Abstract
± 3,4-Methylenedioxymethamphetamine (MDMA) is a chemical derivative of amphetamine that has become a popular drug of abuse and has been shown to deplete serotonin in the brains of users and animals exposed to it. To date, most studies have investigated the effects of MDMA on adult animals. With a majority of users of MDMA being young adults, the chances of the users becoming pregnant and exposing the fetuses to MDMA are also a concern. Evidence to date has shown that developmental exposure to MDMA results in learning and memory impairments in the Morris water maze, a task known to be sensitive to hippocampal disruption, when the animals are tested as adults. Developmental MDMA exposure leads to hypoactivity in the offspring as adults but does not affect outcome on tests of anxiety. MDMA administration decreases pup weight, increases corticosterone and brain-derived neurotrophic factor levels during treatment while decreasing brain levels of serotonin; a decrease that initially dissipates and then reappears in adulthood. Neonatal MDMA exposure increases the sensitivity of the serotonin 1A receptor, a possible mechanism underlying the learning and memory deficits seen. Taken together, the evidence shows that MDMA exposure has adverse effects on the developing brain and behavior. The animal and human data on developmental MDMA exposure are reviewed and their public health implications discussed.
Keywords: anxiety; developmental effects; memory; ± 3,4-methylenedioxymethamphetamine; serotonin
Introduction
±3,4-Methylenedioxymethamphetamine (MDMA), N-methyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane, is a derivative of amphetamine, and is structurally related to mescaline, methamphetamine, and fenfluramine (Fig. 1). MDMA administration primarily activates serotonergic neurons, with secondary activation of noradrenergic and dopaminergic terminals (Rothman et al., 2001). Known as a club or designer drug, MDMA is primarily taken at group gatherings known as ‘raves’ and other group settings. MDMA is mildly hallucinogenic with users reporting visual and auditory disturbances and an increase in the intensity of sensory inputs (Davison and Parrott, 1997). Psychological effects of the drug begin 20–60min after oral ingestion and include a general euphoric state, emotional openness, reduction of negativity, and a decrease of inhibitions (Green et al., 2003) resulting in its characterization as an emphathogen or enactogen. Physiological effects include elevated heart rate, blood pressure, nystagmus, endocrine activation, insomnia, and sometimes hyperthermia (Davison and Parrott, 1997).
Fig. 1.
Comparison of the chemical structure of ±3,4-methylenedioxymethamphetamine (MDMA), mescaline, fenfluramine and amphetamine.
History and prevalence of use
MDMA was first reported in 1912 in a patent application as a precursor to potentially therapeutic compounds (Cohen, 1998; Bernschneider-Reif et al., 2006). The United States Army conducted the first studies on the lethality of MDMA and behavioral changes in animals during the 1950s, although the results were not declassified until 1969 (Hardman et al., 1973). The psychoactive effects of MDMA were first described in the late 1970s (Shulgin and Nichols, 1978) and soon thereafter, some therapists began to use it to enhance openness and communication with their patients (Grinspoon and Bakalar, 1986). In 1985, MDMA was classified as a Schedule I substance in the USA because it lacked an approved therapeutic use and was judged to have high abuse potential (Lawn, 1986). MDMA is also classified as a controlled substance in the European Union and Canada (Green et al., 2003).
Since 1989, the first year MDMA was included in the Monitoring the Future study published by the National Institute on Drug Abuse, the prevalence of MDMA use has increased significantly, although recently there has been a decline. For example, in 1989, 1.9% of 19 to 20-year-old individuals reported using MDMA within the last 12 months whereas use in this age group peaked in 2001 at 11% (Johnston et al., 2005a, b). By 2004, prevalence of previous year use in this age group had declined to 4.2%. When prevalence data for lifetime use for 2004 were compiled, 8% of high school seniors, 14% of college seniors (approximately 21–22 years), and 20% of 25 to 26-year-old individuals reported having tried MDMA. In the European Union, MDMA prevalence of self-reported use ranges from 0.6 to 8.8% of 15 to 34-year-old individuals depending on the country (EMCDDA, 2004). With the majority of MDMA users being young adults, including women, the risk of exposure to fetuses becomes a concern, as users could become pregnant and continue to take the drug during part or all of gestation (Ho et al., 2001).
Adult pharmacology of ± 3,4-methylenedioxymethamphetamine
As the majority of investigations into the mechanism of action of MDMA has occurred in adult rats, a brief overview of these findings is presented. In adult rats, MDMA acts primarily on serotonergic neurons, followed by actions on noradrenergic and dopaminergic neurons (Green et al., 2003; Baumann et al., 2007). The number of MDMA injections and dose varies between experiments; however, the range of doses is normally 5–60 mg/kg/day. For most experiments MDMA is administered on a single day, although in some cases dosing lasts for 4 consecutive days. The first study to show the effects of MDMA on serotonin (5-HT) used hippocampal slices and it was determined that both enantiomers of MDMA released radiolabeled 5-HT equally well (Johnson et al., 1986). Using in-vivo microdialysis, it has been shown that peripherally administered MDMA increases central extracellular 5-HT levels (Gough et al., 1991; Yamamoto et al., 1995; Gudelsky and Nash, 1996; Sabol and Seiden, 1998; Shankaran and Gudelsky, 1999; Mechan et al., 2002). Although MDMA initially induces increased extracellular levels of 5-HT, large doses or multiple exposures lead to long-lasting reductions in brain 5-HT levels.
The first experiment to show reductions of 5-HT after MDMA administration in rats was by Battaglia et al. (1987) and this effect has been replicated extensively (for review, see Green et al., 2003). Reductions in 5-HT can persist for at least 8 weeks in the hypothalamus and at least 16 weeks in the hippocampus and striatum (Scanzello et al., 1993). In addition to 5-HT deficits, alterations in the serotonin transporter (SERT) have been observed. Paroxetine binding, a marker of SERT activity, is reduced after MDMA administration (Battaglia et al., 1988; Scanzello et al., 1993). Decreases in paroxetine binding induced by MDMA administration persist for 32 weeks in the cortex and striatum and are lower than control values in the hippocampus for at least 1 year (Battaglia et al., 1988). MDMA not only decreases 5-HT levels and SERT activity, but also decreases the rate-limiting enzyme in 5-HT synthesis, tryptophan hydroxylase (TPH). Stone et al. (1987) were the first to show that TPH activity was decreased in the striatum, frontal cortex, hippocampus, and hypothalamus 15 min after MDMA administration (Stone et al., 1987) and these decreases last up to 2 weeks (Schmidt and Taylor, 1987). It seems that the reductions in TPH are due to a metabolite of MDMA, as acute intracerebroventricular administration of MDMA produced no reductions in TPH; however, a 1 h intracerebroventricular infusion of MDMA did reduce TPH activity (Schmidt and Taylor, 1988), presumably because after 1 h metabolite concentrations have risen. It is unclear if the decreases in 5-HT are attributable to downregulation of 5-HT synthesis in neurons or if the depletions are the result of a loss of 5-HT terminals through axotomy. It has been shown that treatment with MDMA four times in a single day diminishes 5-HT reactivity in the cerebral cortex, hypothalamus, and globus pallidus 2 weeks after administration (Fischer et al., 1995). The 5-HT levels return to normal in the globus pallidus and perhaps reach higher-than-normal levels in the hypothalamus 1 year after MDMA administration, whereas decreases continue to be seen in the neocortex 1 year after exposure (Fischer et al., 1995). It has been suggested that the recovery seen in 5-HT is aberrant owing to the increased innervation seen in the hypothalamus. Citalopram binding was increased in the hypothalamus of MDMA-treated animals whereas decreased binding was seen in the cortex, suggesting that the recovery of 5-HT neurons is region specific. Although this study suggests that MDMA damages serotonergic neurons, as a 5-HT antibody is used to label the neurons, it is difficult to determine if the decrease in immunoreactivity is because of the downregulation of 5-HT synthesis or distal axotomy. The recent finding that SERT levels are unchanged while binding is decreased in MDMA-treated animals suggests that 5-HT neurons remain intact after MDMA exposure (Rothman et al., 2003). Much work is left to be done to resolve the mechanism of 5-HT loss after MDMA exposure.
MDMA has been shown to displace 8-OH-DPAT [8-hydroxy-2-(di-n-propylamino) tetralin] binding to the 5-HT1A receptor (Giannaccini et al., 2007). Furthermore, MDMA antagonized GTPγS stimulation by the 5-HT1A receptor agonists 8-OH-DPAT and 5-carboxamidotryptamine (Giannaccini et al., 2007). Buspirone, a 5-HT1A receptor agonist and D2 receptor antagonist, attenuated the anxiogenic effects of MDMA treatment (Bhattacharya et al., 1998). The 5-HT1A receptor antagonist WAY 100635 has been shown to partially inhibit the increase in social interaction caused by MDMA administration (Morley et al., 2005). When WAY 100635 is given it does not attenuate the locomotor response to MDMA (McCreary et al., 1999). Furthermore, 8-OH-DPAT was unable to substitute for MDMA in drug discrimination paradigms (for review, see Muller et al., 2007), and there were no differences in 8-OH-DPAT binding in MDMA-treated animals compared with controls (McGregor et al., 2003a). Taken together it seems that MDMA may have specific effects on the 5-HT1A receptor that may not equate to a behavioral change.
MDMA stimulates dopamine (DA) release in the striatum and decreases levels of its major metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (Yamamoto and Spanos, 1988; Gough et al., 1991; Nash and Brodkin, 1991; Nash and Yamamoto, 1992; Gudelsky et al., 1994; Yamamoto et al., 1995; Koch and Galloway, 1997; Sabol and Seiden, 1998). Most data suggest that MDMA interacts with the dopamine transporter (DAT) as there is a lack of DA release after the administration of the DAT antagonist, GBR12909, both in vivo (Nash and Brodkin, 1991) and in vitro (Koch and Galloway, 1997), although a contradictory report showed increased DA levels in dialysate after GBR12909 pretreatment (Mechan et al., 2002). Pretreatment with fluoxetine as well as antagonism of the 5-HT2A/2C receptor blocks MDMA-induced DA release, and agonists of 5-HT receptors enhance DA release, suggesting that 5-HT release is important for DA release after MDMA treatment (Gudelsky et al., 1994; Yamamoto et al., 1995; Koch and Galloway, 1997). MDMA treatment also has been shown to cause small (10–25%), long-lasting reductions in DA levels in the striatum for up to 10 weeks (Commins et al., 1987; McGregor et al., 2003b; Clemens et al., 2004; Cohen et al., 2005; Able et al., 2006). It should be noted that in mice, the primary effect of MDMA treatment is to decrease DA levels (for review see Green et al., 2003); however, as few developmental models of MDMA are in mice, this model system will not be discussed in this review.
Other neurotransmitter systems are also known to be affected by MDMA treatment. MDMA alters norepinephrine (NE) release; however, MDMA has not been shown to reduce NE levels in the rat brain, regardless of the dosing regimen employed (Rothman et al., 2001). Recently, MDMA has been shown to increase acetylcholine (ACh) release in vivo in the frontal cortex, hippocampus (Acquas et al., 2001; Nair and Gudelsky, 2006), and the striatum (Acquas et al., 2001), an effect that was also shown in striatal slices (Fischer et al., 2000). The release of ACh was prevented in the prefrontal cortex by the administration of the TPH inhibitor p-chlorophenylalanine or by inhibiting DA release and reuptake using amphetamine (Nair and Gudelsky, 2006); however, hippocampal ACh release was unaffected by either compound. These results suggest that MDMA-induced ACh release is 5-HT and DA-dependent in the prefrontal cortex but other mechanisms are responsible for ACh release in the hippocampus.
Glutamate levels in synaptosomes from Wistar rats that were drug naive or previously exposed to MDMA were unaffected by MDMA exposure, suggesting that glutamate may not be affected by MDMA in the adult rat brain (Bogen et al., 2003). This is further supported by evidence showing no effect of MDMA on striatal glutamate release (Nash and Yamamoto, 1992). Taken together, the results of these studies show that MDMA impacts specific neurotransmitter systems in multiple brain regions (especially 5-HT), whereas it is ineffective for other neurotransmitters, after adult administration.
MDMA increases free radical production, as demonstrated by an increase in lipid peroxidation (Sprague and Nichols, 1995; Colado et al., 1997b) and by the alleviation of 5-HT damage by the nitrone radical trapping agent α-phenyl-N-tert-butyl nitrone (Colado and Green, 1995). Further evidence of this was shown by the increased conversion of salicylate to 2,3-dihydroxybenzoic acid by MDMA (Colado et al., 1997b) that occurs in the presence of free radicals. This MDMA-induced 2,3-dihydroxybenzoic acid formation can be prevented with pretreatment of either ascorbic acid antioxidant (Shankaran et al., 2001) or mazindol (a NE reuptake inhibitor) (Shankaran et al., 1999). Administration of sodium ascorbate or the antioxidant L-cysteine also prevents the long-term 5-HT depletions caused by MDMA exposure (Gudelsky, 1996) and another antioxidant, α-lipoic acid, pretreatment reduces 5-HT depletions in the hippocampus without preventing hyperthermia (Aguirre et al., 1999). Contrary to these findings, another study showed that salicylate does not prevent 5-HT terminal effects (Yeh, 1997). Although this one study may cast doubt on the role of free radical production in MDMA-induced 5-HT reductions, most of the evidence supports the concept of free radical damage.
