7 | 10.3 ± 0.7 | 9.6 ± 0.7 | 9.3 ± 0.7 | 10.0 ± 0.7 |
| Number of animals | 39 | 40 | 38 | 38 | 37 |
| PD 11–15 | |||||
| Time in open, s | 100.4 ± 7.7 | 107.8 ± 7.7 | 110.1 ± 8.0 | 120.0 ± 7.9 | 109.9 ± 8.1 |
| Open entries, n | 8.7 ± 0.7 | 10.1 ± 0.7 | 9.9 ± 0.7 | 10.7 ± 0.7 | 9.8 ± 0.7 |
| Number of animals | 39 | 40 | 35 | 36 | 34 |
| PD 16–20 | |||||
| Time in open, s | 97.6 ± 7.7 | 90.6 ± 7.8 | 94.3 ± 7.8 | 104.1 ± 7.7 | 98.7 ± 7.7 |
| Open entries, n | 9.4 ± 0.7 | 8.2 ± 0.7 | 8.6 ± 0.7 | 9.1 ± 0.7 | 8.8 ± 0.7 |
| Number of animals | 40 | 38 | 38 | 40 | 40 |
Data are least-squares means ± SEM. In each group, males and females were combined since there were no significant treatment × sex interactions.
Locomotor Activity
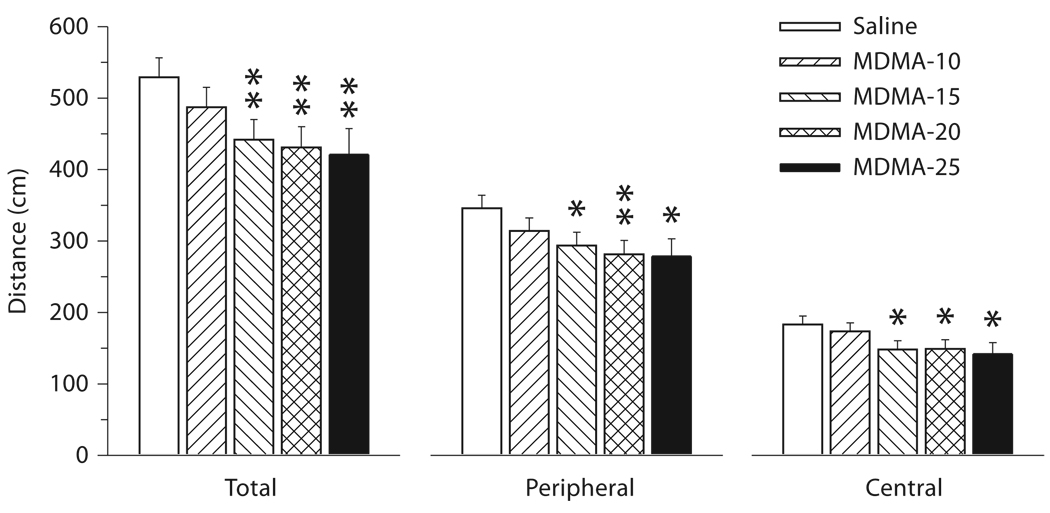
All measures showed similar patterns of significant drug treatment effects. For the measures of horizontal distance, drug treatment effects were evident (d.f. = 4, 554: total distance F = 3.46, p < 0.01, peripheral distance F = 3.03, p < 0.02, and central distance F = 2.70, p < 0.03). Sex was significant for total and peripheral distance (p < 0.001), but not central distance. In addition, there were drug treatment × interval interactions (d .f. = 20, 3,035: total distance F = 2 .42, p < 0.0 0 04, peripheral distance F = 1.80, p < 0.02, central distance F = 2.62, p < 0.0001). One other factor was significant only for central distance and this was the drug treatment × regimen × interval interaction [F(60, 3,195) = 1.44, p < 0.02]. For vertical movement (rears), the drug treatment main effect was not significant, but sex (p < 0.0001) and the drug treatment × interval interaction [F(20, 3,090) = 2.15, p < 0.01] were. For illustrative purposes, the distance measures are shown in figure 1. Pair-wise group comparisons comparing each MDMA group to control showed that for total distance the 3 highest-dose MDMA groups were less active than saline controls, while the MDMA-10 group did not differ significantly. This pattern was repeated for both peripheral and central distances, with no apparent differential peripheral versus central differences in the direction or magnitude of the MDMA effects.
