Abstract
Cellular signalling pathways are critical in regulating the balance between latency and lytic replication of herpesviruses. Here, we investigated the effect of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway on replication of two gamma-2 herpesviruses, murine gammaherpesvirus-68 (MHV-68) and human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus (HHV-8/KSHV). We found that de novo infection of MHV-68 induced PI3K-dependent Akt activation and the lytic replication of MHV-68 was enhanced by inhibiting the PI3K-Akt pathway with both chemical inhibitors and RNA interference technology. Inhibiting the activity of Akt using Akt inhibitor VIII also facilitated the reactivation of KSHV from latency. Both lytic replication and latency depend on the activity of viral transactivator RTA and we further show that the activity of RTA is increased by reducing Akt1 expression. The data suggest that the PI3K-Akt pathway suppresses the activity of RTA and thereby contributes to the maintenance of viral latency and promotes tumorigenesis.
The phosphatidylinositol 3-kinases (PI3Ks) are a family of enzymes (classes I, II and III) that produce lipid second messengers by phosphorylation of plasma membrane phosphoinositides at the 3′OH of the inositol ring. One major function of PI3K is to produce PtdIns(3,4,5)P3 (PIP3) from PtdIns(4,5)P2 (PIP2) (Vanhaesebroeck et al., 2001). PIP3 is crucial for the activation of Akt, a serine/threonine kinase that has a wide range of substrates. Akt can delay apoptosis by inactivating a number of pro-apoptotic proteins, including the Bcl2 family member BAD, caspase-9 and glycogen synthase kinase-3 beta (GSK-3β). Akt has also been found to regulate apoptosis through transcriptional control of pro- and anti-apoptotic genes. In recent years, the PI3K-Akt pathway has emerged as an important regulator of mammalian cell proliferation and survival. Several components of this pathway are dysregulated in a wide spectrum of human cancers (Cooray, 2004; Vivanco & Sawyers, 2002).
Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus (HHV-8/KSHV) is aetiologically linked with several types of tumours. There is accumulating evidence suggesting that the PI3K-Akt pathway is involved in the development of virus-associated neoplasms. It has been demonstrated that Akt is activated in KSHV-associated Kaposi's sarcoma (KS) (Sodhi et al., 2004) and primary effusion lymphoma (PEL) (Uddin et al., 2005). K1 and the G protein-coupled receptor of KSHV were reported to activate the PI3K-Akt pathway (Montaner et al., 2001; Tomlinson & Damania, 2004). The mammalian target of rapamycin (mTOR) is a downstream effector of Akt (Hay, 2005). The mTOR inhibitor rapamycin was reported to cure cutaneous KS in a clinical setting (Stallone et al., 2005) and was efficacious against PEL in culture and in a murine xenograft model (Sin et al., 2007). However, it remains unclear if the PI3K-Akt pathway directly affects viral replication.
Herpesviruses are characterized by two distinct phases of their life cycle: lytic replication and latency. Both phases contribute to associated viral pathogenesis. Cell lines are available to study latency and reactivation but there is no robust system to study de novo lytic infection of human gammaherpesviruses. Murine gammaherpesvirus 68 (MHV-68), which is closely related to human gammaherpesviruses (Virgin et al., 1997) and replicates to a high level in many cell lines, has been used as a model system for this purpose. We therefore explored the role of PI3K-Akt signalling during viral lytic replication using MHV-68 infection of permissive fibroblasts (NIH3T3) and epithelial cells (293T), and its role in reactivation using a B cell line latently infected with KSHV.