Neuroendocrine effects
MDMA administration alters neuroendocrine systems. Corticosterone (CORT) is increased beginning 30 min after MDMA treatment and returns to control levels by 6 h; an effect that was dose-dependent (Nash et al., 1988). Prolactin levels peak 1 h after MDMA administration and return to baseline levels by 4 h (Nash et al., 1988). Two weeks after a three dose × 2 h administration of MDMA, CORT and prolactin levels in MDMA-treated animals had an attenuated increase in response to an MDMA challenge compared with animals that were pretreated with saline (Baumann et al., 2007). Renin and aldosterone are increased in plasma after MDMA exposure (Burns et al., 1996), and it has been shown that MDMA treatment increases oxytocin and vasopressin levels in hypothalamic cultures (Forsling et al., 2001, 2002). The long-term impact of altered neuroendocrine and neurotransmitter systems is not known, but may be involved in residual cognitive effects of the drug (see below).
Cognitive and psychiatric effects associated with adult human and rat exposure
In humans, it is difficult to assess the effects of MDMA exposure on cognition, as many who abuse MDMA also take other drugs, including cannabis, alcohol, and other stimulants (Klugman and Gruzelier, 2003). Recently a meta-analysis of several MDMA studies has shown that MDMA users show moderate deficiencies in cognitive function compared with drug naive controls, using lenient stringency conditions in which controls were matched only based on the cognitive tests they conducted (Kalechstein et al., 2007). However, when higher stringency conditions were used, in which controls were matched by age, education, and estimated preexposure intelligence, MDMA abusers showed larger deficits (Kalechstein et al., 2007). Delayed verbal recall and decreases in spatial associative learning were observed in MDMA users who were abstinent for at least 1 week (Zakzanis and Campbell, 2006). These effects were evident when comparing MDMA users to polydrug users who did not use MDMA or to drug-naive controls (Bolla et al., 1998; Parrott et al., 1998; Klugman and Gruzelier, 2003; Zakzanis and Campbell, 2006). Another potential confound with MDMA findings in humans is that many studies do not require abstinence from other drugs for a significant period of time before memory assessment. As it is problematic to administer a compound that may reduce participants’ cognitive ability, more studies must be carried out using model systems to test the psychiatric and cognitive effects of MDMA. To date, few studies have examined the effects of MDMA on the cognitive ability of animals.
In adult rats, a single day, four-dose exposure (15 mg/kg/dose) to MDMA induces a combination of path integration and spatial deficits in the Cincinnati water maze (CWM), as assessed by increased latency and errors of commission in learning to find the escape (Able et al., 2006). Path integration learning is the ability of an animal to navigate using intrinsic cues to relocate its start position or a goal within the environment, such as when foraging for food (Etienne and Jeffery, 2004). This form of learning can be assessed using tests that require finding a specific location in the absence of spatial cues. An example of such a test is the CWM (Vorhees, 1987) (Fig. 2), which can be used with or without visual spatial cues, depending on whether the emphasis is on path integration learning or a combination of path integration and spatial navigation.
Fig. 2.
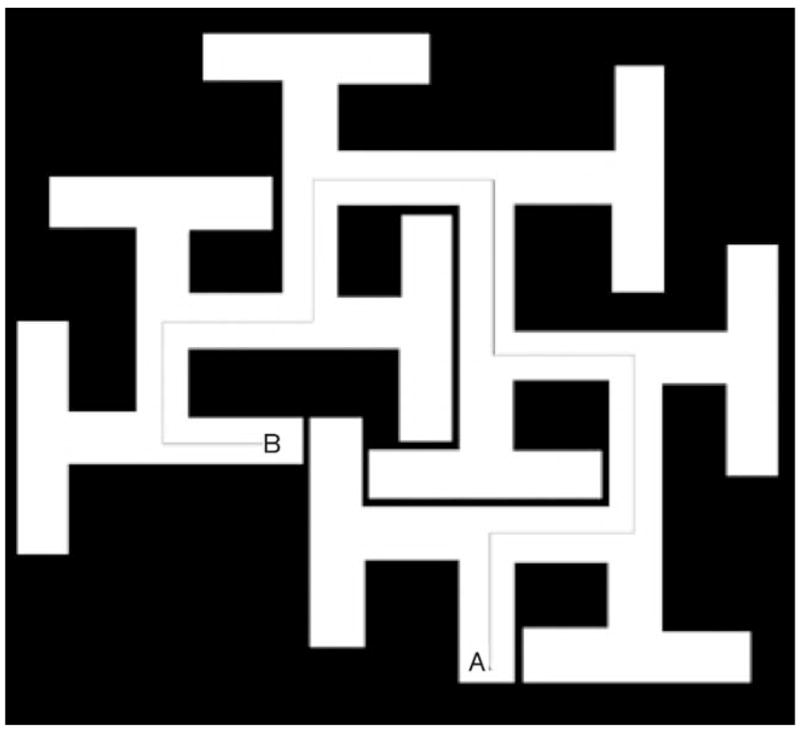
The Cincinnati water maze, a test of path integration learning. Animals are generally started at point ‘B’ with the escape ladder located at point ‘A’.
In the Morris water maze (MWM), where the emphasis is on use of spatial navigation to escape, MDMA-treated animals spend less time in the target quadrant relative to saline-treated controls during memory or probe trials, suggesting that reference memory is disrupted by MDMA exposure. MDMA and saline-treated animals, however, performed similarly in latency and path length on learning trials when the hidden platform was present, suggesting spatial learning is spared and the deficit limited to retention (Sprague et al., 2003; Able et al., 2006). Reduced time spent observing a novel object, in a test of novel object recognition (NOR), also suggests memory impairment in MDMA-treated animals, using a 15-min retention interval (Morley et al., 2001); however, a longer 1-h retention interval showed no differences (Morley et al., 2001; Able et al., 2006). Active avoidance learning was found to be impaired in a low-anxiety subset of Wistar rats treated with MDMA (Ho et al., 2004), whereas another study showed no passive or active avoidance learning effects from the drug (Timar et al., 2003). MDMA also disrupts ethanol preference in rats (Cole et al., 2003) and decreases locomotor activity (Timar et al., 2003) 3 days after administration. The effect on locomotor activity was confirmed in another experiment that tested rats 1 week after MDMA and found reduced diurnal and nocturnal activity (Wallace et al., 2001). No locomotor effects were, however, observed at longer intervals (several weeks) after MDMA administration (Timar et al., 2003).
Disruptions of other behaviors have also been reported after MDMA administration. A single dose of MDMA increased the time spent in the open arms of the elevated plus maze 30 min after injection; however, these effects were not present when the animals were tested 12 days later (Ho et al., 2004). By contrast, MDMA decreased time spent in open arms of the elevated plus maze 15 weeks after MDMA exposure, suggesting an anxiogenic effect develops over time (Morley et al., 2001).
Pharmacokinetics
In adult humans, the maximum concentrations of MDMA in plasma in naive users after oral administration occur at 2 h and the elimination half-life of MDMA is between 8 and 9 h (De la Torre et al., 2004). In adult rats after intravenous drug administration, the elimination half-life of MDMA is considerably faster at 73 min (Cho et al., 1990). In neonatal rats, MDMA reaches a maximum plasma level much sooner and the animals clear the drug more quickly than humans, but the drug stays in the system longer than in adult rats. For example, on postnatal day (P)1 a subcutaneous dose of 20 mg/kg reaches maximum plasma concentration at 14 min with an elimination half-life of approximately 3 h (Williams et al., 2004). P11 animals reach maximum plasma concentrations at 13 min and have an elimination half-life of approximately 4 h (Williams et al., 2004). For pups exposed in utero via subcutaneous treatment to the dam, brain concentrations of MDMA were at their maximum levels at 2 h after a 15 mg/kg dose to the pregnant female, and the elimination half-life in fetal brain was approximately 3 h (maximum dam levels were observed at 90min and the T1/2 was 2.72 h) (Campbell et al., 2006). Although route of administration may affect the pharmacokinetic profile of a drug, the data show that neonatal rats eliminate MDMA more quickly than humans, and that neonates clear the drug a little slower than adult rats. As MDMA is metabolized more quickly in rats than in humans, this would also suggest that 3,4-methylenedioxyamphetamine (MDA), which may contribute to the toxicity in MDMA-treated animals, would be absorbed more rapidly, reach a higher peak, and be cleared more rapidly in the rat. This raises the question of which factor contributes more to the effects of MDMA, the peak or duration of MDMA. If peak is more important, then the more frequent doses would more likely cause severe effects than seen in human users of MDMA. If the duration is, however, more important, the frequent dose model is more important. More work is required in this area to determine which is the proper model of human MDMA use. These data, when considered together, support administering higher, more frequent doses to rodents to provide an equivalent tissue concentration to that seen in humans.
Effects of in-utero ± 3,4-methylenedioxymethamphetamine exposure in humans
Few studies have been performed on the effects of MDMA on fetal development in humans. McElhatton et al. (1997, 1999) reported that infants exposed to MDMA in utero had significantly higher incidences of club foot and congenital heart defects than a comparison group reporting no use of MDMA. Fifty-four percent of the 136 women in this study were MDMA-only users; the remainder took other drugs as well primarily cocaine, cannabis, alcohol, or lysergic acid diethylamide. A potential confound to this study was that women who abused MDMA had more elective abortions, which could lead to underrepresentation of high exposure individuals in the drug group, indicating that there may have been birth defect cases that were missed.
A Dutch study examined infants prenatally exposed to MDMA and found that one infant out of 43 had cardiac malformations; however, the study was too small to draw definitive conclusions (Van Tonningen-van Driel et al., 1999). A Canadian study examined the profiles of women who self-reported use of MDMA during pregnancy and called a hotline for information regarding the drug or the ingestion of other drugs during pregnancy (Ho et al., 2001). The findings of this study showed that women who took MDMA during pregnancy are more likely to smoke, consume alcohol, and use other illicit drugs, as well as to procure an abortion, as compared with women who reported no MDMA use during pregnancy. Most women who reported MDMA in this study used it once and claimed they terminated the use after recognition of the pregnancy. This study undoubtedly does not represent the majority of the MDMA-using population as this was a survey of women who voluntarily called to obtain information about MDMA effects during pregnancy and the women in this study were of higher-than-average social economic status. No long-term studies examining the cognitive or neurological consequences of MDMA in humans are available. In one longitudinal study involving prenatal amphetamine (D and D,L-amphetamine) exposure, the children showed a greater tendency to be one grade lower than their chronological age would predict and a larger number of these children required special schooling (Cernerud et al., 1996).
Modeling human drug exposure during pregnancy
A number of factors must be taken into account to model human MDMA exposure in rodents. For example, human and rodent brain development are not temporally congruent as regions of the brain develop postnatally in rodents that develop prenatally in humans. Furthermore, some systems that interact with the brain, such as the hypothalamic-pituitary-adrenal axis, also develop later in rodents. Other considerations for a model are how much drug should be given to animals to mimic exposures in humans. Women who abuse MDMA throughout pregnancy are likely to be heavy users because lighter users tend to quit in early pregnancy, as observed for other stimulants, such as cocaine (Richardson, 1999). Accordingly, modeling heavier use patterns may provide the best evidence of what worst-case effects might be. If high doses show no effects, then testing lower doses becomes unnecessary.
Comparison of human and animal brain development
With regard to brain development, the neonatal rat is approximately equivalent to second and third trimester human development (Bayer et al., 1993; Rice and Barone, 2000; Clancy et al., 2001, 2007a). Neurogenesis, especially of granule cells, which are critical to integration of hippocampal function (they are part of the trisynaptic system of the temporal lobe), continues in the dentate gyrus until P19 (Bayer et al., 1993), and myelination in many structures such as the striatum continues well into postnatal life (Rice and Barone, 2000). Although exact comparisons of neurotransmitter system development across species is not possible, it is known that the glutamatergic system develops predominantly postnatally in the rat and predominantly prenatally in the human (Herlenius and Lagercrantz, 2004). For the purpose of this discussion, neonatal exposure to MDMA is defined as exposure in animals younger than P21 and is a model of the second half of human intrauterine development as detailed by Clancy et al. (2007b) using interspecies growth and development algorithms (www.translatingtime.net). Additionally, the neonatal rat goes through a period of low circulating glucocorticoid levels known as the stress hyporesponsive period (SHRP) similar to reduced cortisol release in the second trimester of human fetal development (Brosnan, 2001).
