Abstract
Platelet activation must be tightly controlled to provide an effective, but not excessive, response to vascular injury. Cytosolic calcium is a critical regulator of platelet function, including granule secretion, integrin activation, and phosphatidylserine (PS) exposure. Here we report that the novel protein kinase C isoform, PKCθ, plays an important role in negatively regulating Ca2+ signaling downstream of the major collagen receptor, glycoprotein VI (GPVI). This limits PS exposure and so may prevent excessive platelet procoagulant activity. Stimulation of GPVI resulted in significantly higher and more sustained Ca2+ signals in PKCθ−/− platelets. PKCθ acts at multiple distinct sites. PKCθ limits secretion, reducing autocrine ADP signaling that enhances Ca2+ release from intracellular Ca2+ stores. PKCθ thereby indirectly regulates activation of store-operated Ca2+ entry. However, PKCθ also directly and negatively regulates store-independent Ca2+ entry. This pathway, activated by the diacylglycerol analogue, 1-oleoyl-2-acetyl-sn-glycerol, was enhanced in PKCθ−/− platelets, independently of ADP secretion. Moreover, LOE-908, which blocks 1-oleoyl-2-acetyl-sn-glycerol-induced Ca2+ entry but not store-operated Ca2+ entry, blocked the enhanced GPVI-dependent Ca2+ signaling and PS exposure seen in PKCθ−/− platelets. We propose that PKCθ normally acts to restrict store-independent Ca2+ entry during GPVI signaling, which results in reduced PS exposure, limiting platelet procoagulant activity during thrombus formation.
Keywords: Calcium Channels, Gene Knockout, Phosphatidylserine, Platelet, Protein Kinase C (PKC)
Introduction
The central role of platelets in both physiological hemostasis and pathological thrombosis means that platelet activation must be tightly controlled. Blood vessel damage or atherosclerotic plaque rupture exposes subendoethial matrix proteins, in particular collagens, to flowing blood, leading to platelet adhesion and activation (1, 2). Activated platelets expose phosphatidylserine (PS),2 which is an efficient surface for assembly of the tenase (FIXa/VIIIa) and prothrombinase (FXa/Va) complexes (3), generating a burst of thrombin that is responsible for producing a stable hemostatic clot or an occlusive thrombus.
PS-exposing platelets are essential to thrombus growth in arterioles and, to a lesser extent, in venules (4). Restricting platelet PS exposure may therefore restrict thrombus growth.
A rise in intracellular calcium concentration ([Ca2+]i) is required for many platelet responses. Sustained Ca2+ signaling is required for platelets to expose PS and accelerate thrombin generation (5–8). Loss of plasma membrane asymmetry takes several minutes, so a sustained increase in intracellular Ca2+ is required. If [Ca2+]i is restored to basal levels, membrane asymmetry can be restored (9). Tight control of Ca2+ signaling therefore closely regulates PS exposure and platelet-dependent thrombin generation, and any factor that negatively regulates sustained Ca2+ signaling may reduce PS exposure and limit occlusive thrombus development.
Most platelet activators induce Ca2+ release from intracellular Ca2+ stores via the second messenger, inositol 1,4,5-trisphosphate (10). The initial transient increase in [Ca2+]i due to Ca2+ release is amplified Ca2+ entry from the extracellular medium. Ca2+ entry is also likely to be required to provide a sufficiently sustained signal to induce prolonged PS exposure, because agonist-induced Ca2+ release is usually transient (11). Store-operated Ca2+ entry (SOCE), a major pathway for Ca2+ entry into platelets and other nonexcitable cells, is activated by a decrease in the Ca2+ content of the intracellular Ca2+ stores (12–14). Platelets also express at least one store-independent Ca2+ entry pathway, activated by diacylglycerol (DAG) (15). The relative contributions of these different Ca2+ entry pathways to agonist-induced Ca2+ signaling and Ca2+-dependent platelet responses, in particular PS exposure, are less well understood. Moreover, how Ca2+ signaling is regulated during platelet activation, to provide sufficient signal without excessive PS exposure, is not known.
Protein kinase C (PKC) isoforms have long been known to play important roles in platelet activation. Although often considered a positive regulator of platelet activation, PKC may also negatively regulate some signaling events (16). In particular, it has been suggested that PKC negatively regulates platelet Ca2+ signaling (17). We have previously reported that the novel PKC isoform, PKCθ, negatively regulates platelet activation downstream of the major collagen receptor, glycoprotein VI (GPVI), with PKCθ−/− platelets displaying enhanced collagen-related peptide (CRP)-induced integrin activation (18) and granule secretion (19) at low agonist concentrations. Here, we have investigated whether PKCθ regulates GPVI-dependent Ca2+ signaling in mouse platelets. In particular, we report that PKCθ negatively regulates store-independent Ca2+ entry, which leads to higher sustained [Ca2+]i levels in PKCθ−/− platelets and enhanced PS exposure, revealing a novel mechanism by which platelet procoagulant activity may be tightly controlled.
EXPERIMENTAL PROCEDURES
Materials
Unless stated, all materials were from Sigma and were of analytical grade. Cross-linked CRP was from Professor Richard Farndale (Biochemistry, University of Cambridge, UK). Fura-PE3 was from TefLabs (Austin, TX). Annexin V-fluorescein isothiocyanate was obtained from Abcam (Cambridge, UK). Bisindolylmaleimide I (BIM), LOE-908, and MRS-2279 were from Tocris Bioscience (Bristol, UK). AR-C69931M-X was a kind gift from AstraZeneca.
Mice
PKCθ−/− C57B6/J mice have been previously described (20). No compensatory change in expression of other PKC isoforms has been observed in PKCθ−/− platelets (18), and we have found no differences in GPVI surface expression between PKCθ−/− and WT platelets (data not shown). Wild-type C57B6/J mice were used as control. Use of mouse platelets was approved by local research ethics committee at the University of Bristol, UK, and mice were bred for this purpose under UK Home Office license (PPL 30/2386) held by A. W. P.