Fig. 1.
Locomotor activity: horizontal distance (least-squares means ± SEM in centimeters ) summed over a 1-hour test session in 10-min intervals for each treatment regimen. Group sizes, summed across regimens and sexes (male/female), were: saline = 78/79, MDMA-10 = 79/76, MDMA-15 = 74/72, MDMA-20 = 69/70, and MDMA-25 = 62/59. * p < 0.05, ** p < 0.01 compared to saline controls.
For all variables, the drug treatment × interval interaction was further analyzed using slice effect ANOVA for each interval. Drug treatment (irrespective of treatment regimen) was significant for all variables for interval 1 (analyzed in 10-min intervals). In interval 1, the MDMA groups were less active than saline controls. For central distance, the 3-way interaction of drug treatment × regimen × interval was further analyzed by slice effect ANOVA for each interval of each regimen. For all 4 regimens, drug treatment effects were found only for interval 1. Pairwise group comparisons are shown in figure 2. For the PD 1–5 and PD 6–10 regimens, all four MDMA-treated groups were less active; however, for the PD 11–15 regimen, the effect was less pronounced with only the MDMA-15 and MDMA-25 groups showing significant reductions in central distance. For the PD 16–20 regimen, no MDMA-related hypoactivity during interval 1 was observed, and the MDMA-25 group showed a small but significant increase.
Fig. 2.
Locomotor Activity: horizontal distance (least-squares means ± SEM in centimeters) in the center of the arena during the first 10-min interval of the 60-min test shown separately for each regimen with males and females combined. Group sizes, summed across sexes, for each regimen are: PD 1–5: saline = 38, MDMA-10 = 36, MDMA-15 = 32, MDMA-20 = 24, MDMA-25 = 9; PD 6–10: saline = 40, MDMA-10 = 40, MDMA-15 = 39, MDMA-20 = 38, MDMA-25 = 37; PD 11–15: saline = 39, MDMA-10 = 40, MDMA-15 = 38, MDMA-20 = 37, MDMA-25 = 35; PD 16–20: saline = 40, MDMA-10 = 39, MDMA-15 = 39, MDMA-20 = 40, MDMA-25 = 40. * p < 0.05, ** p < 0.01 compared to saline controls.
Novel Object Recognition
An analysis of time spent investigating the 2 identical objects during familiarization showed no drug treatment or regimen effects. Similarly, during retention testing with 1 familiar and 1 novel object, no significant drug treatment or regimen effects were obtained (not shown).
Straight Channel
There were no significant effects of drug treatment (main effects or interactions) or regimen on straight channel swimming times, indicating that neither MDMA nor age of treatment had any effect on basic swimming ability or motivation to escape (not shown).
Cincinnati Water Maze
Analyses of CWM performance showed no drug treatment effects (either main effects or interactions) for errors, latency to escape, or start returns (not shown). In addition, there were no significant effects of regimen on any of these measures. There were significant effects of sex for errors (p < 0.0001), latency (p < 0.0001), and start returns (p < 0.0001). On this test, as we have found previously, females (regardless of treatment group) performed significantly better than males (i.e. females had fewer errors, shorter latencies, and fewer start returns).
MWM – Phase 1
Analyses on learning trials focused on latency, path length and cumulative distance from the goal during all 3 test phases. There was good agreement among these variables, and we have previously shown them to be highly correlated among themselves [Vorhees and Williams, 2006]; therefore, data for path length are reported to illustrate the findings.