Dose relevance: humans to rats
Several dose factors are relevant when developing a model of human exposure to psychostimulants. For example, in humans, the amount of MDMA obtained in a single use (tablet), the number of tablets taken on any given occasion, the frequency of use per day or week, as well as drug absorption, distribution, metabolism, and elimination, must be taken into consideration. Although it is difficult to directly match across species for each of these parameters, there are methods for estimating some of these. Comparison of drug effects could be made on a strict weight-adjusted (milligram per kilogram) basis, as has been suggested by Baumann et al. (2007); however, this may underestimate the bioavailability of the drug. To account for differences in bioavailability, scaling methods have been proposed to account for differences in body size, disposition, excretion, metabolic rate, and related factors (Lin et al., 1999). If one uses the Mordenti and Chappell interspecies scaling formula of Dosehuman= Doseanimal (Weighthuman/Weightanimal)0.7 (Mordenti and Chappell, 1989; for drugs of abuse, see also Green et al., 2003), a P11 pup weighing 25 g and receiving a 10 mg/kg dose of MDMA would be equivalent to a 58 mg dose taken by a 60 kg human. Newer models of interspecies scaling suggest that the exponent in the equation should be either 3/4 (West et al., 2002) or 2/3 (White and Seymour, 2005), and therefore the amount administered to a neonatal rat would be equivalent to a human dose of between 29 mg and 85 mg, respectively. It should be noted that some have cautioned that predictions made on the basis of interspecies scaling formulas may not always be accurate (Mahmood, 1999); however, these reservations are based on a small study rather than on the large number of species and other data used to develop the scaling algorithms reviewed by White and Seymour (2005). Notwithstanding the caveat that not all drugs fit the rule, the scaled doses of MDMA administered to pups are well within the range of doses taken by MDMA abusers. As MDMA is typically taken multiple times in a day and the tablets usually contain about 100 mg of MDMA (Schifano et al., 2006) it could be argued that multiple doses are required in a day to better mimic human use. This is further supported using data from plasma samples from active MDMA users who attended a ‘rave’ the previous night that showed a range of plasmatic MDMA levels from approximately 0.1 to 0.9 mg/l up to 10 h after drug ingestion (Irvine et al., 2006). P11 rats given a 20 mg/kg dose of MDMA show plasma levels of 1 mg/l 10 h after administration (Williams et al., 2004). This shows that the higher doses of MDMA administered to animals model similar levels of MDMA found in humans.
Others suggest that rats self-administer MDMA at doses lower than those predicted from interspecies scaling formulas and these lower doses are more indicative of pharmacologically active doses and should therefore be used to model the range of human use (Baumann et al., 2007). This view, however, does not account for the increased clearance of the drug by rats, which may remove MDMA before 5-HT depleting actions have occurred. Furthermore, although an excellent model for some drugs, self-administration studies do not always mimic the human condition accurately. For instance, with alcohol, animals will not self-administer high levels of alcohol, which is quite different than humans (Cunningham et al., 2000). Heroin self-administration in rats can go as high as 3 mg/kg (Dai et al., 1989), well above the range of heroin use generally reported by many users (0.06–0.3 mg/kg) yet less than what very heavy users administer (6 mg/kg) (Uchtenhagen et al., 1999). Furthermore, it should be noted that self-administration studies examine the dose at which the drug becomes sufficiently rewarding to reinforce responding. It is likely that self-administration studies do not model the chronic drug abusers who escalate doses to obtain the euphoric effects of MDMA. Drug discrimination experiments are designed to determine the lowest dose that an animal can distinguish a drug compared with a vehicle; therefore, this is not a model of abuse. These data in conjunction with the pharmacokinetic data between rats and humans indicate that self-administration is not an entirely reliable guide for the doses that should be administered to animals to mimic the use patterns of addicted humans.
The general range of doses administered to neonatal animals thus far has been from 5 to 20 mg/kg/dose. Important for studies that have examined the effects of MDMA during gestation, it has been shown that substituted amphetamines readily cross the placental barrier (Burchfield et al., 1991; Campbell et al., 2006). This is not surprising as all amphetamines readily cross the blood–brain barrier and the blood–brain barrier is more restrictive than the placental barrier. Once across the placenta there is a direct correlation between amniotic and fetal brain MDMA levels (Campbell et al., 2006).
Effects of prenatal ± 3,4-methylenedioxymethamphetamine exposure in animals
A review primarily of the age-related acute effects of MDMA has recently been published (Piper, 2007). Piper discusses the effects of MDMA at different ages on core temperature, body weight, emergence of the serotonin syndrome at different ages, and effects on sexual behavior. Furthermore, discussion of some of the longer-term effects is present; however, the Piper review differs from this review in that here the focus is on the long-term effect of early MDMA exposure, especially with reference to effects on learning and memory. The reader is referred to Piper (2007) for a discussion of other aspects of the effects of MDMA, especially for effects during adolescent stages of development.
Biochemical effects
Table 1 summarizes the studies that have examined the effects of prenatal and neonatal exposure to MDMA in animals. Some of the initial studies on MDMA exposure in the developing animal are problematic because of study design issues. Of the limited data on developmental MDMA exposure, routes of administration, frequency of exposure, strain and species of animal used, and control groups included in each study varied widely. This has made synthesis of the effects of developmental MDMA exposure difficult owing to the lack of a consistent model; therefore limiting the generalizations that can be drawn from these experiments. Another factor limiting generalizations is that very few laboratories have published more than one experiment on developmental MDMA, making it difficult to find consistencies or follow-up experiments that replicated findings or tested hypotheses.
Table 1.
Studies that have examined the effects of developmental MDMA exposure
| Reference | Dose (mg/kg) | Exposure | Species (strain) | Frequency | Route | Control | Parameter examined | Findings |
|---|---|---|---|---|---|---|---|---|
| Winslow and Insel (1990) | 0.5, 1.0, or 10 | P10 (all doses) P0–2 (10 mg/kg) E12–14 (10 mg/kg) |
Rat (SD) | Once (P10); 1/day or 2/day in prenatal and neonatal study | s.c. | Saline | Ultrasonic vocalizations with TFMPP, 8-OH-DPAT, or DOI challenge; grid cell crossing; temperature; 5-HT, 5-HIAA, and 3H-paroxetine binding in cortex | ↓ Ultrasonic vocalizations during acute MDMA exposure on P10 ↑ Grid cell crossing after acute MDMA exposure of 1.0 and 10 mg/kg on P10 ↓ Latency to geotaxic response after acute MDMA exposure of 1.0 and 10 mg/kg on P10 ↓ Ultrasonic vocalizations at 0.5 and 3 h after exposure; no Δ at 6 h; ↑ at 10 and 24 h ↓ Ultrasonic vocalizations on P8, 11, and 14 after MDMA exposure on P0–2; no Δ on P5 No Δ after prenatal exposure No Δ in grid cell crossing and rate of weight gain on P5, 8, 11, and 14 in P0–2 exposed group ↓ 3H-paroxetine binding and 5-HT concentration in P0–2 exposed group ↓ 5-HIAA concentration in group dosed b.i.d. on P0–2; no Δ in group dosed once a day No Δ in the prenatal exposure group in 5-HT, 5-HIAA, and 3H-paroxetine ↓ DA in group exposed b.i.d. P0–2 No Δ in ultrasonic vocalizations after TFMPP, 8-OH-DPAT, or DOI challenge |
| St Omer et al. (1991) | 2.5 or 5.0 | Every other day E6–18 | Rat (CD) | 1/day | Oral | Distilled water | Gestation length, litter size, body weight of offspring, surface righting, negative geotaxis, olfactory orientation, milk induced behavior, forelimb grip strength, passive avoidance, activity, 5-HT and 5-HIAA concentrations | No Δ in length of gestation, litter size, and body weight of offspring No Δ in surface righting ↓ Negative geotaxis in females dosed with 2.5 mg/kg on P7 and 10. No Δ in animals dosed with 10 mg/kg and males dosed with 2.5 mg/kg ↑ Olfactory orientation in males on P9 and 10 and in females on P11 No Δ in milk induced behaviors on P6 No Δ in forelimb grip strength No Δ in passive avoidance retention No Δ in figure 8 activity No Δ in cerebrum 5-HT uptake sites on P29 ↓ 5-HT in caudate nucleus and hippocampus in animals dosed with 10 mg/kg; no Δ in 2.5 mg/mg group No Δ in 5-HT in frontal cortex No Δ in 5-HIAA in frontal cortex, caudate nucleus, and hippocampus |
| Bronson et al. (1994b) | 8, 16, 24, and 32 | E14 and P1 | Chicken | Single | In ova | Distilled water | In-ova motility, distress vocalization, wing extension, tremor, flat body posture, bursting forward movements, loss of righting reflex and convulsant like kicking after MDMA challenge | ↓ In ova motility in 32 mg/kg treated chicks; no Δ in 8 or 16 mg/kg treated chicks ↑ Distress vocalization and wing extension after 24 mg/kg MDMA challenge on P1 ↑ Flat body posture in naive animals on P1; ↑ flat body posture in animals pretreated with 8 and 16 mg/kg compared with naive animals; ↓ with pretreatment with 32 mg/kg ↑ Bursting activity in naive animals and animals pretreated with 8 or 16 mg/kg after challenge; no Δ in 32 mg/kg pretreatment group ↑ Loss of righting reflex in animals pretreated with 16 mg/kg after challenge; no Δ in naive or 8 and 32 mg/kg pretreated group No Δ in tremors or convulsant like kicking No Δ in brain, liver, or body weight |
| Kramer et al. (1994) | 10−3–10−9 mol/l | E17 synaptosomes | Rat (SD) | Single | Culture media | Baseline levels | 5-HT retention | Dose dependent ↓ in 5-HT concentration from MDMA concentrations of 10−3–10−7 mol/l with the exception of 10−4 (no Δ) No Δ at concentration of 10− 8 |
| Bronson et al. (1994a) | 8, 16, or 24 | E14 | Chicken | Single | In ova | Distilled water | Body weight, liver weight, and brain weight | No Δ in brain, liver, or body weight |
| Broening et al. (1994) | 10, 20, and 40 | P10 | Rat (SD) | Single | Orally | Saline | 5-HT, 5-HIAA, DA concentration; 5-HT reuptake sites | No Δ in 5-HT and 5-HIAA concentration on P17 frontal cortex, hippocampus, and caudate putamen ↓ 5-HT concentration 3–24 h after administration in frontal cortex, hippocampus, and caudate putamen No Δ in DA concentration in caudate putamen No Δ in 5-HT reuptake |
| Broening et al. (1995) | 20 or 40 | P10 | Rat (SD) | Single | Orally | Saline | Total thermal response, rectal temperature at different ambient temperatures, 5-HT concentration | No Δ total thermal response Minor fluctuations in temperature between the MDMA and control groups were found during the 8 h monitoring period No Δ in 5-HT concentration or uptake sites in frontal cortex, hippocampus, and caudate putamen No Δ in 3H-paroxetine binding |
| Colado et al. (1997a) | 20 | E14–17 | Rat (Wistar) | 2/day | s.c. | Saline | Litter size; offspring weight; 5-HT, 5-HIAA, and DA concentration in telencephalon, lipid peroxidation | ↓ Litter size No Δ in P1 body weight No Δ in 5-HT, 5-HIAA, or DA concentration No Δ in lipid peroxidation |
| Aguirre et al. (1998) | 20 | Every other day from E6–20 or P14 | Rat (Wistar) | Once | s.c. | Saline | 5-HT and DA concentration | No Δ in 5-HT in hippocampus, frontal cortex, striatum, and hypothalamus No Δ in DA in striatum and hypothalamus |
| Whitworth et al. (2001) | 10 μmol/l | E17–P13 | Rat (SD) thalamo-cortical neuron cultures | Once | Culture media | NA | 5-HT uptake; SERT expression; SERT levels with and without cocaine | MDMA regulated SERT levels similarly to 5-HT ↓ 5-HT uptake, SERT expression, and surface expression of SERT when MDMA and cocaine are combined compared with MDMA alone |
