Abstract
Vaccination with Dryvax elicits a broad humoral response against many viral proteins. Human vaccinia immune globulin was used to screen the secreted proteins from cells infected with Dryvax or the candidate smallpox vaccine LC16m8 to determine whether the protective humoral response included antibodies against secreted viral proteins. Many proteins were detected, with the primary band corresponding to a band of 28 or 30 kDa in cells infected with Dryvax or LC16m8, respectively. This was identified as the vaccinia virus complement protein (VCP), which migrated more slowly in LC16m8-infected cells due to post-translational glycosylation. Vaccinia virus deleted in VCP, vVCPko, protected mice from a lethal intranasal challenge of vaccinia Western Reserve strain. Mice vaccinated with purified VCP demonstrated a strong humoral response, but were not protected against a moderate lethal challenge of vaccinia virus, suggesting that the humoral response against VCP is not critical for protection.
Smallpox, the most serious infectious disease in human history, was eradicated from human circulation in the 1970s after a concerted international effort relying on the extensive use of effective vaccines. Traditional vaccines against smallpox used in the campaign to eradicate the disease are based on vaccinia virus and provide high levels of protection. Unfortunately, they are also associated with a spectrum of rare but serious adverse reactions, some of which can result in death (reviewed by Greenberg & Kennedy, 2008). Efforts to develop new attenuated smallpox vaccines are hampered by the lack of systematic knowledge of the correlates of protection.
The protective humoral response against smallpox is contained in vaccinia immune globulin (VIG), an enriched solution of gamma globulin harvested from individuals vaccinated with traditional vaccinia virus-based vaccines. In order to identify the components of the protective immune response, a number of laboratories, including ours, have identified vaccinia virus proteins recognized by VIG. Both Western blot analysis of protein extracts from infected cells (Jones-Trower et al., 2005) and microarray analysis of a bacterially expressed peptide expression library derived from vaccinia virus (Davies et al., 2005) showed that a large number of viral proteins are recognized by VIG.
As smallpox disease has only an acute phase, patient outcome is decided by the race between the immune system to prevent and clear the disease and the ability of the infection to overwhelm the host. In order to increase their chance of successful infection, orthopoxviruses attack the host immune response with a number of virally encoded immunomodulatory proteins, many of which are secreted from infected cells (Johnston & McFadden, 2003; Seet et al., 2003). We wanted to determine whether the protective antibody response represented by VIG included a response directed against the secreted vaccinia virus proteins and whether that response was important in providing protection against disease (Empig et al., 2006; Hashizume et al., 1985).
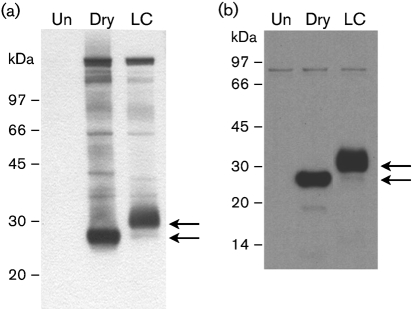
To determine whether proteins secreted from vaccinia virus-infected cells were targets of the protective humoral response, supernatants from cells infected at an m.o.i. of 1.0 in the presence of Opti-MEM (Invitrogen) with either Dryvax (Dry), a licensed vaccine used in the smallpoxe-radication campaign (Jones-Trower et al., 2005), or the candidate smallpox vaccine LC16m8 (provided by Kaketsuken, Japan) were analysed by Western blot. Supernatants from BS-C-1 (ATCC CCL-26) cells infected with LC16m8 or Dry were concentrated by centrifugation (2000 r.p.m., RTH-750 rotor, 4 °C) through an Amicon YM-30 filter and separated on 4–12 % (v/v) polyacrylamide gels. The proteins were transferred to nitrocellulose and analysed by Western blot using VIG reference lot 1 (gift of Dorothy Scott, CBER, FDA) as the primary antibody. When visualized by using an enhanced chemiluminescence-based detection system (Roche), the blots showed a number of co-migrating secreted proteins that were not present in uninfected cells, with major reactive bands from Dry and LC16m8 of approximately 28 kDa and approximately 30 kDa, respectively (Fig. 1a).
