Abstract
We have previously shown that ± 3,4-methylenedioxymethamphetamine (MDMA) treatment from P11 to P20 in rats produces deficits in cognitive ability when these animals are tested in adulthood. The purpose of this experiment was to explore the neuroendocrine and neurochemical changes produced by MDMA treatment on P11. We examined monoamines in the hippocampus and striatum and the serotonin transporter in the hippocampus as well as pituitary and adrenal output following administration of MDMA (10 mg/kg, 4 times) on postnatal day 11. Significant depletions in serotonin were evident in the hippocampus 1 h and in the striatum 24 h after the first dose and remained reduced 78 h later. No changes in serotonin transporter were observed following MDMA treatment, although females had lower levels than males. No changes in dopamine were detected. The metabolites of serotonin and dopamine had different profiles than the parent compounds after MDMA administration. Plasmatic ACTH was elevated immediately following MDMA and remained elevated for at least 1 h after the last dose and returned to baseline by 24 h. Corticosterone was increased after the first dose and remained increased for at least 24 h, and returned to baseline by 30 h. The decreases in serotonin in regions important for learning and memory in conjunction with elevated levels of corticosterone during a period of stress hyporesponsiveness suggest that these initial responses to MDMA may contribute to the long-term learning and memory deficits following neonatal MDMA exposure.
Keywords: 5-HT, 5-HIAA, ACTH, Corticosterone, DOPAC, Dopamine, Ecstasy, Hippocampus, MDMA, Neostriatum, 3H-paroxetine binding, Serotonin transporter, Thymus
1. Introduction
Little information has been acquired regarding the short- and/or long-term effects of ±3,4-methylenedioxymethamphetamine (MDMA) exposure during gestation in humans, although exposure is known to occur [23]. One group has suggested that there may be an increased risk for congenital anomalies, including heart defects, although this study employed a very small population [38]. Given the high use of MDMA among young adults [27,28], who are of child-bearing age, it is likely that there is a significant number of fetuses exposed to MDMA.
We have modeled human third trimester MDMA exposure in the neonatal rat since hippocampal development, especially the dentate granule cell layer, is similar to human third trimester hippocampal development [7,50]. From these studies, we have demonstrated that there are deficits in Morris water maze learning (spatial learning) and in Cincinnati water maze learning (path integration) when MDMA is administered from postnatal day (P)11–20 [10,57,62] but not from P1 to P10 [10]. Decreases in body weight are observed following MDMA [10,57,62]; however, the reduction in body weights alone does not account for the learning deficits [62]. Furthermore, the learning deficits do not appear to be the result of either the inability to incorporate new learning, since working memory is unaffected [57], or long-term monoamine changes in adulthood [10]. Therefore, potential explanations for the MDMA-induced learning deficits are still unknown.
In adult animals, MDMA produces reductions in 5-HT and the serotonin transporter (SERT) following either single or multiple doses [6,8,9,19,47,52]. Although dramatic depletions are observed in neurotransmitter levels after MDMA administration, few cognitive deficits have been identified in adult animals [24,41,52]. In developing animals, multiple administrations of MDMA from P1 to P4 produce long-term reductions in 5-HT and SERT [39,64]; however, this period is not associated with cognitive deficits [10]. Little is known about the neurochemical and neuroendocrine changes produced by MDMA beginning on P11, the first day when neonatal animals showed vulnerability to long-term cognitive changes. Of the extant literature of animals similar in age, a single administration of MDMA (gavage) on P10 produced transient decreases in 5-HT in the frontal cortex, striatum, and hippocampus for 24 h; however, levels returned to baseline within 72 h and no changes were observed in SERT [8]. Others have demonstrated that a single injection of MDMA on P14 produced no decrease in 5-HT, SERT, or dopamine (DA) in various brain regions when measured 7 days later [2]. Multiple dose administrations of MDMA from P11 to P20 has been shown to reduce 5-HT on P21 as well as increase levels of DOPAC and brain derived neurotrophic factor [32]. Nonetheless, no data exist for the immediate effects of multiple MDMA administrations on monoamine levels when administration starts on P11. Therefore, the first goal of this study was to determine 5-HT, 5-HIAA, and 3H-paroxetine binding in the hippocampus and 5-HT, 5-HIAA, DA, and DOPAC levels in neostriatum using multiple subcutaneous injections over a 6-h period on P11 (a dosing regimen commonly used in adult animals).
In adult humans, increases in cortisol have been demonstrated following MDMA [36,44] and increases in corticosterone (CORT) have been noted in adult animals [1,12,42,68]. Low doses of MDMA appear to produce transient increases in CORT [12,42], whereas higher doses have been shown to produce elevated CORT for 16 h [68]. CORT and ACTH reactivity in neonates following MDMA has not been determined. Therefore, the second goal of this study was to examine the pituitary and adrenal response to MDMA in developing animals. This is especially important since high levels of glucocorticoids are known to alter hippocampal development during the neonatal period [18], when the adrenal–cortical response is greatly attenuated following various stimuli [51,54]. We also examined thymus weight since this organ will involute when high levels of glucocorticoids are present in adult animals [5,30], although neonatal animals may not have a similar response [26].
This is the first study to investigate the immediate neurochemical and neuroendocrine changes induced by MDMA in developing animals on P11. This day was selected since animals are first vulnerable to learning and memory deficits induced by MDMA at this age [10]. In this study, we used a 4-dose regimen (1 dose every 2 h) for MDMA, rather than a 2-dose regimen for several reasons. The first was to be consistent with adult animal dosing regimens. Secondly, 4 doses extends the bioavailability of MDMA, since the half-life in P11 rats is less [63] than the half-life in humans [15,43] and humans tend to take multiple doses of the drug [19,45]. Thirdly, this regimen produces greater or comparable deficits in MWM learning without as much weight loss as the same amount of drug given in 1 or 2 daily doses (i.e., 1 dose produced a 19% increase in latency, 2 doses a 31% increase, and 4 doses a 35% increase; unpublished data); similar results were seen with methamphetamine at this age [56]. The last reason to investigate this dosing regimen is so that we can readily compare the effects of methamphetamine and MDMA by employing identical drug administration paradigms. Samples were obtained 1 h following the first dose on P11 as well as 7, 24, 30, or 78 h later (i.e., 1, 18, 24, and 72 h following the 4th dose), using a within-litter design with sex as a factor since most studies have not investigated potential sex differences (cf. Refs. [2,8,9,39]).