| Broening et al. (2001) | 5, 10, or 15 | P1–10 or P11–20 | Rat (SD) | 2/day | s.c. | Saline | Straight channel swimming; CWM; MWM cued, acquisition, reversal, and reduced platform; 5-HT, DA, and NE concentration at adulthood | ↓ Weight gain during dosing period No Δ straight channel swimming No Δ in CWM errors and latency in animals treated P1–10 ↑ CWM errors and latency in animals treated with 10 and 20 mg/kg on P11–20; no Δ in animals treated with 5 mg/kg No Δ in MWM in animals treated P1–10 ↑ Path length, cumulative distance from target, and latency to goal in animals treated P11–20 with 20 mg/kg during acquisition phase of MWM ↑ Path length and cumulative distance in 10 mg/kg group treated P11–20 during acquisition phase of MWM; no Δ 5 mg/kg No Δ reversal phase of MWM ↑ Latency, path length, and cumulative distance in animals treated P11–20 during reduced phase ↑ Average distance and ↓ platform crossings during acquisition, reversal, and reduced phase probe trials in 20 mg/kg group treated P11–20 ↑ Average distance in reversal probe trial in 5 mg/kg group treated on P11–20 ↓ Platform crossing in acquisition and reversal probe trials in 5 mg/kg group treated P11–20 ↑ Average distance in reduced probe trials in 10 mg/kg group; ↓ Platform crossings on third probe trial during reversal P1–10 group: ↓ 5-HT (all groups) and NE (10 and 20 mg/kg groups) in hippocampus; no Δ in 5-HT, DA or NE in frontal cortex P11–20 group: ↓ 5-HT and NE in the hippocampus; ↓ 5-HT in frontal cortex; no Δ in NE or DA in frontal cortex |
| Meyer and Ali (2002) | 10 | P1–4 | Rat (SD) | 2/day | s.c. | Saline | 3H-paroxetine binding; 5-HT, 5-HIAA, DA, DOPAC and HVA concentration | ↓3H-paroxetine binding in hippocampus on P25 and 60 ↓3 H-paroxetine binding in neocortex on P60; no Δ P25 ↓ 5-HT concentration in hippocampus; no Δ in 5-HIAA or HVA No Δ in 5-HT, 5-HIAA, DA, DOPAC, or HVA in neocortex |
| Won et al. (2002) | 40 | E5–12 | C57Bl/6 | 2/day | s.c. | Saline | 5-HT, 5-HIAA, DA, and DOPAC in reaggregate tissue culture | ↑ 5-HT in reaggregates ↑ 5-HIAA in reaggregates and culture media No Δ in DA in reaggregates ↑ DOPAC in culture media and reaggregates |
| Kelly et al. (2002) | 20 | E15–18; P10; P15; P20; or P10–13 | Rat (SD) | 2/day | s.c. | Untreated | 3H-paroxetine and SERT binding at P40; glucouse utilization | No Δ in 3H-paroxetine uptake No Δ in SERT ↑ Glucose utilization in locus coeruleus, inferior olive, nucleus ambiguous, trigeminal nucleus, subiculum of hippocampus, anterior thalamus, medial hypothalamus, septal nucleus, globus pallidus in animals treated E15–18; no Δ with postnatal treatment |
| Williams et al. (2003) | 20 | P11–20 | Rat (SD) | 2/day | s.c. | Saline, weighed only, and large litters | Straight channel swimming; CWM; MWM acquisition, shifted and reduced hidden platform and probe trials; MWM cued; CORT and ACTH baseline and following forced swim | ↓ Weight gain P12–28 No Δ straight channel swimming ↑ Latency in males on trials 4 and 5 of CWM ↑ Errors in males during CWM ↑ Latency, path length, and cumulative distance during acquisition phase of MWM ↑ Average distance during MWM acquisition probe trials; no Δ in crosses ↑ Cumulative distance during reversal phase of MWM; no Δ latency and path length No Δ reversal probe trials ↑ Path length and cumulative distance in reduced platform MWM trials; no Δ latency ↑ Average distance on probe trials during reduced platform trials; no Δ in crossings No Δ MWM cued trials No Δ in CORT and ACTH in baseline and forced swim |
| Koprich et al. (2003b) | 15 | E14–20 | Rat (SD) | 2/day | s.c. | Saline | Locomotor activity, tyrosine hydroxylase immunoreactivity; DA, DOPAC, HVA, 5-HT, and 5-HIAA on P3 and P21 | ↓ Body weight on P1, no Δ beginning on P3 due to culling method ↑ Locomotor activity on P21 ↑ TH immunoreactivity in striatum, nucleus accumbens and frontal cortex on P21 no Δ in monoamine concentrations in FC on P3 or P21 ↓ HVA in striatum on P3 and P21, ↓ DOPAC, 5-HIAA in striatum on P21; no Δ for rest of monoamines on P3 or P21 in striatum ↓ 5-HIAA in nucleus accumbens; no Δ remaining in remaining monoamines examined |
| Koprich et al. (2003a) | 20 | P11–20 | Rat (SD) | 2/day | s.c. | Saline | DA, DOPAC, HVA, 5-HT and 5-HIAA concentrations on P21; BDNF concentrations on P21 | ↓ Weight gain on P21 ↓ 5-HT in FC; ↓ 5-HT in striatum of females and in hippocampus of males; no Δ in the brain stem ↑ 5-HIAA in striatum, FC, brain stem, and hippocampus on P21 no Δ DA ↑ DOPAC in striatum; ↑ DOPAC in FC of females; no Δ DOPAC in brain stem and hippocampus ↑ HVA in striatum, frontal cortex, hippocampus and brain stem ↑ BDNF in hippocampus, striatum, FC, and brain stem |
| Vorhees et al. (2004) | 5, 10, and 20 | P11–20 | Rat (SD) | 2/day | s.c. | Saline | Straight channel swimming; MWM acquisition and probe; Barnes maze counter balanced so of litter were run before MWM and were run after MWM; working memory in MWM | ↓ Weight gain during dosing No Δ straight channel swimming or cued MWM No Δ Barnes maze ↑ Latency, path length, cumulative distance in MWM on P60 ↑ Average distance in probe trials in 20 mg/kg treated males that went through MWM first No Δ in working memory No Δ in MWM for animals that went through Barnes maze first |
| Meyer et al. (2004) | 10 | P1–4 | Rat (SD) | 2/day | s.c. | Saline | Body temperature; cleaved caspase-3 on P5; 5-HT, 5-HIAA, DA, DOPAC, HVA concentrations on P25; paroxetine binding on P25 and P60; SERT immunoreactivity on P90 | No Δ temperature at ambient temperatures of 31 or 371C during dosing ↓ Body weight during dosing ↑ Cleavage of caspase-3 in rostral forebrain and hippocampus on P5 ↓ 5-HT in the hippocampus on P25; no Δ 5-HIAA No Δ 5-HT, 5-HIAA, DA, DOPAC or HVA concentrations in the frontal cortex on P25 ↓ Paroxetine binding on P25 and P60 in hippocampus ↓ Paroxetine binding on P60, no Δ on P25 in neocortex ↓ SERT immunoreactivity in primary visual cortex and somatosensory cortex ↑ SERT in caudate putamen and nucleus accumbens shell on P90 No Δ SERT immunoreactivity on P90 in hippocampus, lateral hypothalamus or frontal cortex |
| Cohen et al. (2005) | 20 | P11–20 | Rat (SD) | 2/day | s.c. | Saline | Interaction of neonatal dosing and adult dosing (~P90 1 day 10 mg/kg 4 × day) on MWM, CWM, Zero Maze, NOR, locomotor activity; and 5-HT, 5-HIAA, DA, and DOPAC concentrations | No Δ zero maze ↑ Latency, path length and cumulative distance in MWM acquisition for neonatal treatment; no interaction with adult treatment ↑ Latency, path length and cumulative distance in reversal phase of MWM in animals treated with MDMA as neonates and as adults ↑ Errors in CWM; no Δ latency and no adult MDMA interaction ↓ Time spent observing the novel object; no adult MDMA interaction ↓ Center and total distance in locomotor activity; no adult MDMA interaction ↓ 5-HIAA in neonatal animals in hippocampus; no Δ for neonatal treatment for 5-HT No Δ neonatal treatment on 5-HT, 5-HIAA, DA, or DOPAC in striatum and prefrontal cortex as adults |
| Galineau et al. (2005) | 10 | E13–20 | Rat (Wistar) | 1/day | s.c. | Saline | DA, DOPAC, HVA, 5-HT and 5-HIAA concentrations at E14, 16, 18, 20, P0, 7, 14, and 21, [125I]PE2I and [3H] MADAM binding at previously mentioned points starting with E18; DA release in striatum after tyramine stimulation at P70; 5-HT release in the hippocampus after fenfluramine stimulation at P70; sucrose preference at P70 | No Δ in whole brain concentrations of DA, DOPAC, or HVA at all times examined ↓ 5-HT and 5-HIAA in whole brain at P0; no Δ at other points examined No Δ [125I]PE2I or [3H] MADAM binding in raphe nucleus, hypothalamus, thalamus, hippocampus, or cortex ↓ DA release after tyramine stimulation at P70 ↓ 5-HT release after FEN stimulation on P70 ↓ Sucrose preference on P70 |
| Williams et al. (2005) | 10 | P11 | Rat (SD) | 4/day | s.c. | Saline | Thymus weights; CORT; ACTH; DA, DOPAC, 5-HT, and 5-HIAA concentrations; paroxetine binding at 1, 7, 24, 30, and 78 h following the first dose | ↓ Thymus weight in females at 78 h after first dose; no Δ in males and at other time points ↑ CORT at 1, 7, and 30; no Δ 78 h after first dose ↓ 5-HT in hippocampus at all time points ↓ 5-HIAA in hippocampus at 6, 24 and 30 h; no Δ at 1 or 78 h ↓ 5-HT at 24, 30 and 78 h in the striatum ↓ 5-HIAA at 24 and 30 h in the striatum No Δ in DA in the striatum; ↓ DOPAC in the striatum at 1, 6, and 24 h No Δ in paroxetine binding |
| Crawford et al. (2006) | 20 | P11-20 | Rat (SD) | 2/day | s.c. | Saline | 5-HT, 5-HIAA, DA, DOPAC concentrations on P90; PKA activity; [35S]GTPγS binding following NPA, 5-HT, or R(+)-8-OH-DPAT stimulation on P90 | ↓ 5-HT in the PFC and hippocampus; no Δ in the striatum; no Δ 5-HIAA concentrations ↓ DA in PFC; ↓ striatum DA in males, no Δ in females; no Δ DOPAC ↓ PKA activity in PFC and hippocampus; no Δ in the striatum No Δ in basal [35S]GTPγS in PFC, striatum, hippocampus No Δ in efficacy or potency of NPA-stimulated [35S]GTPγS binding in the PFC or striatum ↑ 5-HT stimulated [35S]GTPγS binding in the PFC ↓ 5-HT stimulated [35S]GTPγS binding in the striatum ↑ Efficacy of R(+)-8-OH-DPAT and 5-HT stimulated [35S]GTPγS binding in the hippocampus |
| Piper and Meyer (2006) | 10 | P1–4 | Rat (SD) | 2/day | s.c. | Saline | Object recognition memory; temperature dysregulation and serotonin syndrome after MDMA challenge (10 mg/kg) at P100; SERT binding at P70 and P105 | No Δ in object recognition memory ↑ Temperature dysregulation and serotonin syndrome after MDMA challenge No Δ in SERT binding in cortex and hippocampus on P70 or P105 |
| Schaefer et al. (2006) | 10 | P11 | Rat (SD) | 4/day | s.c. | Saline | Plasmatic CORT concentration; DA, DOPAC, 5-HT and 5-HIAA concentrations 24 h after first dose | ↑ CORT on P12 ↓ 5-HT and 5-HIAA in the striatum and hippocampus on P12 No Δ DA in striatum; ↓ DOPAC |
| Skelton et al. (2006) | 20 | P11–20 | Rat (SD) | 2/day | s.c. | Saline | Spatial and path integration learning and memory on P30, 40, 180, and 360 | ↓ Weight gain during dosing ↑ Latency and errors in the CWM on P30 and P40; no Δ on P180 or 360 ↑ Path length, latency, and cumulative distance during acquisition, reversal and reduced phase of the MWM at all ages ↓ Time in target quadrant during probe trials during all phases of probe trials |
| Vorhees et al. (2007) | 40 | P11–20 | Rat (SD) | 1/day; 2/day 4/day |
s.c. | Saline | Spatial and path integration learning; NOR; locomotor activity; zero maze | ↓ Weight gain during dosing ↑ Latency and errors in the CWM for the 1 × and 2 × groups, no Δ in the 4 × group ↑ Path length, latency, and cumulative distance during acquisition, in 2 × and the 4 × groups ↑ Path length, latency, and cumulative distance during reversal phase of the MWM ↑ Latency to entry on 10th trial of Barnes maze in 1 × and 2 × groups ↓ Horizontal activity in all groups No Δ in zero maze or NOR |
| Dasari et al. (2007) | 10 μmol/l, 100 μmol/l, 1 mmol/l | First to third instar stages | D. melanogaster | Constant | Contained in agar | MDMA free agar | Survival, time to pupation, mouth hook, and lower body contractions, evoked responses, 5-HT and DA levels | ↓ Survival in 1 mmol/l group Delay in time to pupation in 1 mmol/l and 10 μmol/l group ↓ Mouth hook and lower body contractions ↑ Bursting behavior in muscles M6 and M7 ↓ Evoked responses in sensory neurons No Δ in 5-HT or DA levels |
ACTH, adrenocorticotrophic hormone; BDNF, brain-derived neurotrophic factor; b.i.d, twice daily; CORT, corticosterone; CWM, Cincinnati water maze; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; FC, frontal cortex; FEN, fenfluramine; 5-HIAA, 5-hydroxyindoleacetic acid; 8-OH-DPAT, [8-hydroxy-2-(di-n-propylamino) tetralin; 5-HT, serotonin; HVA, homovanillic acid; [125I]PE2I, (E)-N-3-Iodoprop-2-enyl)-2b-carbomethoxy-3b-(p-tolyl)-nortropane; [3H] MADAM, N,N-dimethyl-2-(2-amino-4-methylphenylthio)benzylamine; MDMA, ± 3,4-methylenedioxymethamphetamine; MWM, Morris water maze; NE, norepinephrine; NOR, novel object recognition; NPA, R(−)-propylnorapomorphine; PFC, prefrontal cortex; s.c, subcutaneously; SERT, serotonin transporter; TFMPP, 3-trifluoromethylphenylpiperazine.