Fig. 1.
The major secretory protein recognized by VIG is VCP. (a, b) Concentrated supernatants from uninfected cells (Un) or cells infected with Dryvax (Dry) or LC16m8 (LC) were analysed by Western blot using VIG (a) or anti-VCP monoclonal antibody (b) as the primary antibody. The 28 and 30 kDa bands for Dry and LC16m8, respectively, are indicated by arrows.
The 28 kDa primary VIG-recognized protein secreted from Dry-infected cells was identified by using mass spectrometry analysis. The proteins in the supernatant from Dry-infected cells were separated on a one-dimensional Bis-Tris 12 % gel and the approximately 28 kDa band was excised and digested with trypsin (Vassilev et al., 2001). The peptide was extracted from the gel slice and desalted through Zip-Tipμ-C18 (Millipore). Liquid chromatography–electrospray ionization tandem mass spectrometry analysis (LC-MS/MS) of the tryptic peptides was performed according to the manufacturer's specifications, using a capillary HPLC (Surveyor HPLC, Thermo Electron) coupled to the nanospray source of an LTQ mass spectrometer (Thermo Electron) through a flow splitter. The Sequest algorithm was used to interpret MS/MS data and searched against a non-redundant protein database (Eng et al., 1994). The results indicated that the excised band of protein was the vaccinia virus complement control protein (VCP), corresponding to the product of the C3L open reading frame (ORF) of vaccinia virus strain Copenhagen (VACV-COP-028 at http://www.poxvirus.org), with a molecular mass of 28 629 Da.
To determine whether the major reactive band secreted from LC16m8-infected cells was also VCP, supernatants from Dry- and LC16m8-infected cells were analysed by Western blot analysis using a monoclonal antibody to VCP as the primary antibody (Isaacs et al., 2003). Both the 28 kDa band in Dry-infected cells and the 30 kDa band in LC16m8-infected cells were recognized by this antibody (Fig. 1b). Comparison of the VCP sequences from Dry and LC16m8 using NetNGlyc 1.0 (available at http://www.cbs.dtu.dk/services/NetNGlyc) revealed that VCP from LC16m8 has one predicted consensus N-glycosylation site at residue 185 that is not present in the Dry VCP. The post-translational modification of LC16m8 VCP was confirmed by performing Western blot analysis using VIG on supernatants from infected cells treated with 10 μg tunicamycin ml−1 (Sigma), which inhibits N-linked glycosylation. In cells infected with LC16m8 in the presence of tunicamycin, a new, intense band at 28 kDa was observed, and the previously observed 30 kDa protein was absent (data not shown).
To determine whether the post-translational modification of LC16m8 VCP ablated the immune response against the protein, mice were vaccinated with LC16m8, and hyperimmune serum was collected and used as the primary antiserum in Western blot analysis of the secreted proteins from vaccinia virus-infected cells. The LC16m8 hyperimmune serum contained high amounts of vaccinia virus-specific antibody, as determined by ELISA, against the intracellular mature virus form of vaccinia virus (data not shown). Western blot analyses demonstrated that LC16m8-derived serum recognized a distinct set of proteins, both in the presence and in the absence of tunicamycin. The major reactive protein in both Dry- and LC16m8-infected cell supernatants was VCP.
VCP inhibits both the classical pathway and the alternative pathway of complement activation. This interaction prevents antibody-mediated virus neutralization, thereby suppressing the host immune response. In variola virus, the VCP analogue appears to be one of the key host immune-evasion genes, called smallpox inhibitor of complement enzymes (SPICE) (reviewed by Dunlop et al., 2003). A comparative analysis of the VCP gene and the SPICE gene has shown that only a few amino acid differences might be responsible for their host specificity (Rosengard et al., 2002). SPICE appears to be a ubiquitous immunomodulator in variola virus, as it is present in all 47 strains of variola virus that have had their sequence determined to date (http://www.poxvirus.org).