2. Methods
2.1. Subjects
Nulliparous female and male Sprague–Dawley CD IGS rats (Charles River Laboratories, Raleigh) were mated in hanging wire cages following an acclimation period to the vivarium of at least 1 week. The day a sperm plug was detected was considered embryonic day 0 (EO). Fourteen days after being placed with the males, the females were transferred to polycarbonate cages (45.7 × 23.8 × 20.3 cm). The offspring were the test subjects for these experiments. The vivarium was maintained on a 14-h light/10-h dark cycle (lights on at 0600 h) and food and water were freely available. All procedures were approved by the Cincinnati Children’s Research Foundation’s Laboratory Animal Care and Use Committee and the vivarium was fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Beginning on E21, females were checked twice daily for the presence of a litter. The day of birth was considered postnatal day 0 (P0), and on P1, litters were standardized to 10 pups with 5 males and 5 females. Pups were weighed on P7 and uniquely identified by an ear punch on this day.
2.2. Drug administration
±3,4-Methylenedioxymethamphetamine (MDMA; National Institute on Drug Abuse, Bethesda, MD) was expressed as the freebase and dissolved in sterile saline at a concentration of 10 mg/3 ml. This volume of vehicle alleviates potential problems of dermal irritation. MDMA was administered to animals at a dose of 10 mg/kg through a subcutaneous route in the dorsum. Beginning at 1000 h on P11, pups began a series of drug administrations every 2 h for 6 h for a total of 4 doses of MDMA or saline (SAL). Injection sites were varied at each time point. Temperature was not monitored in this study, since others have demonstrated that MDMA does not produce a hyperthermic response in neonatal animals [9,40] and we did not want to confound the neuroendocrine effects with the manipulations required for temperature monitoring.
2.3. Sampling procedure
This was a mixed factor experiment with treatment as a between factor and time course and sex as within factors. Litters were randomly assigned to either receive MDMA or SAL (n = 8 litters/treatment). Male and female pairs were euthanized at the following time points measured from the first treatment on P11: 1, 7, 24, 30, and 78 h. Each animal was removed from the litter, brought to a different room (<25 s), decapitated, and trunk blood collected in tubes containing 2% EDTA (0.05 ml) for CORT and ACTH determination. Animals were not weighed after the last dose in order to minimize potential increases in CORT and ACTH.
The brain was rapidly removed, placed on ice, and the neostriatum and hippocampi were dissected using a brain block (Zivic-Miller, Pittsburgh, PA) and then frozen on dry ice. The brain was first sliced coronally at the optic chiasm and the neostriatum was dissected freehand from a block of tissue that was 2 mm thick and immediately rostral to this first coronal cut. The remaining forebrain was sliced sagitally and the hippocampus was removed from the cerebral hemispheres bilaterally and pooled. Tissues were placed in preweighed tubes and then reweighed with tissue for determination of tissue weight. All tissues were stored at −80 °C until assayed for monoamines (DA, DOPAC, 5-HT, and 5-HIAA).
The thymus gland was also removed from each animal, freed of fatty tissue, weighed and the weight recorded. Thymus tissue was collected because of the known involution that can occur when high levels of glucocorticoids are present [5,30]. Thymus weights are expressed as a percentage of body weight measured at the last administration of MDMA or SAL on P11.
2.4. Endocrine assessment
The trunk blood was centrifuged (1399 RCF) for 25 min at 4 °C, plasma collected, and stored at −80 °C until assayed. Prior to the determination of hormone, plasma was diluted 3:1 in assay buffer for CORT and 4:1 for ACTH and assayed in duplicate using commercially available EIAs for CORT (ALPCO Diagnostics, Windham, NH) and ACTH (Peninsula Laboratories, Inc., San Carlos, CA). Each EIA has little cross-reactivity with other hormones or precursors.
2.5. Monoamine determination
For the determination of 5-HT and 5-HIAA in the hippocampus and striatum and for DA and DOPAC in the striatum, high-pressure liquid chromatography was used as described previously [61]. Briefly, tissues were thawed and diluted with 50 volumes of 0.2 N perchloric acid, homogenized, and centrifuged at 10,000 × g for 10 min. Samples (20 μl) were injected into a C18 column (5 μ, 100 × 2 mm) connected to an LC-4B amperometric detector (Bioanalytical Systems, West Lafayette, IN) with a reference electrode maintained at an oxidation potential of +0.60 V. The mobile phase consisted of 35 mM citric acid, 54 mM sodium acetate, 50 mg/l disodium ethylenediamine tetraacetate, 70 mg/l octane sulfonic acid sodium salt, 6% methanol, 6% acetonitrile, pH 4.0. The flow rate was 0.4 ml/min. Chromatograms were recorded and integrated and neurotransmitter concentrations were calculated from standard curves generated for each analyte. The lower limit of detection of the assay for 5-HT is 0.5 pg.
2.6. Serotonin transporter binding assay
3H-Paroxetine binding to the 5-HT transporter was performed as described previously [16,46] with modifications. Briefly, hippocampi were homogenized in 40 volumes ice-cold 50 mM Tris–HCl buffer (pH 7.4) and centrifuged (25,000 × g, 10 min at 4 °C). The supernatants were discarded and pellets washed thrice by resuspension in 40 volumes of buffer followed by centrifugation as described above. The final pellet was suspended in 50 mM Tris–HCl buffer containing 5 mM KCl, 120 mM NaCl, 2 mM EDTA (pH 7.4) at a mean protein concentration of 1 mg/ml, and stored at −80 °C until assayed. The binding reaction was comprised of 2 nM 3H-paroxetine (specific activity of 21.5 Ci/mmol), incubated with hippocampal homogenate (75 μg protein) in a final volume of 500 μl, carried out for 90 min at 25 °C. Nonspecific binding was assessed with 100 μM fluoxetine (Sigma, St. Louis, MO). Reactions were terminated by the addition of cold assay buffer followed by rapid filtration through Whatman GF/B filters presoaked in polyethyleneimine (PEI) to reduce nonspecific filter binding. The filters were washed twice with buffer and the entrapped radioactivity was counted by a liquid scintillation counter (Beckmann, LS7600) for 1 min. All samples were run in triplicate. Membrane protein concentrations were measured using Coomassie Plus Protein Assay Reagent Kit (Pierce Biochemicals).