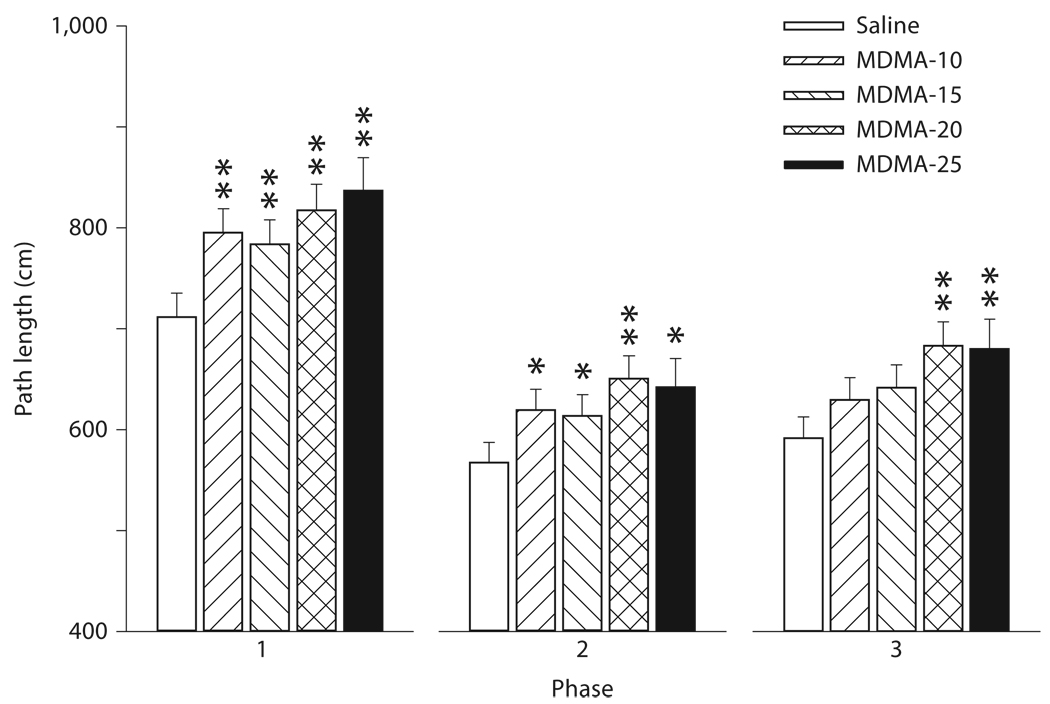
MDMA treatment significantly impaired learning to find the hidden platform as measured by path length during phase 1 [acquisition; F(4, 634) = 4.22, p < 0.003]. Other significant factors were sex (p < 0.0003), regimen (p < 0.03), day (p < 0.0001) and regimen × day (p < 0.004). When further analyzed, the regimen × day effect was primarily attributable to a regimen effect on day 1 (p < 0.001). The day-1 regimen effect, averaged across groups, is illustrated in figure 3 a. As can been seen, the PD 6–10 and PD 11–15 regimens had longer path lengths than those in the PD 1–5 regimen and the PD 11–15 regimen had longer path lengths than the PD 16–20 regimen. The sex effect was caused by the fact that across all groups and regimens, males performed better than females (ordinary mean ± SEM averaged across days: males = 740.0 ± 12.8 cm, females = 841.2 ± 17.2 cm). In terms of the hypothesis under investigation, there were no drug × regimen, drug × regimen × sex, drug × regimen × day, or 4 -way drug × regimen × sex × day effects. The significant main effect of drug treatment, averaged across regimens, is illustrated in figure 4. As can be seen, during phase 1, all four MDMA-treated groups had significantly increased path lengths compared to saline controls by approximately 15%.
Fig. 3.
