Abstract
We previously demonstrated that postnatal day 11–20 ±3,4-methylenedioxymethamphetamine (MDMA) exposure reduces locomotor activity and impairs path integration and spatial learning independent of the effects on activity. The effects were seen when the drug was administered twice per day, but the optimal dosing regimen is unknown. We tested whether the same total daily dose of MDMA administered in different patterns would equally affect later behavior. A split-litter design (15 litters) was used with one male/female pair per litter receiving one of four treatment regimens. All offspring received four injections per day on P11–20 as follows: 40 × 1 (40 mg/kg MDMA × 1 + saline × 3), 20 × 2 (20 mg/kg MDMA × 2 + saline × 2), 10 × 4 (10 mg/kg MDMA × 4), or Saline (saline × 4). Does were spaced 2 h apart. Group 40 × 1 received MDMA as the first daily dose followed by three saline doses; group 20 × 2 received MDMA as the first and last dose and saline for the middle two doses; group 10 × 4 received MDMA for all four doses; and the saline group received saline for all four doses. Regardless of dose schedule, all groups treated with MDMA exhibited reduced locomotor activity. No MDMA effects were found on swimming ability in a straight channel. Modest MDMA effects were found on Barnes maze performance. The major findings were that the 40 × 1 and 20 × 2 MDMA groups showed impaired Cincinnati multiple T-water-maze learning and the 10 × 4 and 20 × 2 MDMA groups showed impaired Morris water maze learning. The results suggest that MDMA dose distribution has a long-term differential effect on different types of learning. Dose distribution warrants greater attention in the design of developmental drug studies along with the standard considerations of dose and age.
Keywords: MDMA, ecstasy, brain development, learning and memory
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA) has become a widely abused drug in recent years especially among adolescents and younger adults, who have annual prevalence rates ranging from 4 to 12% over the last decade (Johnston et al., 2005). Knowledge of the effects of MDMA on the mature brain is steadily increasing (Green et al., 2003), but little is known about its effects on the developing brain despite the fact that use during pregnancy has been documented (Ho et al., 2001; McElhatton et al., 1999). The current data are limited to recreational users who tend to limit drug use to the first trimester.
When prenatal cocaine was first investigated, most users showed recreational use patterns. These users showed primarily a first trimester exposure history. As cocaine use became more widespread, users could be identified, who fell into two distinct groups. One was a more educated, lighter using group that obtained prenatal care and used primarily in the first trimester and a second, less educated, heavier using group that did not obtain prenatal care and used throughout pregnancy (Richardson et al., 1999). At present, the data on prenatal MDMA use only captures information on recreational users (Ho et al., 2001; McElhatton et al., 1999). There are no data on chronic MDMA users who may use higher doses or for longer durations during pregnancy.
There are several studies in animals suggesting long-term neuronal effects from exposure to MDMA during early brain development (Kelly et al., 2002; Koprich et al., 2003; Meyer and Ali, 2002; Meyer et al., 2004; Winslow and Insel, 1990; Won et al., 2002), and several studies reporting no significant effects (Broening et al., 1994, 1995; St. Omer et al., 1991). One problem is that these models vary widely in the exposure ages investigated. A number of these have exposed rats to MDMA during stages of brain development analogous to human first or first and part of second trimesters. There are few models of second to third trimester equivalent exposure.
There is considerable evidence that human second to third trimester is analogous to rodent neonatal brain development (Bayer et al., 1993; Clancy et al., 2001; Clancy et al., in press; Herlenius and Lagercrantz, 2004; Rice and Barone, 2000; West and Pierce, 1987). In developing an exposure model for MDMA that is comparable to second to third trimester human brain development, we focused on hippocampal development because of this structure's involvement in learning and memory. Proliferation in the dentate gyrus is highest from postnatal day (P) 1–20. Experiments using bromodeoxyuridine (BrdU) incorporation as an index of neurogenesis show increased rates of labeling on P6 with a peak on P9. This is followed by a decline to P13 and gradual tapering off through P20. Stable, adult neurogenesis rates are reached between P20 and P30 (Liu et al., 2003). We previously found that rats exposed to MDMA exhibit long-term spatial learning and memory deficits in the Morris water maze (MWM) and path integration learning deficits in the Cincinnati water maze (CWM) when exposure occurred on P11–20, but not when exposure occurred on P1–10 (Broening et al., 2001), a period that overlaps with the period of high BrdU labeling in the dentate. We subsequently showed that these impairments were not due to changes in growth, injection stress, or litter effects, and that cued learning in the MWM was not affected (Williams et al., 2003c). We also demonstrated that the effects of P11–20 MDMA treatment on MWM were dose-related, selective for reference memory while sparing working memory (Vorhees et al., 2004), and affected other hippocampally-dependent forms of learning, such as novel object recognition (Cohen et al., 2005). We also showed that the P11–20 MDMA effects on learning were not a function of maternal selective attention for control animals compared to drug exposed animals, since the effects of MDMA on learning and memory were the same regardless of whether a within-litter or between-litter study design was used [cf., (Broening et al., 2001; Williams et al., 2003c)].
A potentially significant but largely unexplored area in developmental drug studies is the effect of dose distribution or schedule of drug administration. For example, in adult animals amphetamines induce different effects depending on the number and spacing of doses. Giving four doses at 2-h interval has become a common method of administration in adult rats for methamphetamine. This regimen increases the efficacy of the drug for reducing of brain monoamines while minimizing mortality (Sonsalla et al., 1989, 1991) compared to single or twice daily higher dose administrations. Developmentally, the same daily dose of methamphetamine given as four or two divided doses on P11–20 causes comparable effects on MWM acquisition and reversal learning, but four divided doses has larger effects on learning with a small platform, whereas the same total daily dose given as two divided doses has little effect on small platform learning (Vorhees et al., 2000). There are no data on the effects of dose distribution for developmental MDMA.
It is not possible to generalize from methamphetamine to MDMA because, despite structural similarities, these drugs have different pharmacological and neurotoxicological effects [cf. (Green et al., 2003; O'Callaghan and Miller, 2002)]. A fundamental question is whether dose schedule is an important factor in determining the long-term effects of drugs after developmental exposure. Given that amphetamine administration to adult rats shows that dose schedule determines the extent to which monoamines are released, and monoamine release in turn determines the extent of long-term monoamine depletions [e.g., (Colado et al., 2004; O'Dell et al., 1991)], we sought to investigate this issue with MDMA in developing animals on behavioral outcomes. Accordingly, the purpose of the present experiment was to determine how dose schedule affects later behavior after P11–20 MDMA exposure to determine the optimal dose distribution for future experiments.