VCP analogues are found in the central African monkeypox virus strains Congo and Zaire, but not in the west African strains from Liberia and Sierra Leone or in the west African strain responsible for the 2003 monkeypox outbreak in the USA. The increased virulence of the monkeypox strains from the Congo basin in comparison to those isolated from west Africa suggests that VCP may contribute to the pathogenicity of monkeypox (Chen et al., 2005). The VCP protein is present in two vaccinia virus-based smallpox vaccines used extensively in the campaign to eradicate smallpox, Lister and New York City Board of Health (Dry).
As VCP appears to be ubiquitous in smallpox and the monkeypox virus strains that are most lethal in humans, we were interested to see whether the strong immune response elicited against the protein after vaccination contributes to protective efficacy. Because VCP was non-essential for growth in tissue culture (Kotwal & Moss, 1988; Kotwal et al., 1990), a gene-knockout strategy using an enhanced green fluorescent protein (EGFP) cassette flanked on the 5′ and 3′ ends by vaccinia virus sequences immediately upstream and downstream of the VCP ORF was utilized. The VCP deletion mutant was introduced into the clonal isolate of Dry designated DV-3. This clonal isolate was isolated in our laboratory to serve as a vaccine strain in animal-model work, as it has similar immunogenicity and protective capability to the Dry vaccine in the mouse intranasal challenge model (Meseda and others, unpublished data). The EGFP cassette was transfected into DV-3-infected cells and a recombinant DV-3 clone that expressed EGFP (vVCPko) was isolated and purified by iterative rounds of plaque purification on BS-C-1 cells. The genomic structure of vVCPko was confirmed by PCR; no VCP protein was detected from cells infected with vVCPko by VCP-specific antibody in Western blot analysis of infected-cell supernatants, and the haemolysis-inhibitory activity of the VCP protein was absent (Fig. 2a). The DV-3 and vVCPko viruses were indistinguishable in a one-step growth curve in BS-C-1 cells (data not shown), using a procedure described previously (Williams et al., 1999).
Fig. 2.
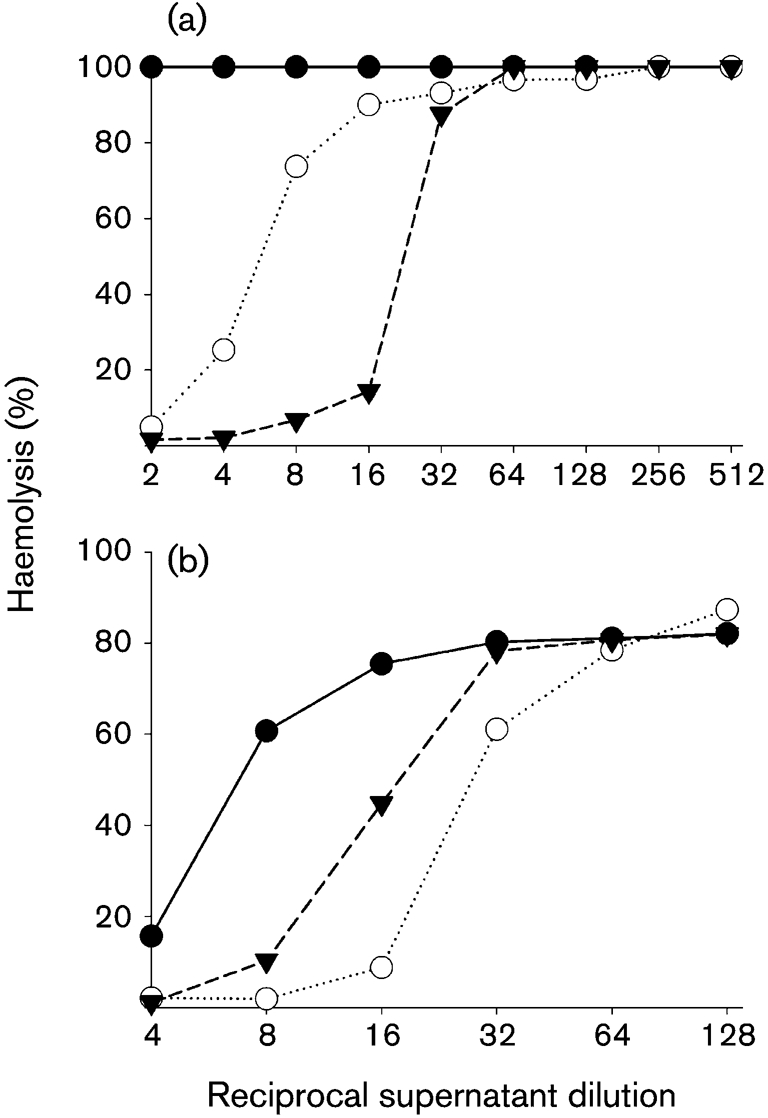
Haemolysis activity of recombinant viruses and elicited antisera. (a) Concentrated cell supernatant from cells infected with DV-3 (▾), vVCPko (•) or vVCP–FLAG (○) were used in a haemolysis-inhibition assay as described by Morgan (2000). (b) Concentrated supernatant from cells infected with DV-3 was pre-incubated with buffer alone (▾), normal mouse serum (○) or pooled serum from mice immunized with 50 μg VCP–FLAG (•). The percentage haemolysis was calculated by using the mean OD405 value for red blood cells lysed in water as 100 %.