Preparation of Fura-PE3-loaded Mouse Platelets
Washed mouse platelets were prepared essentially as described previously (21) and suspended in a modified Tyrode's/HEPES buffer (134 mm NaCl, 0.34 mm Na2HPO4, 2.9 mm KCl, 12 mm NaHCO3, 20 mm HEPES, 5 mm glucose, and 1 mm MgCl2, pH 7.3) supplemented with 0.35% bovine serum albumin, 1 μm prostaglandin E1, 10 μm indomethacin, 0.02 units/ml apyrase (grade VII)). Platelets were incubated with Fura-PE3 (3 μm) for 30 min at room temperature. Acid citrate dextrose (20 mm citric acid, 110 mm sodium citrate, 5 mm glucose) was added (1:9), and platelets were collected by centrifugation and resuspended in modified HEPES/Tyrode's, supplemented with indomethacin and apyrase, to a concentration of 1 × 108 platelets/ml.
[Ca2+]i Measurements
Platelets were stimulated at 37 °C with continuous stirring. Fura-PE3 was excited alternately at 340 and 380 nm, and fluorescence emission was detected at 510 nm. Fluorescence signals were corrected for autofluorescence. Fluorescence ratios, R (340/380), were calibrated in terms of [Ca2+]i using the following equation: [Ca2+]i = Kd·(S0/Ss)·(R − Rmin)/(Rmax − R), where S0 and Ss are the fluorescence at 380 nm in the absence of Ca2+ and in saturating Ca2+, respectively (22), and Kd = 290 nm (23). Where indicated, the area under the Ca2+ trace (AUC) above basal was calculated for 3 min after stimulation. For store-operated Ca2+ entry, AUC was calculated for 3 min after re-addition of Ca2+ (see Fig. 3A), corrected by subtraction of the increase in fluorescence due to leaked Fura-PE3.
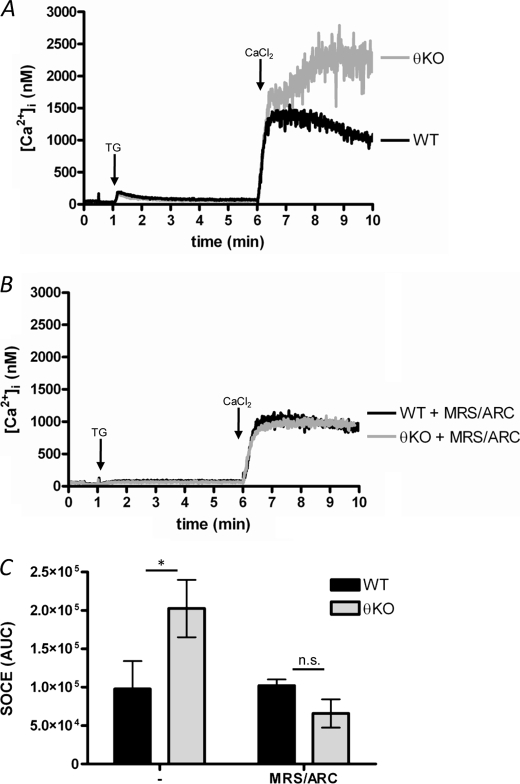
FIGURE 3.
Store-operated Ca2+ entry is not directly regulated by PKCθ independently of ADP secretion. A and B, SOCE was activated by treating platelets with thapsigargin (TG; 1 μm) for 5 min in the absence of extracellular Ca2+ (200 μm EGTA added). SOCE was then assessed by addition of 1.2 mm CaCl2. B, platelets were first treated with P2Y antagonists (MRS-2279/ARC). C, quantification of SOCE, as AUC. n.s., not significant; KO, knock-out. * indicates p < 0.05; n = 3.
Mn2+ Quench as a Marker of Divalent Cation Entry
Platelets were stimulated in the presence of MnCl2 (100 μm) and CaCl2 (1 mm), and fluorescence was monitored at an excitation wavelength of 360 nm. As with Fura-2 (24), at this wavelength Fura-PE3 fluorescence is insensitive to changes in Ca2+ concentration but is quenched by Mn2+. Autofluorescence was subtracted from the fluorescence intensity, which was then normalized to initial fluorescence intensity (F/F0). The extent of decrease 3 min after stimulation was measured and corrected by subtraction for the decrease in fluorescence due to Mn2+ or dye leakage (monitored as the quench in the absence of agonist over the same time period).
Flow Cytometry
Annexin V-fluorescein isothiocyanate was used to detect surface PS exposure. Platelets (5 × 107/ml) were stimulated in the presence of CaCl2 (2 mm) for 10 min. Annexin V binding was detected by flow cytometry, with platelets gated by their forward and side scatter profile. Analysis of 20,000 platelets was performed using a FACSCalibur (BD Biosciences). Data were analyzed using WinMDI version 2.8.
Statistical Analyses and Data Presentation
Where presented, mean data are given ± S.E. Statistical significance was determined by two-way analysis of variance with Bonferroni post-test, performed using Prism 4.0 (GraphPad Software). p < 0.05 was considered significant.
RESULTS
PKCθ Negatively Regulates CRP-induced Ca2+ Signaling and PS Exposure
The role of PKCθ in GPVI-dependent Ca2+ signaling was investigated in Fura-PE3-loaded and washed mouse platelets. The platelets were treated with indomethacin (10 μm) to prevent any thromboxane generation, because this may also be regulated by PKCθ (25).