MATERIALS AND METHODS
Subjects
Nulliparous female Sprague–Dawley CD, IGS rats (Charles River, Raleigh, NC) were bred in house to males of the same strain and supplier. Evidence of pregnancy was designated as E0 and most females delivered on E22. Birth was designated P0 and litters were culled using a random number table to four males and four females on P1. The animals were maintained in polycarbonate shoebox cages (46 × 24 × 20 cm) on a 14-h light/ 10-h dark cycle (lights on at 600 h) with food and water available ad libitum in a vivarium at 21 ± 1°C with 50 ± 10% humidity and accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The research protocol was approved by the Cincinnati Children's Research Foundation Animal Care and Use Committee. Within each litter, one male and one female pair was uniquely identified by ear-punch and designated to receive one of four treatments (three drug groups and one saline group). The saline group received four doses of saline (3 ml/kg) every 2 h each day on P11–20 (times 0, 2, 4, and 6 h). All drug groups received a total daily dose of 40 mg/kg ±3,4-methylenedioxymethamphetamine HCl (calculated as the free base and greater than 95% pure, National Institute on Drug Abuse) also in four divided doses as follows: group 40 × 1 (40 mg/kg MDMA at time 0 h, followed by saline at times 2, 4, and 6 h), group 20 × 2 (20 mg/kg at times 0 and 6 and saline at time 2 and time 4 h), group 10 × 4 (10 mg/kg at times 0, 2, 4, and 6 h), and saline (saline at times 0, 2, 4, 6 h). All doses were given subcutaneously in the dorsum and injection sites varied to prevent irritation. Twenty litters were initially dosed creating a total of 160 offspring for enrollment in the experiment, i.e., 20 males and 20 females per group for each of the four groups.
Litters were weaned on P28 and separated into same-sex pairs for the remainder of the experiment. Offspring were weighed once per week and testing began on P60.
Elevated zero maze
On P60, animals were tested in an elevated zero maze as a test of anxiety (Shepherd et al., 1994). Animals were placed in the center of one of the closed areas of the ring-shaped apparatus and behavior was recorded for 5 min with an overhead camera connected to a video recorder. The circular runway was 105 cm in diameter with 10-cm path width; two quadrants of the ring were enclosed by black acrylic sidewalls and two quadrants were open; the latter had a 1 cm clear acrylic curb to prevent slipping off the edge (Williams et al., 2003b). The test was conducted under dim halogen lighting. The dependent measures for this task were: entries and time in the open (front paws outside of the closed area); head dips in open quadrants; and stretch-attend postures (stretching into an open area with torso and hindpaws remaining in a closed area). The maze was cleaned with 70% ethanol between animals.
Locomotor activity
On P61, locomotor activity was measured in a 41 × 41 × 30 cm Accuscan activity monitor equipped with 16 pairs of photodetector-LED beams along the x and y axes for 1 h (Accuscan Electronics with VersaMax software, Columbus, OH). The apparatus was cleaned with 70% ethanol solution between animals. Horizontal activity was recorded in 5-min intervals.
Novel object recognition
On P64–68, animals were tested for novel object recognition (NOR). Square acrylic arenas measuring 41 × 41 cm with 30-cm high walls were used. Each animal was given 10-min exposure to the arena per day for 4 days for habituation. Test arenas were cleaned between animals with 70% ethanol. The test phase took place on the fifth day and was divided in two phases, familiarization and retention. The familiarization phase entailed placing two identical objects 5 cm from the sides of the arena in opposite corners. Rats were placed in the arena between the two objects and given a maximum of 10 min to accumulate 30 s of object exploration (Clark et al., 2000). Object exploration was defined as the animal standing within 1 cm and oriented toward the object. Exploration of an object was defined as the animal sniffing or pawing the object, however climbing on the object was not counted (Clark et al., 2000). The retention phase began 1 h after familiarization. A new object was introduced along with a duplicate of one of the original objects. As in the familiarization phase, the animals had 10 min to complete 30 s of object exploration. A video camera was placed over the testing arena, and behavior was scored by an observer assisted by a scoring program generously provided by Dr. Robert E. Clark (Clark et al., 2000). Time exploring the new object during the test phase was analyzed.
Barnes maze
On P71–80, animals were tested in the Barnes maze (Barnes, 1979) with modifications as described elsewhere (Williams et al., 2003a). The maze is a circular platform 210 cm in diameter mounted 74 cm above the floor with 30 equally spaced 9.5-cm holes (centered 20 cm from the edge) around the perimeter and made of black textured Kydex. The maze rests on a rotating stand and each hole has supports on the underside that permit a drawer-style goal box to be inserted beneath any hole. In addition to the overhead fluorescent lighting, the room was illuminated using a 150 W floodlight mounted directly above the center of the maze and four 300 W halogen floor lamps spaced evenly around the maze with reflectors aimed toward the ceiling. The room contains many cues as well as three large posters mounted on the walls closest to the maze. A curtain separated the maze from the experimenter on the fourth side of the maze. A camera was mounted above the maze with the signal fed to a closed circuit monitor located behind the curtain that allowed the experimenter to observe the animal unobtrusively. The goal box is made of black acrylic and is 16 cm deep. A plastic start ring (20 cm in diameter and 27 cm in height) was positioned in the center of the maze at the beginning of each trial. Rats received one trial per day for 10 days with a 4 min time limit per trial. The only motivation for the animal was to escape the bright arena by entering the darkened goal box. On Day 1, each rat received goal-box training by being confined in the goal box for 4 min before the first trial. Immediately after confinement, Trial 1 was given by placing the animal in the start ring for 15 s. The start ring was removed to begin the trial. Each animal's performance was recorded using a Polytrack video-tracking system (San Diego Instruments, San Diego, CA). Data recorded included latency to reach the goal, path length to the goal, and cumulative distance traveled to reach the goal. The ITI was 30 s spent in the goal box. After each trial, the maze was cleaned, rotated randomly, and the goal box placed beneath a new hole located in the same spatial position as the original goal. Animals not entering the goal box within the 4-min time limit were placed in the goal-box for 30 s.
