Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel belonging to the ATP-binding cassette transporter superfamily. CFTR is gated by ATP binding and hydrolysis at its two nucleotide-binding domains (NBDs), which dimerize in the presence of ATP to form two ATP-binding pockets (ABP1 and ABP2). Mutations reducing the activity of CFTR result in the genetic disease cystic fibrosis. Two of the most common mutations causing a severe phenotype are G551D and ΔF508. Previously we found that the ATP analog N6-(2-phenylethyl)-ATP (P-ATP) potentiates the activity of G551D by ∼7-fold. Here we show that 2′-deoxy-ATP (dATP), but not 3′-deoxy-ATP, increases the activity of G551D-CFTR by ∼8-fold. We custom synthesized N6-(2-phenylethyl)-2′-deoxy-ATP (P-dATP), an analog combining the chemical modifications in dATP and P-ATP. This new analog enhances G551D current by 36.2 ± 5.4-fold suggesting an independent but energetically additive action of these two different chemical modifications. We show that P-dATP binds to ABP1 to potentiate the activity of G551D, and mutations in both sides of ABP1 (W401G and S1347G) decrease its potentiation effect, suggesting that the action of P-dATP takes place at the interface of both NBDs. Interestingly, P-dATP completely rectified the gating abnormality of ΔF508-CFTR by increasing its activity by 19.5 ± 3.8-fold through binding to both ABPs. This result highlights the severity of the gating defect associated with ΔF508, the most prevalent disease-associated mutation. The new analog P-dATP can be not only an invaluable tool to study CFTR gating, but it can also serve as a proof-of-principle that, by combining elements that potentiate the channel activity independently, the increase in chloride transport necessary to reach a therapeutic target is attainable.
Keywords: ABC Transporter, Chloride Channels, Chloride Transport, Cystic Fibrosis, Ion Channels
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR)2 chloride channel is a major player in salt and water transport across epithelia. Like all members of the ATP-binding cassette (ABC) family, CFTR has two nucleotide-binding domains (NBDs), which contain the Walker A and B motifs and the highly conserved signature sequence. A regulatory (R) domain, unique to CFTR, needs to be phosphorylated by protein kinase A (PKA) for the channel to function. Experimental evidence suggests that the two NBDs of CFTR dimerize in a head-to-tail configuration (1), as in other ABC transporters, forming two ATP-binding pockets (ABPs) with two ATP molecules sandwiched in the interface. ABP1 is formed by the Walker A and B motifs of NBD1, and the signature sequence of NBD2 and ABP2 is formed by the Walker A and B motifs of NBD2 and the signature sequence of NBD1. Interestingly, two ABPs of CFTR assume distinct functional roles in controlling CFTR gating. Zhou et al. (2) showed that ABP2 is the site critical for channel opening by ATP, whereas the role of ABP1 is limited to help with the stabilization of the open channel conformation. Furthermore, although ABP1 seems unable to hydrolyze ATP, ATP hydrolysis at NBD2 is associated with the closing of the channel (3–5).
Loss-of-function mutations of CFTR result in the lethal genetic disease cystic fibrosis (CF) (6). CF is characterized by the absence of cAMP-stimulated Cl− current across a variety of epithelia, including the nasal epithelium (7), airway epithelia (8), pancreatic ducts (9), sweat glands (10), and the intestine (6). More than 1500 disease-associated mutations have been identified, and they can be grouped into at least four classes according to the different mechanisms by which they cause channel dysfunction: defective protein production, defective protein processing (for example ΔF508), defective activation and regulation (ΔF508, G551D), and defective conductance.