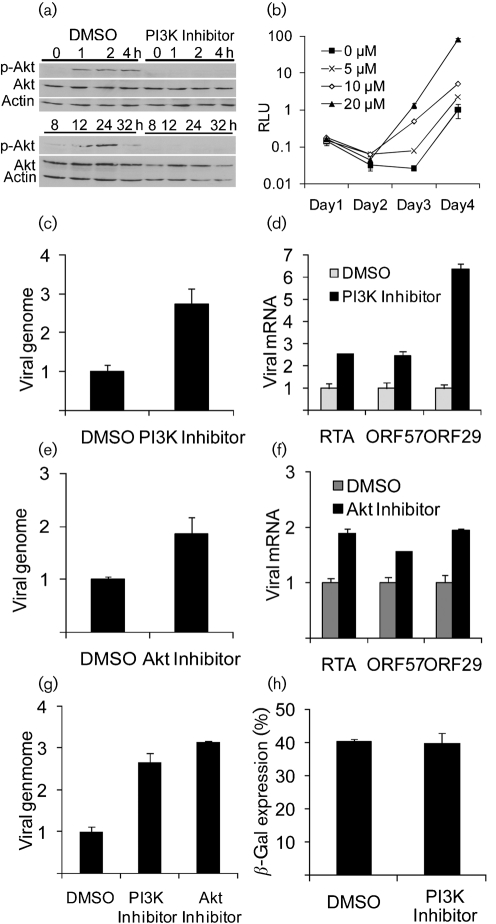
We first investigated whether MHV-68 infection led to activation of the PI3K-Akt cascade by examining the level of phosphorylated Akt. As shown in Fig. 1(a), in MHV-68-infected NIH3T3 cells, phosphorylated Akt was detected in a biphasic pattern between 1 and 32 h post-infection (p.i.), with no change in the total level of Akt protein. In cells treated with the PI3K inhibitor, LY294002, no phosphorylated Akt was detected. These results suggest that MHV-68 infection activated Akt in a PI3K-dependent manner; the two phases of activation possibly correlated with viral binding/entry and viral gene expression, which is consistent with the observation in KSHV infection (Sadagopan et al., 2007). We then studied the role of the PI3K-Akt pathway in MHV-68 de novo infection by examining whether the PI3K inhibitor affected virus production. In this experiment, we used a recombinant MHV-68 virus (M3FL) that contained a viral M3 promoter-driven firefly luciferase cassette incorporated in a non-essential region of the viral genome, for efficient quantification by measuring the luciferase activity. The construction of M3FL and the validation of this method by comparing with plaque assay were reported previously (Hwang et al., 2008). NIH3T3 cells were infected at 0.02 p.f.u. per cell with M3FL in the presence of various concentrations of LY294002 and then the supernatants were sampled and refilled (with inhibitors) every day for 4 days. The harvested supernatants containing the M3FL virus were inoculated onto fresh 293T cells and 24 h later, the luciferase activity in the cells was measured. As shown in Fig. 1(b), the virus production was enhanced at days 3 and 4 p.i. by the PI3K inhibitor in a dose-dependent manner. We investigated the effect of residual inhibitor present in the harvested supernatants on the virus production in fresh 293T cells. The result indicated that the residual inhibitor present in the inoculum, and therefore added to the 293T cell culture, did not contribute to the observed enhancement effect in Fig. 1(b) (data not shown). To confirm the enhancement effect, we used Q-PCR to measure the levels of viral DNA and mRNA in wild-type MHV-68 infection. Consistent with the previous growth curve, the viral DNA copy number was also increased by treatment with the PI3K inhibitor (Fig. 1c). In accordance with above results, transcript levels of immediate-early (RTA), early (ORF57) and late (ORF29) genes were all enhanced when PI3K activity was inhibited (Fig. 1d). Since PI3K has many downstream effectors, in order to test if the effect was mediated by Akt inhibition, we used Akt inhibitor VIII (Calbiochem), which specifically blocks Akt1 and Akt2 activity (Lindsley et al., 2005). Similar to our previous data using the PI3K inhibitor, MHV-68 DNA replication (Fig. 1e) and gene expression (Fig. 1f) were enhanced in the presence of Akt inhibitor VIII. The enhancing effects by both inhibitors were also observed in 293T cells (Fig. 1g). The viral DNA copy numbers were increased, compared with mock treatment, by the treatment with either inhibitor. These results indicate that inhibition of the PI3K-Akt pathway enhances viral gene expression, DNA replication and production of infectious virus. We also found that rapamycin did not promote MHV-68 replication in NIH3T3 cells (data not shown), which suggests that mTOR might not be the mediator of the enhancement effect of Akt inhibition on de novo infection.
Fig. 1.