2.7. Statistics
Data were analyzed with mixed-model analyses of variance (ANOVA) utilizing the general linear modeling procedure for all parameters with the exception of 3H-paroxetine binding. The between-litter main effect was treatment group (MDMA or SAL) and the within-subject factors were sex, time of day (for body weights during dosing), and time of sampling. The experimental unit was the litter (n = 8/treatment). If Sex was not a significant factor, either as a main effect or an interaction, the mean of the male and female pair within a litter was used for analysis. The Greenhouse–Geisser correction was used in instances in which symmetry of the variance–covariance matrices were significantly non-spherical. Because of low protein content in some of the samples for 3H-paroxetine binding, we had an incomplete data set for a mixed model ANOVA and therefore, a between-factor analysis was used for 3H-paroxetine binding data (n = 6–8/treatment/time point). Significant main effects were analyzed further to determine differences using the step-down F-test procedure of Kirk [31]; simple effect ANOVAs were used to further analyze significant interactions. Significance was set at P ≤ 0.05.
3. Results
3.1. Weights
Body weights at the first and last dose were analyzed by ANOVA. The main effect of Treatment was not significant, but as expected, males weighed more than females, Sex, F(1,14) = 20.9, P < 0.0004. The interactions of Treatment × Dose Time, F(1,14) = 9.2, P < 0.009, and Sex × Dose Time, F(1,14) = 4.9, P < 0.05, were significant. The animals treated with MDMA did not demonstrate an increase in body weights over the period of dosing (mean ± SEM for first and last dose = 26.62 ± 0.69 and 26.34 ± 0.75, respectively), whereas animals treated with SAL did show significant increases in body weight (mean ± SEM for first and last dose = 25.44 ± 1.02 and 26.04 ± 1.02, respectively). No other body weights were taken so as not to influence the levels of CORT and ACTH.
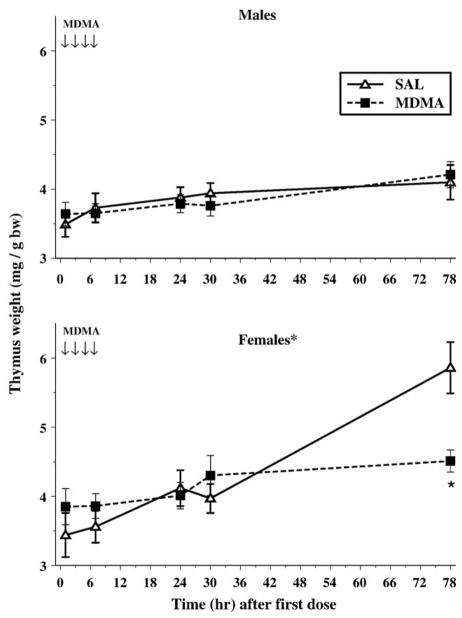
For thymus weights, only the main effects of Sampling Time, F(4,56) = 15.1, P < 0.0001, and Sex, F(1,14) = 20.5, P = 0.0005, were significant. The percentage of thymus weight to body weight increased for all groups and females had a greater percentage of thymus weights relative to males. The highest order interaction to show significance was Treatment × Sex × Sampling Time, F(4,56) = 6.1, P = 0.0007. Investigation of this interaction showed that the females given MDMA who were sampled at the 78-h time point had a smaller percentage thymus weight than females given SAL (Fig. 1). No differences in males were seen at any time point.
Fig. 1.
The relative thymus weights (mg/g body weight) ± SEM are displayed for males (top) and females (bottom). Females had larger thymuses proportionate to body weight. MDMA-treated females had smaller relative thymus weights when examined 78 h after the first dose. The arrows represent the time points when MDMA was administered on P11. *P < 0.05.
3.2. Hormones
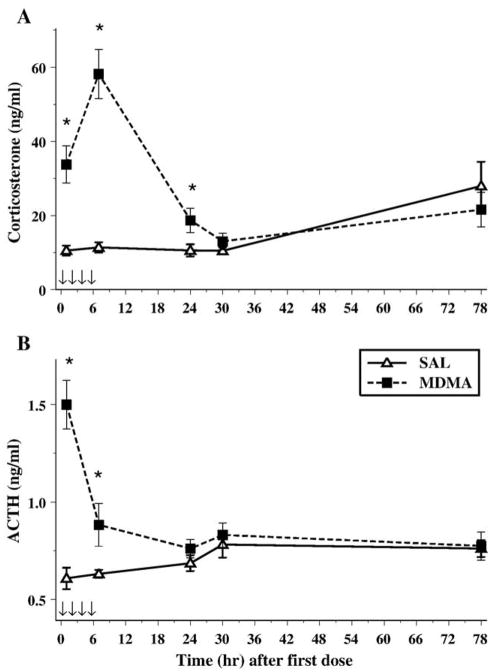
Plasma was assessed for ACTH and CORT at each of the five time points. For CORT, hormone levels varied across time points, F(4,56) = 11.5, P < 0.0001, and CORT increases were induced by MDMA-treatment, F(1,14) = 25.3, P < 0.0002. CORT levels were affected by the interaction of Treatment and Sampling Time, F(4,56) = 15.2, P < 0.0001. Animals treated with MDMA had elevated CORT levels at 1, 7, and 24 h after the first dose (Fig. 2A). No sex differences or any other significant interactions were found for CORT.
Fig. 2.
Corticosterone (A) and ACTH (B) following MDMA (10 mg/kg) on P11. The arrows represent the time points when MDMA was administered on P11. As can be seen in panel A, MDMA produced an increase in CORT 1 h after the first dose that continued to be elevated at least 24 h after the first dose. ACTH displayed a different pattern, such that it was only increased 1 h after the first dose and last dose (7 h after first). *P < 0.05.
The levels of ACTH varied across sampling time points, F(4,56) = 4.8, P < 0.01, and ACTH increases were induced by MDMA-treatment, F(1,14) = 18.3, P < 0.001. Similar to CORT, the interaction of Treatment × Sampling Time, F(4,56) = 10.2, P < 0.0001, revealed that MDMA-induced elevations in ACTH were only significantly different from ACTH levels in SAL-treated animals at the 1- and 7-h time points (Fig. 2B). No differences were noted for Sex and no other interactions were noted.