MWM treatment regimen effects: path length (least-squares means ± SEM in centimeters) to find the submerged platform averaged across trials, sexes and treatment groups to illustrate the main effect of treatment regimen (age). a Regimen effects during phase 1 (acquisition) of testing on day 1. b Regimen effects during phase 2 (reversal) of testing on day 2. Group sizes averaged across treatment groups and sexes for each regimen were: PD 1–5: = 139, PD 6–10 = 194; PD 11–15 = 189; PD 16–20 = 198. ** p < 0.01 between the regimens connected by brackets.
Fig. 4.
MWM treatment effects: path length (LS means ± SEM in centimeters) during the 3 phases of MWM averaged across days, trials, and regimens. Group sizes averaged across days, trials, and regimens were: saline = 157, MDMA-10 = 155, MDMA-15 = 149, MDMA-20 = 138, and MDMA-25 = 121. * p < 0.05, ** p < 0.01 compared to saline controls.
Variables analyzed on the probe trials were average distance from the platform site, percent time and distance in the target quadrant, number of site crossovers, and mean search distance (distance in the target quadrant minus distance in each of the other quadrants subtracted separately and divided by 3). Analyses of these variables for the phase-1 probe trial showed no significant effects of drug treatment or regimen. Sex was significant because males performed significantly better than females (average distance from platform site: males = 126.9 ± 1.7 cm, females = 139.0 ± 1.7 cm).
MWM – Phase 2
For phase 2 (reversal), the ANOVA on path length showed a drug treatment effect [F(4, 626) = 2.51, p < 0.04]. Other factors were a significant effect of sex (p < 0.0001), day (p < 0.001), regimen × day (p < 0.03), and a nearly significant effect of regimen (p < 0.052). In terms of the hypothesis, there were no significant interactions of drug treatment × regimen. Further analysis of the regimen × day effects showed that regimen was significant only on day 2 (p < 0.004). The day-2 regimen effect was that the PD 6–10 and PD 11–15 regimens had longer path lengths than the PD 16–20 regimen (fig. 3 b). The drug treatment effect irrespective of regimen for phase 2 is shown in figure 4. As for phase 1, in phase 2 all four MDMA-treated groups had longer swim paths to the goal than saline controls.
For the probe trial conducted after phase-2 training, there were no drug treatment effects and no drug treatment × regimen interactions (not shown). Sex was significant as before.
MWM – Phase 3
The ANOVA on path length revealed an effect of drug treatment [F(4, 607) = 3.12, p < 0.015]. Sex was also significant (p < 0.0001); regimen was not. In terms of the hypothesis, there were no significant interactions between drug treatment × regimen. The effects of drug treatment, irrespective of regimen, are shown in figure 4. During this phase, the MDMA-20 and MDMA-25 groups differed significantly in path length to the goal compared to saline controls, whereas the MDMA-10 and MDMA-15 groups did not.
For the probe trial conducted after phase 3, there were no effects of drug treatment or drug treatment × regimen (not shown). Sex was significant as before.
Learning curves of path length for MWM phase 1 are shown in figure 5. Drug group differences emerged rapidly, and were already apparent in all MDMA groups on day 1. Figure 5 (inset) shows the emergence of these differences when plotted by trial for day 1. As can be seen, the MDMA-treated groups started out on trial 1 at performance levels no different from those of saline controls. However, thereafter saline controls showed greater reductions in path lengths than the MDMA-treated groups. These data reveal that the MDMA groups did not start the task with any preexisting swimming impairment that might explain their deficits in terms of performance effects. Rather, the MDMA-treated groups began the test comparably to controls, but failed to eliminate off-target swim paths to the goal as quickly as saline controls.
Fig. 5.
MWM phase 1 (acquisition) learning curves. Main panel: mean ± SEM path lengths (cm) shown for each treatment group averaged across 4 daily trials, regimens, and sexes. Inset: path length shown by trial on day 1 averaged across regimens and sexes (ordinate = cm). Significant treatment group differences are shown in figure 4. Group sizes for each group by regimen with sexes combined are (in regimen order: PD 1–5/6–10/11–15/16–20): saline = 38/40/39/40; MDMA-10 = 36/40/40/39; MDMA-15 = 33/39/38/39; MDMA-20 = 23/38/37/40; MDMA-25 = 9/37/35/40.