Straight channel
On P82, animals were tested in a 15 × 244 cm straight swimming channel with a wire escape ladder mounted at one end. Each rat received four consecutive trials on the test day. On each trial, the rat was placed at one end facing the end wall and timed until it grasped the escape at the opposite end (maximum time = 2min/trial).
Cincinnati water maze
On P85–89, animals were tested in the Cincinnati water maze (CWM). The CWM was developed as previously described (Vorhees, 1987). The CWM consists of nine closed T-shaped cul-de-sacs that branch from a central channel extending from the starting point to the goal where an escape ladder is positioned. The arms of the Ts and the channels are 15 cm wide and the walls are 51 cm high. The water was 25 ± 1 cm deep and maintained at room temperature (22 ± 1)°C. Testing was performed under red light (single 25 W bulb) to reduce the use of extramaze (distal spatial) cues. To begin each trial, an animal was placed in the start position (position “B”) and allowed to find the goal (position “A”). Two trials per day were administered with a 5-min limit per trial and a minimum 5-min intertrial interval. Errors and latency to escape were scored. An error is defined as a whole-body entry into one of the arms of a T. Animals were tested for five consecutive days.
Morris water maze
The MWM used was 210 cm in diameter, made of stainless steel, and painted flat black (Vorhees and Williams, 2006). The maze was surrounded on all sides by white curtains that could be opened or closed to reveal or obscure prominent room cues. In addition to the indigenous room cues, the three near walls (representing arbitrarily the N, E, and W points) each had large geometric figures mounted on them. Each phase consisted of four trials per day for five successive days with the curtains open; this was followed by one additional day for a single probe trial. The limit was 2 min per trial and the intertrial interval (ITI) was 15 s spent on the platform. Animals not finding the platform within 2 min were placed on the platform. The platforms (either 10 × 10 cm or 5 × 5 cm) were made of clear acrylic with thin nylon screening attached to the surface. During testing, the platform was positioned 1.5–2 cm below the surface of the water and was camouflaged by virtue of being transparent against a black background. Water temperature was 21 ± 1°C.
During acquisition testing, the 10 × 10 cm2 platform was located in the SW quadrant halfway between the center and the sidewall. Rats were started at one of four positions located distal to the quadrant containing the platform in a random order with the restraint that they received one trial from each of the four starting positions per day. The start positions used were: NW, N, E, and SE. These positions were used to eliminate short path solutions such as those that are possible if S or W start positions were used. The day after the last acquisition trial, each rat was given a single 30-s probe trial. For the probe trial, rats were started from a position they had never been started from before (NE).
On days P99–104, rats were tested in the MWM in the reversal phase with a reduced size platform (Rev-Red). For this phase, the platform was moved to the NE quadrant and the 10 × 10 cm platform was replaced with a 5 × 5 cm platform to increase the accuracy required for the animal to locate the goal. The same procedure used for acquisition was used for reversal (5 days, 4 trials/day). Start positions were SE, S, W, and NW. On the sixth day, the platform was removed and a 30-s reversal probe trial was administered with the start position being SW. Performance was recorded using a Polytrack video tracking system (San Diego Instruments, San Diego, CA). For the learning trials, path length, cumulative distance from the platform, latency, and first bearing were analyzed. For the probe trials, dependent measures were time and distance in the target quadrant, number of target site crossings, average distance from the platform site, and first bearing. The tracker recorded the animal's position every 55 ms. First bearing was determined based on the animal's average heading during the first 13 cm of tracking on each trial relative to a direct line from start to goal.
Statistical procedures
Because the experiment used a split-litter design, offspring were matched on multiple factors by virtue of being littermates. To ensure that litter effects were controlled for in the analyses, litter was treated as a random factor (block) in a complete randomized block model analysis of variance (ANOVA). In this model, group and sex were between factors within each block and litter was the block factor. Measures taken repetitively on the same animal, such as time interval for locomotor testing or day for maze testing, were treated as repeated measure (within) factors. For locomotor activity, the ANOVA was a 2-between, 1-within randomized block model. In this model, treatment group had four levels (dose distribution), sex had two levels (male/female), and test interval had 12 levels (5-min intervals). For novel object recognition, the model was the same except that there was no repeated measure factor. For the maze tests, the model was the same as for locomotor activity except that the repeated measure factor was day. Data were analyzed using SAS Proc Mixed (SAS Institute, Cary, NC). Each model was checked for best fit against covariance matrix models provided by Proc Mixed. In most cases, best fit parameters indicated that the autoregressive-1 [AR(1)] covariance structure was optimal, but in a few cases compound symmetry (CS) was the better fit. Proc Mixed provides adjusted degrees of freedom that do not match those used in standard ANOVAs and can be fractional. Significant interactions were analyzed using simple-effect slice ANOVAs at each level of the repeated measure factor. Pairwise group comparisons of main effects or of simple-effects were conducted using the step-down Bonferroni method to control for multiple comparisons (SAS Multest).
RESULTS
General characteristics
Fifteen litters were used in this experiment. Twenty litters began treatment but five litters were not retained past weaning. The five eliminated litters were removed from the experiment because one animal in each of these litters died or became ill. The split-litter design is optimized when all blocks within the design are complete, i.e., when all cells of the design have offspring for each group-sex combination within each litter. For three of the eliminated litters one of the 20 × 2 MDMA-treated pups died, in another litter it was one of the 10 × 4 MDMA-treated pups that died, and in the fifth litter a saline-treated pup became ill and was therefore euthanized. Of the four MDMA-treated offspring that died, two were males and two were females. Three of the four deaths occurred on P12 (the day after treatment began) and one occurred on P20 (the last day of treatment). No seizures were observed.