Effect of the PI3K-Akt inhibitors on MHV-68 replication in NIH3T3 and 293T cells. (a) Akt phosphorylation following MHV-68 infection. NIH3T3 cells were treated with DMSO or PI3K inhibitor (20 μM) for 1 h and infected with MHV-68 at 3.0 p.f.u. per cell. Cell lysates prepared at specific time points p.i. were subjected to Western blotting using phospho-Akt (Ser473) (top panel), total Akt (middle panel) and actin (bottom panel) antibodies. (b) Virus production in NIH3T3 cells (treated with LY294002) assayed by luciferase activity. RLU, Relative light units. (c, e) Q-PCR assay of viral DNA in NIH3T3. (d, f) RT-Q-PCR analysis of viral transcripts in NIH3T3. (c–f) Cells were treated with DMSO or PI3K inhibitor (20 μM) (c, d) or 5 μM of Akt inhibitor VIII (e, f) for 1 h and then infected with MHV-68 at 0.1 p.f.u. per cell. Forty-eight hours later, total DNA was extracted and subjected to Q-PCR (c, e). Total RNA was extracted 16 (d) or 48 (f) h p.i. and subjected to reverse transcription and followed by Q-PCR assays specifically amplifying the spliced cDNA products (d, f). (g) Q-PCR assay of viral DNA in 293T. Cells were treated with 20 μM PI3K inhibitor or 5 μM Akt Inhibitor VIII for 1 h before being infected with MHV-68 at 0.1 p.f.u. per cell. Forty-eight hours later, total DNA was extracted and subjected to Q-PCR. In (c–g), the levels of viral genomes and viral cDNAs were normalized against cellular β-actin DNA and cDNA, respectively. (h) The percentage of β-gal-expressing NIH3T3 cells. In (c–h), the columns represent relative values to those of DMSO-treated samples (with the value of DMSO-treated sample as 1). Experiments were carried out multiple times independently. For each panel, the result of a representative experiment is shown; error bars show sd of triplicates.
To address whether PI3K activity has any effect on viral entry into cells, we used a recombinant virus (MHV-68/β-gal) whose genome contains a β-galactosidase (β-gal) cassette driven by a cytomegalovirus (CMV) immediate-early gene promoter (CMV-IEp). NIH3T3 cells were pretreated with LY294002 and then infected at 0.1 p.f.u. per cell. At 12 h p.i., the cells were fixed to examine the expression of β-gal. If the β-gal recombinant virus enters a cell and the viral genome enters the nucleus leading to β-gal expression, the cell produces a blue colour after being fixed and treated with X-Gal, the substrate of β-gal (Tiwari et al., 2007). Thus, individual cells which turn blue can be counted indicating that each blue cell has been successfully infected. As shown in Fig. 1(h), we found that the PI3K inhibitor had no significant effect on the percentage of β-gal-expressing cells. The result indicates that PI3K activity does not affect the early steps of the viral life cycle, such as viral entry, uncoating and delivery of the viral genome into the nucleus.
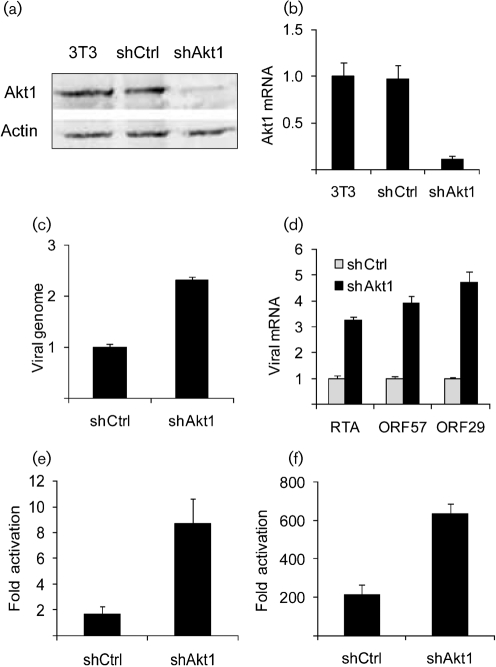
To confirm the results obtained using the chemical inhibitors further, we used RNA interference (RNAi) technology to specifically reduce the expression of Akt. We generated a retrovirus vector pSR-shAkt1 expressing a short hairpin RNA sequence against Akt1, which makes up the majority of total cellular Akt (Liu et al., 2006). pSR-shAkt1 was constructed by cloning the annealed primers that contain the shAkt1 sequence (5′-GATCCGTGACCATGAACGAGTTTGATTCAAGAGATCAAACTCGTTCATGGTCACTTTTTTACGCGTG-3′ and 5′-AATTCACGCGTAAAAAAGTGACCATGAACGAGTTTGATCTCTTGAATCAAACTCGTTCATGGTCACG-3′) into pSIREN-RetroQ (pSR, Clontech Laboratories). A control vector, pSR-shCtrl, expressing a control siRNA that