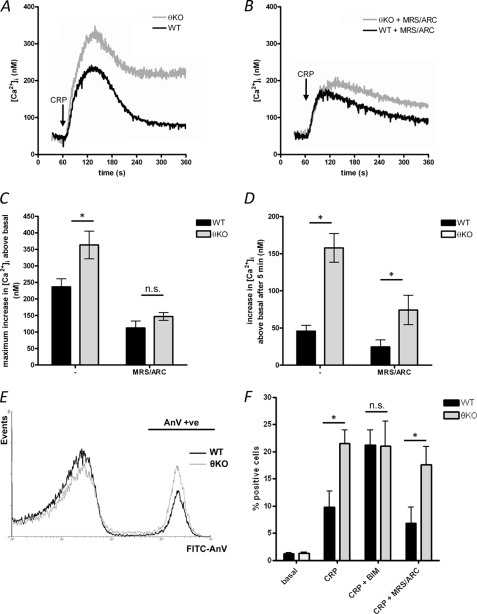
A low concentration of the GPVI-selective agonist, CRP (1 μg/ml), induced an increase in [Ca2+]i in wild-type (WT) platelets, which reached a peak within 2 min and then declined toward a sustained level (Fig. 1A). In PKCθ−/− platelets, the peak increase was significantly greater than seen in WT (363 ± 42 nm above basal in PKCθ−/− compared with 236 ± 24 nm in WT; n = 4; p < 0.01). Furthermore, the sustained [Ca2+]i level was significantly greater in PKCθ−/− platelets (in PKCθ−/− platelets, [Ca2+]i was 158 ± 19 nm above basal, 5 min after stimulation, compared with 46 ± 8 nm in WT; n = 4; p < 0.001). These data indicate that PKCθ negatively regulates GPVI-dependent Ca2+ signaling.
FIGURE 1.
PKCθ negatively regulates GPVI-dependent Ca2+ signaling and PS exposure. A, washed platelets from wild-type (WT) and PKCθ−/− (θKO) mice were stimulated by CRP (1 μg/ml) in the presence of extracellular Ca2+ (1 mm CaCl2 added). Platelets had been treated with indomethacin to prevent thromboxane synthesis, which may also be affected by PKCθ. [Ca2+]i was monitored by Fura-PE3 fluorescence. B, platelets were treated with MRS-2279 (10 μm; MRS-2279) and AR-C69931M-X (1 μm; ARC), antagonists of P2Y1 and P2Y12, respectively, prior to stimulation. C and D, maximum increase in [Ca2+]i above basal (C) and the increase in [Ca2+]i above basal 5 min after stimulation (i.e. at 360 s in A and B) were determined. Data are means ± S.E. of four independent experiments. * indicates p < 0.05 for WT versus PKCθ−/− platelets. E, platelets were stimulated with CRP (5 μg/ml) in the presence of annexin V-fluorescein isothiocyanate for 10 min. The annexin V-positive population is indicated (AnV +ve). The histogram is representative of nine independent experiments. F, mean data (± S.E.) showing the percentage of annexin V-positive platelets (n = 9). In some experiments, platelets were treated with BIM (10 μm, 10 min) or MRS-2279 + ARC prior to stimulation (n = 4 for CRP + BIM and CRP + MRS-2279/ARC). n.s., not significant.
We have previously shown that CRP-induced granule secretion is greater in PKCθ−/− platelets, under some conditions (18, 19). To test whether enhanced ADP secretion could explain the enhanced Ca2+ signal, platelets were treated with MRS-2279 (10 μm) and AR-C69931M-X (1 μm), antagonists of P2Y1 and P2Y12, respectively. Although P2Y antagonism abolished the enhancement in peak Ca2+ increase (Fig. 1, B and C), [Ca2+]i was still sustained at a greater level in PKCθ−/− platelets (Fig. 1, B and D; the increase in [Ca2+]i 5 min after stimulation was 74 ± 20 nm in PKCθ−/− platelets, compared with 24 ± 10 nm in WT; n = 4; p < 0.05). These data indicate that ADP signaling is not required for the enhanced sustained [Ca2+]i level.
PS exposure on the platelet surface forms an assembly site for coagulation complexes and accelerates thrombin generation. PS exposure requires a sustained increase in [Ca2+]i. CRP induced a proportion of WT platelets to expose PS (9.8 ± 2.9% were stained positively with annexin V; n = 9). This proportion was doubled in PKCθ−/− platelets (21.5 ± 2.5%; n = 9; Fig. 1E). Consistent with these data, pretreatment of WT platelets with the broad spectrum PKC inhibitor BIM (10 μm) increased the proportion of annexin V-positive platelets (21.3 ± 2.7%; n = 4). In contrast, BIM had no additional effect on PKCθ−/− platelets (21.0 ± 4.6%; n = 4; Fig. 1F).
Pretreatment with MRS-2279 and AR-C69931M-X partially reduced annexin V binding in WT platelets (6.8 ± 2.9% were annexin V-positive; n = 4). Even with P2Y1/P2Y12 blockade, however, annexin V binding was significantly enhanced in PKCθ−/− platelets (17.6 ± 3.4%; n = 4; p < 0.05). These data suggest that PKCθ negatively regulates CRP-induced PS exposure in an ADP-independent manner.
PKCθ Negatively Regulates CRP-induced Ca2+ Release Indirectly by Reducing ADP Secretion
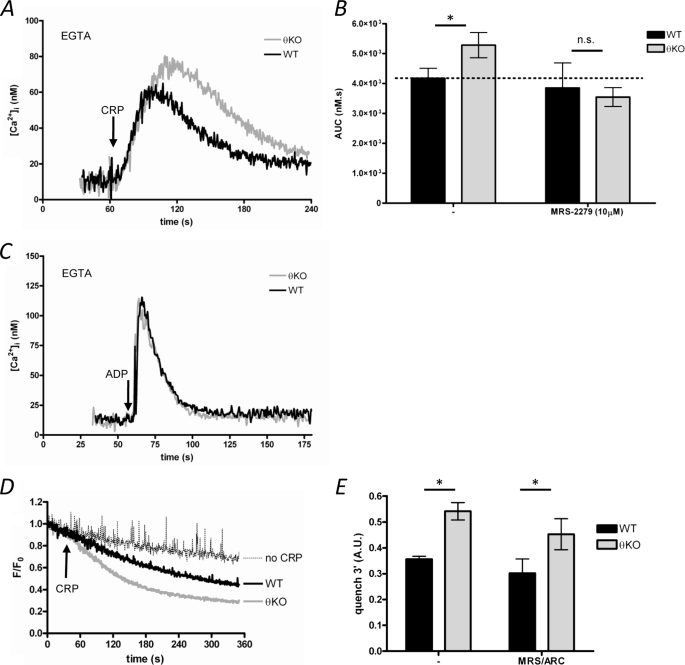
CRP induced an increase in [Ca2+]i in the absence of extracellular Ca2+ (200 μm EGTA added), indicating that CRP induces Ca2+ release from intracellular Ca2+ stores (Fig. 2A). In PKCθ−/− platelets, CRP-induced Ca2+ release was enhanced (Fig. 2, A and B). However, the enhanced Ca2+ release in PKCθ−/− platelets was blocked by MRS-2279, indicating that it required P2Y1 (Fig. 2B). Because PKCθ does not directly regulate ADP-induced Ca2+ release (Fig. 2C), these data suggest that enhanced ADP secretion in PKCθ−/− platelets is responsible for the observed increase in Ca2+ release. However, this also suggests that altered Ca2+ release does not underlie the enhanced sustained Ca2+ signal seen in the presence of extracellular Ca2+.
