Abstract
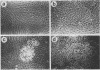
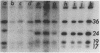
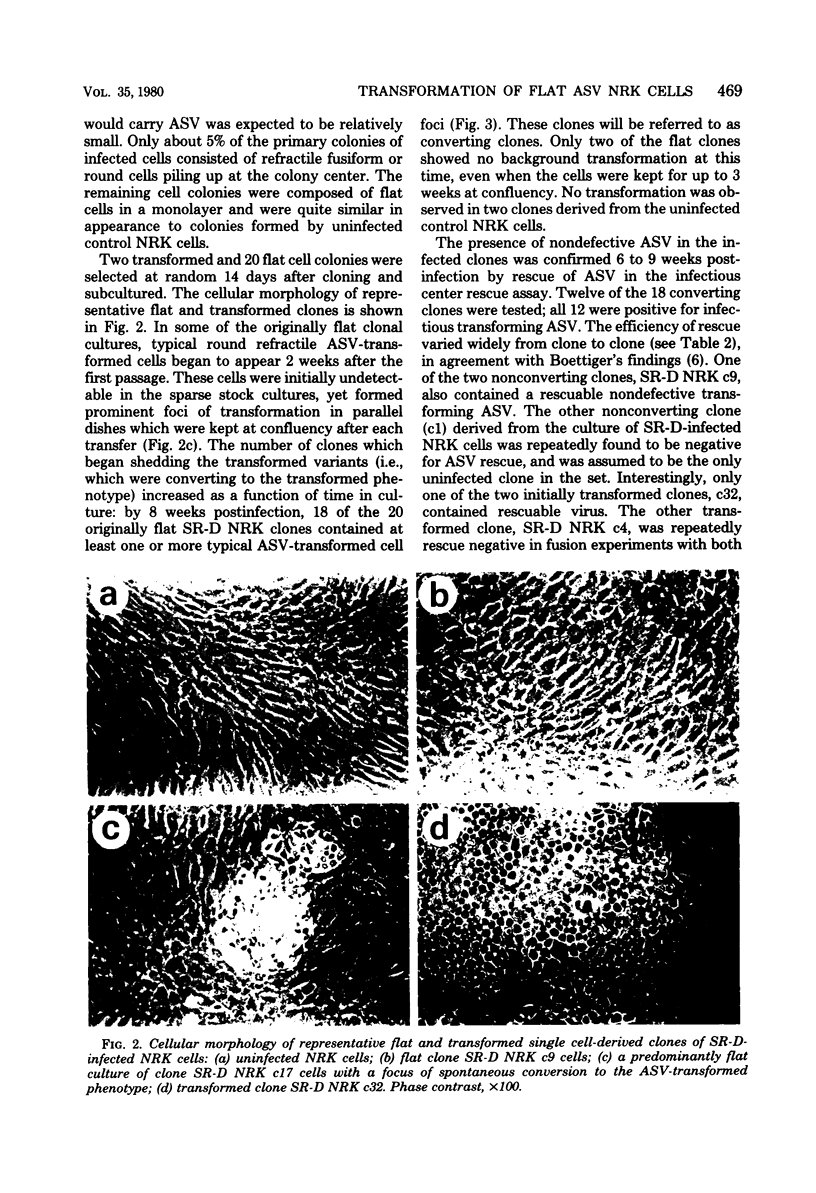
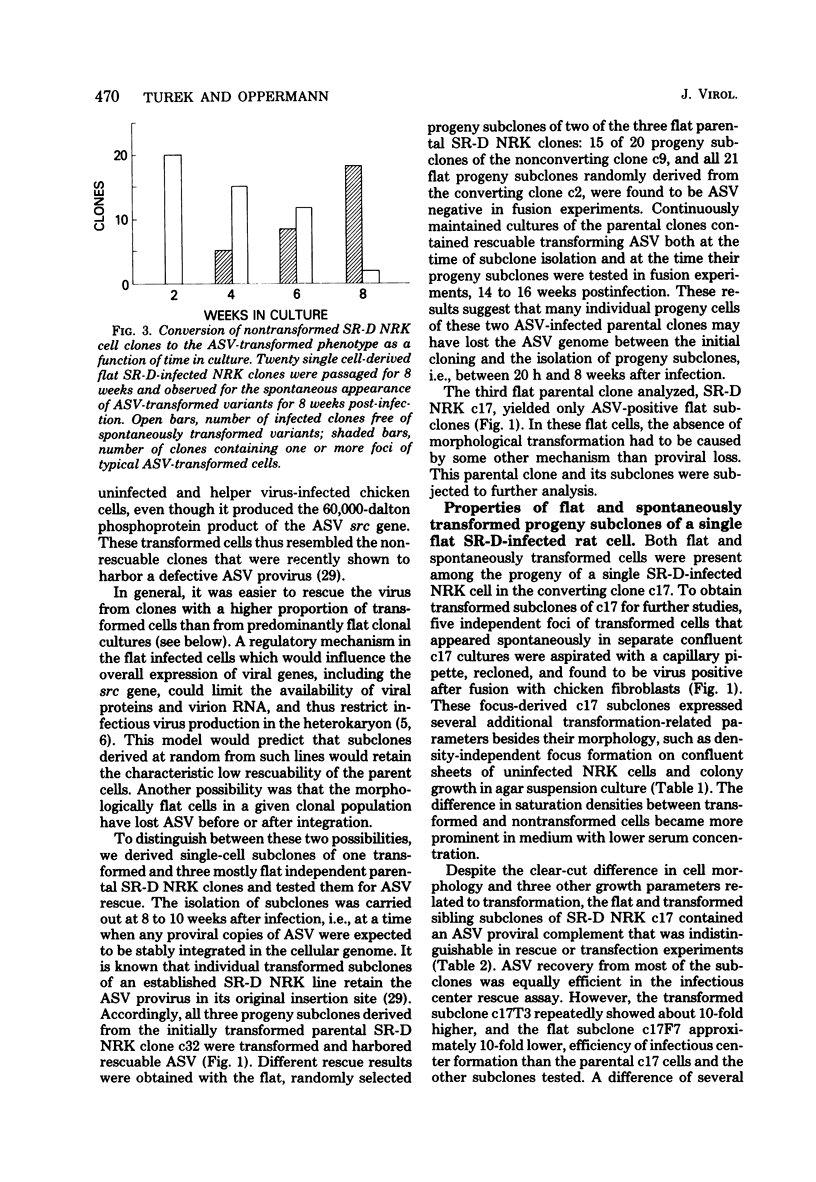
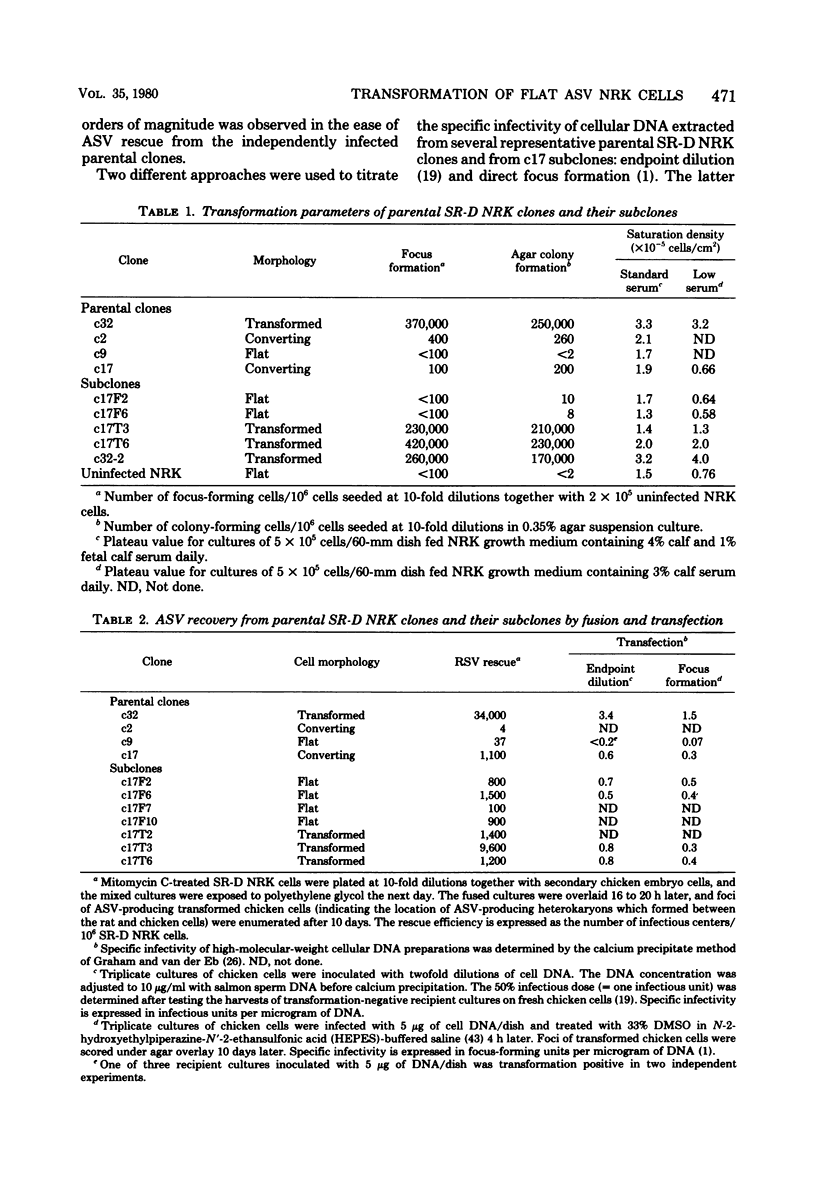
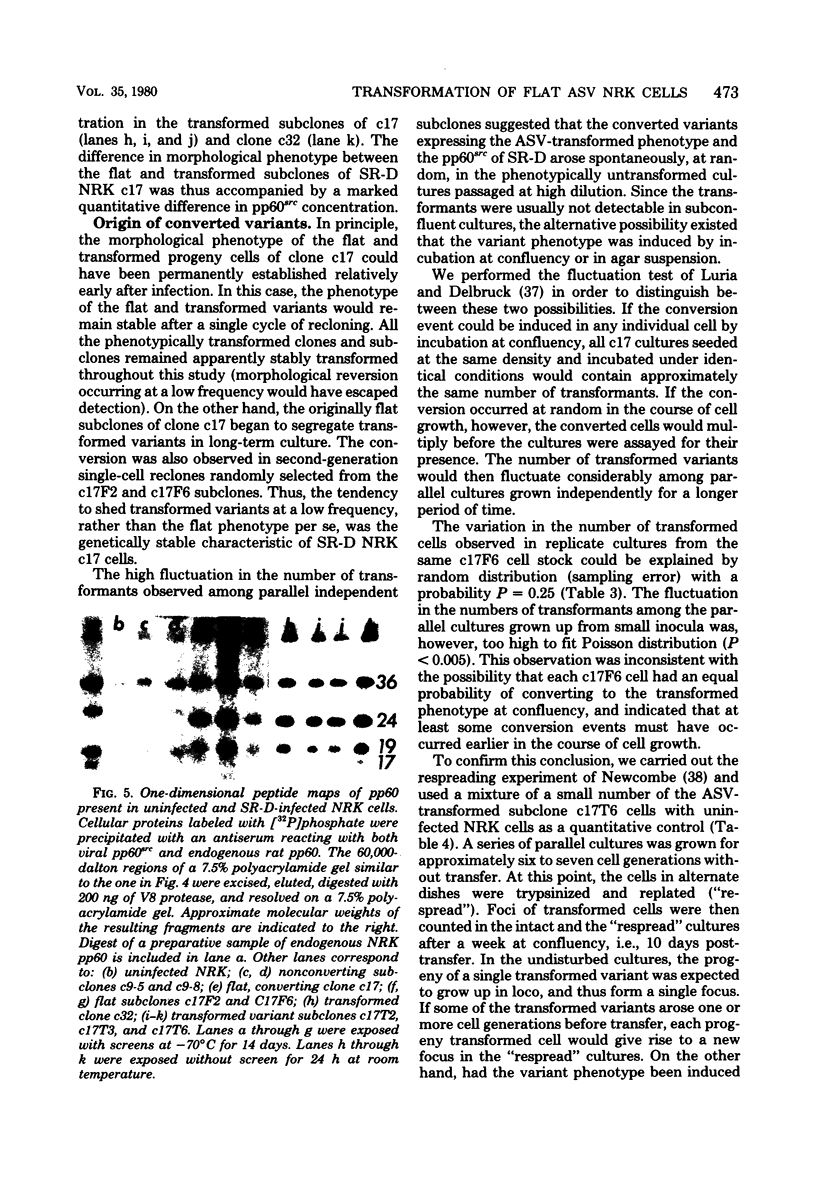
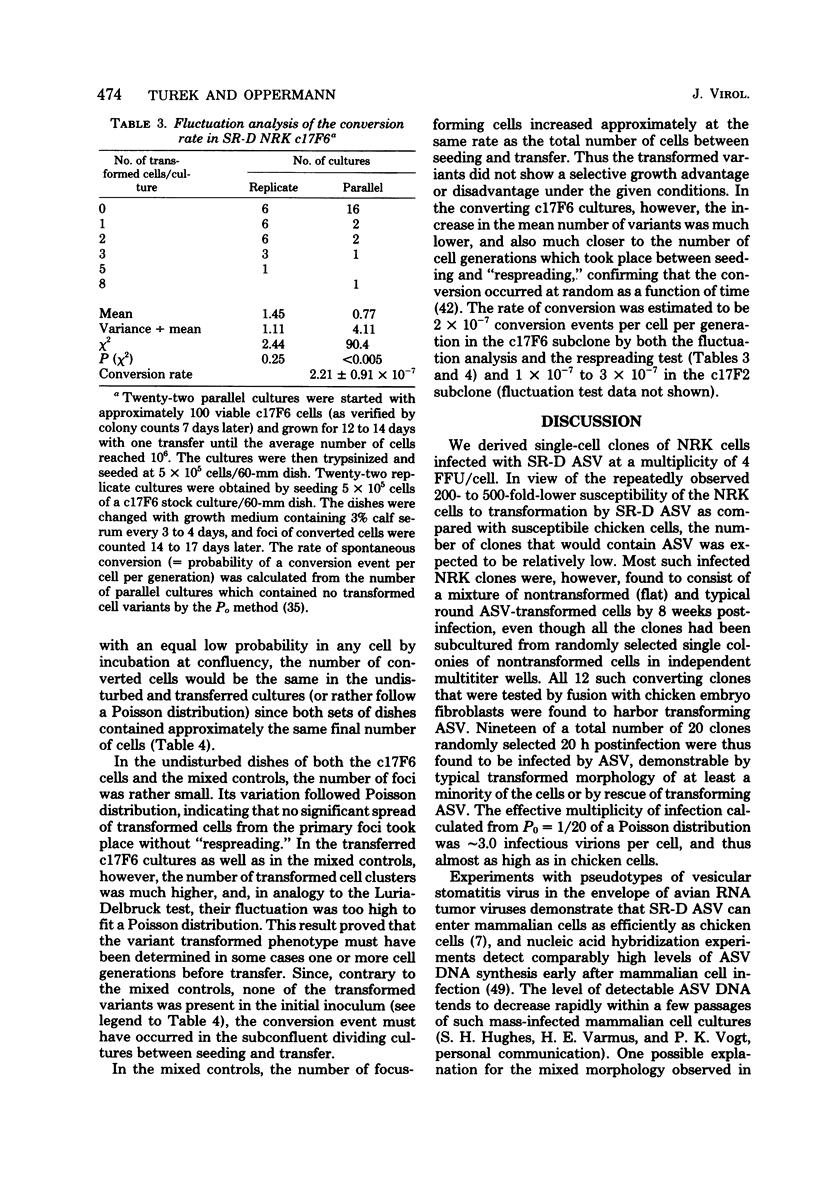
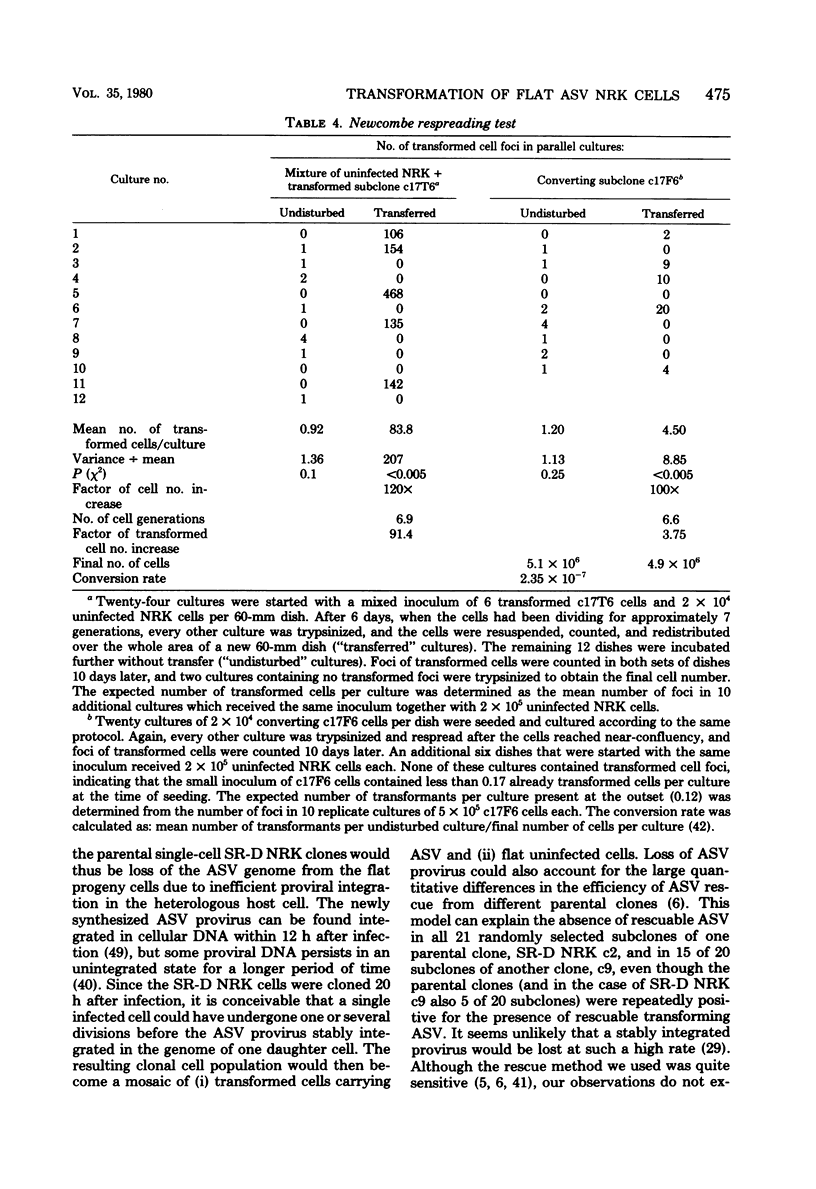
Normal rat kidney (NRK) fibroblasts were infected with the Schmidt-Ruppin strain (SR-D) of avian sarcoma virus (ASV) and cloned 20 h after infection without selection for the transformed phenotype. Most infected clones initially exhibited the flat, nontransformed morphology that is characteristic of uninfected NRK cells. In long-term culture, however, the majority of the SR-D NRK clones began segregating typical ASV-transformed cells. Transforming ASV could be rescued by fusion with chicken embryo fibroblasts from most of the infected clones tested. Three predominantly flat, independently infected clones were further analyzed by subcloning 8 to 10 weeks after infection. Most flat progeny subclones derived at random from two of these “parental” SR-D NRK clonal lines did not