has no sequence homology to any gene transcript was constructed similarly using annealed primers (5′-GATCCGCGTACGCGGAATACTTCGATTCAAGAGATCGAAGTATTCCGCGTACGCTTTTTTACGCGTG-3′ and 5′-AATTCACGCGTAAAAAAGCGTACGCGGAATACTTCGATCTCTTGAATCGAAGTATTCCGCGTACGCG-3′). Retroviruses derived from pSR-shAkt1 and pSR-shCtrl were used to infect NIH3T3 cells. After selection with puromycin, cell populations with stable expression of shAkt1 and shCtrl were established. Successful inhibition of Akt1 expression in 3T3-shAkt1 cells was confirmed by both Western blot with Akt1 antibody (Fig. 2a) and Q-PCR with primers specific for Akt1 mRNA (Fig. 2b). The 3T3-shCtrl and 3T3-shAkt1 cells were then infected with MHV-68. Similar to the results obtained from pharmacological inhibition of the PI3K-Akt pathway, specific inhibition of Akt1 expression increased both viral DNA replication (Fig. 2c) and expression of immediate-early, early and late genes (Fig. 2d). These results confirmed that inhibition of the PI3K-Akt pathway enhances viral replication upon MHV-68 de novo infection in NIH3T3 cells.
Fig. 2.
Effect of the inhibition of Akt1 expression on MHV-68 replication. (a) Western blot analysis of Akt1 protein expression. Antibodies against total Akt and β-actin were used. (b) RT-Q-PCR analysis for the Akt1 transcript. (c) Q-PCR analysis of MHV-68 DNA. (d) RT-Q-PCR analysis of MHV-68 transcripts. 3T3-shCtrl and 3T3-shAkt1 cells were infected with MHV-68 at 0.1 p.f.u. per cell. Total RNA was harvested at 24 h p.i. and total DNA was extracted at 48 h p.i. for quantification. In (b–d), columns represent relative values to those of the corresponding control samples. (e, f) Effect of inhibition of Akt1 expression on RTA trans-activation of lytic gene promoters. Cells were transfected with RTA promoter (e) or ORF57 promoter (f) reporter in the absence or presence of RTA protein expression plasmid (0.5 ng was used in transfections done in 96-well plates). The fold change in activation was calculated by dividing reporter activity in the presence of RTA by that in the absence of RTA. In all reporter assays, an internal control of pRL-SV40 (Promega) was included in the transfection for normalization. The cell lysates were harvested at 24 h post-transfection for Dual luciferase assay (Promega). Experiments were carried out multiple times independently. For each panel, the result of a representative experiment is shown; error bars show sd of triplicates.
MHV-68 lytic replication is initiated by the viral transactivator, RTA, an immediate-early gene which regulates expression of downstream viral lytic genes (Wu et al., 2001). We have shown, in Fig. 1, that Akt affects the early stages of viral replication, which is consistent with the effect on RTA. Therefore, we sought to determine if Akt acts through RTA. RTA has been previously shown to activate the ORF57 promoter (p57) (Pavlova et al., 2005) as well as auto-activating its own promoter (pRTA) (Rickabaugh et al., 2005). In this experiment, we compared RTA trans-activation of pRTA and p57 (promoter activity in the presence of RTA compared with that in the absence of RTA) in 3T3-shAkt1 and 3T3-shCtrl cells using a luciferase-based reporter assay. As shown in Fig. 2(e) (pRTA) and Fig. 2(f) (p57), RTA substantially activated both promoters to higher levels in 3T3-shAkt1 than in 3T3-shCtrl. The results suggest that Akt negatively regulates the RTA activity. As RTA is an essential immediate-early gene, an increase in RTA activity by Akt inhibition would be sufficient to enhance all downstream stages of viral replication.