FIGURE 2.
PKCθ negatively regulates Ca2+ entry but does not directly regulate Ca2+ release. A, washed platelets were stimulated with CRP in the absence of extracellular Ca2+ (200 μm EGTA added). B, platelets were treated with the P2Y1 antagonist, MRS-2279, then stimulated as in A. Ca2+ release was analyzed as the area under the Ca2+ trace (AUC) above basal (see “Experimental Procedures”). Treatment with MRS-2279 blocked the enhanced Ca2+ release. C, platelets were stimulated by ADP (10 μm) in the absence of extracellular Ca2+ (200 μm EGTA added). D, divalent cation entry was monitored using Mn2+-dependent quench of Fura-PE3 fluorescence. The quench in the absence of CRP is indicated. E, analysis of the quench experiments (see “Experimental Procedures”) indicates that Mn2+ entry is increased in PKCθ−/− platelets, even in the presence of P2Y antagonists (MRS-2279/ARC). * indicates p < 0.05. KO, knock-out; n.s., not significant.
PKCθ Negatively Regulates CRP-induced Ca2+ Entry Independently of ADP Secretion
To confirm that PKCθ regulates a Ca2+ entry pathway, Mn2+-induced quench of Fura-PE3 fluorescence was used as a marker for Ca2+ entry. The extent of Mn2+ quench was greater in PKCθ−/− platelets compared with WT (Fig. 2E). This enhancement was also unaffected by P2Y antagonism (Fig. 2F). Together, these data indicate that PKCθ negatively regulates a Ca2+ entry pathway following stimulation of GPVI.
PKCθ Does Not Directly Regulate SOCE
SOCE, a major Ca2+ entry pathway in nonexcitable cells, was activated by treating platelets with the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor, thapsigargin (1 μm; 5 min), in the absence to extracellular Ca2+ (200 μm EGTA added). SOCE was assessed following addition of CaCl2 (1.2 mm). The Ca2+ signal was much greater in PKCθ−/− platelets (Fig. 3A). However, P2Y antagonists blocked the apparent enhancement, suggesting that enhanced ADP secretion was responsible for this, rather than a direct effect on SOCE itself (Fig. 3, B and C). Furthermore, these data suggest PKCθ may instead regulate a store-independent Ca2+ entry pathway.
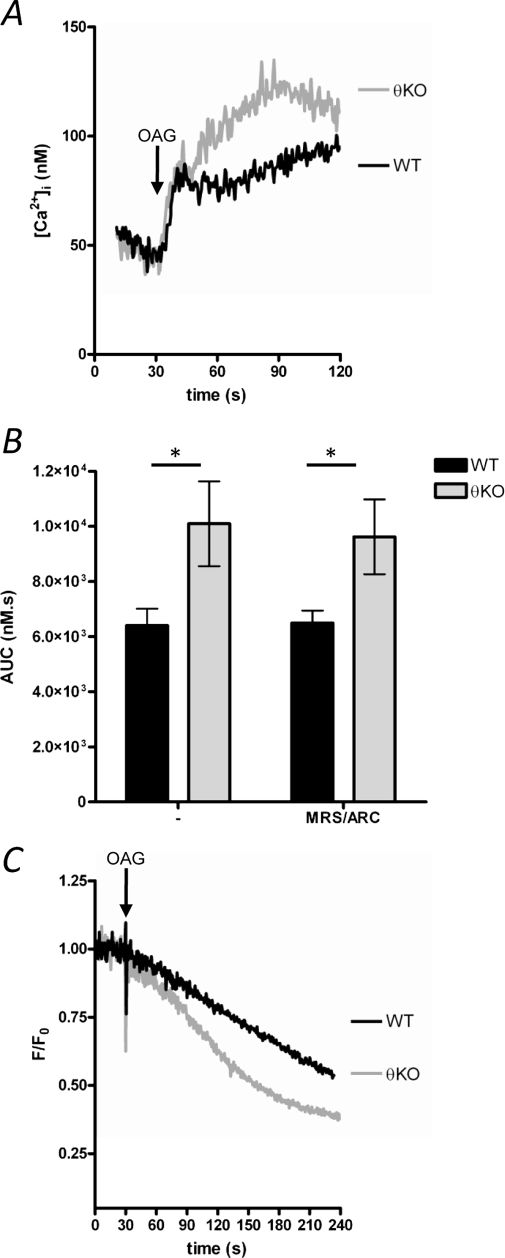
PKCθ Negatively Regulates Store-independent Ca2+ Entry
Store-independent Ca2+ entry pathways (also known as noncapacitative Ca2+ entry) have been described in many cells, including platelets (15). In many cells, Ca2+ entry can be directly activated by DAG analogues, such as OAG. In WT platelets, OAG (20 μm) induced a sustained increase in [Ca2+]i (Fig. 4A) that was unaffected by P2Y blockade (Fig. 4B). OAG-induced Ca2+ entry was significantly greater in PKCθ−/− platelets (Fig. 4A) but also unaffected by P2Y antagonists (Fig. 4B). Furthermore, OAG-induced Mn2+ entry was enhanced in PKCθ−/− platelets independently of P2Y signaling (Fig. 4, C and D). These data indicate that PKCθ negatively regulates OAG-induced store-independent Ca2+ entry.