G551D, the glycine-to-aspartate missense mutation at position 551 in the signature sequence of NBD1 (ABP2), is the third most common CF-associated mutation. The mutant CFTR protein can traffic normally to the apical membrane and is normally phosphorylated by cAMP-dependent protein kinase (11–13), but it has an extremely low open probability (Po), ∼100 times smaller than that of WT (14). Although ATP fails to open G551D channels, the high affinity ATP analog N6-(2-phenylethyl)-ATP (P-ATP) can enhance the G551D-CFTR activity by ∼7-fold mainly by increasing the open time (15). Interestingly, 2′-deoxy-ATP (dATP), an ATP analog with a similar affinity to ATP, has been reported to increase the activity of G551D to a similar extent as P-ATP (16). Because the Po of G551D channels is ∼100-fold smaller than WT, a 7-fold increase of the Po by these ATP analogs is not expected to restore the activity of the mutant channel to normal levels (compare with Ref. 17).
The most common mutation associated with CF is the deletion of phenylalanine 508 (ΔF508). Several physiological defects have been demonstrated for the ΔF508-CFTR channel: First, most of the mutant proteins are retained in the endoplasmic reticulum and targeted for degradation (processing defect) (18, 19); second, the small fraction of the mutant channels that reached the plasma membrane responds poorly to cAMP stimulation (functional defect) (20–22) and third, the mutant protein is unstable in the plasma membrane (stability defect) (23, 24). The trafficking problem can be partially corrected experimentally by incubating the cells at a lower temperature (27 °C) (25) or in the presence of chemical chaperones (26). Several studies found that in cell-attached patches the maximal Po of ΔF508-CFTR was less than that of WT-CFTR, the difference being due to a prolonged closed time for ΔF508-CFTR (20–22). However, studies performed in cell-free systems, including excised inside-out patches and reconstituted lipid bilayers, quote very different maximal Po ranging from 0.1 to 0.4 for ΔF508-CFTR (17, 21, 27–31). It should be noted that these studies may be liable to quantitative errors due to technical difficulties in quantifying ΔF508-CFTR gating. Without a reliable method of estimating the number of functional channels, the Po as well as kinetic parameters cannot be calculated accurately. The use of ATP analogs that increase the activity of the channel may help resolve this issue.
In this study, we first confirmed the effects of dATP on G551D channels reported previously by Cai et al. (16). We show that dATP, similar to P-ATP, potentiates the activity of the channel mainly by increasing the open time of the channels. Interestingly, we found that 3′-deoxy-ATP has a much smaller effect on G551D-CFTR currents in comparison to dATP. Because the modifications present in P-ATP and dATP are located in different parts of the ATP molecule and likely interact with different amino acids in the binding pocket, we hypothesized that an ATP analog that contains both modifications could have an additive effect on G551D channels, resulting in a significantly higher increase of the activity. The analog N6-(2-phenylethyl)-2′-deoxy-ATP (P-dATP) was custom-synthesized. This novel analog has an extra ring added to the N6 position of the adenine ring and a hydroxyl group removed from the 2′ position of the ribose.
Indeed, P-dATP enhances the G551D-CFTR current by ∼36-fold mainly by prolonging the open time of the channel, with a small but significant increase in the opening rate. This remarkable increase in the activity G551D is achieved by P-dATP binding mainly to ABP1. This result corroborates not only our previous finding that the Po of G551D-CFTR is very small (14) but also suggests that the increase of Po necessary to rescue the defective G551D channel function is indeed achievable. P-dATP also enhances ΔF508-CFTR activity dramatically, mainly by increasing the opening rate of the channel. The magnitude (∼20-fold) of the effect of P-dATP on ΔF508-CFTR allowed reliable determination of the number of active channels present in a patch; the Po of ΔF508-CFTR channels can then be accurately measured. Thus P-dATP can serve as a tool to do rigorous kinetic analysis of CFTR mutants with low open probabilities.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis
All mutation constructs were generated using the QuikChange XL kit (Stratagene, La Jolla, CA) as described previously (14, 15). The entire CFTR cDNA was sequenced to confirm the mutation (DNA core, University of Missouri). The ΔNBD2-CFTR construct was a generous gift from Dr. K. Kirk (University of Alabama). In this construct amino acids from NBD2 and the C-terminal are deleted (32).