3.3. Hippocampus
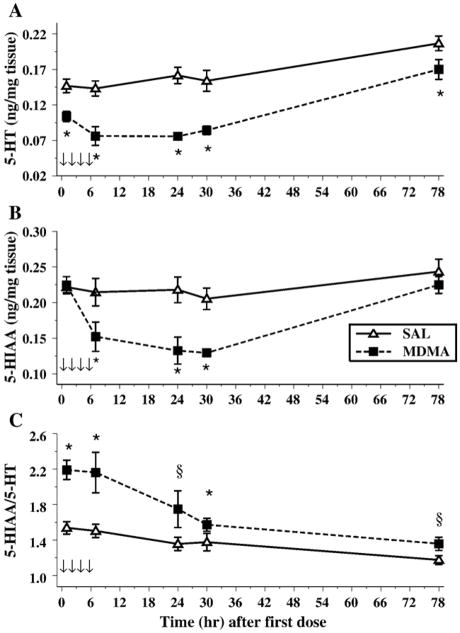
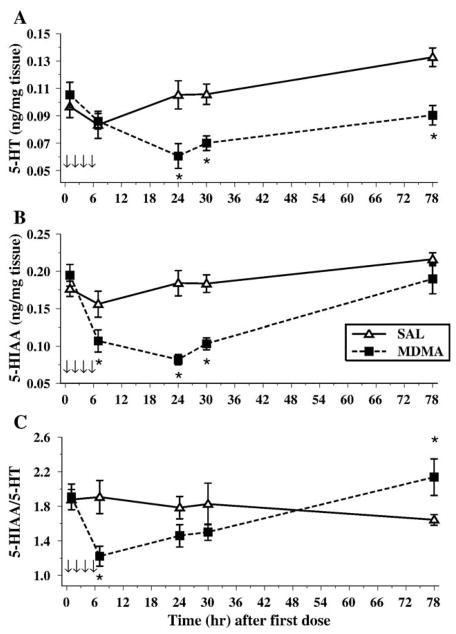
In the hippocampus, we examined 5-HT and its metabolite, 5-HIAA, as well as the 5-HT transporter (SERT). For 5-HT, the levels were different over the Sampling period, F(4,56) = 26.6, P < 0.001. MDMA administration also produced an overall decrease in levels, Treatment, F(1,14) = 34.9, P < 0.0001. The interaction of Treatment × Sampling Time approached significance, P < 0.08 as did Sex, P < 0.09. No other effects were noted for 5-HT (Fig. 3A).
Fig. 3.
The concentrations of 5-HT (A), 5-HIAA (B), and the 5-HIAA/5-HT (C) ratio in the hippocampus of animals exposed to 4 doses of MDMA on P11 (arrows). Decreases in 5-HT were observed at all time points following MDMA exposure, although only the 7-, 24-, and 30-h time points were decreased for 5-HIAA. For the ratio, a significant increase was observed in MDMA animals, and this was dependent on sex, since females displayed this pattern for all time points, whereas males had elevated levels only at particular time points. *P < 0.05. §P < 0.05 females only.
The levels of 5-HIAA were altered across time intervals [Sampling Time, F(4,56) = 12.1, P < 0.0001]. The administration of MDMA produced decreases in 5-HIAA overall, F(1,14) = 9.7, P < 0.008, and the decreases differed as a function of time, Treatment × Sampling Times, F(4,56 = 4.9, P < 0.002. The level of 5-HIAA did not decrease until 7 h and remained reduced through 30 h; however, no differences were observed 78 h after the first dose. No Sex differences or other interactions were found (Fig. 3B).
Examination of the 5-HIAA/5-HT ratio showed that MDMA increased the ratio of 5-HIAA/5-HT, Treatment, F(1,14) = 22.1, P < 0.0003. Sampling Time also was a significant factor, F(4,56) = 10.1, P < 0.0001 (Fig. 3C). A significant interaction of Treatment × Sex × Sampling Time, F(4,56) = 3.0, P < 0.04, was also observed. Further analysis of the interaction revealed that, for males and females, the overall pattern was similar but the details differed. MDMA produced a significant increase in the 5-HIAA/5-HT ratio for females at all time points; for males, they had higher 5-HIAA/5-HT ratios at 1, 7, and 30 h only after the first MDMA administration.
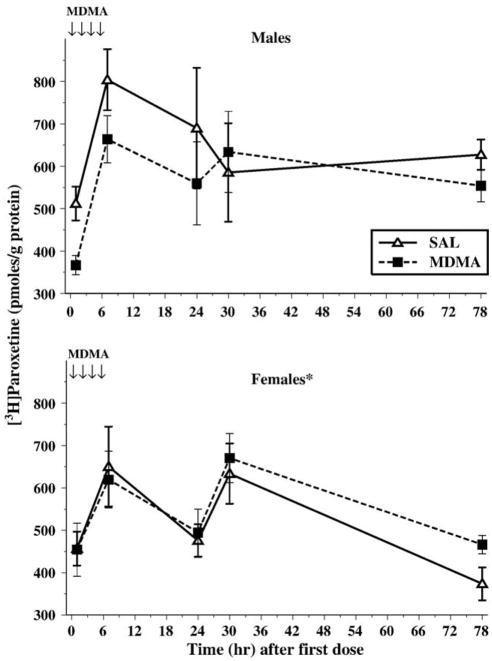
For 3H-paroxetine binding in the hippocampus, no main effect of MDMA treatment was observed. Males had higher levels of 3H-paroxetine binding than the females, F(1,127) = 5.4, P < 0.03. The mean ± SEM for males was 597.8 ± 25.8 pmol bound/g protein, and for females, it was 525.5 ± 20.8 pmol bound/g protein. The Treatment × Sex interaction approached significance, P < 0.07, and was the result of the MDMA-treated males having less 3H-paroxetine binding than SAL males. The 3H-paroxetine binding profile also changed over the Sampling Time, F(4,127) = 7.9, P < 0.0001. As can be seen in Fig. 4, there are time-dependent increases in SERT even in saline-treated controls. No other interactions were significant.