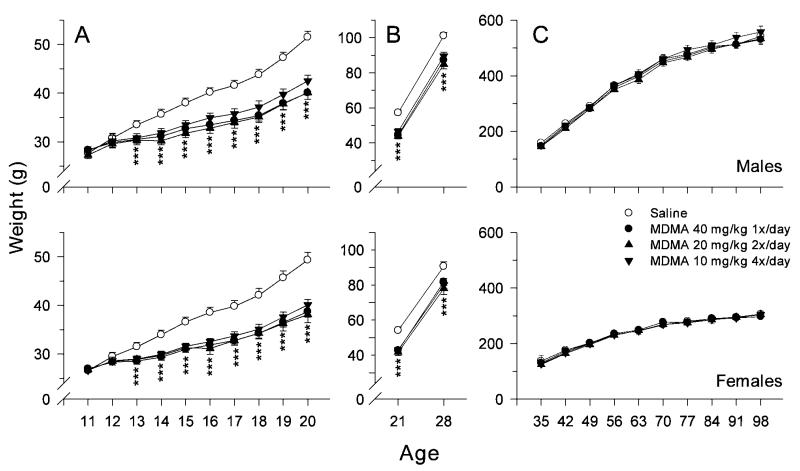
An analysis of body weights during treatment showed that there was an effect of group, sex, day and group × day (group: F(3110) = 48.57, P < 0.0001; group × day: F(27, 999) = 11.01, P < 0.001). Simple effect slice ANOVAs on each day showed that there were no group differences on P11–12. Group differences were significant on P13–20 (Fig. 1A). On these days, all three MDMA-treated groups showed significant weight decrements compared to saline controls, however, there were no significant differences among the three MDMA-treated groups. These same weight differences were observed after the end of treatment prior to weaning (P21 and 28) (group: F(3, 98) = 63.22, P < 0.001). As during treatment, the MDMA groups each weighed less than the saline group and the MDMA groups did not differ from one another (Fig. 1B). From the first week after weaning (P35) until the end of the experiment (P98) no effect of group, group × sex, group × day, or group × sex × day effects were found (Fig. 1C). Significant factors in this analysis were sex, day, and sex × day. These were due to growth and sexually dimorphic growth. Overall, the effect of MDMA on body weight gain was transient with recovery occurring rapidly (i.e., by P35) and MDMA were comparable to saline controls throughout the remainder of the experiment.
Fig. 1.
Mean ± SEM body weights during different ages in rats treated with one of three dosing regimens of MDMA. A: Offspring body weights during dosing on P11–20. B: Offspring body weights after dosing up to the day of separation of the pups from the dams. C: Offspring body weights (g) from 1 week after weaning to the end of the experiment. 40 × 1 = 40 mg/kg MDMA given s.c. once per day at time 0 h and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given s.c. twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4= 10 mg/kg MDMA given s.c. once per day at times 0, 2, 4, and 6 h; Saline = saline given s.c. four times per day at times 0, 2, 4, and 6 h. ***P < 0.001 compared to saline. Group sizes (male/female): Saline = 15/15, 40× 1 = 15/15, 20 × 2 = 15/15, and 10 × 4 = 15/15.
Elevated zero maze
No group or group × sex effects were found on the zero-maze for time spent in open, number of open quadrant entries, number of head dips, or number of stretch-attend movements. There was a group trend for time in open [F(3, 87) = 2.15, P < 0.10]. An inspection of the group means suggests that the MDMA 10 × 4 group spent less time in the open than did the saline or the other MDMA-treated groups: (mean ± SEM) saline = 71.7 ± 10.4; MDMA 40 × 1 = 66.8 ± 13.4, MDMA 20 × 2 = 76.7 ± 10.1, MDMA 10 × 4 = 51.7 ± 10.8.
Locomotor activity
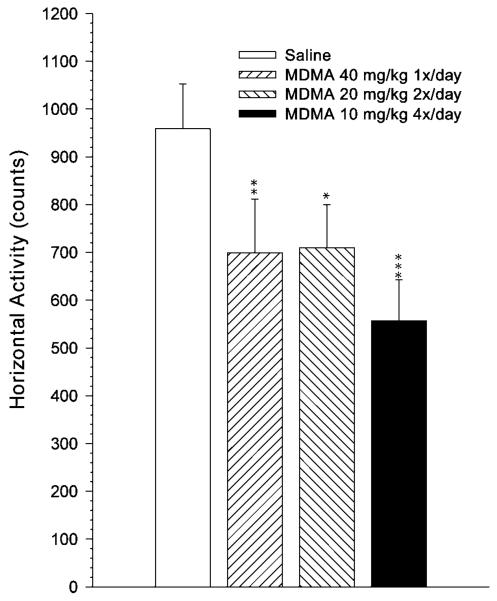
Analysis of horizontal activity showed a main effect of group [F(3, 85.7) = 4.52, P < 0.01] and no interaction of group with interval, sex or interval and sex. Horizontal activity averaged across intervals is depicted in Figure 2. Pairwise comparisons revealed that each of the MDMA-treated groups differed from the saline group. Comparisons among the MDMA-treated groups using unadjusted means showed that despite the apparent lower activity of the 10 × 4 MDMA group there were no significant differences among the three drug groups.
Fig. 2.
Mean ± SEM horizontal locomotor activity summed over a 1 h test session recorded in 5-min intervals. 40 × 1 = 40 mg/kg MDMA given once per day at time 0 h and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4 = 10 mg/kg MDMA given once per day at times 0, 2, 4, and 6 h; Saline = saline given four times per day at times 0, 2, 4, and 6 h. All injections were given s.c. *P < 0.05, **P < 0.01 compared to saline controls. Group sizes (male/female): Saline = 13/13; 40 × 1 = 13/13; 20 × 2 = 12/12; 10 × 4 = 13/13.
Novel object recognition
During the familiarization stage of training, there were no significant group differences for time spent investigating two identical objects. During the test phase, there were no group effects found for preference for the novel object. There was also no effect of sex or group × sex (not shown).
Barnes maze
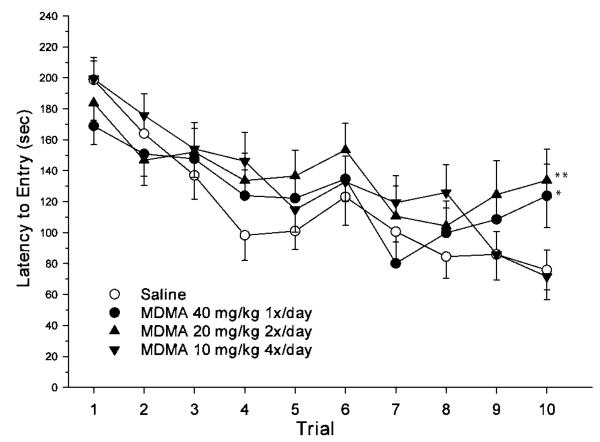
Only one group effect was found on one measure of Barnes maze performance. This was a significant group × day effect on latency to reach the goal [F(27, 1008) = 1.61, P < 0.03]. Although all three MDMA-treated groups showed an overall pattern of longer latencies than saline controls, pairwise comparison revealed significant differences between saline and the 20 × 2 MDMA- and 40 × 1 MDMA-treated groups only on Trial 10 (Fig. 3). Measures of path length and cumulative distance from the goal showed no significant effect of group or group-related interactions.