Cell Culture
Chinese hamster ovary (CHO) naïve cells and C127 cells stably expressing ΔF508-CFTR were grown at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. CHO cells were cultured in 35-mm tissue culture dishes 1 day before transfection. CFTR cDNA was cotransfected with pEGFP-C3 (Clontech, Palo Alto, CA) encoding green fluorescent protein using PolyFect transfection reagent (Qiagen) according to the manufacturer's protocols. Cells expressing ΔF508-CFTR were placed at 27 °C for at least 2 days to allow for the protein to traffic to the membrane.
Electrophysiological Recording and Data Analysis
All CFTR currents were recorded at room temperature with an EPC9 amplifier (HEKA, Lamberecht/Pfalz, Germany). The pipette resistance was 3–5 mΩ in the bath solution. For whole cell recordings, the membrane potential was held at 0 mV. A 200-ms voltage ramp (−100 mV to 100 mV) was applied every 5 s. Currents were filtered at 1 kHz with a built-in 4-pole Bessel filter and digitized to the computer at a sampling rate of 2 kHz. The bath solution contained (in mm) 145 NaCl, 5 KCl, 2 MgCl2, 1 CaCl2, 5 glucose, and 5 HEPES (pH 7.4 with NaOH), and 20 mm sucrose was added to the bath solution to prevent activation of swelling-induced currents. The pipette solution contained (in mm) 10 EGTA, 10 HEPES, 20 tetraethyl ammonium-chloride, 2 MgCl2, 10 MgATP (or 100 μm P-dATP), 5 glucose, and CsOH to adjust pH. For excised inside-out recordings, the membrane potential was held at −50 mV for all experiments. Data were filtered at 100 Hz with an eight-pole Bessel filter (Warner Instrument, Hammed, CT). The pipette solution contained (in mm) 140 N-methyl-d-glucamine chloride, 2 MgCl2, 5 CaCl2, and 10 HEPES (pH 7.4, with N-methyl-d-glucamine). After a tight seal was obtained, the patch was excised into a perfusion solution containing (in mm): 150 N-methyl-d-glucamine chloride, 10 EGTA, 10 HEPES, 8 Tris, and 2 MgCl2 (pH 7.4 with N-methyl-d-glucamine).
The steady-state mean currents were calculated with Igor (Wavemetrics, Lake Oswego, OR). Recordings with up to four channel open steps were further filtered off-line at 50 Hz with a digital filter and used for single channel kinetic analysis using a program developed by Dr. Csanády (33). Briefly, current traces were baseline-corrected, idealized, and fitted to a three-state model C ↔ O ↔ B, where O is the open state, C is the closed states, and B is a blocked state induced by an intrinsic blocker (34). The rate constants rCO, rOC, rOB, and rBO are extracted by a simultaneous fit to the dwell-time histograms of all conductance levels. The mean open time was calculated as To = (1/rOC)(1 + rOB/rBO), and the Po = 1/(1+ rOC/rCO + rOB/rBO). For channels with very low activity, the number of channels in the membrane patch could not be ascertained. Although the analysis program allows us to determine the mean open time of the channel (To) accurately, the opening rate (rCO) is very sensitive to the number of channels present in the patch. For G551D channels we used the maximum number of opening steps in the presence of P-dATP as the number of channels in the patch to determine the -fold increase of the opening rate induced by the ATP analogs (dATP, 3′-dATP, and P-dATP). We recognize that our analyses likely underestimate the number of channels present in the patch, resulting in an overestimation of the opening rate, and perhaps an underestimation of the -fold increase of the opening rate. Nevertheless, the general conclusions of the current work are not affected by these technical imperfections. In the case of ΔF508 channels, P-dATP completely rectifies the gating defect, so the number of channels in the presence of this analog can be accurately determined (see “Results”), and the opening rate and Po should be reliable.
Results are shown as mean ± S.E. Student's t-tests were performed using Excel (Microsoft). p < 0.01 was considered significant.