Fig. 4.
For the serotonin transporter (SERT), males (top) treated with MDMA tended to have less SERT than males treated with saline (P < 0.07). Females (bottom) had lower levels of SERT compared to males, regardless of treatment. The arrows represent the time points when MDMA was administered on P11. *P < 0.05.
3.4. Striatum
In the striatum, the levels of DA, DOPAC, 5-HT, and 5-HIAA were assessed. For DA, only the main effect of Sampling Time was significant, F(4,56) = 11.7, P < 0.0001. This effect can be accounted for by the increase in DA content observed over time (Fig. 5A).
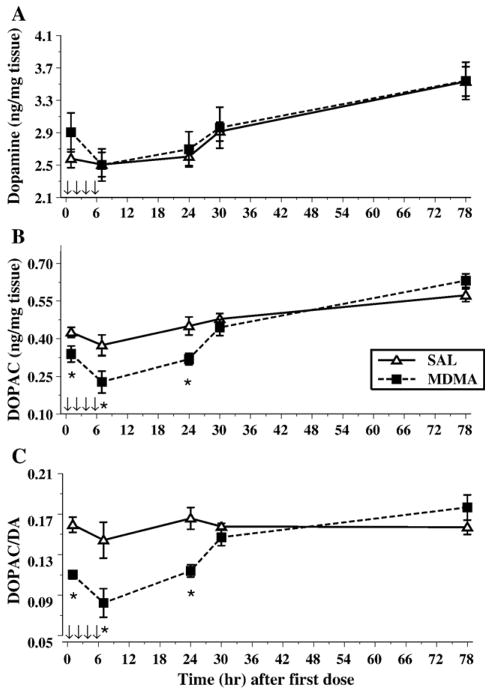
Fig. 5.
Depicted are the levels of dopamine (A), DOPAC (B), and the ratio of DOPAC/DA (C) in the neostriatum following MDMA administration (10 mg/kg) on P11. The arrows represent the time points when MDMA was administered. No treatment-related effects were noted for dopamine; however, levels did increase over time. A reduction in DOPAC was noted during the first 24 h after the first dose with a return to saline control levels, and a similar pattern was detected for the ratio of DOPAC/DA. *P < 0.05.
The levels of DOPAC were also influenced by the Sampling Time, F(4,56) = 25.3, P < 0.0001. Treatment with MDMA resulted in a decrease of DOPAC, Treatment, F(1,14) = 14.6, P < 0.002, and this decrease interacted with Sampling Time, F(4,56) = 3.3, P < 0.03, such that decreases were observed at 1, 7, and 24 h after the first dose, with levels similar to controls after that point (Fig. 5B). No sex differences were found or any other interactions.
The DOPAC/DA ratio was decreased by MDMA treatment, F(1,14) = 18.2, P < 0.001. As observed with DOPAC, there was a significant effect of Sampling Time, F(4,56) = 8.4, P < 0.001, and the interaction of Treatment × Sampling time was significant F(4,56) = 6.2, P < 0.005. The interaction showed that DOPAC/DA ratios were decreased for at least 24 h and returned to SAL control levels by 30 h and remained similar to SAL at 78 h (Fig. 5C).
A different pattern of 5-HT levels was observed in the striatum relative to the hippocampus. Both Sampling Time, F(4,56) = 6.0, P < 0.002, and Treatment, F(1,14) = 10.1, P < 0.007, affected the 5-HT levels. Treatment with MDMA produced a reduction in levels; however, unlike the hippocampus where reductions were immediate and lasted over the 72-h time period, in the striatum, the reductions were slower, first appearing at the 24-h time point and persisted throughout the 72-h time point, Treatment × Sampling Time, F(4,56) = 6.7, P < 0.0007 (Fig. 6A). No other significant main effects or interactions were present.
Fig. 6.
The concentrations of 5-HT (A), 5-HIAA (B), and the ratio of 5-HIAA/5-HT (C) are shown. Unlike the hippocampus, striatal levels of 5-HT did not display a decrease until 24 h, and then remained decreased until 78 h after the first dose. A different pattern emerged for 5-HIAA, such that differences were only noted at 7, 24, and 30 h. The ratio of 5-HIAA/5-HT was decreased at 7 h, but it displayed an increased rate at 78 h. *P < 0.05.
Similar to changes in the hippocampus, the levels of 5-HIAA in striatum were also affected by Treatment, F(1,14) = 12.5, P < 0.004, and Sampling Time, F(4,56) = 18.59, P < 0.0001. The MDMA-treated animals had lower levels of 5-HIAA than animals treated with SAL. These reduced levels in MDMA-treated animals were only observed at the 7-, 24-, and 30-h times, Treatment × Sampling Time, F(4,56) = 9.51, P < 0.0001 (Fig. 6B). No other differences were detected.
For the 5-HIAA/5-HT ratio in the striatum, only the MDMA Treatment × Sampling Time interaction was significant, F(4,56) = 5.6, P < 0.003. Sampling Time approached significance, P < 0.06. As can be seen in Fig. 6C, there was a reduction at 7 h with levels lower (but not significantly) at 24 h. No differences were noted at 30 h; however, there was an increase in the 5-HIAA/5-HT ratio in the MDMA group at 78 h.
4. Discussion
This study was designed to investigate the short-term effects of MDMA following 1 day of drug administration, similar to what is used in adult animals. Several effects following MDMA on P11 were obtained. Firstly, the data demonstrate that a prolonged decrease in monoamines (>3 days) occurs even after a single day of 4-dose MDMA administration, an effect that has not been shown in neonates previously. Secondly, although long-lasting decreases in 5-HT were observed, SERT, as assessed by 3H-paroxetine binding, was unaltered in contrast to what is seen after P1–4 treatment [39,64]. Thirdly, this is the first study showing that rapid HPA axis activation occurs following MDMA in developing animals as demonstrated by the increases in plasmatic CORT and ACTH. These increases occur during a portion of development termed the stress hyporesponsive period [51,54]. Fourthly, there appears to be dissociation between the release of ACTH and CORT, since CORT remained elevated for longer than normal feedback inhibition would predict. These data demonstrate that multiple administrations of MDMA induce neurochemical changes that may be the precursors of long-term changes that ultimately result in cognitive deficits.