Fig. 3.
Latency (mean ± SEM) to enter the goal box on each day of testing in the Barnes maze (1 trial/day). Groups: 40 × 1 = 40 mg/kg MDMA given once per day at time 0 h and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4 = 10 mg/kg MDMA given once per day at times 0, 2, 4, and 6 h; Saline = saline given four times per day at times 0, 2, 4, and 6 h. All injections were given s.c. *P < 0.05, **P < 0.01 compared to saline controls. Group sizes (male/female): Saline = 15/15; 40 × 1 = 15/15; 20 × 2 = 15/15; 10 × 4 = 15/15.
Straight channel
Straight channel swimming times were assessed to ensure that animals were acclimated to swimming before entering the water mazes and that all groups had comparable motoric abilities and motivation to escape from the water and that there was a findable escape. Analyses of straight channel swimming times showed no group, sex, or group × sex effects (not shown). The only significant factor was trial, which was the result of all groups swimming progressively faster across successive trials.
Cincinnati water maze
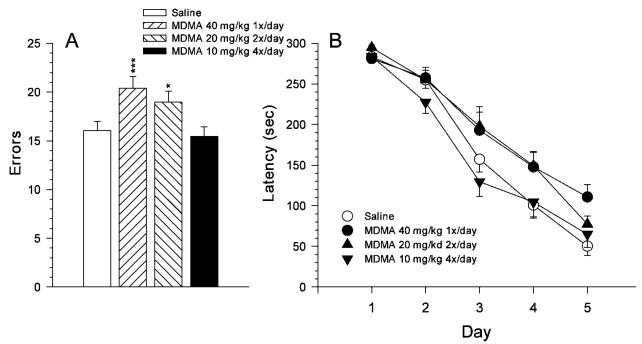
An analysis of CWM showed a significant group effect on errors [F(3, 98) = 6.96, P < 0.001]. There were no interactions between group and other factors on this measure (sex or day). Pairwise Bonferroni comparisons (Fig. 4A) revealed that the 40 × 1 and 20 × 2 MDMA-treated groups committed more errors than saline controls, whereas the 10 × 4 MDMA group was not different from saline controls. For latency to find the escape, both the group main effect [F(3, 98) = 3.65, P < 0.02] and the group 3 day interaction [F(12, 448) = 2.10, P < 0.02] were significant. There were no other significant effects except for day. The group × day interaction is depicted in Figure 4B. As can be seen, the pattern on latencies is similar to that found for errors inasmuch as the 40 × 1 and 20 × 2 MDMA-treated groups had longer latencies compared to saline controls or the 10 × 4 MDMA group. The interaction was attributable to the fact that group differences were not apparent on Day 1 when all groups were discovering the location of the escape ladder, but as the saline controls improved, the 40 × 1 and 20 × 2 MDMA-treated groups lagged behind, especially on Days 3–5 (except that on Day 5 the 20 × 2 MDMA-treated group improved more than the 40 × 1 MDMA-treated group did).
Fig. 4.
Mean (±SEM) performance in the Cincinnati water maze (CWM). A: Mean number of errors (2 trials/day) averaged across test days. B: Mean latency to reach the escape ladder per day. Groups: 40 × 1 = 40 mg/kg MDMA given once per day at time 0 and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4 = 10 mg/kg MDMA given once per day at times 0, 2, 4 and 6 h; Saline = saline given four times per day at times 0, 2, 4, and 6 h. All injections were given s.c. *P < 0.05, ***P < 0.001 compared to saline controls. Group sizes (M/F): Saline = 15/15; 40 × 1 = 15/15; 20 × 2 = 15/15; 10 × 4 = 15/15.
Morris water maze—Acquisition
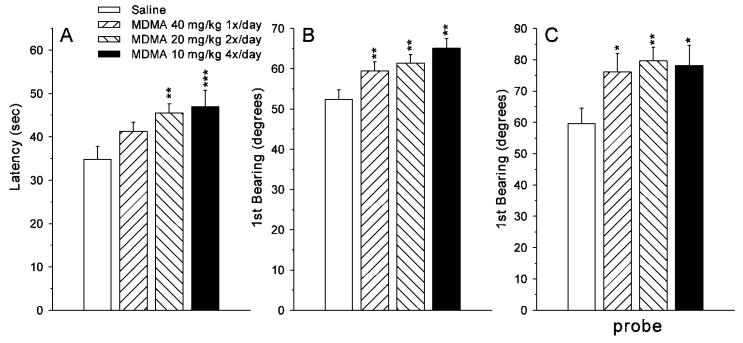
Analyses of latency, path length, and cumulative distance from the goal during initial learning (acquisition) showed the same pattern for each measure and these measures were highly correlated with one another (Vorhees and Williams, 2006). Accordingly, for simplicity, data are shown only for latency (Fig. 5A). There were main effects of group [F(3101) = 5.15, P < 0.01], sex [F(1101) = 13.8, P < 0.0001], and day [F(4346) = 66.26, P < 0.0001] but no interactions among the factors. Pairwise step-down Bonferroni comparisons showed that the 10 × 4 and 20 × 2 MDMA-treated groups had longer latencies than saline controls, while the 40 × 1 MDMA-treated group was intermediate and did not differ significantly from saline controls or the other MDMA-treated groups. A dependent measure that does not correlate as highly with latency (Vorhees and Williams, 2006) is first bearing to the goal, which is the angle between the animal's initial heading after starting a trial (within the first 13 cm) relative to a direct line to the center of the goal. Analysis of first bearing data also showed a significant main effect of group [F(3, 98.9) = 9.79, P < 0.0001] as well as effects of sex and day but no interactions. Bonferroni step-down pairwise group comparisons on first bearing revealed that each MDMA-treated group was significantly further off-course at the beginning of each trial than were saline controls (Fig. 5B). For all measures, males performed better than females (sex main effect).