Reagents
Mg-ATP, 2′-deoxy-ATP, 3′-deoxy-ATP, chlorophenylthio-cAMP, and PKA were purchased from Sigma. (2′-3′)-dideoxy-ATP was purchased from USB (Cleveland, OH). Forskolin and genistein were purchased from Alexis (Plymouth Meeting, PA). P-ATP was purchased from Biolog Life Science Institute (Bremen, Germany) and P-dATP was custom-synthesized by the same institute.
RESULTS
G551D-CFTR Gating by 2′- and 3′-deoxy-ATP
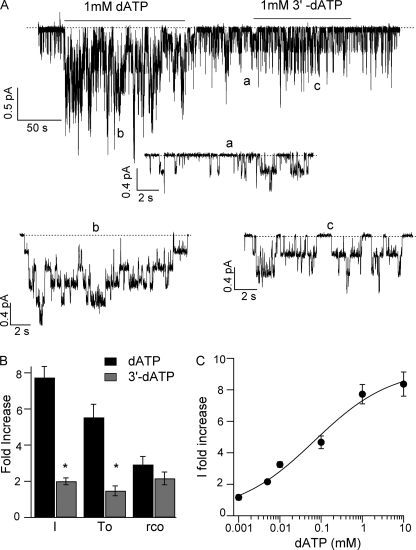
We transfected CHO cells with cDNA encoding G551D-CFTR, and performed patch-clamp experiments on the transfected cells in the inside-out mode to test the effect of dATP and 3′-deoxy-ATP (3-dATP). Fig. 1A shows the effect of 1 mm dATP and 1 mm 3-dATP on G551D-CFTR currents pre-activated with ATP plus PKA. Upon removal of ATP plus PKA, the current remains unchanged as reported previously (14, 15). Application of 1 mm dATP enhanced G551D-CFTR currents by 7.7 ± 0.6-fold (n = 12), and the potentiation effect was readily reversible when dATP was removed. In contrast, in the same patch, application of 1 mm 3-dATP increased the currents of G551D-CFTR only by 2.0 ± 0.2-fold (n = 6). Kinetic analysis for data obtained in patches with fewer channels show that the main effect of dATP is to increase the open time of the channels by 5.5 ± 0.7-fold (n = 12) (Fig. 1B). A small but significant increase in the opening rate was also observed (2.9 ± 0.5-fold, n = 12). These results with dATP are essentially the same as reported by Cai et al. (16). Interestingly, 3-dATP had very small effect on the open time of G551D-CFTR (1.5 ± 0.3-fold increase, n = 5), whereas the increase in the opening rate (2.2 ± 0.3, n = 5) was similar to the one induced by dATP. The ATP analog di-deoxy-ATP (both 2′- and 3′-hydroxyl groups removed) showed similar potentiation effect as 2′-deoxy-ATP (data not shown). Thus, the two hydroxyl groups on the ribose ring of ATP play different roles in the observed effects on G551D-CFTR. Of note, this result in G551D-CFTR is quite different from those reported by Aleksandrov et al. (35) for WT-CFTR, where they demonstrate little difference between the effect of 2′- and 3′-deoxy-ATP.
FIGURE 1.
Effect of dATP and 3-dATP on G551D-CFTR activity. A, a representative trace of G551D-CFTR from an inside-out patch showing the channel activity in the presence of 1 mm dATP or 1 mm 3-dATP. a, b, and c represent expanded traces of A, as marked. B, summary of the -fold increase in the mean current (I), the open time (To), and the opening rate (rCO) induced by 1 mm dATP (n = 12) and 1 mm 3-dATP (n = 5). *, p < 0.001 versus dATP. C, dATP dose-response relationship for G551D. Currents were activated with 1 mm ATP plus PKA, and the mean currents at different [dATP] were normalized to the current level in the absence of dATP (washout) (n = 5–12 for each data point).