FIGURE 4.
Store-independent Ca2+ entry is negatively regulated by PKCθ. A, platelets were stimulated with the DAG analogue, OAG (20 μm), in the presence of extracellular CaCl2 (1 mm). At this concentration, OAG does not induce Ca2+ release (data not shown). B, quantification of OAG-induced Ca2+ signaling, as AUC. Treatment with P2Y antagonists (MRS-2279/ARC) did not affect OAG-induced Ca2+ signaling. * indicates p < 0.05; n = 4. C, OAG-induced Mn2+ entry was monitored by quench of Fura-PE3 fluorescence. Trace is representative of three independent experiments. KO, knock-out.
Role of Store-independent Ca2+ Entry in CRP-induced Ca2+ Signaling
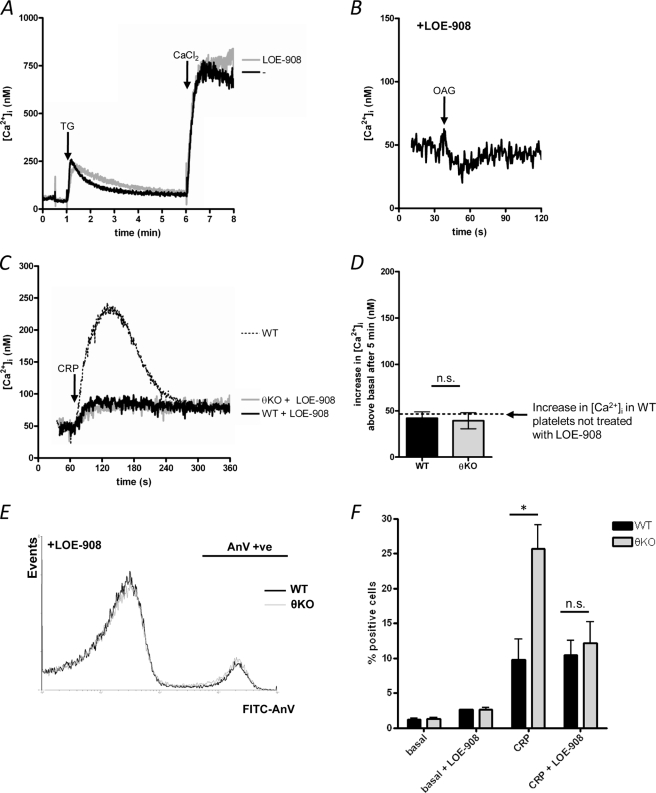
LOE-908 is a blocker of some nonselective cation channels (26, 27). LOE-908 (30 μm) blocked OAG-induced store-independent Ca2+ entry (Fig. 5A),but at this concentration has no effect on SOCE (Fig. 5B). LOE-908 significantly reduced the CRP-induced Ca2+ signal in WT platelets, although the sustained [Ca2+]i level reached after 5 min was not affected (Fig. 5, C and D). Importantly, the Ca2+ signal was no different between WT and PKCθ−/− platelets in the presence of LOE-908 (Fig. 5C). The increase in [Ca2+]i above basal, 5 min after stimulation, was 42 ± 7 nm in WT compared with 39 ± 9 nm in PKCθ−/− platelets (p > 0.05; Fig. 5, C and D). These data suggest that store-independent Ca2+ entry is required for the higher, sustained [Ca2+]i in PKCθ−/− platelets.
FIGURE 5.
Role of store-independent Ca2+ entry in GPVI-dependent Ca2+ signaling and PS exposure. A, WT platelets were treated with LOE-908 (30 μm) or the vehicle (Tyrode's) prior to activation of SOCE. No significant difference in SOCE was seen between LOE-908-treated and untreated cells (n = 3). TG, thapsigargin. B, WT platelets treated with LOE-908 were stimulated with OAG. In contrast to untreated cells (see Fig. 4A), no Ca2+ signal was seen in LOE-908-treated cells (n = 3). C, WT and PKCθ−/− (θKO) platelets were treated with LOE-908 and then stimulated with CRP. The dotted line indicates the Ca2+ signal seen in untreated WT cells, for comparison. The increase in [Ca2+]i, 5 min after stimulation, is shown in D. The dashed line indicates the [Ca2+]i after 5 min in untreated, CRP-stimulated WT platelets (see Fig. 1D). E, PS exposure was monitored in LOE-908-treated WT and PKCθ−/− platelets by annexin V binding, as in Fig. 1E. The annexin V-positive population is indicated (AnV +ve). F, mean data (± S.E.) showing the percentage of annexin V positive platelets. Control data (basal, CRP) are the same as Fig. 1F, for comparison. LOE-908 blocks the enhanced PS exposure in CRP-stimulated PKCθ−/− platelets. * indicates p < 0.05 for WT versus PKCθ−/−. n.s., not significant; FITC, fluorescein isothiocyanate.
PKCθ Negatively Regulates PS Exposure
LOE-908 had little effect on CRP-induced PS exposure in WT platelets, consistent with its lack of effect on the sustained [Ca2+]i in these cells. Importantly, however, there was no difference in PS exposure between WT and PKCθ−/− platelets in the presence of LOE-908 (10.5 ± 2.1% in WT, 12.1 ± 3.1% in PKCθ−/−, p > 0.05; Fig. 5E). These data are summarized in Fig. 5F. These data strongly suggest that PKCθ negatively regulates CRP-induced Ca2+ signaling by reducing store-independent Ca2+ entry, which in turn reduces platelet PS exposure.