Fig. 5.
Morris water maze (hidden platform) performance during the acquisition phase of learning (mean ± SEM) averaged across trials, days, and sex. A: latency (s) to reach the platform; B: first bearing angle (or angle of discrepancy) measured from the animal's heading taken 13 cm after the start of a trial relative to a direct lay line to the goal: C: First bearing on the probe trial given the day after the last day of acquisition. Groups: 40 × 1 = 40 mg/kg MDMA given once per day at time 0 h and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4 = 10 mg/kg MDMA given once per day at times 0, 2, 4, and 6 h; Saline = saline given four times per day at times 0, 2, 4, and 6 h. All injections were given s.c. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline controls. Group sizes (male/female): Saline = 15/15; 40 × 1 = 15/15; 20 × 2 = 15/15; 10 × 4 = 15/15.
On the probe trial, administered the day after the end of acquisition, there was a group effect on first bearing [F(3, 97) = 2.67, P = 0.05]. The first bearing effects are illustrated in Figure 5C. As can been seen, all of the MDMA groups were further off-course on the probe trial than saline controls. For other measures recorded on the probe trial, there was a trend toward a group main effect on average distance to the goal site (P < 0.08), but no effect on number of goal site crossings or percent time in the target quadrant.
Morris water maze—Reversal
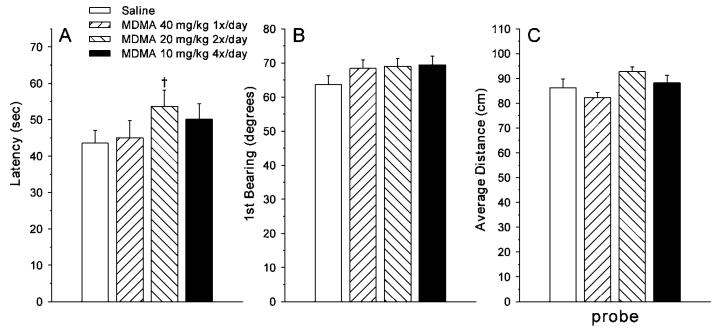
For reversal learning, the analysis of latency showed a main effect of group [F(3, 98.7) = 2.75, P < 0.05]. Bonferroni step-down pairwise comparisons showed only a trend toward longer latencies in the 20 × 2 MDMA-treated group compared to saline controls (Fig. 6A). For first bearing, there was a trend toward a group main effect [F(3, 98.1) = 2.29, P < 0.08] and a significant group × day interaction [F(12, 387) = 2.11, P < 0.02]. Overall, the pattern was that the 10 × 4 and 20 × 2 MDMA groups began the reversal phase performing worse (longer latencies) than the 40 × 1 MDMA group or controls, but by the end of reversal testing, the 10 × 4 MDMA group was intermediate in performance, while the 20 × 2 MDMA group continued to be the worst performing group.
Fig. 6.
Morris water maze (hidden platform) performance during the reversal phase of learning (mean ± SEM) averaged across trials, days, and sex. A: Latency (s) to reach the platform; B: First bearing (or angle of discrepancy) of the animal's heading taken 13 cm after the start of a trial relative to a direct lay line to the goal; C: Average distance from the platform site on the probe trial given the day after the last day of reversal. Groups: 40 × 1 = 40 mg/kg MDMA given once per day at time 0 h and saline given three times per day at times 2, 4, and 6 h; 20 × 2 = 20 mg/kg MDMA given twice per day at times 0 and 6 h and saline given twice per day at times 2 and 4 h; 10 × 4 = 10 mg/kg MDMA given once per day at times 0, 2, 4, and 6 h; Saline = saline given four times per day at times 0, 2, 4, and 6 h. All injections were given s.c. There was a significant treatment group effect on the probe trial (panel C) but a posteriori pairwise group comparisons showed that no treated group differed significantly from saline controls; the only significant pairwise comparison was between the 40 × 1 and 20 × 2 MDMA groups (P < 0.05). Group sizes (male/female): Saline = 15/15; 40 × 1 = 15/15; 20 × 2 = 15/15; 10 × 4 = 15/15.
On the probe trial administered the day after the last day of reversal learning, there were main effects of group on average distance to the goal site [F(3, 97) = 3.27, P < 0.03], and percent distance traveled in the target quadrant [F(3, 97) = 3.77, P < 0.02], but no effect on first bearing or number of platform site crossings. The effects on average distance and percent distance in the target were similar and average distance can be seen in Figure 6C. None of the pairwise step-down Bonferroni comparisons were significant between any of the MDMA groups and saline controls. The only significant comparison was between the 20 × 2 and 40 × 1 MDMA groups, the meaning of which is unclear.
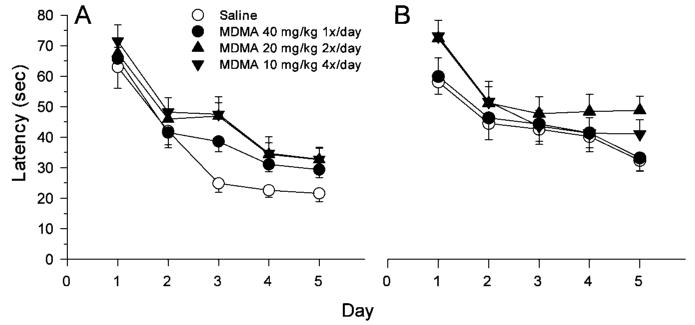
Learning curves for both acquisition and reversal are shown in Figure 7. As the data illustrate, all groups began the acquisition phase performing equally, indicating that there were no preexisting performance deficits in the MDMA-treated groups compared to saline controls. Performance improved on Day 2 to approximately equal degrees by all groups, but on Day 3 saline controls showed continued improvement before leveling off on Days 4 and 5. Performance in the MDMA-treated groups, by contrast, plateaued on Day 3 and showed little improvement thereafter, never reaching the level of performance seen in the saline group. On reversal, the deficit seen in the MDMA 10 × 4 and 20 × 2 groups continued, whereas the 40 × 1 MDMA-treated group reached control levels of performance and remained comparable to controls throughout the reversal learning phase. The 10 × 4 MDMA group improved on Day 2 of reversal and reached control levels of performance by Day 3, but then remained at this level while controls showed further improvement on Day 5. By contrast, the 20 × 2 MDMA group showed no improvement in performance after Day 2 of reversal and never approached control levels of performance.