The virulence of DV-3 and vVCPko was measured by determining their LD50 in mice. Groups of five mice were infected intranasally with the appropriate virus, using doses from 103 to 107 p.f.u. per mouse. The LD50 was calculated by using the method of Reed & Muench (1938). In two independent determinations, both DV-3 and vVCPko were attenuated with an LD50 of >106 p.f.u., compared with an LD50 of 4×104 p.f.u. for vaccinia virus strain Western Reserve (WR). Although VCP has been implicated in virulence by measuring the lesion size on rabbits using recombinant viruses made in the virulent WR strain (Isaacs et al., 1992), the vVCPko deletion was made in the DV-3 vaccine, derived from the attenuated Dry strain. Sera collected from mice vaccinated with DV-3 or vVCPko and evaluated for vaccinia IgG 3 weeks post-immunization using an ELISA against mature virions had similar IgG end-point antibody titres (data not shown).
To determine whether the immune response against VCP was a critical component of the protective immune response, DV-3 and vVCPko were used in three replicate experiments to immunize mice prior to stringent challenge with 25 or 100 LD50 vaccinia virus WR at 3 weeks post-vaccination (Table 1). Male, 8–12-week-old, age-matched BALB/cByJ mice (Jackson Laboratories) were housed at the CBER animal facility with food and water provided ad libitum. Groups of five mice each were immunized with 106 p.f.u. Dry, DV-3 or vVCPko virus via tail scarification. Intranasal challenge with vaccinia virus WR strain was performed as described previously (Meseda et al., 2005). Mice treated with PBS succumbed at the 25 LD50 dose within 7 days. Most mice immunized with DV-3 (12 of 13 mice) or vVCPko (14 of 15 mice) survived the challenge dose of 25 LD50. When vaccinated mice were challenged with 100 LD50 vaccinia virus WR, seven of 15 mice vaccinated with vVCPko and 11 of 15 mice vaccinated with DV-3 survived. Although DV-3 appeared to be slightly more protective at the 100 LD50 challenge, the differences between the two groups were not statistically significant (Fisher's exact test or χ2 test). These results demonstrate that vaccination with a virus lacking VCP had the same level of protection as vaccination with DV-3 against challenge with vaccinia virus WR in the intranasal mouse model.
Table 1.