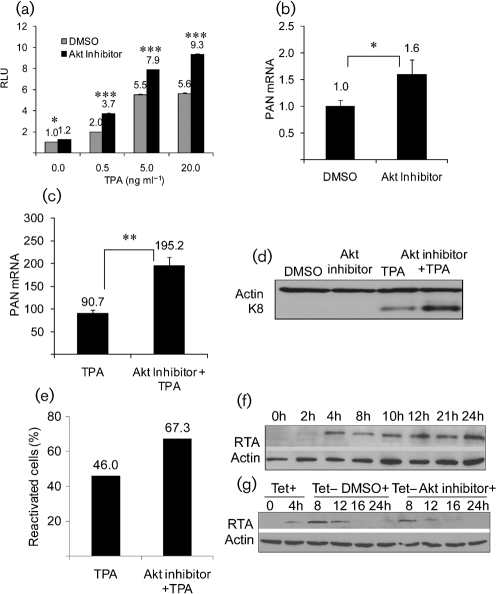
Finally, we examined whether reactivation from latency was also modulated by Akt activity using a cell line, BC-3, which is latently infected with KSHV. Our data showed that inhibition of Akt enhanced MHV-68 lytic replication. We therefore determined whether inhibition of Akt could affect KSHV reactivation. RTA is conserved between MHV-68 and KSHV and is required for virus reactivation (Lukac et al., 1998, 1999; Sun et al., 1998; Xu et al., 2005). We transfected BC-3 cells with an RTA-responsive luciferase reporter (pPAN-69Luc) (Song et al., 2001; Yu et al., 2007) as a sensitive indicator of RTA expression and, therefore, reactivation. Treatment of Akt inhibitor VIII (2 μM) only marginally increased the luciferase activity compared with the DMSO-treated cells (data not shown). We then examined whether inhibition of Akt has any effect on the reactivation induced by the phorbol ester, TPA. TPA is a commonly used chemical for reactivation (Renne et al., 1996; zur Hausen et al., 1979) and we have shown previously that it induces reactivation through the Raf/MEK/ERK pathway (Yu et al., 2007). After transfection with pPAN-69Luc, cells were treated with Akt inhibitor VIII (2 μM) or DMSO for 1 h and subsequently with different doses of TPA for 24 h before harvesting cell lysates. As shown in Fig. 3(a), in the absence of Akt inhibitor, the luciferase activity in cells increased in a dose-dependent manner with TPA treatment and reached a maximal level at 5 ng ml−1. In addition, TPA-induced luciferase activity could be significantly enhanced by the Akt inhibitor at all concentrations tested. To confirm this effect, we examined the expression of the RTA responsive viral lytic transcript PAN and the early viral lytic protein K8. The Akt inhibitor increased the PAN transcript (Fig. 3b, c). Levels of K8 protein were also enhanced by the Akt inhibitor in combination with TPA (Fig. 3d). Flow cytometry was used to further investigate reactivation at a single cell level using antibodies against early protein ORF59 (Brown et al., 2005). The percentage of reactivated cells increased after treatment with the Akt inhibitor in the presence of TPA (Fig. 3e), but not by the Akt inhibitor alone (data not shown). Taken together, our data showed that inhibition of Akt stimulates viral lytic gene expression by modulating RTA activity in KSHV-infected BC-3 cells, therefore suggesting that the inhibition of Akt facilitates KSHV reactivation from latency.
Fig. 3.
Effect of Akt inhibitor on KSHV reactivation in BC-3 cells and RTA stability. (a) The RTA-inducible promoter activity during viral reactivation. BC-3 cells were co-transfected with pPAN-69Luc and pRL-SV40, followed by DMSO or Akt inhibitor VIII, and various dosages of TPA (0.5–20 ng ml−1). Dual luciferase assays were performed 24 h after drug treatment. Relative luciferase activities are shown; the DMSO-treated sample without TPA is expressed as 1.0. (b, c) RT-Q-PCR assay of a KSHV lytic gene transcript. (d) Western blot analysis of a KSHV lytic protein. BC-3 cells were treated with DMSO, Akt inhibitor VIII (2 μM), TPA (5 ng ml−1) or both for 20 h. Total RNA and proteins were harvested to measure the levels of PAN transcripts (normalized to levels of β-actin transcripts) (b, c) and to examine the expression of the K8 protein (d). (e) Flow cytometry analysis of reactivated cells. BC-3 cells were treated with TPA (0.5 ng ml−1) or in combination with Akt inhibitor VIII (2 μM) for 20 h; cells were incubated with anti-ORF-59 antibody and subsequently with secondary antibody. (f) FLAG–RTA expression induced by tetracycline in 293RTA cell. Tetracycline (1 μg ml−1) was used to induce the expression of FLAG–RTA. Western blot was performed using anti-FLAG and anti-actin antibodies for samples at different time points after induction. (g) Effect of Akt inhibitor VIII on FLAG–RTA stability. Tetracycline (1 μg ml−1) was added to the cell culture and was maintained for 4 h. The medium was then changed to withdraw tetracycline, and 5 μΜ Akt inhibitor VIII was added. Whole-cell lysates at different time points were analysed by Western blot. In (a–c), columns represent relative values to those of the corresponding control samples. (*P<0.05; **P<0.001; ***P<0.0001). Experiments were carried out multiple times independently. For each panel, the result of a representative experiment is shown; error bars show sd of triplicates.