In previous studies, we have administered MDMA from P11 to P20 and detected cognitive deficits in adulthood [10,57,62]. It should be recognized that the single-day approach used herein was not intended to be comparable to our previous data, but only to provide data on the neurochemical and neuroendocrine changes after MDMA on P11 and the following 3 days. Single-day drug administration was also selected since this may be when critical changes are initiated, especially considering that neurotransmitter and hormonal systems are rapidly developing during the neonatal period. Interestingly, data from Koprich et al. [32] show that 24 h after P11–20 MDMA administration, hippocampal 5-HT is reduced by approximately 31%, whereas in the present study, 24 h after the last dose on P11 hippocampal 5-HT levels were reduced by approximately 45%. The comparison between these data suggests that the effect of MDMA may diminish as drug administration continues. Further studies will have to be performed to determine the effects of multiple days of dosing.
In the present study, both the hippocampus and neostriatum were investigated since they are involved in spatial learning [25,58,60], and the hippocampus is still undergoing neurogenesis [7,49]. Although the striatum is not undergoing neurogenesis on P11, synaptogenesis and myelination are occurring (see [55]) and this region is susceptible to damage by MDMA in adult animals [19]. We found that the reductions in 5-HT and 5-HIAA were region and time specific. For example, in the hippocampus, there was an immediate reduction in 5-HT following MDMA that showed only partial recovery 78 h later. On the other hand, 5-HT in the neostriatum was not reduced until 24 h after the first MDMA injection at which point there was a 42% decrease and little recovery occurred. By comparison, following a single 40 mg/kg gavage administration of MDMA (identical to the total dosage given here) on P10, 5-HT decreases (up to 77%) were not region specific [8]. Furthermore, in that study, reductions in 5-HT were observed 3 h following administration in all regions (the first time point examined), but no differences were noted at 72 h. The present data suggest that multiple injections of MDMA on a single day are more effective at producing protracted reductions in 5-HT and produce effects that are regionally specific compared to a single large dose of the drug.
Although MDMA disrupted the 5-HT system as evidenced by the changes in 5-HT, 5-HIAA, and the ratio of 5-HIAA/5-HT, we did not observe changes in 3H-paroxetine binding. Sex differences were detected, and differences in SERT during the early postnatal period have been reported previously, although these effects were not consistent among the various ages examined [48]. There was also a trend for the MDMA-treated males to have less 3H-paroxetine binding, although this has to be interpreted with caution. The overall lack of change in SERT is in agreement with the study by Broening et al. [8]. In contrast, others have demonstrated a reduction in SERT after 4 days of MDMA administration from P1 to P4 [39,64]. Whether the differences in these studies are related to the age of the animals or to the difference in dosing regimens is not certain; however, it is likely that the age of the animal was an important factor. For example, administration of MDMA from P10 to P13 produced no changes in SERT when examined 2 weeks later but administration from P25 to P29 resulted in decreased binding [29].
It is unlikely that the variation in 3H-paroxetine binding at some of the time points in this study was from differences in tissue dissection, since it would be expected that 5-HT levels in comparably dissected hippocampi would also vary widely. However, examination of Fig. 3A demonstrates that the variation in 5-HT levels was very low. Interestingly, regardless of treatment, there was a sharp increase in 3H-paroxetine binding between the 1-h time point and the 7-h time point. Following a single episode of electroconvulsive shock in adult animals, it has been demonstrated that there was also a rapid increase in 3H-paroxetine binding that was still apparent even 24 h and 14 days later [22]. The rapid increase in binding sites in this study may be related to the injections the animals received, handling, or increased maternal attention. Alternatively, it has been demonstrated that during development, there is a dramatic increase in 3H-paroxetine binding between P15 and P22 [48]; hence, there may have been both developmental and precocious development of SERT in P11-treated animals. Further investigation into the cause of the P11 increase in 3H-paroxetine binding over a 6-h time period will require further investigation.
Early developmental influences can alter the response of the hypothalamic–pituitary–adrenal (HPA) axis (see Ref. [3]). These changes are thought to be the result of early increases in glucocorticoids such as CORT, as well as perturbations of the 5-HT system; however, the basis of these changes is not fully understood. The HPA axis, and especially glucocorticoids, modulates learning and memory as well as a number of physiological and behavioral responses [13,34,35], and therefore any deviation in the development of this system is likely to lead to long-term changes in how the animal responds to later stressors or how the animal performs during learning tasks. We found that CORT levels remained elevated for at least 24 h after the initial exposure to MDMA, an effect that was unanticipated given that MDMA was administered during the stress hyporesponsive period (SHRP) [51,54]. The SHRP is associated with rapid growth of neurons, especially within the hippocampus, and it has been demonstrated that peak levels of BrdU-labeled cells in the dentate gyrus occur between P9 and P11 [33]. It has been hypothesized that the SHRP is neuroprotective against the adverse effects that high levels of glucocorticoids can exert during this stage of development [51]. With the exception of females treated with MDMA and examined 78 h later, no differences in thymus weights were observed, contrary to what would be seen in adults with prolonged CORT increases [5,30]. The lack of effect is in agreement with the notion that the thymus is not regulated by glucocorticoids until later in development [26].
The protracted increase of CORT produced by MDMA is likely to interfere with neuronal development [17,33,65] and development of the HPA axis. Interestingly, the prolonged increase in CORT occurred without protracted ACTH increases, suggesting a disconnection between the two. During the period of drug administration, it can clearly be seen that ACTH in MDMA-treated animals is increased almost threefold over levels in SAL-treated animals 1 h after the first dose. Nevertheless, 1 h after the last dose of MDMA, there is a precipitous fall in ACTH, suggesting that the increased levels of CORT are exerting negative feedback control as would be expected. However, CORT elevations without ACTH increases were not expected at the 24-h time point. Therefore, other factors must be important for the prolonged increase in CORT. For example, there may have been direct stimulation of the adrenal gland by MDMA-induced 5-HT release. It is known that in neonates, 5-HT administration can induce an increase in CORT release [21]. We did not measure levels of 5-HT in the blood of these animals; therefore, it is unknown if this could account for the protracted CORT increase.