Effects of P-dATP on G551D-CFTR
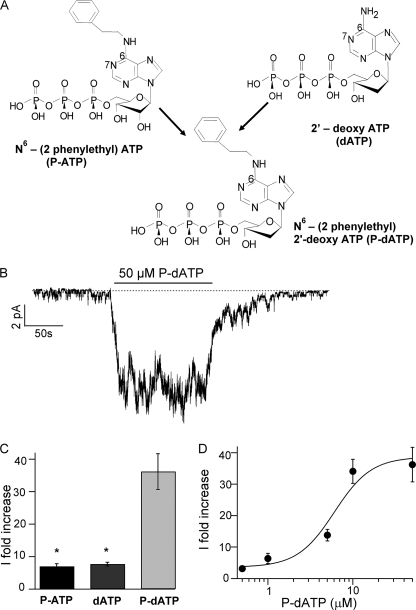
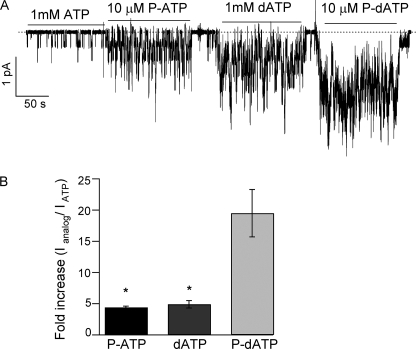
The two ATP analogs (P-ATP and dATP) that significantly increase the activity of G551D-CFTR have different chemical modifications (an extra ring for P-ATP and a hydroxyl group removed for dATP) in different parts of the ATP molecule (adenine ring and ribose, respectively). We reasoned that if these two different modifications act independently, an analog that has both modifications (Fig. 2A, an extra ring in the N6 position of the adenine ring and the hydroxyl group in the 2′ position of the ribose removed) may have a greater effect on G551D-CFTR currents. This new analog, like P-ATP, should also exhibit a high binding affinity with the added benzene ring. Indeed, Fig. 2B shows that at 50 μm P-dATP can enhance the G551D-CFTR current by 36.2 ± 5.4-fold (n = 9). Fig. 2C summarizes the magnitude of current increase by P-ATP, dATP, and P-dATP. The observation that the -fold increase by P-dATP is approximately the product of -fold increases by P-ATP and dATP suggests an energetic mechanism of an additive nature (see “Discussion”). The dose-response relationship for P-dATP (Fig. 2D) demonstrates that the apparent affinity for P-dATP is indeed similar to that of P-ATP on G551D (15). The effect of P-dATP in WT channels is shown in supplemental Fig. S1.
FIGURE 2.
Effect of P-dATP on G551D-CFTR activity. A, the ATP analog P-dATP was designed to include an extra ring in the 6 position of the adenine ring (same modification as P-ATP) and a hydroxyl group missing in the 2 position of the ribose. B, 50 μm P-dATP can increase G551D-CFTR currents by 36.2 ± 5.4-fold (n = 9). C, summary of the maximum -fold increase in G551D-CFTR activity by the ATP analogs P-ATP, dATP, and P-dATP. *, p < 0.0001 versus P-dATP. D, P-dATP dose-response relationship for G551D. Currents were activated with 1 mm ATP plus PKA, and the mean currents at different [P-dATP] were normalized to the current level in the absence of P-dATP (washout) (n = 5–17 for each data point).
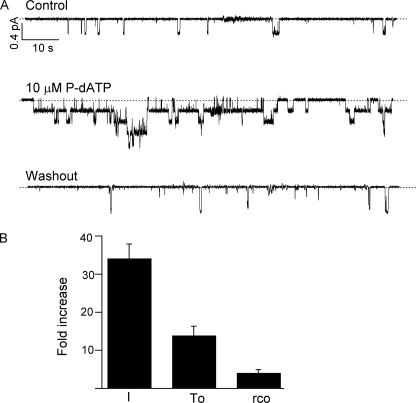
In patches showing very few sporadic openings, one can see the significantly prolonged openings of G551D-CFTR in the presence of 10 μm P-dATP (Fig. 3A). Kinetic analysis from patches with <5 channels (a difficult task given the huge -fold increase in the activity in response to P-dATP) reveals that the main effect of the new analog is to increase the open time of the channel by 13.9 ± 2.5-fold (n = 6), but a modest increase in the opening rate is also observed (4.1 ± 0.9-fold, n = 6).