In rats that were administered MDMA (2.5 or 5 mg/kg) every other day from embryonic day (E)6 to 18, reductions of 5-HT were observed in the caudate nucleus and the hippocampus of the offspring at P21, whereas levels of 5-HT in the frontal cortex and 5-hydroxyindoleacetic acid (5-HIAA) levels in all other regions examined were unaltered (St Omer et al., 1991). When MDMA (10−3–10− 9 mol/l) was applied to synaptosomes from E17 rat embryos, a dose-dependent decrease in 5-HT was observed (Kramer et al., 1994). Similarly, E14–17 MDMA (20 mg/kg; 2/day) exposure in rats decreased 5-HT levels in the dorsal telencephalon of the MDMA-exposed offspring on P7 (Colado et al., 1997a). Mice exposed to 40 mg/kg MDMA from E5 to E12 twice a day had a significant increase in 5-HT and 5-HIAA in mesencephalic-striatal tissue cultures (Won et al., 2002). Although some studies in rats show that MDMA reduces 5-HT, other studies have shown few effects of MDMA exposure on the developing animal, this is likely because of methodological differences. For instance, no changes in 5-HTor DA levels in various brain regions were observed in rat offspring whose mothers were exposed to 20 mg/kg MDMA once per day every other day beginning at E6 (Aguirre et al., 1998). Furthermore, when rats were exposed to MDMA (0.5, 1, or 10 mg/kg) twice a day from E12 to E15, no differences were seen in hippocampal levels of 5-HT, 5-HIAA, DA, DOPAC, homovanillic acid, or NE of their offspring when assayed on P21 (Winslow and Insel, 1990). Offspring of rats exposed to MDMA (15 mg/kg) twice a day from E14 to E20 showed no changes in 5-HT levels in several brain regions on P3 or P21; however, there was an MDMA-induced decrease in striatal 5-HIAA levels and subsequently decreased 5-HT turnover in the striatum on P21 with similar effects seen in DA metabolism and turnover (Koprich et al., 2003b). Although no alterations in DA were observed in animals exposed to MDMA from E14 to E20 in the Koprich et al. study, the MDMA-exposed animals had an increase in tyrosine hydroxylase immunoreactivity in the striatum, nucleus accumbens, and frontal cortex on P21 (Koprich et al., 2003b). A dose of 10mg/kg once a day from E14 to E20 in rats decreased the 5-HT and 5-HIAA content of the whole brain in the offspring on P0; however, 5-HT and 5-HIAA, as well as DA and DOPAC levels, were unaltered at all other days examined [every other day from E6 to P0 and then every 7th day from P0 to P21 (Galineau et al., 2005)]. In that same study, no changes were observed in DAT or SERT binding from E18 to P70 (Galineau et al., 2005). Regardless of the earlier lack of effect on total neurotransmitter levels, using microdialysis, DA or 5-HT-stimulated release on P70 by tyramine or fenfluramine, respectively, was augmented in prenatally MDMA-exposed animals (Galineau et al., 2005). No differences in paroxetine binding were observed in the offspring of rats exposed to 20 mg/kg MDMA twice a day from E15 to E18 when examined on P40, although there was an increase in glucose utilization in the brains of offspring on P90 (Kelly et al., 2002). It is of interest that doses of MDMA that produce 5-HT depletions in adult animals do not produce the same effects in the offspring of dams exposed to comparable MDMA doses. This is likely owing to the fact that the 5-HT system does not reach adult function in a rat until adolescence. During the prenatal and perinatal period, 5-HT may play a neurotrophic role in the developing brain (Azmitia, 1999). Thus, MDMA exposure may not directly affect serotonergic function, but may alter proper brain development. The need for a consistent exposure model to mimic early human development is required to truly ascertain the developmental effects of MDMA.
General characteristics and behavior
The effects of prenatal MDMA exposure include reduced body weights in the offspring of rats exposed twice daily to MDMA (10 mg/kg) from E12 to E15 (Winslow and Insel, 1990). On P9, there were no alterations in ultrasonic vocalizations, grid line crossings (a form of locomotor activity), or negative geotaxis in the female offspring of MDMA-treated rats compared with control female offspring (Winslow and Insel, 1990). There were no differences in litter size, length of gestation, or body size of offspring of rats exposed to MDMA (2.5 or 5 mg/kg) from E6 to E18 compared with the offspring of the control animals that received distilled water (St Omer et al., 1991). Behaviorally, the offspring showed sex-dependent effects of in utero MDMA exposure, suggesting short-term disruptions of sensory function (long-term effects in these behaviors were not examined). Negative geotaxis was measured once a day from P7 to P10 and female offspring of MDMA exposed rats showed a decrease in rotation only on P7; however no effects were observed in the MDMA-exposed male offspring on any test day. Effects were not dose dependent with the offspring from the females exposed to the lower dose of MDMA showing effects on 2 of the 5 days tested, indicating that this may be an artifact of testing and not a true effect of the drug. Male offspring of MDMA-treated rats showed an increase in olfactory orientation on P9 and P10, while female offspring of MDMA-exposed rats had increased orientation on P11. Other behaviors examined in that study, including surface righting, grip strength, and milk-induced behaviors, were unaffected in MDMA-exposed offspring (St Omer et al., 1991). MDMA exposure (20 mg/kg) twice a day to dams from E14 to E17 reduced litter size compared to the saline-exposed control rats (Colado et al., 1997a). The offspring of rats exposed from E14 to E20 to 15 mg/kg MDMA twice daily showed decreased locomotor activity relative to controls during the first 5 min on P21 followed by increased locomotor activity relative to saline exposed controls over the rest of the 20 min test period compared to saline-treated controls (Koprich et al., 2003b). MDMA administration (10 mg/kg 1/day) from E14 to E20 also decreased sucrose preference when the offspring were tested at P70 (Galineau et al., 2005).
Effects of neonatal exposure to MDMA in animals
General characteristics
As defined above, neonatal exposure to MDMA in rats up to approximately P21 is roughly analogous to the second half of gestation in humans. It has been shown that MDMA administration to neonatal rats decreases the rate of weight gain (Broening et al., 1995, 2001; Koprich et al., 2003a; Williams et al., 2003; Meyer et al., 2004; Vorhees et al., 2004; Skelton et al., 2006). This anorectic effect of MDMA continues several weeks after administration of the drug is discontinued; however, weights are similar by adulthood (Broening et al., 2001; Williams et al., 2003; Vorhees et al., 2004; Skelton et al., 2006). In adults it has been shown that MDMA causes a hyperthermic effect when ambient temperatures are over approximately 21°C. In developing animals, MDMA does not affect the thermal response of the animals. For example, at room temperatures of 25°C, MDMA exposure to P10 rats caused a mild, nonsignificant decrease in rectal temperatures whereas when room temperatures are lowered (10°C), a variable response to MDMA treatment is observed, that is, an increase in temperature relative to controls occurs initially and then hypothermia starts 6 h after MDMA administration, compared with saline-treated animals maintained at the same room temperature (Broening et al., 1995). For rats younger in age (i.e. P1–4) and exposed to MDMA, no alterations were observed in their thermal response regardless of the temperature at which the animals were housed (31 or 37°C) (Meyer et al., 2004).
Biochemistry
MDMA treatment of neonatal animals seems to disrupt the serotonergic system. For example, MDMA exposure from P0 to P2 decreased 5-HT and 5-HIAA levels and paroxetine binding in the cortex, hippocampus, striatum, and hypothalamus when examined on P21 (Winslow and Insel, 1990). Decreases in cortical DA levels were also observed after MDMA administration (Winslow and Insel, 1990). MDMA treatment on P10 resulted in decreased 5-HT in the frontal cortex 3–24 h after dosing, but no effects were observed 1 week later (Broening et al., 1994, 1995). MDMA exposure from P1 to P4 decreased 5-HT levels in the hippocampus on P25 (Meyer et al., 2004). Paroxetine binding was decreased in the hippocampus on P25 and P60 and decreased in the neocortex on P25; however, SERT immunoreactivity was unchanged in these regions at P90, again suggesting that the function and/or effect on the 5-HT system fluctuates over time (Meyer et al., 2004). On P11, MDMA (10 mg/kg/dose) treatment to rats induces reductions in hippocampal 5-HT at 1 and 7 h after the first of four doses given at 2 h intervals on a single day as well as at 24, 30, and 78 h after the first dose (Williams et al., 2005). Reductions in 5-HIAA were seen at 24 and 30 h after the first dose in the hippocampus. In the striatum, reductions in 5-HT did not emerge until 24 h after the first dose, whereas 5-HIAA reductions were observed 7 h after the first dose. No effects on dopamine were observed in the striatum; however, there were reductions in DOPAC seen at 6, 24, and 30 h after the first dose (Williams et al., 2005). The 24 h reductions in 5-HT and 5-HIAA were replicated in a subsequent study (Schaefer et al., 2006). After behavioral testing (approximately P105), the study by Broening et al. (2001) examined monoamine levels in adult animals exposed to 5, 10, or 20 mg/kg twice a day of MDMA from either P1 to P10 or P11 to P20. MDMA exposure induced small reductions in 5-HT in the frontal cortex and hippocampus as well as hippocampal NE 85 days after MDMA treatment. The reductions in hippocampal and frontal cortex 5-HT were also observed in a subsequent study where rats did not perform behavioral tasks (Crawford et al., 2006). In addition, a reduction of DA in the prefrontal cortex and striatum was also observed in this study. Unlike earlier exposure periods, twice a day MDMA (20 mg/kg) exposure on P10–13, P15–18, P20–23, or P25–28 did not affect paroxetine binding in the neocortex when rats were examined at P40 (Kelly et al., 2002). Similar to the findings of Kelly et al., paroxetine binding was also unchanged 1–78 h after MDMA exposure on P11 (Williams et al., 2005). These results suggest that MDMA works through serotonin systems in adulthood and during development, but these show different degrees of severity. This hypothesis is further supported by the finding that neonatally MDMA-treated animals had enhanced G-protein activity after 5-HTstimulation in the hippocampus and prefrontal cortex as adults, and an increase of G-protein activity after (+)-8-OH-DPAT stimulation in the hippocampus, suggesting an increase in sensitivity of the 5-HT1A receptor (Crawford et al., 2006). The 5-HT1A receptor has been implicated in learning and memory and is a possible mechanism for learning and memory deficits in MDMA-treated animals (Meneses and Perez-Garcia, 2007). In particular, increases in the 5-HT1A receptor were observed in older rats that had deficits in the MWM (Topic et al., 2007). At high doses, the 5-HT1A receptor agonist 8-OH-DPAT leads to decreased performance in a passive avoidance task, whereas administration of 5-HT1A receptor antagonists facilitated passive avoidance learning (for review, see Meneses and Perez-Garcia, 2007). The 5-HT1A receptor has been implicated in MDMA pharmacology in adolescent animals, as animals exposed to MDMA during adolescence had lower serotonin syndrome scores after a challenge dose of 8-OH-DPAT during adulthood (Piper et al., 2006). In adulthood, the effects of MDMA on the 5-HT1A receptor are not firmly established (for review, see Muller et al., 2007); however, it must be noted that the serotonin system is still not fully developed during the perinatal period (Herlenius and Lagercrantz, 2004). A recent study has shown most new neurons in the developing hippocampus express the 5-HT1A receptor and that the 5-HT1A receptor system is still developing in the dentate gyrus at P16 (Patel and Zhou, 2005). Taken together with the increased sensitivity of the 5-HT1A receptor after neonatal MDMA exposure, these data support the hypothesis that the 5-HT1A receptor is involved in MDMA-induced learning and memory deficits.