We also examined the effect of the inhibition of Akt activity on RTA stability using a 293 cell line, T-REx293RTA, (T-REx system, Invitrogen) which expresses FLAG-tagged KSHV RTA in response to tetracycline (Brown et al., 2005). As shown in Fig. 3(f), we established that treatment of these cells with tetracycline for 4 h was sufficient for detection by Western blot. We therefore investigated the effect of tetracycline withdrawal at 4 h followed by addition of Akt inhibitor VIII or DMSO on the levels of RTA. As shown in Fig. 3(g), inhibition of Akt did not result in any increase in RTA protein levels. This result suggests that inhibition of Akt does not increase the stability of RTA.
Our study, using MHV-68 infection of permissive fibroblasts and epithelial cells and KSHV reactivation in a latently infected B-cell line, implicates the serine/threonine kinase Akt in regulating viral lytic replication. It also suggests that this occurs at least in part through modulation of the trans-activation function (and subsequent increase in expression) of the viral transcription factor RTA. RTA is a central player in initiating lytic replication during de novo productive infection as well as reactivation from latency. Therefore, through modulating this key viral protein, the cellular PI3K-Akt pathway regulates the switch between lytic replication and latency of the gamma-2 herpesvirus life cycle. One possibility is that RTA is phosphorylated upon Akt activation and this modification reduces the trans-activation activity of RTA. However, we and others have observed that phosphorylation of RTA, at least at certain sites, positively regulates RTA activity. Thus, it is not a straightforward task to distinguish the effect of phosphorylation on RTA activity. Alternatively, inhibition may be mediated via phosphorylation of a cellular protein. Interestingly, we found that Akt becomes activated in a PI3K-dependent manner at a very early stage of MHV-68 replication. At this point, it remains to be addressed how Akt becomes activated upon infection.
In a previous report, LY294002 was shown to decrease RTA (BRLF1)-stimulated reactivation of EBV (Darr et al., 2001). In addition, this report showed that PI3K activation was required for activation of promoters of immediate-early gene BZLF1 and early gene BMRF1, but not that of another early gene SM. SM is directly activated by RTA. Although EBV and KSHV are closely related human herpesviruses, they also belong to different subcategories of gammaherpesvirus: EBV is in the gamma-1 category, while KSHV and MHV-68 are gamma 2 viruses. In KSHV, the homologue of BZLF1 does not initiate viral gene expression (Izumiya et al., 2003; Lin et al., 1999). MHV-68 does not encode a homologue of BZLF1. The roles of PI3K in their reactivation processes could therefore be different. It is also possible that the signalling networks vary among cell types and that the PI3K pathway regulates the virus distinctively in different cellular environments (Darr et al., 2001).
Upon ligand binding to several plasma membrane receptors, the PI3K-Akt pathway becomes activated and mediates several important cellular functions in response to extracellular stimuli. One of the most well-studied functions of this pathway is to promote cell survival. Constitutive activation of the PI3K-Akt pathway has been found in many human cancers, including breast cancer (Sun et al., 2001a, b), ovarian cancer (Philp et al., 2001; Shayesteh et al., 1999; Sun et al., 2001b) and thyroid carcinoma (Ringel et al., 2001). This pathway is also found to be activated in tumours associated with human herpesviruses, KHSV (Sodhi et al., 2004; Uddin et al., 2005) and Epstein–Barr virus (EBV) (Morrison et al., 2004). In addition, in several tumour-derived latently infected B-cell lines, the PI3K-Akt pathway is constitutively activated (Uddin et al., 2005). There is, therefore, a strong correlation between activation of the PI3K-Akt pathway, cell transformation and latent infection. Studies have identified several viral proteins that are capable of activating the PI3K-Akt pathway (Dawson et al., 2003; Montaner et al., 2001; Swart et al., 2000; Tomlinson & Damania, 2004), providing a plausible molecular mechanism for tumorigenesis associated with KHSV and EBV. Our results are therefore consistent with a hypothesis that activation of the Akt pathway promotes viral persistence by negatively regulating viral lytic replication.