MWM – Swim Speed
On all 3 test phases, for both learning and probe trials, swimming speed was also analyzed. There were no drug treatment effects on swimming speed seen in any of these analyses (not shown). This further indicates that MDMA-treated animals do not have a performance deficit that would interfere with their ability to learn the task; rather, the data suggest that a central deficit led to the observed spatial navigation impairments.
MWM – Cued
There were no significant drug treatment impairments found on cued trials; in fact, the opposite was seen, and all the drug groups reached the visible platform more quickly than saline controls [F(4, 614) = 2.90, p < 0.03; saline = 14.2 ± 0.5, MDMA-10 = 12.7 ± 0.5, MDMA-15 = 12.7 ± 0.5, MDMA-20 = 13.2 ± 0.5, MDMA-25 = 12.2 ± 0.6 s]. This reinforces the evidence that MDMA-treatment did not impair swimming performance even though it impaired spatial navigation ability.
Discussion
The major findings from this experiment were that neonatal MDMA treatment during any 1 of 4 intervals from PD 1–20 causes dose-dependent impaired learning in the MWM when tested as adults. The data demonstrated that these allocentric (spatial) deficits were independent of swimming speed, were present during all 3 phases of the test (acquisition, reversal, and shifted-reduced platform trials), and were selective, in that other types of learning were not affected. The effects were consistent inasmuch as they were seen on all measures (path length, latency, and cumulative distance). By contrast, cued learning was unimpaired by MDMA as previously reported with a 10-day dosing regimen [Williams et al., 2003b]. Unexpectedly, egocentric learning tested in the CWM was not significantly affected by the shorter regimen of MDMA treatment, nor was novel object recognition despite the fact that we have seen MDMA-induced deficits in both of these tests when the treatment interval was longer (PD 11–20) [Williams et al., 2003b; Cohen et al., 2005]. There was also no effect observed on indices of anxiety in the elevated zero maze. On the other hand, MDMA-treated groups were less active in a test of locomotor activity and this effect was largely confined to the first 10 min. The activity reduction was modest and was relatively uniform with no differential effect on central versus peripheral exploration, and activity levels were comparable to saline controls for the last 50 min. Therefore, the slight reduction in activity on land is unlikely to explain any deficits that were observed in MWM performance. Moreover, if a reduction in activity were to have an effect on swimming then it should have affected straight channel, CWM, and MWM alike; in fact, only the learning trials of the MWM were affected, swimming speed was not, making a general hypoactivity explanation for the MWM learning deficits implausible.
The central hypothesis of this experiment arose from data showing that there is a developmental profile of MDMA-induced corticosterone release that closely matches the SHRP. There are also SHRP data showing that this is a vulnerable stage of development if animals are exposed to stressors that can produce high glucocorticoid levels. It has been noted that stressors applied during the SHRP can lead to long-term biochemical and behavioral changes [Vazquez, 1998]. On the basis of observations about the SHRP, we anticipated that an exposure period to MDMA that preceded the SHRP (i.e. PD 1–5) would show a diminished learning and memory deficit. Similarly, we anticipated that exposure periods after the peak of the SHRP (PD 16–20) would also show a diminished response to the drug. This prediction was not confirmed inasmuch as all 4 exposure periods induced statistically comparable impaired allocentric learning, although a nonsignificant trend consistent with increased effects during the SHRP was observed in the group means. Also, in contrast to our predictions and previous data with longer exposure periods, none of the 4 exposure periods impaired egocentric learning or novel object recognition, even though we have shown impairment on both of these tests after PD 11–20 MDMA treatment [Cohen et al., 2005; Vorhees et al., 2007]. We surmise that egocentric learning and novel object recognition were significantly affected by both dose and duration of exposure in our previous experiments, rather than dose alone. Why this might be the case given that our PD 11–15 and PD 16–20 dosing regimens completely cover the PD 11–20 exposure interval is not clear, but suggests that there must be a cumulative damaging effect with the 10 days of treatment that does not occur with separate 5-day treatment periods even with total drug exposure similar between the 10-day and 5-day regimens. An interesting question would be whether a different 10-day exposure period, such as PD 6–15 or PD 7–16, etc., would more closely resemble the findings obtained herein or would more closely resemble those we have reported previously after PD 11–20 treatment. Regardless, the data point to an interesting observation: the effects of developmental MDMA treatment are not easily predicted based on treatment period alone, and that to characterize long-term effects from early drug treatments requires investigation of exposure period, dose, and duration of exposure. The permutations that arise from the combinations of 3 factors suggest significant complexity in predicting the effects of developmental drug treatments on brain function.