FIGURE 3.
P-dATP-dependent gating of G551D-CFTR in excised patches. A, single-channel current traces for G551D-CFTR in the presence and absence of 10 μm P-dATP. B, summary of the -fold increase in the mean current (I), the open time (To), and the opening rate (rCO) for G551D-CFTR in the presence or absence of 10 μm P-dATP (n = 6).
Structural Nature of the Interaction between P-dATP and G551D-CFTR
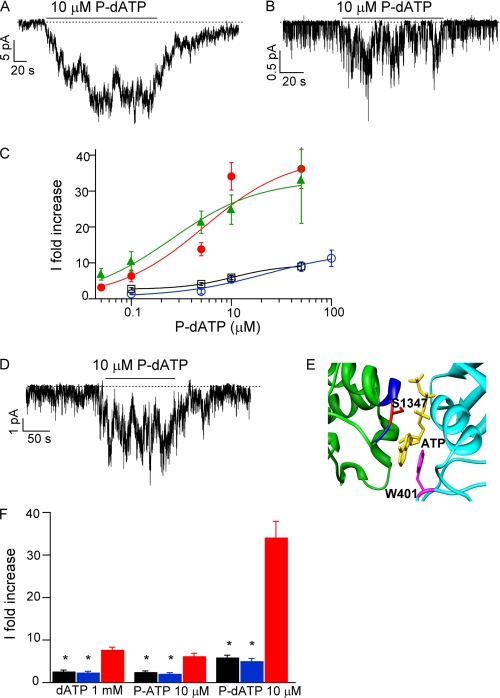
To further understand the effect of P-dATP on G551D channels, we made mutations that lower the apparent binding affinity of ATP in each ABP, W401G in ABP1, and Y1219G in ABP2 (2). Previously we have shown that P-ATP potentiates G551D-CFTR by binding to ABP1 (15). Because both P-ATP and P-dATP have similar binding affinity, and both analogs mainly increase the open time of the channel, we expect that P-dATP likely also acts mainly on ABP1. The dose-response relationships of P-dATP for G551D/Y1219G-CFTR (Fig. 4C) show that P-dATP increases the current of the G551D/Y1219G mutant to a somewhat similar extent as for G551D channels. On the other hand, the dose-response relationship of P-dATP for the W401G/G551D mutant (Fig. 4C) shows a significant rightward shift compared with that of G551D-CFTR accompanied with a considerable reduction of the maximal effect of P-dATP. These results suggest that P-dATP, like P-ATP, binds mainly to ABP1 to increase the current of G551D-CFTR channels.
FIGURE 4.
Potential interactions of ATP analogs with the binding pockets of G551D-CFTR. Representative current traces of G551D/Y1219G (A), W401G/G551D (B), and G551D/S1347G (D) in the presence of 10 μm P-dATP. C, P-dATP dose-response relationships for G551D (red, ●), W401G/G551D (blue, ○), G551D/Y1219G (green, ▴), and G551D/S1347G (black, ■). Currents were activated with 1 mm ATP plus PKA, and the mean currents at different [P-dATP] were normalized to the current level in the absence of P-dATP (washout) (n = 5–17 for each data point). E, CFTR dimer model depicting the location of two of the amino acids that interact with the ATP molecule (yellow) in ABP1 (39). Cyan represents part of NBD 1, green represents part of NBD2, and the signature sequence is depicted in blue. This figure was prepared using UCSF Chimera (48). F, comparison between the -fold increase in the current for G551D (red), W401G/G551D (blue), and G551D/S1347G (black) channels in the presence of saturating concentrations of dATP, P-ATP, and P-dATP. *, p < 0.001 versus G551D.