MDMA also induces the release of the stress hormone CORT during the P11–20 dosing period. This period of drug administration includes the latter portion of the SHRP when there is an attenuation of stress-induced release of CORT, a mechanism believed to protect the developing animal from the possible neurodegenerative effects of high levels of circulating glucocorticoids. MDMA causes an immediate increase in CORT levels in the plasma of developing rats on P11, and these levels are elevated for 24 h after the first of four doses on P11 (Williams et al., 2005). These CORT alterations do not seem to last into adulthood, as baseline and stressed animals exposed neonatally to MDMA do not have alterations in CORT release under basal conditions or after a 15 min forced swim compared with neonatally saline-treated animals (Williams et al., 2003). A possible hypothesis for the learning deficits involves the increases of CORT caused by MDMA exposure and subsequent development of regions important for learning and memory. It has been shown that CORT affects learning in an ‘inverted U’ manner (i.e. low or high levels of CORT have detrimental effects on learning) (Lupien and McEwen, 1997). Animals treated with methamphetamine (MA) from P11 to P20 show spatial learning and memory deficits when tested as adults (Vorhees et al., 2000). During the dosing period, MA administration increases CORT in the animals during testing (Williams et al., 2000) an effect also seen after MDMA exposure as noted above.
Increases in brain-derived neurotrophic factor (BDNF) were observed on P21 after P11–20 MDMA exposure (20 mg/kg) in rats in the frontal cortex, striatum, brain stem, and hippocampus (Koprich et al., 2003b). BDNF is a protein that has been shown to be important for neuronal survival and learning and memory (Leibrock et al., 1989; Lindsay et al., 1994; Linnarsson et al., 1997). A relationship between CORT and BDNF was also observed, which could explain the BDNF effects discovered in the MDMA-treated animals (Roskoden et al., 2004). Increases in BDNF have also been shown in MA-treated animals on P15 and P20 (Skelton et al., 2007). MDMA (10 mg/kg) exposure from P1 to P4 increased the cleavage of caspase-3, an apoptotic marker, in the rostral forebrain on P5, suggesting that MDMA may be inducing apoptosis in developing neurons (Meyer et al., 2004). Although these are significant areas to continue to explore for the mechanisms underlying MDMA-induced learning and memory deficits, other mechanisms should also be investigated.
Behavior
Acutely, MDMA exposure (0.5, 1, or 10 mg/kg) has been shown to cause increases in grid cell crossings and diminish ultrasonic vocalizations as well as reduce negative geotaxis performance of P10-treated rats (Winslow and Insel, 1990). Exposure from P0 to P2 with 10mg/kg of MDMA also reduced ultrasonic vocalizations of the pups when examined on P8, 11, and 14; however, there were no general effects on activity compared with saline-treated controls (Winslow and Insel, 1990). No other studies have examined the immediate effects of neonatal MDMA exposure, but instead have concentrated on possible long-term effects.
Areas of the brain important for cognitive function develop rapidly during the second half of gestation in humans and in part for this reason, animal models of the second half of human gestation (approximately P1–20 in rats) have focused more on assessing cognitive abilities. Rats exposed from P1 to P4 to MDMA (10 mg/kg 2/day) were unaffected in object recognition memory when examined on P68 (Piper and Meyer, 2006). These animals were also given an adult challenge dose of MDMA and the MDMA-treated animals showed a decrease in behaviors associated with the serotonin syndrome (head weaving, forepaw treading, and low body posture among others) compared with saline-treated animals, which supports the hypothesis that 5-HT1A receptor function may be altered in these animals (Piper and Meyer, 2006). Other studies have examined the effect of neonatal exposure to MDMA on other forms of learning and memory in adulthood. The first of these studies examined learning and memory after either P1–10 or P11–20 MDMA exposure (Broening et al., 2001). Rats were exposed twice a day to three different doses of MDMA (5, 10, or 20 mg/kg/dose) and tested in the MWM and CWM. The MWM assesses spatial learning and memory (Morris, 1981; Morris et al., 1982), whereas the CWM (Vorhees, 1987) assesses path integration (when tested under infrared lighting conditions) or a combination of path integration and spatial abilities (under visible lighting conditions). Animals treated with MDMA from P1 to P10 showed no deficits in CWM learning (using visible lighting), that is, animals completed the maze with similar latencies and committed a similar number of errors in finding the platform, compared with saline-treated littermates (Broening et al., 2001). Contrary to P1–10 exposure, rats treated from P11 to P20 with either 10 or 20 mg/kg twice per day of MDMA showed increased latency and errors in the CWM, whereas animals treated with 5 mg/kg performed similarly to saline-treated controls. Sensorimotor deficits were not observed in MDMA-treated animals from either treatment age as evidenced by asymptotic performance in MWM visible (cued) platform trials and a straight channel swimming task used to assess swimming speed. In the MWM, animals treated from P1 to P10 with MDMA, regardless of dose, showed no deficits compared with saline-treated animals; however, after P11–20 MDMA administration the 20 mg/kg MDMA group showed increases in cumulative distance, latency, and path length during the MWM hidden platform acquisition phase. The 10 mg/kg group in the P11–20 exposure period had increased path length and cumulative distance, and similar to CWM effects, the 5 mg/kg group showed no differences in performance compared with saline-treated animals. During reversal trials in the MWM, where the platform is moved to the opposite quadrant, MDMA exposure had no significant effect on performance, with only a trend toward increased latency in the 20 mg/kg group. For the final phase of the MWM testing, the platform was moved to the original position and reduced in size from 10 × 10 to 5 × 5 cm (for a description of the testing procedures, see Vorhees and Williams, 2006), therefore requiring higher spatial navigation accuracy. Animals treated from P11 to P20 with all doses of MDMA showed increases in latency, path length, and cumulative distance compared with saline-treated animals (Broening et al., 2001). These data support the view that MDMA, during critical periods of brain development, alters the ability of animals to learn in both spatial and path integration tasks and these effects are dose-dependent and persist into adulthood. It should also be noted that deficits did not appear in the 5 mg/kg group until a high degree of accuracy was required and this suggests that tests that rats can easily solve, may underestimate the impact of neonatal drug exposures. As the SHRP does not begin in the neonatal rat until P4 (Sapolsky and Meaney, 1986), it is possible that MDMA treatment before this (before P10) primes animals for early-onset SHRP; whereas dosing that commenced after P10 (on P11), during the SHRP, provided no protection. Interestingly, P1–10 dosing decreases 5-HT levels in adult animals similarly to P11–20 dosing (Broening et al., 2001); however, the impact that this reduction in 5-HT in the P1–10 animals has on the various receptor systems, including 5-HT1A receptors is unknown. Therefore, it would be worthwhile investigating 5-HTreceptors and in particular the 5-HT1A receptor after P1–10 MDMA treatment to compare it with the effects after P11–20 treatment.
Reference memory can be assessed by removing the platform from the MWM and determining the amount of time an animal searches in the area of the former platform location (i.e. probe trial), the average distance from the platform, or related measures of spatial preference. In the Broening et al. (2001) study, the 20 mg/kg MDMA group had a longer average distance from the platform site during probe trials on all phases of the MWM, whereas the 5 mg/kg group had a longer average distance from the platform site during the reversal phase and the 10 mg/kg group during the reduced platform phase probe trials (Broening et al., 2001).
As the MDMA-treated animals lost weight during development and were exposed to the stress of injection, a subsequent study examined the effects of either injection stress or undernutrition on the MDMA-induced learning and memory deficits in the CWM and MWM (Williams et al., 2003). To simulate the weight loss and undernutrition experienced by MDMA-exposed rats, a large litter design was used with 16 animals per litter during the period of drug administration, and to control for injection stress some litters were only weighed at each treatment time from P11 to P20. In addition, this experiment used entire litters that were either treated or control, whereas the earlier study used the split-litter design, with different offspring within a litter receiving different treatments. In the whole-litter experiment, MDMA exposure from P11 to P20 induced increased latency and errors in the CWM (using visible lighting) in the male offspring but the effect in female offspring was not significant. During the acquisition phase of the MWM, MDMA-treated animals (20 mg/kg) had increased latency, path length, and cumulative distance to the platform, whereas animals from the saline-treated large litters and saline-treated standard size litters performed essentially identically to animals from standard sized litters that were only weighed (in both males and females). The reversal trials showed that MDMA-treated animals had an increase in cumulative distance from the platform with trends for increased latency and path length to the platform. Again, standard-size saline-treated and large saline-treated control litters showed no differences compared with standard size weighed-only litters. Finally, during the reduced-platform phase of the MWM, MDMA-treated animals showed increased path length and cumulative distances with a trend toward increased latency to the escape platform compared with all control groups. In the probe trials, MDMA-treated offspring had longer average distances from the platform site at the end of the acquisition and reduced phase trials and were similar to saline-treated animals during the reversal phase. During the reversal probe, large litter offspring had a longer average distance from the platform than weighed animals. This was the only measure in which the large litter saline-treated animals differed from the weighed or standard-size saline-treated animals (Williams et al., 2003). The results of this study supported the earlier findings of Broening et al. (2001), showing that MDMA has effects that are independent of injection stress, undernutrition/reduced growth, and that regardless of whether the experimental design used a within-litter or between-litter approach, the effects of MDMA were the same. The large litter design likely stressed the offspring during the dosing period; however, the levels of stress may not have reached the threshold that leads to learning and memory deficits in and of themselves.
To obtain convergent evidence that MDMA exposure from P11 to P20 produced spatial learning and memory deficits and was not related to some difference in swimming ability or sensory impairment while in the water, a land-based spatial task was employed in an ensuing study (Vorhees et al., 2004). The Barnes maze tests spatial learning without swimming and can be run as either an appetitive or aversive task (Barnes, 1979). In the Vorhees et al. (2004) study, half of the animals, counterbalanced for treatment, were either tested first in the MWM (using a 5 × 5 cm platform) and then the Barnes maze or vice versa, and these tasks were then followed by a working memory (match-to-sample) test in the MWM (Vorhees et al., 2004). MDMA-treated animals showed increases in latency, path length, and cumulative distance during MWM testing at all doses administered (5, 10, or 20 mg/kg); however, no effects were observed in the Barnes maze or the working memory MWM (Vorhees et al., 2004). Interestingly, if animals were tested in the Barnes maze before being tested in the MWM, there was an amelioration of the MWM deficits. This suggests there may be some positive transfer of spatial learning strategies from one task to the other. As in earlier studies, no deficits were observed in the cued (visible platform) phase of the MWM or in the ability of the animals exposed to MDMA to swim a straight channel as rapidly as saline-treated animals, thereby further solidifying the hypothesis that MDMA-induced learning and memory deficits are not due to sensorimotor deficits (Vorhees et al., 2004). As the MDMA-treated offspring did not differ in the Barnes maze, it remains unclear whether the spatial navigation/reference memory impairments are specific to swimming tasks, or, instead, are because the Barnes maze was less sensitive to the drug’s effects. This point can only be resolved by further experiments.
The earlier studies examined the learning and memory ability of the animals when they were young adults; however, no data were available showing at what age the learning and memory effects began or how long they lasted. To test the emergence and relative permanence of the learning and memory effects of P11–20 MDMA exposure, animals began testing in spatial and path integration learning at four distinct ages; P30, P40, P180, or P360 (Skelton et al., 2006). These ages represent adolescence (P30 and P40) and middle-age (P180 and P360), respectively, in rats. At P30 and P40, MDMA-treated animals showed increases in latency and errors to find the goal in the CWM (under 25W red light), similar to earlier studies; however, the animals tested on P180 or P360 did not show these deficits. In the MWM, the MDMA-treated animals, regardless of when the animals began testing, showed increases in latency, path length, and cumulative distance during all phases of hidden platform training. During probe trials, MDMA treatment groups showed decreased time in the target quadrant for animals beginning testing on P30, but not in animals that began testing at P40, whereas P180 and P360 tested animals showed deficits depending on the phase of the MWM procedure. MDMA-treated animals tested on P180 showed decreased time in the target quadrant during the acquisition and reversal phases of probe trial testing compared with saline-treated animals, whereas at P360 MDMA-treated animals showed deficits during the acquisition and reduced platform probe trials (Skelton et al., 2006). It seems that the function of the 5-HT receptors during memory consolidation changes as animals age: downregulation is seen in younger animals, whereas upregulation is observed in aged animals (Meneses and Perez-Garcia, 2007). It is possible that MDMA-induced 5-HT1A-receptor alterations could ameliorate the path integration deficits seen at earlier ages. This is appealing as 5-HT perturbations by various drugs in adulthood can alter path integration learning. For example, both MDMA and fenfluramine produce deficits in CWM learning, and both of these drugs deplete 5-HT stores (Williams et al., 2002; Able et al., 2006).