Protective effect of vaccination with DV-3 and vVCPko in the intranasal vaccinia virus WR challenge model
| Group | Vaccine | Challenge | No. surviving mice | Total | ||
|---|---|---|---|---|---|---|
| VACC-33 | VACC-34 | VACC-40 | ||||
| A | PBS | 25 LD50 | 0/5 | 0/5 | 0/5 | 0/15 |
| B | DV-3 | 25 LD50 | 5/5 | 4/4 | 3/4 | 12/13 |
| C | DV-3 | 100 LD50 | 3/5 | 3/5 | 5/5 | 11/15 |
| D | vVCPko | 25 LD50 | 5/5 | 4/5 | 5/5 | 14/15 |
| E | vVCPko | 100 LD50 | 2/5 | 4/5 | 1/4 | 7/15 |
Although the response to VCP was not critical for protective efficacy, it may still contribute to the amelioration of disease. To measure the contribution of VCP to the protective response, we constructed a recombinant vaccinia virus containing VCP linked to a FLAG affinity tag, purified the VCP protein on a 10 ml column of anti-FLAG M2 agarose (Sigma) using the AKTA system (GE Healthcare) with an elution programme for affinity chromatography. The protein was eluted by using 10 mM FLAG peptide (Sigma). After elution, a protein with the appropriate molecular mass was the only major band on a protein gel stained with Sypro Ruby Red (Invitrogen). The eluted material was concentrated by using a 10k MWCO Amicon (Millipore) centrifugal filter device and its identity was verified by Western blot analysis with either anti-VCP or anti-FLAG M2 (Sigma) as the primary antibody. The concentration of the protein was determined by using the BCA method (Pierce). The purified VCP–FLAG protein was active, as measured by its ability to inhibit red-cell lysis in a haemolysis assay as described by Morgan (2000).
Mice were immunized subcutaneously twice with 5, 10 or 50 μg purified VCP–FLAG mixed with Freund's complete adjuvant. Boosts of similar doses were performed 3 weeks later with Freund's incomplete adjuvant. PBS was administered to control mice by the same route. Test sera were obtained from blood samples collected 6 weeks after the first immunization and shown to react with purified VCP–FLAG protein or VCP protein when used as the primary antibody in Western blot analysis. Sera from control mice did not react with VCP. The immunized mice were challenged with a low dose (5 LD50) of vaccinia virus WR. None of the mice immunized with VCP survived the low-dose challenge, whereas all five mice in the control group vaccinated with DV-3 survived. In addition, no group-specific protection was observed when immunized mice were challenged with 1 LD50 vaccinia virus WR (data not shown). The antibody response generated against VCP was effective against VCP activity, as incubation of the sera with purified VCP shifted the curve demonstrating the haemolysis activity of VCP (Fig. 2b).
Although vaccination with either Lister or New York City Board of Health strains of vaccinia virus elicited a strong antibody response against VCP and we demonstrated that the elicited immune response in mice interfered with the activity of VCP, we were unable to demonstrate a role for this response in protective efficacy. The existence of a humoral response against a viral antigen does not guarantee that the antibodies have a role in protective efficacy. The immune response against VCP may not be important for protection, as antibodies against VCP, a secreted protein, cannot neutralize the virus. Alternatively, the antibodies against VCP may play a role in providing protection against smallpox infection, but may not be critical in the vaccinia virus WR challenge mouse model, or the mouse model using intranasal challenge with vaccinia virus WR may be too stringent to detect the subtle role of the response against VCP.
The normal response elicited by poxvirus vaccines includes antibodies directed against a wide assortment of proteins. The generation of more effective immunotherapeutics and subunit vaccines will rely on systematic investigation of the contribution of the immune response against specific viral proteins towards disease prevention. Although VCP appears to influence the virulence of smallpox and monkeypox infections in humans, the potent humoral response elicited against the protein does not appear critical for protection in mice. This implies that effective immunotherapeutics, such as pooled humanized antibodies against poxvirus proteins, may not require antibodies against VCP for maximum effectiveness.
Acknowledgments
We are grateful to Nga Nguyen (CBER core facility) for mass spectrometry analysis and to Joseph Campbell for assistance with the animal studies. We offer thanks to Dorothy Scott (CBER, FDA) for human VIG and to John D. Lambris (University of Pennsylvania) for the anti-VCP antibodies. This work was supported by an Interagency Agreement (Y1-AI-6153-01) between NIAID and the FDA.