Acknowledgments
We thank Fuqu Yu, Elaine Wong and Vaithilingaraja Arumugaswami for insightful discussion and Seungmin Hwang for technical support in Q-PCR analysis. This study was supported by grants CA091791, CA127042 and PO1DE019085 from the National Institutes of Health and the National Science Foundation grant 30570075. L. P. was supported by UCLA Fogarty AIDS International Training and Research Program.
References
- Brown, H. J., McBride, W. H., Zack, J. A. & Sun, R. (2005). Prostratin and bortezomib are novel inducers of latent Kaposi's sarcoma-associated herpesvirus. Antivir Ther 10, 745–751. [PubMed] [Google Scholar]
- Cooray, S. (2004). The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol 85, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Darr, C. D., Mauser, A. & Kenney, S. (2001). Epstein–Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J Virol 75, 6135–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, C. W., Tramountanis, G., Eliopoulos, A. G. & Young, L. S. (2003). Epstein–Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-Kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem 278, 3694–3704. [DOI] [PubMed] [Google Scholar]
- Hay, N. (2005). The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8, 179–183. [DOI] [PubMed] [Google Scholar]
- Hwang, S., Wu, T.-T., Tong, L. M., Kim, K. S., Martinez-Guzman, D., Colantonio, A. D., Uittenbogaart, C. H. & Sun, R. (2008). Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo. J Virol 82, 12498–12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya, Y., Lin, S.-F., Ellison, T., Chen, L.-Y., Izumiya, C., Luciw, P. & Kung, H.-J. (2003). Kaposi's sarcoma-associated herpesvirus K-bZIP is a coregulator of K-Rta: physical association and promoter-dependent transcriptional repression. J Virol 77, 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.-F., Robinson, D. R., Miller, G. & Kung, H.-J. (1999). Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein–Barr virus. J Virol 73, 1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, C. W., Zhao, Z., Leister, W. H., Robinson, R. G., Barnett, S. F., Defeo-Jones, D., Jones, R. E., Hartman, G. D., Huff, J. R. & other authors (2005). Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett 15, 761–764. [DOI] [PubMed] [Google Scholar]
- Liu, X., Shi, Y., Birnbaum, M. J., Ye, K., De Jong, R., Oltersdorf, T., Giranda, V. L. & Luo, Y. (2006). Quantitative analysis of anti-apoptotic function of Akt in Akt1 and Akt2 double knock-out mouse embryonic fibroblast cells under normal and stressed conditions. J Biol Chem 281, 31380–31388. [DOI] [PubMed] [Google Scholar]
- Lukac, D. M., Renne, R., Kirshner, J. R. & Ganem, D. (1998). Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252, 304–312. [DOI] [PubMed] [Google Scholar]
- Lukac, D. M., Kirshner, J. R. & Ganem, D. (1999). Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol 73, 9348–9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner, S., Sodhi, A., Pece, S., Mesri, E. A. & Gutkind, J. S. (2001). The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/Protein kinase B. Cancer Res 61, 2641–2648. [PubMed] [Google Scholar]
- Morrison, J. A., Gulley, M. L., Pathmanathan, R. & Raab-Traub, N. (2004). Differential signaling pathways are activated in the Epstein–Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res 64, 5251–5260. [DOI] [PubMed] [Google Scholar]
- Pavlova, I., Lin, C. Y. & Speck, S. H. (2005). Murine gammaherpesvirus 68 Rta-dependent activation of the gene 57 promoter. Virology 333, 169–179. [DOI] [PubMed] [Google Scholar]
- Philp, A. J., Campbell, I. G., Leet, C., Vincan, E., Rockman, S. P., Whitehead, R. H., Thomas, R. J. & Phillips, W. A. (2001). The phosphatidylinositol 3′-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res 61, 7426–7429. [PubMed] [Google Scholar]
- Renne, R., Zhong, W., Herndier, B., McGrath, M., Abbey, N., Kedes, D. & Ganem, D. (1996). Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med 2, 342–346. [DOI] [PubMed] [Google Scholar]
- Rickabaugh, T. M., Brown, H. J., Wu, T. T., Song, M. J., Hwang, S., Deng, H., Mitsouras, K. & Sun, R. (2005). Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 RTA reactivates murine gammaherpesvirus 68 from latency. J Virol 79, 3217–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel, M. D., Hayre, N., Saito, J., Saunier, B., Schuppert, F., Burch, H., Bernet, V., Burman, K. D., Kohn, L. D. & Saji, M. (2001). Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 61, 6105–6111. [PubMed] [Google Scholar]