The extrapolation of dose across species is a complex matter because of metabolic rates, pattern of metabolite production, volume of distribution and other factors that differ significantly between humans and rats. We have previously discussed how the current doses compare (favorably) to reported human MDMA doses when interspecies dose-scaling formulas are used [Skelton et al., 2008]. However, these scaling approaches have limitations as noted elsewhere [De La Torre and Farre, 2004]. Another way doses may be compared is based on plasma drug concentrations. A recent human study measured plasma MDMA 8–10 h after the start of a rave party at which MDMA was taken. Ten hours is close to the biological half-life of MDMA in humans [Green et al., 2003]. In this study, the average plasma MDMA concentration in the highest using subgroup at 1 half-life post-exposure was approximately 750 ng/ml [Irvine et al., 2006]. Based on our previous pharmacokinetic study in young rats, where the biological half-life of MDMA at PD 1 was 2.75 h and at PD 11 was 4 h, the approximate plasma concentration of MDMA 1-half-life postexposure to 20 mg/kg was 1,000 ng/mg at PD 1 and 2,200 ng/ml at PD 11 [Williams et al., 2004]. This comparison suggests that although our doses are higher than those in this particular human study, they are in the same range of our 20 mg/kg group. Therefore, since we found effects at the 2 lower doses of 10 and 15 mg/kg of MDMA, the plasma levels in rats at these doses would be even closer to the human values [Irvine et al., 2006]. Offsetting this in the opposite direction is the fact that we gave repeated doses whereas our neonatal pharmacokinetic data are based on single doses. However, human users also take multiple doses to obtain the 750 ng/ml concentrations, suggesting that a multiple-dose model such as ours bears a reasonable resemblance to human use patterns.
The doses MDMA users take per occasion vary widely. In a review of the literature Parrott [2005] found that novice users typically take 1 tablet, regular users 2–3 tablets, and heavy users 10–25 tablets per occasion. In surveys of confiscated ecstasy tablets, analytical assays show that tablets are relatively consistent and contain 75–100 mg of MDMA. Using the 100 mg/tablet for comparisons purposes, this would translate into doses of 100, 200–300, and 1,000–25,000 mg per occasion of ingestion of MDMA for novice, regular, and heavy users, respectively. Assuming a body mass of 60 kg for a pregnant woman, these weight-adjusted doses would be 1.7, 3.3–5, and 16.7–41.7 mg/kg/occasion. These extrapolations suggest that the current experiment is modeling the heavy use pattern, but a very realistic pattern nevertheless.
Together with our previous data on the acute and long-term effects of developmental MDMA [for a recent review, see [Skelton et al., 2008] these studies demonstrate that MDMA has clear long-term adverse effects on brain function. This suggests that similar effects may occur in children exposed during pregnancy to MDMA.
Acknowledgments
This research was supported by the following grants from the US National Institutes of Health: DA006733 and DA021394 (C.V.V.), and DA014269 (M.T.W.). We thank Mary S. Moran for assistance with data analyses.