In modeling human consumption of a drug in animal models, the question of dose distribution is seldom examined. To determine if the daily distribution of MDMA from P11 to P20 has an effect on the learning of adult animals, animals were dosed from P11 to P20, once a day with 40 mg/kg MDMA (1 × MDMA and three times with saline), twice a day with 20 mg/kg MDMA (2 × MDMA and twice with saline), or four times a day with 10 mg/kg MDMA (4 × MDMA), or four times a day with saline only, followed by behavioral testing during adulthood (Vorhees et al., 2007). MDMA-treated animals performed similarly to control animals in the elevated zero maze (a test of anxiety) and during NOR. Hypo-activity was observed in all three treatment groups. Two separate tasks were run for assessing spatial learning with increases in latency to reach the goal observed in the 1 × MDMA and 2 × MDMA groups in the Barnes maze, whereas the 4 × MDMA and 2 × MDMA groups showed increases in path length, cumulative distance, and latency in the acquisition phase of the MWM. During the reversal phase of the MWM, MDMA-treated animals showed an increase in latency, path length, and cumulative distance to the goal. Decreased time in the target quadrant during probe trials in the MWM was also seen in the MDMA-treated groups. Increases in latency and errors during CWM testing were observed in the 1 × MDMA and the 2 × MDMA groups, whereas the 4 × MDMA group was unaffected in the CWM. These data suggest that although MDMA disrupts both path integration and spatial learning, the different degree of effects on the two tasks perhaps indicates that various brain regions are differentially affected by dose pattern (Vorhees et al., 2007). Although this study showed an effect in the Barnes maze not previously observed, there were procedural differences between the studies that could explain the difference in outcome. A similar explanation may apply to the NOR differences between this study and another study by Cohen et al. (2005) discussed below.
To examine whether P11–20 MDMA exposure interacts with adult MDMA exposure, rats that were administered MDMA (twice daily) from P11 to P20 with 20 mg/kg/dose or the saline vehicle were subsequently treated with a 1 day, four-dose regimen of 10 mg/kg MDMA or saline when the animals were adults (Cohen et al., 2005). Four groups were thereby created (two neonatal treatments × two adult treatments) and the animals were tested in the following tasks: CWM, MWM, elevated zero maze, NOR, and spontaneous locomotor activity. As shown previously, neonatal MDMA exposure increased latency, path length, and cumulative distance during acquisition and reversal in the MWM. In the reversal phase of the MWM, adult animals that had been exposed to MDMA as adults and neonates showed increases in latency, path length, and cumulative distance, whereas neonatal MDMA-only or adult MDMA-only did not produce such deficits. Animals exposed to MDMA as neonates had an increased number of errors committed in escaping the CWM, replicating earlier findings. This study also showed that neonatal treatment with MDMA decreased time spent with a novel object in the NOR task, which is a hippocampally mediated nonspatial memory test (Clark et al., 2000). Furthermore, neonatal MDMA induced hypoactivity regardless of adult treatment (Cohen et al., 2005).
To summarize the studies utilizing the P11–20 exposure model on later behavior, MDMA treatment from P11 to P20 disrupts spatial and path integration learning, induces hypoactivity, and disrupts spatial reference and object recognition memory, when the animals are tested as adults. The learning effects emerge early after exposure and last at least through midlife at 1 year of age. Anxiety levels seem to be unaffected by neonatal MDMA treatment, but adult animals exposed to another MDMA dose exhibit increased anxiety. When taken together with the exposure of animals during earlier time periods (i.e., before P11), the data demonstrate that there is a critical period during the second 10 days of life for the cognitive effects of MDMA. The data are also consistent inasmuch as there are now five separate experiments showing adult spatial and path-integration learning deficits after neonatal MDMA exposure. At present it is unknown how these cognitive deficits are mediated, but the leading hypothesis, based on current data, is that the 5-HT1A receptor may be involved. Not only are the effects on the 5-HT1A receptor persistent, they are seen in areas of the brain known to be involved in the types of learning and memory affected by MDMA. This ‘guilt by association’ connection is far from establishing cause-and-effect, although it is sufficiently promising to warrant further examination.
Conclusion
In summary, it has been shown that MDMA treatment during the rat developmental period analogous to the second half of human brain development results in long-term learning and memory impairments. The data suggest that the effects of developmental exposure to these drugs are greater, in terms of effects on learning and memory and especially hippocampally mediated types of learning and memory, than adult exposure. Interestingly, the developing brain, while still sensitive to MDMA mediated 5-HT depletions, does not show the degree or persistence of 5-HT depletions that adult rats exposed to MDMA have. Nevertheless, it has been established that the neonatal effects of MDMA are independent of the anorectic effects of the drug across a wide span of doses and dose patterns, and are observable over a wide range of test ages, independently of litter and injection stress influences. Currently, the available data suggest that there are two possible mechanisms responsible for MDMA-induced learning and memory deficits. The first is an increase in the sensitivity of the 5-HT1A receptor, whereas another possible mechanism could be increased CORT release during the stress hyporesponsive period that in turn could overstimulate glucocorticoid receptors in the hippocampus and other regions. As the biochemical studies related to the behavioral deficits have been limited to date, there are other possible mechanisms related to the effects of neonatal MDMA treatment, and other hypotheses should also be investigated.
Acknowledgments
This research was supported by the following grants from the US National Institutes of Health: DA006733 and DA021394 (C.V.V.), DA014269 (M.T.W.), and training grant ES007051 (M.R.S.).
References
- Able JA, Gudelsky GA, Vorhees CV, Williams MT. 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol Psychiatry. 2006;59:1219–1226. doi: 10.1016/j.biopsych.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquas E, Marrocu P, Pisanu A, Cadoni C, Zernig G, Saria A, Di CG. Intravenous administration of ecstasy (3,4-methylendioxymethamphetamine) enhances cortical and striatal acetylcholine release in vivo. Eur J Pharmacol. 2001;418:207–211. doi: 10.1016/s0014-2999(01)00937-2. [DOI] [PubMed] [Google Scholar]
- Aguirre N, Barrionuevo M, Lasheras B, Del Rio J. The role of dopaminergic systems in the perinatal sensitivity to 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J Pharmacol Exp Ther. 1998;286:1159–1165. [PubMed] [Google Scholar]
- Aguirre N, Barrionuevo M, Ramirez MJ, Del RJ, Lasheras B. Alpha-lipoic acid prevents 3,4-methylenedioxy-methamphetamine (MDMA)-induced neurotoxicity. Neuro Report. 1999;10:3675–3680. doi: 10.1097/00001756-199911260-00039. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bernschneider-Reif S, Oxler F, Freudenmann RW. The origin of MDMA (‘ecstasy’) – separating the facts from the myth. Pharmazie. 2006;61:966–972. [PubMed] [Google Scholar]
- Bhattacharya SK, Bhattacharya A, Ghosul S. Anxiogenic activity of methylenedioxymethamphetamine (Ecstasy): an experimental study. Biogenic Amines. 1998;14:217–237. [Google Scholar]
- Bogen IL, Haug KH, Myhre O, Fonnum F. Short- and long-term effects of MDMA (‘ecstasy’) on synaptosomal and vesicular uptake of neurotransmitters in vitro and ex vivo. Neurochem Int. 2003;43:393–400. doi: 10.1016/s0197-0186(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (‘Ecstasy’) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (±)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson ME, Barrios-Zambrano L, Jiang W, Clark CR, DeRuiter J, Newland MC. Behavioral and developmental effects of two 3,4-methylenedioxymethamphetamine (MDMA) derivatives. Drug Alcohol Depend. 1994a;36:161–166. doi: 10.1016/0376-8716(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Bronson ME, Jiang W, Clark CR, DeRuiter J. Effects of designer drugs on the chicken embryo and 1-day-old chicken. Brain Res Bull. 1994b;34:143–150. doi: 10.1016/0361-9230(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Brosnan PG. The hypothalamic pituitary axis in the fetus and newborn. Semin Perinatol. 2001;25:371–384. doi: 10.1053/sper.2001.29038. [DOI] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991;265:1968–1973. [PubMed] [Google Scholar]
- Burns N, Olverman HJ, Kelly PA, Williams BC. Effects of ecstasy on aldosterone secretion in the rat in vivo and in vitro. Endocr Res. 1996;22:601–606. doi: 10.1080/07435809609043753. [DOI] [PubMed] [Google Scholar]
- Campbell NG, Koprich JB, Kanaan NM, Lipton JW. MDMA administration to pregnant Sprague-Dawley rats results in its passage to the fetal compartment. Neurotoxicol Teratol. 2006;28:459–465. doi: 10.1016/j.ntt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Cho AK, Hiramatsu M, Distefano EW, Chang AS, Jenden DJ. Stereochemical differences in the metabolism of 3,4-methylenedioxymethamphetamine in vivo and in vitro: a pharmacokinetic analysis. Drug Metab Dispos. 1990;18:686–691. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJS, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Van Nieuwenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS. MDMA (‘ecstasy’), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology (Berl) 2004;173:318–325. doi: 10.1007/s00213-004-1786-x. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: Interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RS. The love drug. Marching to the beat of ecstasy. Binghamton, NY: Haworth Medical Press; 1998. [Google Scholar]
- Colado MI, Green AR. The spin trap reagent alpha-phenyl-N-tert-butyl nitrone prevents ‘ecstasy’-induced neurodegeneration of 5-hydroxytryptamine neurones. Eur J Pharmacol. 1995;280:343–346. doi: 10.1016/0014-2999(95)00298-y. [DOI] [PubMed] [Google Scholar]
- Colado MI, O’Shea E, Granados R, Misra A, Murray TK, Green AR. A study of the neurotoxic effect of MDMA (‘ecstasy’) on 5-HT neurones in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol. 1997a;121:827–833. doi: 10.1038/sj.bjp.0701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, O’Shea E, Granados R, Murray TK, Green AR. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (‘ecstasy’) and p-chloroamphetamine but not the degeneration following fenfluramine. Br J Pharmacol. 1997b;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR, O’Shea E, Marsden CA. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology (Berl) 2003;166:383–390. doi: 10.1007/s00213-002-1374-x. [DOI] [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV. Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [(35)S]GTPgammaS binding in adult rats. Brain Res. 2006;1077:178–186. doi: 10.1016/j.brainres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Dai S, Corrigall WA, Coen KM, Kalant H. Heroin self-administration by rats: influence of dose and physical dependence. Pharmacol Biochem Behav. 1989;32:1009–1015. doi: 10.1016/0091-3057(89)90074-9. [DOI] [PubMed] [Google Scholar]
- Dasari S, Viele K, Turner AC, Cooper RL. Influence of PCPA and MDMA (ecstasy) on physiology, development and behavior in Drosophila melanogaster. Eur J Neurosci. 2007;26:424–438. doi: 10.1111/j.1460-9568.2007.05655.x. [DOI] [PubMed] [Google Scholar]
- Davison D, Parrott AC. Ecstasy (MDMA) in recreational users: self-reported psychological and physiological effects. Human Psychopharmacol. 1997;12:221–226. [Google Scholar]
- De la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- EMCDDA. The state of the drugs problem in the European Union and Norway. Luxemborg: European Monitoring Centre for Drugs and Drug Addiction; 2004. Annual Report 2004. Ref Type: Report. [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (±)3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) J Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A. MDMA (‘ecstasy’) enhances basal acetylcholine release in brain slices of the rat striatum. Eur J Neurosci. 2000;12:1385–1390. doi: 10.1046/j.1460-9568.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- Forsling M, Fallon JK, Kicman AT, Hutt AJ, Cowan DA, Henry JA. Arginine vasopressin release in response to the administration of 3,4-methylenedioxymethamphetamine (‘ecstasy’): is metabolism a contributory factor? J Pharm Pharmacol. 2001;53:1357–1363. doi: 10.1211/0022357011777855. [DOI] [PubMed] [Google Scholar]
- Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, Hutt AJ. The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharmacol. 2002;135:649–656. doi: 10.1038/sj.bjp.0704502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galineau L, Belzung C, Ercem K, Bodard S, Guilloteau D, Chalon S. Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) exposure induces long-term alterations in the dopaminergic and serotonergic functions in the rat. Dev Brain Res. 2005;154:165–176. doi: 10.1016/j.devbrainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Giannaccini G, Betti L, Pirone A, Palego L, Fabiani O, Fabbrini L, et al. Short-term effects of 3,4-methylen-dioxy-metamphetamine (MDMA) on 5-HT(1A) receptors in the rat hippocampus. Neurochem Int. 2007;51:496–506. doi: 10.1016/j.neuint.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Gough B, Ali SF, Slikker W, Jr, Holson RR. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol Biochem Behav. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Can drugs be used to enhance the psychotherapeutic process? Am J Psychother. 1986;40:393–404. doi: 10.1176/appi.psychotherapy.1986.40.3.393. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Effect of ascorbate and cysteine on the 3,4-methylenedioxymethamphetamine-induced depletion of brain serotonin. J Neural Transm. 1996;103:1397–1404. doi: 10.1007/BF01271253. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK, Nash JF. Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists. Eur J Pharmacol. 1994;264:325–330. doi: 10.1016/0014-2999(94)90669-6. [DOI] [PubMed] [Google Scholar]