Fig. 7.
Morris water maze performance during the acquisition and reversal learning phases to show learning curves. Shown are mean (±SEM) latencies to reach the platform averaged across four trials per day with males and females averaged together. A: Acquisition; B: Reversal. See Figures 5 and 6 for significant group differences on each phase. Note that on acquisition, there were no group differences on Day 1 indicating that there were no preexisting treatment effects on the animals prior to testing. This indicates that there were no drug-induced performance deficits in the MDMA-treated offspring, i.e., all groups swam and found the platform on initial trials (by trial-and-error initially) equally well. Group sizes are the same as in Figures 5 and 6.
DISCUSSION
Although people take drugs in various patterns, experimental studies of the long-term effects of drugs seldom explore the effects of dose distribution. Data in adult rats show that the number of doses of methamphetamine (O'Dell et al., 1991) or of MDMA (Colado et al., 2004) determines the amount of dopamine (DA) or serotonin released, respectively. The amount of release increases with each successive dose as does the attendant long-term monoamine reduction. In short, the greater the monoamine release the greater the subsequent reduction and the longer the depletion persists. In the case of developing animals, models of early exposure are usually not based on monoamine markers but instead are based on long-term effects on behavior; yet in these models no tests of dose distribution effects have been undertaken.
Based to some extent on evidence that treatment with methamphetamine given on P11–20, using the same total daily amount of drug but divided and administered as two or four doses, produced different patterns of MWM double-reversal learning deficits (Vorhees et al., 2000), we sought to determine if this was a more general phenomenon. To test this, the present experiment compared three different dosing regimens of a different substituted amphetamine, MDMA. Such data could be important because ecstasy users are known to take the drug in different patterns. Care was taken to match each group for total daily MDMA dose and total number of daily injections. Each MDMA-treated group received four subcutaneous injections of saline each day containing either saline alone or saline mixed with different doses of MDMA. Hence, each group received a combination of MDMA injections (zero, one, two, or four times) and saline alone (four, three, two, or zero times). Using a split-litter design so that all treatment groups were represented within each litter, the experiment tested three different dosing patterns while maintaining litter as the experimental unit of analysis by treating litter as a randomized block within the statistical model. In this way, data could be analyzed with litter as a random factor and treatment group and sex as fixed-effect factors within block without violating the one-offspring-per-condition-per-litter requirement known to be critical in developmental experiments (Holson and Pearce, 1992). Factors such as trial and test interval were treated as repeated measure variables.
With this approach, the data showed that the long-term effects of P11–20 MDMA treatment on learning varied as a function of dose distribution regimen, whereas effects on locomotor activity were independent of dose distribution. In essence, all three MDMA-treated groups showed similar reductions in locomotion. Although the 10 × 4 MDMA group showed a slightly larger reduction in activity than the 40 × 1 and 20 × 2 MDMA groups, pairwise comparisons maximized for statistical power (i.e., direct t-test without control for multiple comparisons) failed to show the 10 × 4 MDMA group to be significantly different from the other MDMA groups.
In contrast to the effects on locomotion, on tests of learning, MDMA dosing regimen contributed significantly to outcome. In the CWM, a test of path integration or ego-centric learning, the group receiving MDMA in a single dose (40 × 1 group) showed the largest deficit in performance for both errors and latency to escape. By contrast, in the MWM, a test of spatial learning and reference memory, the group receiving MDMA in four divided doses (10 × 4) showed the largest deficit on the acquisition phase. This effect was followed in order by the 20 × 2 and 40 × 1 MDMA groups that showed significant but somewhat smaller deficits. On the probe trial used to assess reference memory, all MDMA groups were affected with no dose-pattern-dependent effect. On reversal learning, the effects of MDMA were smaller, as noted in previous studies (Williams et al., 2003c), and more complex; i.e., on reversal, the effects were different on different test days. On initial days, both the MDMA 10 × 4 and 20 × 2 groups were most affected, whereas by the last day the 20 × 2 MDMA group performed worse than the 10 × 4 MDMA group; and the 10 × 4 MDMA group in turn performed worse than the 40 × 1 MDMA group. Hence, the 40 × 1 group that showed the largest effect in the CWM showed the smallest effect in MWM acquisition and showed no difference on MWM reversal.
Given that the MDMA groups all exhibited hypoactivity, it could be suggested that this reduction caused reduced MWM and CWM performance, but this is unlikely for several reasons. First, all animals were tested in a straight swimming channel that required almost no learning. This test was included as an index of motoric proficiency. This test revealed no group differences in swimming speed prior to water maze testing. Second, if a general hypoactivity effect were operating, it would be expected that all MDMA groups would have equal deficits on the two mazes and this was not the case. Unique patterns related to drug group were seen and these showed different outcomes on each maze, making a generic performance deficit from MDMA exposure implausible. Third, it is well established that swimming is relatively immune to differences in general locomotor activity within a broad range of activity differences (Cravens, 1974), suggesting that a contribution from this source is highly unlikely.
Some tests produced no MDMA-related effects. These were the tests of novel object recognition and straight channel swimming. That a drug administered during development was not found to affect all measured outcomes is not surprising and there are multiple reasons for this. One reason may have to do with the drug's selectivity; another may have to do with the CNS structures undergoing development at the time of drug delivery, or it may have to do with critical methodological differences. Novel object recognition, for example, is known to be hippocampally-dependent (Clark et al., 2000), therefore, one would predict that it would be affected if both the MWM and Barnes mazes were affected. Indeed, we have recently shown that P11–20 MDMA administered as 20 × 2 did result in a novel object recognition deficit (Cohen et al., 2005). Although published first, the Cohen et al. (2005) experiment was conducted after the present experiment and the apparatus used in the present novel object recognition test and that used by Cohen et al. (2005) were very different. Indeed, we changed the apparatus in the Cohen et al. (2005) experiment because of concern over whether the test arena used herein was providing the best structure for recognition memory. Here we used a 41 × 41 cm arena. Observations of the animals during testing suggested that animals were spending much of their time in the corners and were not adequately investigating the objects. In the follow-up study (Cohen et al., 2005), we used 91-cm diameter circular arenas and obtained more object exploration and found an MDMA-dependent effect for object recognition.