We next ask if the effect of P-dATP is mediated through an interaction of the ligand with the head of NBD1 as well as the tail of NBD2 (i.e. at the ABP1 dimer interface). It has been shown that CFTR channels completely missing NBD2 (i.e. ΔNBD2-CFTR) exhibit ATP-independent openings similar to those observed in G551D channels (32). Thus, the opening of the gate may not absolutely require the action of NBD2. In theory then the effect of all these nucleotides we have tested may be mediated through an interaction with the NBD1 part of ABP1 independently of NBD2.
If P-dATP interacts with both sides of ABP1 (head of NBD1 and tail of NBD2) to potentiate the activity of G551D-CFTR, we predict that introducing mutations that diminish the interaction of the nucleotide with the binding pocket will result in a decreased effect of P-dATP. In fact, we have already shown that the mutation of amino acid Trp-401 in the head of NBD1 decreases the effect of P-dATP in G551D channels (Fig. 4, B and C). As for nucleotide interaction with the tail of NBD2, a conserved serine in the signature sequence of NBD2 (Ser-1347 in CFTR) has been shown to interact with the γ-phosphate of ATP in several crystal structures of other ABC transporters (36–38). Fig. 4E shows the predicted positions of amino acids Ser-1347 and Trp-401 in a dimer model of the NBDs of CFTR (39). Mutation of the serine at position 1347 to glycine will likely diminish the interaction of the P-dATP molecule with the signature sequence in NBD2. Fig. 4D shows a representative trace for the mutant G551D/S1347G. 10 μm P-dATP can only potentiate the current by 5.9 ± 0.6-fold. In fact the mutation S1347G in NBD2 decreases the potentiation effect of P-dATP to a similar extent as the W401G mutation in NBD1 (Fig. 4C). The summary of the data for these mutants in the presence of all tested ATP analogs (P-ATP, dATP, and P-dATP) is shown in Fig. 4F. Our data indicate that the interaction of the nucleotides with both sides of ABP1 is responsible for the higher activity of G551D channels in the presence of the above mentioned ATP analogs (see “Discussion” for details). Corroborating this idea, the ATP-independent activity exhibited in ΔNBD2-CFTR channels is not affected by the presence of any of these analogs (not shown).
Effect of ATP Analogs on ΔF508-CFTR
The gating defect of ΔF508-CFTR channels is often overlooked, because of the severe trafficking defect caused by the mutation, which needs to be overcome before attempting to study the function of the channel. There are also contradicting reports regarding the actual Po of ΔF508-CFTR channels (17, 21, 27–30). It is important to note that, for a channel with very low Po, it is very difficult to obtain a quantitative understanding of the kinetic defects associated with ΔF508-CFTR when it is uncertain how many channels are present in a patch. An underestimation of the number of channels can easily result in an overestimation of the Po and the opening rate. The novel high affinity ATP analog P-dATP may serve as a tool to accurately determine the Po of ΔF508-CFTR channels, if it can considerably increase the channel activity, as observed for G551D channels. Fig. 5 shows the effect of the ATP analogs P-ATP, dATP, and P-dATP on ΔF508-CFTR channels transiently expressed in CHO cells. ΔF508-CFTR currents elicited by 1 mm ATP (previously activated by ATP plus PKA) can be further increased by P-ATP (10 μm), dATP (1 mm), and P-dATP (10 μm) by 4.4 ± 0.2-fold (n = 9), 4.9 ± 0.6-fold (n = 12), and 19.5 ± 3.8-fold (n = 13), respectively.
FIGURE 5.
Effect of different ATP analogs on ΔF508-CFTR activity. A, a representative trace of ΔF508-CFTR from an inside-out patch showing the channel activity in the presence of 1 mm ATP and then in the presence of saturating concentrations of P-ATP (10 μm), dATP (1 mm), and P-dATP (10 μm). B, summary of the maximum current -fold increase in ΔF508-CFTR activity by the ATP analogs P-ATP, dATP, and P-dATP, compared with 1 mm ATP. *, p < 0.001 versus P-dATP (n = 9–13).