References
- Chen, N., Li, G., Liszewski, M. K., Atkinson, J. P., Jahrling, P. B., Feng, Z., Schriewer, J., Buck, C., Wang, C. & other authors (2005). Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340, 46–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, D. H., Liang, X., Hernandez, J. E., Randall, A., Hirst, S., Mu, Y., Romero, K. M., Nguyen, T. T., Kalantari-Dehaghi, M. & other authors (2005). Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A 102, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop, L. R., Oehlberg, K. A., Reid, J. J., Avci, D. & Rosengard, A. M. (2003). Variola virus immune evasion proteins. Microbes Infect 5, 1049–1056. [DOI] [PubMed] [Google Scholar]
- Empig, C., Kenner, J. R., Perret-Gentil, M., Youree, B. E., Bell, E., Chen, A., Gurwith, M., Higgins, K., Lock, M. & other authors (2006). Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine 24, 3686–3694. [DOI] [PubMed] [Google Scholar]
- Eng, J. K., McCormack, A. L. & Yates, J. R., III (1994). An approach to correlate mass spectral data of peptides with amino acid sequences in a protein databases. J Am Soc Mass Spectrom 5, 976–989. [DOI] [PubMed] [Google Scholar]
- Greenberg, R. N. & Kennedy, J. S. (2008). ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs 17, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume, S., Yoshizawa, H., Morita, M. & Suzuki, K. (1985). Properties of attenuated mutant vaccinia virus, LC16m8, derived from Lister strain. In Vaccinia Viruses as Vectors for Vaccine Antigens, pp. 87–99. Edited by G. V. Quinnan. New York: Elsevier.
- Isaacs, S. N., Kotwal, G. J. & Moss, B. (1992). Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A 89, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs, S. N., Argyropoulos, E., Sfyroera, G., Mohammad, S. & Lambris, J. D. (2003). Restoration of complement-enhanced neutralization of vaccinia virus virions by novel monoclonal antibodies raised against the vaccinia virus complement control protein. J Virol 77, 8256–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, J. B. & McFadden, G. (2003). Poxvirus immunomodulatory strategies: current perspectives. J Virol 77, 6093–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Trower, A., Garcia, A., Meseda, C. A., He, Y., Weiss, C., Kumar, A., Weir, J. P. & Merchlinsky, M. (2005). Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343, 128–140. [DOI] [PubMed] [Google Scholar]
- Kotwal, G. J. & Moss, B. (1988). Analysis of a large cluster of nonessential genes deleted from a vaccinia virus terminal transposition mutant. Virology 167, 524–537. [PubMed] [Google Scholar]
- Kotwal, G. J., Isaacs, S. N., McKenzie, R. &, Frank, M. M. & Moss, B. (1990). Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250, 827–830. [DOI] [PubMed] [Google Scholar]
- Meseda, C. A., Garcia, A. D., Kumar, A., Mayer, A. E., Manischewitz, J., King, L. R., Golding, H., Merchlinsky, M. & Weir, J. P. (2005). Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339, 164–175. [DOI] [PubMed] [Google Scholar]
- Morgan, B. P. (2000). Measurement of complement hemolytic activity. Methods Mol Biol 150, 61–71. [DOI] [PubMed] [Google Scholar]
- Reed, L. J. & Muench, H. (1938). A simple method of estimating fifty per cent endpoints. Am J Hyg 27, 493–497. [Google Scholar]
- Rosengard, A. M., Liu, Y., Nie, Z. & Jimenez, R. (2002). Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc Natl Acad Sci U S A 99, 8808–8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet, B. T., Johnston, J. B., Brunetti, C. R., Barrett, J. W., Everett, H., Cameron, C., Sypula, J., Nazarian, S. H., Lucas, A. & McFadden, G. (2003). Poxviruses and immune evasion. Annu Rev Immunol 21, 377–423. [DOI] [PubMed] [Google Scholar]
- Vassilev, A., Kaneko, K. J., Shu, H., Zhao, Y. & DePamphilis, M. L. (2001). TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 15, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, O., Wolffe, E. J., Weisberg, A. S. & Merchlinsky, M. (1999). Vaccinia virus WR gene A5L is required for morphogenesis of mature virions. J Virol 73, 4590–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]