DISCUSSION
In this study we have shown that PKCθ negatively regulates GPVI-dependent Ca2+ signaling, leading to reduced PS exposure. The negative regulation occurs at two distinct points as follows: 1) negative regulation of dense granule secretion and subsequent ADP-dependent Ca2+ release; 2) negative regulation of store-independent Ca2+ entry. We suggest that under normal conditions, store-independent Ca2+ entry contributes to the initial, transient increase in [Ca2+]i but is then down-regulated by PKCθ. This limits the sustained increase in [Ca2+]i and so reduces PS exposure. This is represented in Fig. 6. The work therefore reveals a novel pathway for regulation of platelet Ca2+ signaling and procoagulant activity, which plays a key role in thrombus growth.
FIGURE 6.
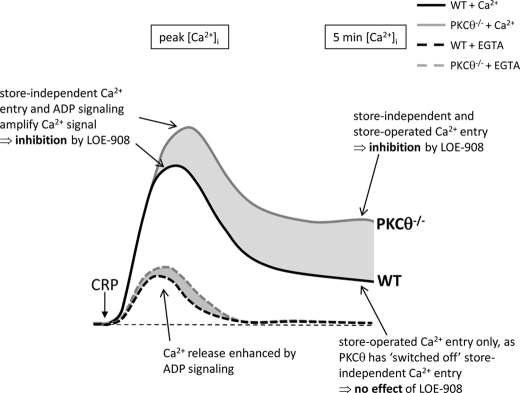
Store-independent Ca2+ entry in GPVI-dependent Ca2+ signaling. Stimulation of GPVI by CRP induces Ca2+ release from intracellular Ca2+ stores and Ca2+ entry. The initial peak includes Ca2+ release (amplified by ADP) and Ca2+ entry (mainly via store-independent Ca2+ entry and so blocked by LOE-908). Following the peak Ca2+ signal, PKCθ down-regulates store-independent Ca2+ entry in WT platelets, and so the sustained Ca2+ signal is not affected by LOE-908. In PKCθ−/− platelets, however, store-independent Ca2+ entry remains active leading to a higher sustained Ca2+ signal. This can be normalized to WT levels by LOE-908.
We have previously shown that PKCθ negatively regulates GPVI-dependent platelet activation (18). Here, we were first able to show that GPVI-dependent Ca2+ signaling is greater in amplitude and sustained at a higher level in PKCθ−/− platelets. The more sustained Ca2+ signal is associated with a PS exposure in a greater proportion of cells. The normal function of PKCθ therefore may be to limit GPVI-dependent Ca2+ signaling and so restrict PS exposure and platelet procoagulant activity during thrombus formation. The sustained Ca2+ signal was not entirely due to enhanced ADP secretion. We have previously demonstrated that, under some conditions, CRP-induced dense granule secretion is enhanced in PKCθ−/− platelets (19) (although this is partly dependent on assay conditions and agonist concentration). However, Ca2+ signaling was sustained at a higher level in PKCθ−/− platelets even in the presence of P2Y receptor antagonists, indicating that ADP signaling was not required.
Similarly, although Ca2+ release from intracellular Ca2+ stores was enhanced in PKCθ−/− platelets, this also does not underlie the more sustained Ca2+ signaling that we have observed. Instead, our data indicate that the enhanced Ca2+ release was secondary to ADP secretion and P2Y1 activation. P2Y1 is coupled via Gαq to phospholipase Cβ, resulting in inositol 1,4,5-trisphosphate formation and subsequent Ca2+ release. PKCθ does not appear to directly regulate Ca2+ release downstream of P2Y1 because ADP-induced Ca2+ was unaffected. In the absence of P2Y1 signaling, CRP-induced Ca2+ release was also unaffected, indicating that PKCθ does directly regulate this process. Therefore, under normal conditions PKCθ acts to reduce dense granule secretion (19), limiting P2Y1-dependent amplification of the initial Ca2+ release. However, this is not responsible for the greater sustained Ca2+ signal.
In contrast, we found that PKCθ negatively regulates Ca2+ entry, independently of ADP secretion. Mn2+ entry, a marker of divalent cation entry, was enhanced, even when P2Y receptors were blocked. Multiple Ca2+ entry pathways have been described in platelets. SOCE is activated by a decrease in the Ca2+ content within the intracellular Ca2+ stores (12) and can regulate PS exposure (28). Although we found that thapsigargin-activated SOCE was enhanced in PKCθ−/− platelets, it is known that part of the apparent SOCE signal may in fact be due to dense granule release (29). When the contribution of autocrine P2Y signaling was blocked, SOCE was not significantly different between PKCθ−/− and WT platelets. This indicates that SOCE is not directly regulated by PKCθ. Rather, increased dense granule secretion may further enhance Ca2+ release and store depletion, leading to greater SOCE. These data suggest that the negative regulation of CRP-induced Ca2+ entry is not through regulation of SOCE.
Ca2+ entry can occur through store-independent pathways, although much less is known about these pathways in platelets. In common with many cells, DAG analogues induce Ca2+ entry in platelets (15). Interestingly, this Ca2+ entry was enhanced in PKCθ−/− platelets. Furthermore, unlike SOCE, this enhancement was seen in the presence of P2Y antagonists. This means that OAG-activated, store-independent Ca2+ entry pathway is directly regulated by PKCθ, whereas SOCE is not.
OAG-induced Ca2+ entry pathway was blocked by LOE-908, whereas SOCE was unaffected by this drug. This makes LOE-908 a useful tool to discriminate between different Ca2+ entry pathways during platelet activation. LOE-908 significantly reduced CRP-induced Ca2+ signaling. In WT platelets, LOE-908 reduced the initial Ca2+ signal but not the sustained signal. This suggests that normally, store-independent Ca2+ entry amplifies the initial response but is not involved in the sustained Ca2+ signal. Importantly, in the presence of LOE-908, PKCθ−/− platelets showed identical Ca2+ signaling to WT, indicating that the enhanced response requires store-independent Ca2+ entry. We conclude, therefore, that PKCθ negatively regulates store-independent Ca2+ entry following stimulation of GPVI and that normally (i.e. in WT cells) store-independent Ca2+ entry is not involved in the sustained response because it has been switched off by PKCθ.