In conjunction with the elevated CORT, we also showed decreases in 5-HT. This combination may produce lasting changes in brain development and therefore in later learning and memory ability. Serotoninergic neurons appear very early in development and 5-HT possesses neurotrophic properties at this time [4,59]. Depletion of serotonin with either p-chloroamphetamine (PCA) or 5,7-dihydroxytryptamine (5,7-DHT) during the neonatal period disrupts the neurodevelopment of the dentate gyrus, such that there are fewer spines on the dendrites of these neurons in the absence of changes in dendritic length [67]. The reduction in spines following PCA-induced depletion of serotonin can be ameliorated with a 5-HT1a receptor agonist [66], suggesting that the 5-HT1a receptor is important in the development of synapses within the dentate gyrus. Others have found that inhibition of tryptophan hydroxylase with p-chlorophenylalanine from P10 to P20 results in fewer synapses in the hippocampus and deficits in learning and memory when the animals were tested in adulthood [37]. Taken together with data demonstrating that P11–20 MDMA disrupts spatial learning and memory and the findings herein that single-day MDMA administration on P11 decreases 5-HT in regions important in learning and memory (i.e., hippocampus and striatum), these data support an association between early 5-HT disruption and later cognitive deficits. Therefore, following MDMA, it is likely that the combination of both the prolonged elevations in CORT and the reductions in 5-HT alter the ability of animals to learn as effectively as control animals.
The amount of MDMA can vary from 0 mg to at least 150 mg per tablet [11,19,53]. Users generally take 1–4 tablets in a day [19], although greater consumption has been reported [45] even during pregnancy [23]. Therefore, a 60-kg woman who consumed four, 150 mg tablets would use 10 mg/kg of MDMA per occasion. We administered 40 mg/ kg in 4 doses of 10 mg/kg each; therefore, this would be about four times higher than what would be expected in a heavy user of MDMA. However, a non-scaled mg/kg dosage comparison does not account for differences in metabolic rate and rate of elimination of drug between humans and other species and therefore scaling to account for these differences may be a more appropriate basis for comparison (for review, see [19], although see [14]). Using the Mordenti and Chappell’s interspecies scaling formula of Dosehuman = Doseanimal (Weighthuman/Weightanimal)0.7 [19] and assuming a 60-kg woman, a 150-mg tablet, and a 300-g animal, the equivalent rat dose would be 3.68 mg or 12.27 mg/kg. If this same woman were to take 4 doses on a single occasion, then the dose would be for rats 49.08 mg/kg, similar to the 40 mg/kg/day administered herein. The amount of drug administered in this study represents a heavier user of MDMA; however, this may be clinically relevant for a subset of MDMA users and heavier users are the most likely to use stimulants throughout pregnancy (cf. [50]).
In summary, this is the first study to demonstrate that P11 MDMA induces depletions in 5-HT that last up to 3 days after 1 day of drug administration, that MDMA-treated animals show elevated CORT for over 24 h, and that ACTH is increased following MDMA exposure. ACTH increases have only been reported previously in human studies [20]. The increased levels of CORT at 24 h do not appear to be the result of elevated ACTH, suggesting that other factors must be involved in this protracted CORT increase. Taken together, the data implicate 5-HT disruption and adrenal hyperactivity as potential factors that may contribute to the susceptibility of the developing brain to MDMA.
Acknowledgments
Portions of these data were presented at the 12th annual meeting of the International Behavioral Neuroscience Society meeting in San Juan, Puerto Rico. Supported by National Institutes of Health grants DA14269 (MTW), DA11902 (CVV), and DA07427 (GAG) and training grant ES07051 (LAE).
References
- 1.Aguirre N, Frechilla D, García-Osta A, Lasheras B, Del Río J. Differential regulation by methylenedioxymethamphetamine of 5-hydroxytryptamine1A receptor density and mRNA expression in rat hippocampus, frontal cortex, and brainstem: the role of corticosteroids. J Neurochem. 1997;68:1099–1105. doi: 10.1046/j.1471-4159.1997.68031099.x. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre N, Barrionuevo M, Lasheras B, Del Río J. The role of dopaminergic systems in the perinatal sensitivity to 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J Pharmacol Exp Ther. 1998;286:1159–1165. [PubMed] [Google Scholar]
- 3.Andrews MH, Matthews SG. Programming of the hypothalamo–pituitary–adrenal axis: serotonergic involvement. Stress. 2004;7:15–27. doi: 10.1080/10253890310001650277. [DOI] [PubMed] [Google Scholar]
- 4.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 5.Bakker JM, Kavelaars A, Heijnen CJ, Tilders FJH, van Rees EP. Effects of neonatal dexamethasone treatment on hypothalamo–pituitary adrenal axis and immune system of the rat. J Neuroimmunol. 1997;74:69–76. doi: 10.1016/s0165-5728(96)00207-x. [DOI] [PubMed] [Google Scholar]
- 6.Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- 7.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 8.Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- 9.Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (±)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- 10.Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-Methylenedioxymethamphetamine (Ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, San L, de la TR. Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Connor TJ, McNamara MG, Finn D, Currid A, O’Malley M, Redmond AM, Kelly JP, Leonard BE. Acute 3,4-methylenedioxymethamphetamine (MDMA) administration produces a rapid and sustained suppression of immune function in the rat. Immunopharmacology. 1998;38:253–260. doi: 10.1016/s0162-3109(97)00084-2. [DOI] [PubMed] [Google Scholar]
- 13.de Kloet ER, Vreugdenhil E, Oitzl MS, Joδls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 14.de la TR, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.de la TR, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, Segura J, Cami J. Non-linear pharmacokinetics of MDMA (“ecstasy”) in humans. Br J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez F, Aguerre S, Mormede P, Chaouloff F. Influences of the corticotropic axis and sympathetic activity on neurochemical consequences of 3,4-methylenedioxymethamphetamine (MDMA) administration in Fischer 344 rats. Eur J Neurosci. 2002;16:607–618. doi: 10.1046/j.1460-9568.2002.02110.x. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- 18.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 19.Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 20.Grob CS, Poland RE, Chang L, Ernst T. Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res. 1996;73:103–107. doi: 10.1016/0166-4328(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 21.Grota LJ. Serotonin regulation of corticoid secretion in infant rats. Dev Psychobiol. 1981;14:221–228. doi: 10.1002/dev.420140311. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa H, Okamoto Y, Shimizu M, Nishida A, Motohashi N, Yamawaki S. Single or repeated treatment with electroconvulsive shock increases number of serotonin uptake binding sites in the frontal cortex. Neuropsychobiology. 1995;31:1–5. doi: 10.1159/000119164. [DOI] [PubMed] [Google Scholar]