We have previously shown that two doses/day of MDMA administered on P11–20 induces both CWM and MWM deficits, consistent with the present data (Broening et al., 2001; Cohen et al., 2005; Vorhees et al., 2004; Williams et al., 2003c). The current data add to these that one large MDMA dose per day is more effective in altering CWM performance but is not as effective for inducing MWM deficits. The data suggest that two doses per day may be the optimal regimen for investigating effects on both tasks, whereas the four daily dose model is similar to the 2× model for investigating MWM effects, but is less effective for investigating CWM deficits. This raises the question of what the optimal dosing pattern is for MDMA in developmental studies. The current data suggest that no single regimen is likely to span all possible outcomes and as such is a cautionary message about how drug effects are studied and how difficult it is to prove that a given drug dosing regimen is not inducing a unique effect not readily generalized to other dosing regimens.
At present, it is not known why different dosing regimens induced different cognitive deficits, but pharmacokinetic differences would be one factor worth considering. Yet it is difficult to imagine how pharmacokinetic data will explain the effects, since locomotor effects were the same in all groups but cognitive effects varied by task. No matter what the pharmacokinetic patterns, a complex explanation would be required to fit all the data. Nevertheless, the data make clear that different cognitive functions (path integration vs. spatial mapping) have different vulnerabilities to MDMA. The data support the view that path integration is more susceptible to peak drug concentration, since the maximum effect on this function was induced by a single large daily dose of MDMA. On the other hand, spatial mapping was more susceptible to length of MDMA exposure rather than peak concentration, since the maximum effect on this behavior was induced by four divided doses, with the group receiving two divided doses being similar. Some caution in interpreting the present findings are also warranted. Herein rats were tested in the CWM under red light conditions. We have established that rats see sufficiently well under red light conditions to perform nearly as well as under white light. Moreover, we have newer (unpublished) data showing that under infrared conditions, where extramaze cues are completely eliminated, task difficulty increases dramatically and the task becomes very difficult. Eliminating extramaze cues requires animals to rely entirely on strategies other than spatial mapping, such as path integration. We also have data on MDMA (10 mg/kg × 4/day administered on P11–20) that show CWM deficits under infrared conditions (unpublished), even though this same dosing regimen did not have an effect when animals were tested under the red light conditions used here. This suggests that dose-pattern effects may depend on what type of learning is being assessed or the difficulty of the task.
Because different patterns produce similar effects on activity but somewhat different effects on learning, mechanistic studies will need to be directed toward determining which neurotransmitters and receptors are affected in different brain regions for each dose distribution pattern. Preliminary data (unpublished) show that 20 × 2 doses/day on P11–20 of MDMA causes enduring changes in the expression of several proteins in the dentate related to NMDA receptor complexes, including NMDA-NR1, nNOS, and PSD-95. Future studies might profitably compare the effects on these proteins for each of the dosing patterns used here.
ACKNOWLEDGMENTS
We thank Mary S. Moran for assistance with data analyses.
Grant sponsor: U.S. National Institutes of Health; Grant numbers: DA006733, DA021394, DA014269.
REFERENCES
- Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neuro-genesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age dependent sensitivity of rats to the long-term effects of the serotonin neurotoxicant (+/−) -3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-Methylenedioxymethamphetamine (ecstasy) induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJS, Findlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. doi: 10.1385/ni:5:1:79. in press. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (Ecstasy) in rats: Interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Cravens RW. Effects of maternal undernutrition on offspring behavior: Incentive value of a food reward and ability to escape from water. Dev Psychobiol. 1974;7:61–69. doi: 10.1002/dev.420070110. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use Ecstasy (3,4-methylenedoxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Secondary school students. I. U.S. Department of Health and Human Services; Bethesda, MD: 2005. Monitor the future: National survey results on drug use; pp. 1975–2004. [Google Scholar]
- Kelly PAT, Ritchie IM, Quate L, McBean DE, Olverman HJ, Aase JM. Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol. 2002;137:963–970. doi: 10.1038/sj.bjp.0704961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Chen E-Y, Kanaan NM, Campbell NG, Kordower JH, Lipton JW. Prenatal 3,4-methylenedioxymethamphetamine (Ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases forebrain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol Teratol. 2003;25:509–517. doi: 10.1016/s0892-0362(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Liu HKaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- McElhatton PR, Bateman DN, Evans C, Pughe KR, Thomas SH. Congenital anomalies after prenatal ecstasy exposure. Lancet. 1999;354:1441–1442. doi: 10.1016/s0140-6736(99)02423-x. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Ali SF. Serotonergic neurotoxicity of MDMA (ecstasy) in the developing rat brain. Ann N Y Acad Sci. 2002;965:373–380. doi: 10.1111/j.1749-6632.2002.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (“ecstasy”) administration on neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxic effects of substituted amphetamines in rats and mice: Challenges to the current dogma. In: Massaro EJ, editor. Handbook of Neurotoxicity. Vol. 2. Humana; Totowa, NJ: 2002. pp. p269–301. [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: Comparison of a prenatal care and no prenatal care sample. Pediatrics. 1999;104:1–10. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterization of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Riordan DE, Heikkila RE. Competitive and non-competitive antagonists at N-methyl-d-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J Pharmacol Exp Ther. 1991;256:506–512. [PubMed] [Google Scholar]
- Omer VE, Ali SF, Holson RR, Scalzo FM, Slikker W., Jr Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA) exposure in rats. Neurotoxicol Teratol. 1991;13:13–20. doi: 10.1016/0892-0362(91)90022-o. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two Water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to d-methamphetamine: Selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Pierce DR. Perinatal alcohol exposure and neuronal damage. In: West JR, editor. Alcohol and brain development. Oxford University Press; New York: 1987. pp. p120–157. [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev Brain Res. 2003a;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Res. 2003c;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: Studies with 3,4-methylendioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–220. [PubMed] [Google Scholar]
- Won L, Bubula N, Heller A. Fetal exposure to (6)-methylenedoxymethamphetamine in utero enhances the development and metabolism of serotonergic neurons in three-dimensional reaggre-gate tissue culture. Dev Brain Res. 2002;137:67–73. doi: 10.1016/s0165-3806(02)00411-x. [DOI] [PubMed] [Google Scholar]