Negative regulation of store-independent Ca2+ entry by PKC has been proposed in some cultured cell lines (30), although, to the best of our knowledge, this is the first report that describes regulation of store-independent Ca2+ entry by a specific PKC isoform in a native cell type. The mechanism of regulation by PKC is still unclear. TRPC3, which may form part of store-independent channels in some cells (although its expression in platelets is uncertain (31, 32)), is phosphorylated at Ser-712 in a PKC-dependent manner in HEK-293 cells (33), which leads to inhibition of TRPC3. The store-independent channel in platelets, yet to be unambiguously identified, may also be directly phosphorylated. Alternatively, PKCθ may act indirectly, for example by modulating channel expression in the plasma membrane (34, 35).
SOCE is commonly considered to provide the majority of Ca2+ entry in nonexcitable cells (12). This view is strengthened by the large reduction in Ca2+ signaling in Orai1−/− platelets, in which SOCE is almost completely abolished (36). In contrast, LOE-908, which does not affect SOCE under the conditions used in this study, significantly reduced CRP-induced Ca2+ signaling. The effect of LOE-908 was restricted to the earlier portion of the Ca2+ signal, whereas the sustained [Ca2+]i was unaffected. These data suggest that the importance of different Ca2+ entry pathways may be temporally controlled, with DAG-activated/store-independent Ca2+ entry contributing to the early peak and SOCE more important for sustained Ca2+ signaling. Our data suggest that one mechanism behind this temporal control is PKCθ, which may “switch off” store-independent Ca2+ entry (see Fig. 6).
The major functional consequence of this negative regulation of store-independent Ca2+ entry may be to limit platelet PS exposure, which is a key component of thrombin generation during vascular damage. Although tissue factor-exposing cells initiate thrombin generation through the extrinsic coagulation cascade, propagation of thrombin generation occurs on PS-exposing platelets (3). Platelets provide an efficient and regulated procoagulant surface because they are localized to sites of vascular injury and can coordinate assembly of the tenase and prothrombinase complexes. Furthermore, inhibition of these coagulation complexes by plasma protease inhibitors is less effective when the proteases are on cell surfaces than when they are in solution (3). Central in inducing PS exposure is a sustained increase in [Ca2+]i (9, 37, 38). Our data suggest that PKCθ negatively regulates this process by inhibiting store-independent Ca2+ entry and so reducing [Ca2+]i. Importantly, this regulation appears to be the normal situation, because LOE-908 had little effect on PS exposure in WT platelets, despite the large inhibition of the earlier phase of the Ca2+ signal. Interestingly, LOE-908 reduced infarct volume following focal cerebral ischemia in a rat middle cerebral artery occlusion model of stroke (39, 40), highlighting the importance of controlling store-independent Ca2+ entry.
We have previously reported that PKCθ negatively regulates GPVI-dependent granule secretion, integrin activation, and thrombus formation on collagen (18). In contrast, another group has reported that PKCθ positively regulates GPVI-dependent granule secretion, integrin activation, and platelet aggregation (25). With regards to granule secretion, this may reflect agonist concentration (with negative regulation seen at low CRP concentrations by both groups (19)). The alterations in integrin activation are likely to be related to changes in dense granule secretion, because P2Y12 is a major regulator of integrin activation (41). It is important to stress, however, that these previous studies were performed in the absence of extracellular Ca2+, and so the novel regulation of store-independent Ca2+ entry that we describe here cannot account for the regulation of granule secretion. Rather, PKCθ is likely to regulate granule secretion and Ca2+ entry through distinct mechanisms. In summary, we have identified a novel role for PKCθ in reducing GPVI-dependent Ca2+ signaling and PS exposure. This is mediated by reduced Ca2+ entry through a store-independent Ca2+ channel. Because platelet PS exposure is a key determinant of thrombin generation and thrombus formation, PKCθ may act to limit collagen-dependent thrombin generation and hence occlusive thrombus formation at sites of vascular injury.
Acknowledgment
We thank Elizabeth Aitken for expert technical assistance supporting this work.
This work was supported by the British Heart Foundation Programme Grant RG/05/015.
- PS
- phosphatidylserine
- ARC
- AR-C69931M-X
- BIM
- bisindolylmaleimide I
- [Ca2+]i
- intracellular Ca2+ concentration
- CRP
- collagen-related peptide
- DAG
- diacylglycerol
- GPVI
- glycoprotein VI
- OAG
- 1-oleoyl-2-acetyl-sn-glycerol
- PLC
- phospholipase C
- PKC
- protein kinase C
- SOCE
- store-operated Ca2+ entry
- WT
- wild type
- AUC
- area under curve.