- 23.Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use Ecstasy (3,4-methylenedioxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 24.Ho YJ, Pawlak CR, Guo L, Schwarting RK. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res. 2004;149:135–144. doi: 10.1016/s0166-4328(03)00220-1. [DOI] [PubMed] [Google Scholar]
- 25.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inomata T, Nakamura T. Influence of adrenalectomy on the development of the neonatal thymus in the rat. Biol Neonate. 1989;55:238–243. doi: 10.1159/000242924. [DOI] [PubMed] [Google Scholar]
- 27.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students, NIH Publication, 04-5507. I. National Institue on Drug Abuse; Bethesda, MD: 2004. Monitoring the future national survey results on drug abuse, 1975–2003. [Google Scholar]
- 28.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. College students and adult ages 19–45, NIH Publication, vol. 04-5508. II. National Institue on Drug Abuse; Bethesda, MD: 2004. Monitoring the future national survey results on drug abuse, 1975–2003. [Google Scholar]
- 29.Kelly PA, Ritchie IM, Quate L, McBean DE, Olverman HJ. Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol. 2002;137:963–970. doi: 10.1038/sj.bjp.0704961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kioukia-Fougia N, Antoniou K, Bekris S, Liapi C, Christofidis I, Padadopoulou-Daifoti Z. The effects of stress exposure on the hypothalamic–pituitary–adrenal axis, thymus, thyroid hormones and glucose levels. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:823–830. doi: 10.1016/s0278-5846(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 31.Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole Publishing; Pacific Grove: 1995. [Google Scholar]
- 32.Koprich JB, Campbell NG, Lipton JW. Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain-derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res Dev Brain Res. 2003;147:177–182. doi: 10.1016/s0165-3806(03)00219-0. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 34.Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 35.Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- 36.Mas M, Farré M, de la Torre R, Roset PN, Ortuño J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- 37.Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- 38.McElhatton PR, Baterman DN, Evans C, Pughe KR, Thomas SH. Congenital anomalies after prenatal ecstasy exposure. Lancet. 1999;354:1441–1442. doi: 10.1016/s0140-6736(99)02423-x. [DOI] [PubMed] [Google Scholar]
- 39.Meyer JS, Ali SF. Serotonergic neurotoxicity of MDMA (ecstasy) in the developing rat brain. Ann N Y Acad Sci. 2002;965:373–380. doi: 10.1111/j.1749-6632.2002.tb04179.x. [DOI] [PubMed] [Google Scholar]
- 40.Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (“ecstasy”) administration to neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (“Ecstasy”) Eur J Pharmacol. 2001;433:91–99. doi: 10.1016/s0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- 42.Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- 43.Navarro M, Pichini S, Farre M, Ortuno J, Roset PN, Segura J, de la TR. Usefulness of saliva for measurement of 3,4-methylenedioxymethamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 44.Pacifici R, Zuccaro P, Lopez CH, Pichini S, di Carlo S, Farre M, Roset PN, Ortuño J, Segura J, Torre RL. Acute effects of 3,4-methylenedioxymethamphetamine alone and in combination with ethanol on the immune system in humans. J Pharmacol Exp Ther. 2001;296:207–215. [PubMed] [Google Scholar]
- 45.Parrott AC. Recreational ecstasy/MDMA, the serotonin syndrome, and serotonergic neurotoxicity, Pharmacology. Biochemistry and Behavior. 2002;71:837–844. doi: 10.1016/s0091-3057(01)00711-0. [DOI] [PubMed] [Google Scholar]
- 46.Pollier F, Sarre S, Aguerre S, Ebinger G, Mormede P, Michotte Y, Chaouloff F. Serotonin reuptake inhibition by citalopram in rat strains differing for their emotionality. Neuropsychopharmacology. 2000;22:64–76. doi: 10.1016/S0893-133X(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 47.Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn-Schmie-deberg’s Arch Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- 48.Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Brain Res Dev Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- 49.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: comparison of prenatal care and no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- 51.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 52.Sprague JE, Preston AS, Leifheit M, Woodside B. Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiol Behav. 2003;79:281–287. doi: 10.1016/s0031-9384(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 53.Teter CJ, Guthrie SK. A comprehensive review of MDMA and GHB: two common club drugs. Pharmacotherapy. 2001;21:1486–1513. doi: 10.1592/phco.21.20.1486.34472. [DOI] [PubMed] [Google Scholar]
- 54.Vázquez DM. Stress and the developing limbichypothalamicpituitaryadrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 55.Vorhees CV. Methods for detecting long-term CNS dysfunction after prenatal exposure to neurotoxins. Drug Chem Toxicol. 1997;20:387–399. doi: 10.3109/01480549709003895. [DOI] [PubMed] [Google Scholar]
- 56.Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 1120 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 59.Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 60.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 61.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev Brain Res. 2003;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- 63.Williams MT, Brown CA, Skelton MR, Vinks AA, Vorhees CV. Absorption and clearance of +/−3,4-methylenedioxymethamphetamine from the plasma of neonatal rats. Neurotoxicol Teratol. 2004;26:849–856. doi: 10.1016/j.ntt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–220. [PubMed] [Google Scholar]
- 65.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 66.Yan W, Wilson CC, Haring JH. 5-HT1a receptors mediate the neurotrophic effect of serotonin on developing dentate granule cells. Brain Res Dev Brain Res. 1997;98:185–190. doi: 10.1016/s0165-3806(96)00175-7. [DOI] [PubMed] [Google Scholar]
- 67.Yan W, Wilson CC, Haring JH. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]
- 68.Yau JLW, Noble J, Seckl JR. Site-specific regulation of corticosteroid and serotonin receptor subtype gene expression in the rat hippocampus following 3,4-methylenedioxymethamphetamine: role of corticosterone and serotonin. Neuroscience. 1997;78:111–121. doi: 10.1016/s0306-4522(96)00497-6. [DOI] [PubMed] [Google Scholar]