- Sadagopan, S., Sharma-Walia, N., Veettil, M. V., Raghu, H., Sivakumar, R., Bottero, V. & Chandran, B. (2007). Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol 81, 3949–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayesteh, L., Lu, Y., Kuo, W. L., Baldocchi, R., Godfrey, T., Collins, C., Pinkel, D., Powell, B., Mills, G. B. & Gray, J. W. (1999). PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet 21, 99–102. [DOI] [PubMed] [Google Scholar]
- Sin, S.-H., Roy, D., Wang, L., Staudt, M. R., Fakhari, F. D., Patel, D. D., Henry, D., Harrington, W. J., Jr, Damania, B. A. & Dittmer, D. P. (2007). Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 109, 2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi, A., Montaner, S., Patel, V., Gomez-Roman, J. J., Li, Y., Sausville, E. A., Sawai, E. T. & Gutkind, J. S. (2004). Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A 101, 4821–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M. J., Brown, H. J., Wu, T. T. & Sun, R. (2001). Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J Virol 75, 3129–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallone, G., Schena, A., Infante, B., Di Paolo, S., Loverre, A., Maggio, G., Ranieri, E., Gesualdo, L., Schena, F. P. & Grandaliano, G. (2005). Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 352, 1317–1323. [DOI] [PubMed] [Google Scholar]
- Sun, R., Lin, S. F., Gradoville, L., Yuan, Y., Zhu, F. & Miller, G. (1998). A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 95, 10866–10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M., Paciga, J. E., Feldman, R. I., Yuan, Z., Coppola, D., Lu, Y. Y., Shelley, S. A., Nicosia, S. V. & Cheng, J. Q. (2001a). Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERα) via interaction between ERα and PI3K. Cancer Res 61, 5985–5991. [PubMed] [Google Scholar]
- Sun, M., Wang, G., Paciga, J. E., Feldman, R. I., Yuan, Z. Q., Ma, X. L., Shelley, S. A., Jove, R., Tsichlis, P. N. & other authors (2001b). AKT1/PKBα kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol 159, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart, R., Ruf, I. K., Sample, J. & Longnecker, R. (2000). Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol 74, 10838–10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, V., Shukla, S. Y., Yue, B. Y. J. T. & Shukla, D. (2007). Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol 88, 2106–2110. [DOI] [PubMed] [Google Scholar]
- Tomlinson, C. C. & Damania, B. (2004). The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J Virol 78, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, S., Hussain, A. R., Al-Hussein, K. A., Manogaran, P. S., Wickrema, A., Gutierrez, M. I. & Bhatia, K. G. (2005). Inhibition of phosphatidylinositol 3′-kinase/AKT signaling promotes apoptosis of primary effusion lymphoma cells. Clin Cancer Res 11, 3102–3108. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S. J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P. C., Woscholski, R., Parker, P. J. & Waterfield, M. D. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Virgin, H. W., IV, Latreille, P., Wamsley, P., Hallsworth, K., Weck, K. E., Dal Canto, A. J. & Speck, S. H. (1997). Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol 71, 5894–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco, I. & Sawyers, C. L. (2002). The phosphatidylinositol 3-Kinase-AKT pathway in human cancer. Nat Rev Cancer 2, 489–501. [DOI] [PubMed] [Google Scholar]
- Wu, T. T., Tong, L., Rickabaugh, T., Speck, S. & Sun, R. (2001). Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J Virol 75, 9262–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., AuCoin, D. P., Huete, A. R., Cei, S. A., Hanson, L. J. & Pari, G. S. (2005). A Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J Virol 79, 3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F., Harada, J. N., Brown, H. J., Deng, H., Song, M. J., Wu, T.-T., Kato-Stankiewicz, J., Nelson, C. G., Vieira, J. & other authors (2007). Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog 3, e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen, H., Bornkamm, G. W., Schmidt, R. & Hecker, E. (1979). Tumor initiators and promoters in the induction of Epstein–Barr virus. Proc Natl Acad Sci U S A 76, 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]