REFERENCES
- 1.Jackson S. P., Nesbitt W. S., Kulkarni S. (2003) J. Thromb. Haemost. 1, 1602–1612 [DOI] [PubMed] [Google Scholar]
- 2.Varga-Szabo D., Pleines I., Nieswandt B. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 403–412 [DOI] [PubMed] [Google Scholar]
- 3.Monroe D. M., Hoffman M. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 41–48 [DOI] [PubMed] [Google Scholar]
- 4.Kuijpers M. J., Munnix I. C., Cosemans J. M., Vlijmen B. V., Reutelingsperger C. P., Egbrink M. O., Heemskerk J. W. (2008) Microcirculation 15, 269–282 [DOI] [PubMed] [Google Scholar]
- 5.Solum N. O. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2841–2846 [DOI] [PubMed] [Google Scholar]
- 6.Zwaal R. F., Comfurius P., Bevers E. M. (2004) Biochim. Biophys. Acta 1636, 119–128 [DOI] [PubMed] [Google Scholar]
- 7.Arachiche A., Kerbiriou-Nabias D., Garcin I., Letellier T., Dachary-Prigent J. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1883–1889 [DOI] [PubMed] [Google Scholar]
- 8.Smeets E. F., Heemskerk J. W., Comfurius P., Bevers E. M., Zwaal R. F. (1993) Thromb. Haemost. 70, 1024–1029 [PubMed] [Google Scholar]
- 9.Williamson P., Bevers E. M., Smeets E. F., Comfurius P., Schlegel R. A., Zwaal R. F. (1995) Biochemistry 34, 10448–10455 [DOI] [PubMed] [Google Scholar]
- 10.Varga-Szabo D., Braun A., Nieswandt B. (2009) J. Thromb. Haemost. 7, 1057–1066 [DOI] [PubMed] [Google Scholar]
- 11.Heemskerk J. W., Feijge M. A., Henneman L., Rosing J., Hemker H. C. (1997) Eur. J. Biochem. 249, 547–555 [DOI] [PubMed] [Google Scholar]
- 12.Parekh A. B., Putney J. W., Jr. (2005) Physiol. Rev. 85, 757–810 [DOI] [PubMed] [Google Scholar]
- 13.Salido G. M., Sage S. O., Rosado J. A. (2009) Cell. Signal. 21, 457–461 [DOI] [PubMed] [Google Scholar]
- 14.Lewis R. S. (2007) Nature 446, 284–287 [DOI] [PubMed] [Google Scholar]
- 15.Hassock S. R., Zhu M. X., Trost C., Flockerzi V., Authi K. S. (2002) Blood 100, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 16.Harper M. T., Poole A. W. (2007) Biochem. Soc. Trans. 35, 1005–1008 [DOI] [PubMed] [Google Scholar]
- 17.Strehl A., Munnix I. C., Kuijpers M. J., van der Meijden P. E., Cosemans J. M., Feijge M. A., Nieswandt B., Heemskerk J. W. (2007) J. Biol. Chem. 282, 7046–7055 [DOI] [PubMed] [Google Scholar]
- 18.Hall K. J., Harper M. T., Gilio K., Cosemans J. M., Heemskerk J. W., Poole A. W. (2008) PLoS ONE 3, e3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper M. T., Poole A. W. (2009) Blood 114, 489–492 [DOI] [PubMed] [Google Scholar]
- 20.Soriani A., Moran B., de Virgilio M., Kawakami T., Altman A., Lowell C., Eto K., Shattil S. J. (2006) J. Thromb. Haemost. 4, 648–655 [DOI] [PubMed] [Google Scholar]
- 21.Konopatskaya O., Gilio K., Harper M. T., Zhao Y., Cosemans J. M., Karim Z. A., Whiteheart S. W., Molkentin J. D., Verkade P., Watson S. P., Heemskerk J. W., Poole A. W. (2009) J. Clin. Invest. 119, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 23.Vorndran C., Minta A., Poenie M. (1995) Biophys. J. 69, 2112–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. (1989) Biochem. J. 258, 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy B., Jr., Bhavaraju K., Getz T., Bynagari Y. S., Kim S., Kunapuli S. P. (2009) Blood 113, 2557–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krautwurst D., Degtiar V. E., Schultz G., Hescheler J. (1994) Naunyn Schmiedebergs Arch. Pharmacol. 349, 301–307 [DOI] [PubMed] [Google Scholar]
- 27.Krautwurst D., Hescheler J., Arndts D., Lösel W., Hammer R., Schultz G. (1993) Mol. Pharmacol. 43, 655–659 [PubMed] [Google Scholar]
- 28.Bergmeier W., Oh-Hora M., McCarl C. A., Roden R. C., Bray P. F., Feske S. (2009) Blood 113, 675–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper A. G., Mason M. J., Sage S. O. (2009) Cell Calcium 45, 413–420 [DOI] [PubMed] [Google Scholar]
- 30.Venkatachalam K., Zheng F., Gill D. L. (2003) J. Biol. Chem. 278, 29031–29040 [DOI] [PubMed] [Google Scholar]
- 31.Brownlow S. L., Sage S. O. (2005) Thromb. Haemost. 94, 839–845 [DOI] [PubMed] [Google Scholar]
- 32.Carter R. N., Tolhurst G., Walmsley G., Vizuete-Forster M., Miller N., Mahaut-Smith M. P. (2006) J. Physiol. 576, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trebak M., Hempel N., Wedel B. J., Smyth J. T., Bird G. S., Putney J. W., Jr. (2005) Mol. Pharmacol. 67, 558–563 [DOI] [PubMed] [Google Scholar]
- 34.Liu D., Maier A., Scholze A., Rauch U., Boltzen U., Zhao Z., Zhu Z., Tepel M. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 746–751 [DOI] [PubMed] [Google Scholar]
- 35.Cayouette S., Lussier M. P., Mathieu E. L., Bousquet S. M., Boulay G. (2004) J. Biol. Chem. 279, 7241–7246 [DOI] [PubMed] [Google Scholar]
- 36.Braun A., Varga-Szabo D., Kleinschnitz C., Pleines I., Bender M., Austinat M., Bosl M., Stoll G., Nieswandt B. (2009) Blood 113, 2056–2063 [DOI] [PubMed] [Google Scholar]
- 37.Arachiche A., Kerbiriou-Nabias D., Garcin I., Letellier T., Dachary-Prigent J. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1883–1889 [DOI] [PubMed] [Google Scholar]
- 38.Munnix I. C., Strehl A., Kuijpers M. J., Auger J. M., van der Meijden P. E., van Zandvoort M. A., oude Egbrink M. G., Nieswandt B., Heemskerk J. W. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 2673–2678 [DOI] [PubMed] [Google Scholar]
- 39.Christensen T., Wienrich M., Ensinger H. A., Diemer N. H. (2005) Basic Clin. Pharmacol. Toxicol. 96, 316–324 [DOI] [PubMed] [Google Scholar]
- 40.Tatlisumak T., Carano R. A., Takano K., Meiler M. R., Li F., Sotak C. H., Arndts D., Pschorn U., Fisher M. (2000) J. Neurol. Sci. 178, 107–113 [DOI] [PubMed] [Google Scholar]
- 41.Cosemans J. M., Iserbyt B. F., Deckmyn H., Heemskerk J. W. (2008) J. Thromb. Haemost. 6, 1253–1261 [DOI] [PubMed] [Google Scholar]