- Hardman HF, Haavik CO, Seevers MH. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicol Appl Pharmacol. 1973;25:299–309. doi: 10.1016/s0041-008x(73)80016-x. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190 (Suppl 1):S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3,4-methylenedioxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Pawlak CR, Guo L, Schwarting RK. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res. 2004;149:135–144. doi: 10.1016/s0166-4328(03)00220-1. [DOI] [PubMed] [Google Scholar]
- Irvine RJ, Keane M, Felgate P, McCann UD, Callaghan PD, White JM. Plasma drug concentrations and physiological measures in ‘dance party’ participants. Neuropsychopharmacology. 2006;31:424–430. doi: 10.1038/sj.npp.1300896. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bahman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2004. Vol. I: secondary school students. (NIH Publication No. 05-5727) Bethesda, MD: National Institue on Drug Abuse; 2005a. Ref Type: Report. [Google Scholar]
- Johnston LD, O’Malley PM, Bahman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2004. Vol. II: college students and adults ages 19–45. Bethesda, MD: National Institute on Drug Abuse; 2005b. [Google Scholar]
- Kalechstein AD, De La GR, Mahoney JJ, III, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Ritchie IM, Quate L, McBean DE, Olverman HJ. Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol. 2002;137:963–970. doi: 10.1038/sj.bjp.0704961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman A, Gruzelier J. Chronic cognitive impairment in users of ‘ecstasy’ and cannabis. World Psychiatry. 2003;2:184–190. [PMC free article] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Campbell NG, Lipton JW. Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain derived neurotrophic factor in the forebrain and brain-stem of the rat. Dev Brain Res. 2003a;147:177–182. doi: 10.1016/s0165-3806(03)00219-0. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Chen EY, Kanaan NM, Campbell NG, Kordower JH, Lipton JW. Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases forebrain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol Teratol. 2003b;25:509–517. doi: 10.1016/s0892-0362(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Kramer K, Azmitia EC, Whitaker-Azmitia PM. In vitro release of [3H]5-hydroxytryptamine from fetal and maternal brain by drugs of abuse. Brain Res Dev Brain Res. 1994;78:142–146. doi: 10.1016/0165-3806(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Lawn JC. Schedules of controlled substances; scheduling of 3,4-methlenedioxymethamphetamine (MDMA) into schedule I of the controlled substances act. Federal Register. 1986;51:36552–36560. [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371–378. [PubMed] [Google Scholar]
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Mahmood I. Allometric issues in drug development. J Pharm Sci. 1999;88:1101–1106. doi: 10.1021/js9902163. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, Cunningham KA. Pharmacological studies of the acute and chronic effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B/1D) receptors. J Pharmacol Exp Ther. 1999;290:965–973. [PubMed] [Google Scholar]
- McElhatton PR, Bateman DN, Evans C, Pughe KR, Worsley AJ. Does prenatal exposure to ecstasy cause congenital malformations? A prospective follow-up of 92 pregnancies. Br J Clin Pharmacol. 1997;45:184. [Google Scholar]
- McElhatton PR, Bateman DN, Evans C, Pughe KR, Thomas SH. Congenital anomalies after prenatal ecstasy exposure. Lancet. 1999;354:1441–1442. doi: 10.1016/s0140-6736(99)02423-x. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Clemens KJ, Van der PG, Li KM, Hunt GE, Chen F, Lawrence AJ. Increased anxiety 3 months after brief exposure to MDMA (‘Ecstasy’) in rats: association with altered 5-HT transporter and receptor density. Neuropsychopharmacology. 2003a;28:1472–1484. doi: 10.1038/sj.npp.1300185. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Gurtman CG, Morley KC, Clemens KJ, Blokland A, Li KM, et al. Increased anxiety and ‘depressive’ symptoms months after MDMA (‘ecstasy’) in rats: drug-induced hyperthermia does not predict long-term outcomes. Psychopharmacology (Berl) 2003b;168:465–474. doi: 10.1007/s00213-003-1452-8. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G. 5-HT(1A) receptors and memory. Neurosci Biobehav Rev. 2007;31:705–727. doi: 10.1016/j.neubiorev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Ali SF. Serotonergic neurotoxicity of MDMA (ecstasy) in the developing rat brain. Ann N Y Acad Sci. 2002;965:373–380. doi: 10.1111/j.1749-6632.2002.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (‘ecstasy’) administration to neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics in new drug development. New York: Pergamon Press; 1989. pp. 42–96. [Google Scholar]
- Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (‘ecstasy’) Eur J Pharmacol. 2001;433:91–99. doi: 10.1016/s0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- Morley KC, Arnold JC, McGregor IS. Serotonin (1A) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:648–657. doi: 10.1016/j.pnpbp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. 3,4-Methylenedioxymethamphetamine enhances the release of acetylcholine in the prefrontal cortex and dorsal hippocampus of the rat. Psychopharmacology (Berl) 2006;184:182–189. doi: 10.1007/s00213-005-0271-5. [DOI] [PubMed] [Google Scholar]
- Nash JF, Brodkin J. Microdialysis studies on 3,4-methylenedioxymethamphetamine-induced dopamine release: effect of dopamine uptake inhibitors. J Pharmacol Exp Ther. 1991;259:820–825. [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Parrott AC, Lees A, Garnham NJ, Jones M, Wesnes K. Cognitive performance in recreational users of MDMA of ‘ecstasy’: evidence for memory deficits. J Psychopharmacol. 1998;12:79–83. doi: 10.1177/026988119801200110. [DOI] [PubMed] [Google Scholar]
- Patel TD, Zhou FC. Ontogeny of 5-HT1A receptor expression in the developing hippocampus. Brain Res Dev Brain Res. 2005;157:42–57. doi: 10.1016/j.devbrainres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Piper BJ. A developmental comparison of the neurobehavioral effects of ecstasy (MDMA) Neurotoxicol Teratol. 2007;29:288–300. doi: 10.1016/j.ntt.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Meyer JS. Increased responsiveness to MDMA in adult rats treated neonatally with MDMA. Neurotoxicol Teratol. 2006;28:95–102. doi: 10.1016/j.ntt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Vu HL, Safain MG, Oliver AJ, Meyer JS. Repeated adolescent 3,4-methylenedioxymethamphetamine (MDMA) exposure in rats attenuates the effects of a subsequent challenge with MDMA or a 5-hydroxytryptamine(1A) receptor agonist. J Pharmacol Exp Ther. 2006;317:838–849. doi: 10.1124/jpet.105.095760. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KK. Adolescent pregnancy and substance use. J Obstet Gynecol Neonatal Nurs. 1999;28:623–627. doi: 10.1111/j.1552-6909.1999.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Otten U, Schwegler H. Early postnatal corticosterone administration regulates neurotrophins and their receptors in septum and hippocampus of the rat. Exp Brain Res. 2004;154:183–191. doi: 10.1007/s00221-003-1656-5. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carrol FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Jayanthi S, Wang X, Dersch CM, Cadet JL, Prisinzano T, et al. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synapse. 2003;50:233–239. doi: 10.1002/syn.10266. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Seiden LS. Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 1998;806:69–78. doi: 10.1016/s0006-8993(98)00720-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Scanzello CR, Hatzidimitriou G, Martello AL, Katz JL, Ricaurte GA. Serotonergic recovery after (±)3,4-(methylenedioxy) methamphetamine injury: observations in rats. J Pharmacol Exp Ther. 1993;264:1484–1491. [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. A comparison of monoamine and corticosterone levels 24 h following (+) methamphetamine, (±)3,4-methelynedioxymethamphetamine, cocaine, (+) fenfluramine, or (±)methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Deluca P, Oyefeso A, Ghodse AH. Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994–2003) J Psychopharmacol. 2006;20:456–463. doi: 10.1177/0269881106060147. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Taylor VL. Depression of rat brain tryptophan hydroxylase activity following the acute administration of methylenedioxymethamphetamine. Biochem Pharmacol. 1987;36:4095–4102. doi: 10.1016/0006-2952(87)90566-1. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Taylor VL. Direct central effects of acute methylenedioxymethamphetamine on serotonergic neurons. Eur J Pharmacol. 1988;156:121–131. doi: 10.1016/0014-2999(88)90154-9. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology (Berl) 1999;147:66–72. doi: 10.1007/s002130051143. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J Neurochem. 1999;72:2516–2522. doi: 10.1046/j.1471-4159.1999.0722516.x. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Ascorbic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxyl radical formation and the behavioral and neurochemical consequences of the depletion of brain 5-HT. Synapse. 2001;40:55–64. doi: 10.1002/1098-2396(200104)40:1<55::AID-SYN1026>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Nichols DE. Characterization of three new psychomimetics. In: Stillman RC, Willette RE, editors. The psychopharmacology of hallucinogens. Oxford: Pergamon Press; 1978. [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+) methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Nichols DE. The monoamine oxidase-B inhibitor L-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J Pharmacol Exp Ther. 1995;273:667–673. [PubMed] [Google Scholar]
- Sprague JE, Preston AS, Leifheit M, Woodside B. Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiol Behav. 2003;79:281–287. doi: 10.1016/s0031-9384(03)00092-1. [DOI] [PubMed] [Google Scholar]
- St Omer VE, Ali SF, Holson RR, Duhart HM, Scalzo FM, Slikker W., Jr Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA) exposure in rats. Neurotoxicol Teratol. 1991;13:13–20. doi: 10.1016/0892-0362(91)90022-o. [DOI] [PubMed] [Google Scholar]
- Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;26:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Timar J, Gyarmati S, Szabo A, Furst S. Behavioural changes in rats treated with a neurotoxic dose regimen of dextrorotatory amphetamine derivatives. Behav Pharmacol. 2003;14:199–206. doi: 10.1097/00008877-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Topic B, Willuhn I, Palomero-Gallagher N, Zilles K, Huston JP, Hasenohrl RU. Impaired maze performance in aged rats is accompanied by increased density of NMDA, 5-HT1A, and alpha-adrenoceptor binding in hippocampus. Hippocampus. 2007;17:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- Uchtenhagen A, Dobler-Mikola A, Steffen T, Gutzwiller F, Blatter R, Pfeifer S. Med Prescr of Narcotics. Basel: Karger; 1999. Prescription of narcotics for heroin addicts. Main results of the Swiss National Cohort Study. [Google Scholar]
- Van Tonningen-van Driel M, Garbis-Berkvens JM, Reuvers-Lodewijks WE. Pregnancy outcome after ecstasy use; 43 cases followed by the Teratology Information Service of the National Institute for Public Health and Environment (RIVM) Ned Tijdschr Geneeskd. 1999;143:27–31. [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of ±-methylenedioxymethamphetamine on spatial vs. path integration learning: effects of dose distribution. Synapse. 2007;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Alterations in diurnal and nocturnal locomotor activity in rats treated with a monoamine-depleting regimen of methamphetamine or 3,4-methylenedioxymethamphetamine. Psychopharmacology (Berl) 2001;153:321–326. doi: 10.1007/s002130000578. [DOI] [PubMed] [Google Scholar]
- West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci U S A. 2002;99 (Suppl 1):2473–2478. doi: 10.1073/pnas.012579799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Seymour RS. Allometric scaling of mammalian metabolism. J Exp Biol. 2005;208:1611–1619. doi: 10.1242/jeb.01501. [DOI] [PubMed] [Google Scholar]
- Whitworth TL, Herndon LC, Quick MW. Psychostimulants differentially regulate serotonin transporter expression in thalamocortical neurons. J Neurosci. 2001;21:RC192. doi: 10.1523/JNEUROSCI.22-01-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, et al. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, McCrea AE, Wood SL, Vorhees CV. Administration of D,L-fenfluramine to rats produces learning deficits in the Cincinnati water maze but not the Morris water maze: relationship to adrenal cortical output. Neurotoxicol Teratol. 2002;24:783–796. doi: 10.1016/s0892-0362(02)00318-5. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, et al. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown CA, Skelton MR, Vinks AA, Vorhees CV. Absorption and clearance of ± 3,4-methylenedioxymethamphetamine from the plasma of neonatal rats. Neurotoxicol Teratol. 2004;26:849–856. doi: 10.1016/j.ntt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV. 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–220. [PubMed] [Google Scholar]
- Won L, Bubula N, Heller A. Fetal exposure to (±)-methylenedioxymethamphetamine in utero enhances the development and metabolism of serotonergic neurons in three-dimensional reaggregate tissue culture. Brain Res Dev Brain Res. 2002;137:67–73. doi: 10.1016/s0165-3806(02)00411-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Nash JF, Gudelsky GA. Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther. 1995;273:1063–1070. [PubMed] [Google Scholar]
- Yeh SY. Effects of salicylate on 3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity in rats. Pharmacol Biochem Behav. 1997;58:701–708. doi: 10.1016/s0091-3057(97)90010-1. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z. Memory impairment in now abstinent MDMA users and continued users: a longitudinal follow-up. Neurology. 2006;66:740–741. doi: 10.1212/01.wnl.0000200957.97779.ea. [DOI] [PubMed] [Google Scholar]