References
- Akaike M, Kato N, Ohno H, Kobayashi T. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol. 1991;13:317–322. doi: 10.1016/0892-0362(91)90077-a. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age dependent sensitivity of rats to the long-term effects of the serotonin neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy) induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV. Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [35S]GTP-gammaS binding in adult rats. Brain Res. 2006;1077:178–186. doi: 10.1016/j.brainres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 2004. 2003;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3,4-methylenedoxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Irvine RJ, Keane M, Felgate P, McCann UD, Callaghan PD, White JM. Plasma drug concentrations and physiological measures in ‘dance party’ participants. Neuropsychopharmacology. 2006;31:424–430. doi: 10.1038/sj.npp.1300896. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. college students and adults ages 19–45. Vol. II. Bethesda: National Institute on Drug Abuse - US Department of Health and Human Services; 2007. Monitoring the Future: national survey results on drug use, 1975–2006. [Google Scholar]
- Kelly PAT, Ritchie IM, Quate L, McBean DE, Olverman HJ, Aase JM. Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol. 2002;137:963–970. doi: 10.1038/sj.bjp.0704961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Chen E-Y, Kanaan NM, Campbell NG, Kordower JH, Lipton JW. Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases fore-brain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol Teratol. 2003;25:509–517. doi: 10.1016/s0892-0362(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- McElhatton PR, Bateman DN, Evans C, Pughe KR, Thomas SH. Congenital anomalies after prenatal ecstasy exposure. Lancet. 1999;354:1441–1442. doi: 10.1016/s0140-6736(99)02423-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnager S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Ali SF. Serotonergic neurotoxicity of MDMA (ecstasy) in the developing rat brain. Ann NY Acad Sci. 2002;965:373–380. doi: 10.1111/j.1749-6632.2002.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (‘ecstasy’) administration on neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3,4-methylenedioxymeth-amphetamine) or ecstasy. J Psychopharmacol. 2005;19:71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−methylphenidate administration in the neonatal rat. J Neurochem. 2006a;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Herring NR, Grace CE, Skelton MR, Johnson HL, Vorhees CV, Williams MT. A comparison of preweaning 5-methoxy-diisopropyltryptamine and (±)3,4-methylenedioxymethamphetamine administration on postweaning anxiety, learning and locomotor activity in rats. Abstr Soc Neurosci, Atlanta, October 2006. 2006b;98:1369–1378. [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short-and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterization of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11–20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berlin) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Developmental effects of 3,4-methylenedioxymethamphetamine: a review. Behav Pharmacol. 2008;19:91–111. doi: 10.1097/FBP.0b013e3282f62c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Omer VE, Ali SF, Holson RR, Scalzo FM, Slikker W., Jr Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA) exposure in rats. Neurotoxicol Teratol. 1991;13:13–20. doi: 10.1016/0892-0362(91)90022-o. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of +/−3,4-methylenedioxymethamphetamine on spatial versus path integration learning: effects of dose distribution. Synapse. 2007;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Pierce DR. Perinatal alcohol exposure and neuronal damage. In: West JR, editor. Alcohol and Brain Development. New York: Oxford University Press; 1987. pp. 120–157. [Google Scholar]
- Williams MT, Brown CA, Skelton MR, Vinks AA, Vorhees CV. Absorption and clearance of ±3,4-methylenedioxymethamphetamine from the plasma of neonatal rats. Neurotoxicol Teratol. 2004;26:849–856. doi: 10.1016/j.ntt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Res. 2003b;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: the effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylendioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–220. [PubMed] [Google Scholar]
- Won L, Bubula N, Heller A. Fetal exposure to (±)-methylenedoxymethamphetamine in utero enhances the development and metabolism of serotonergic neurons in three-dimensional reaggregate tissue culture. Dev Brain Res. 2002;137:67–73. doi: 10.1016/s0165-3806(02)00411-x. [DOI] [PubMed] [Google Scholar]