yield virus upon fusion with chicken embryo fibroblasts, although a nondefective transforming ASV was repeatedly recovered from the parental clones. This observation suggested that most, but not all, daughter cells in these SR-D NRK clones lost the ASV provirus after cloning. The progeny of the third independent parental cell clone, c17, gave rise to both flat and transformed subclones that carried ASV. In this case, ASV recovery by fusion and transfection from the progeny subclones was equally efficient regardless of the transformation phenotype of the cells. The 60,000-dalton phosphoprotein product of the ASV src gene was, however, expressed at high level only in the transformed variants. The results of a Luria-Delbruck fluctuation analysis and of Newcombe's respreading test indicated that the event leading to the spontaneous conversion to the transformed state occurred at random in dividing cultures of these flat ASV NRK cells at a rate predicted for somatic mutation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Vogt P. K. Integration of different sarcoma virus genomes into host DNA: evidence against tandem arrangement and for shared integration sites. Proc Natl Acad Sci U S A. 1979 May;76(5):2465–2469. doi: 10.1073/pnas.76.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., DeMars R. Somatic cell mutation. Detection and quantification of x-ray-induced mutation in cultured, diploid human fibroblasts. Mutat Res. 1973 May;18(2):199–224. doi: 10.1016/0027-5107(73)90037-7. [DOI] [PubMed] [Google Scholar]
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Battula N., Temin H. M. Infectious DNA of spleen necrosis virus is integrated at a single site in the DNA of chronically infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):281–285. doi: 10.1073/pnas.74.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Love D. N., Weiss R. A. Virus envelope markers in mammalian tropism of avian RNA tumor viruses. J Virol. 1975 Jan;15(1):108–114. doi: 10.1128/jvi.15.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D. Reversion and induction of Rous sarcoma virus expression in virus-transformed baby hamster kidney cells. Virology. 1974 Dec;62(2):522–529. doi: 10.1016/0042-6822(74)90412-7. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Virogenic nontransformed cells isolated following infection of normal rat kidney cells with B77 strain Rous sarcoma virus. Cell. 1974 Sep;3(1):71–76. doi: 10.1016/0092-8674(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Hayman M. J., Vogt P. K. Properties of mammalian cells transformed by temperature-sensitive mutants of avian sarcoma virus. Cell. 1977 Jul;11(3):513–521. doi: 10.1016/0092-8674(77)90069-1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L., Lau A. F., Krzyzek R. A., Faras A. J. The src gene product of transformed and morphologically reverted ASV-infected mammalian cells. Nature. 1979 Sep 20;281(5728):195–198. doi: 10.1038/281195a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Boettiger D., Green T. L., Burgess M. B., Devlin H., Parsons J. T. Arrangement of integrated avian sarcoma virus DNA sequences within the cellular genomes of transformed and revertant mammalian cells. J Virol. 1980 Feb;33(2):760–768. doi: 10.1128/jvi.33.2.760-768.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Silverman L. Linkage of the endogenous avian leukosis virus genome of virus-producing chicken cells to inhibitory cellular DNA sequences. Cell. 1978 Oct;15(2):573–577. doi: 10.1016/0092-8674(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Friis R. R. Differential expression of transformation in rat and chicken cells infected with an avian sarcoma virus ts mutant. Virology. 1973 Nov;56(1):369–374. doi: 10.1016/0042-6822(73)90314-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Hillová J. Production virale dans les fibroblastes de poule traités par l'acide désoxyribonucléique de cellulex XC de rat transformées par le virus de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 14;272(24):3094–3097. [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau A. F., Krzyzek R. A., Brugge J. S., Erikson R. L., Schollmeyer J., Faras A. J. Morphological revertants of an avian sarcoma virus-transformed mammalian cell line exhibit tumorigenicity and contain pp60src. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3904–3908. doi: 10.1073/pnas.76.8.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Complementation rescue of Rous sarcoma virus from transformed mammalian cells by polyethylene glycol-mediated cell fusion. J Virol. 1977 Jul;23(1):133–141. doi: 10.1128/jvi.23.1.133-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O., Zadrazil S. Problems of RSV rescue from virogenic mammalian cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1077–1083. doi: 10.1101/sqb.1974.039.01.123. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Machala O., Donner L., Sovovã Comparative study of RSV rescue from RSV-transformed mammalian cells. Int J Cancer. 1971 Nov 15;8(3):391–400. doi: 10.1002/ijc.2910080306. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Popovic M., Sainerová H., Mach O., Shoyab M., Baluda M. A. Incomplete viral genome in a non-virogenic mouse tumour cell line (RVP3) transformed by Prague strain of avian sarcoma virus. Int J Cancer. 1977 Jun 15;19(6):851–858. doi: 10.1002/ijc.2910190617. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Hu S. S. The genetic structure of RNA tumor viruses. Annu Rev Genet. 1977;11:203–238. doi: 10.1146/annurev.ge.11.120177.001223. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]