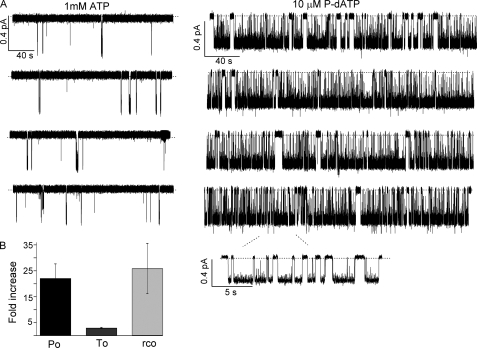
Fig. 6 shows a continuous trace representing the activity of one single ΔF508-CFTR channel in the presence of 1 mm ATP (16 min) and then in the presence of 10 μm P-dATP (30 min, not all shown). The high activity observed in the presence of the novel analog (Po = 0.77) assures that there is only one channel present in this patch, therefore the kinetic parameters obtained for this channel in the presence of ATP (To = 600 ms, rCO = 0.03 s−1, and Po = 0.02) should be reliable. Kinetic analysis obtained from 6 traces having less than 5 channels in the presence of 10 μm P-dATP indicates that the Po of the channels is increased mainly by increasing the opening rate by 25 ± 9-fold, reaching a value close to that of WT channels (1.3 ± 0.5 s−1), with a smaller increase of the open time (3.0 ± 0.2-fold, n = 6). Using the maximum number of opening steps in the presence of P-dATP as the number of functional channels in the patch, we can then calculate the Po of ΔF508-CFTR in the presence of 1 mm ATP. The average Po obtained from 6 patches is 0.031 ± 0.007, ∼15 times smaller than the Po of WT channels under the same conditions.
FIGURE 6.
P-dATP-dependent gating of ΔF508-CFTR in an excised patch. A, a continuous single-channel recording of ΔF508-CFTR activity in the presence of 1 mm ATP and in the presence of 10 μm P-dATP. B, summary of the -fold increase in the open probability (Po), the open time (To), and the opening rate (rCO) for ΔF508-CFTR induced by 10 μm P-dATP compared with 1 mm ATP (n = 6).
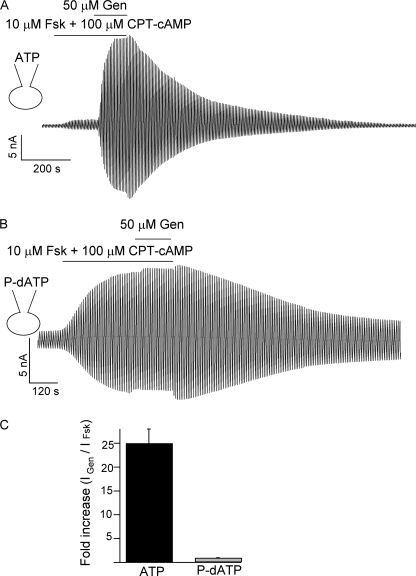
To confirm that P-dATP indeed completely rectifies the gating defect associated with ΔF508-CFTR, we carried out whole cell experiments with P-dATP in the pipette (thus in the cell). We reasoned that, if the Po of ΔF508-CFTR is indeed close to 0.8, in whole cell experiments, genistein, a well known CFTR potentiator, will no longer potentiate the cAMP-dependent ΔF508-CFTR currents. Instead of 10 mm ATP usually used in our whole cell experiments, we filled the pipette with a solution containing 100 μm P-dATP. Fig. 7 shows the striking difference in the effect of genistein. The currents from cells dialyzed with pipette solutions containing ATP can be enhanced by ∼20-fold with 50 μm genistein (Fig. 7A). On the other hand, when ATP was replaced by P-dATP inside the cell, genistein did not further increase the currents activated by forskolin plus chlorophenylthio-cAMP (Fig. 7B), corroborating the data in excised patches showing that P-dATP already maximizes the activity of ΔF508-CFTR.
FIGURE 7.
Effect of genistein on ΔF508-CFTR whole cell currents. A, a continuous whole cell current trace of ΔF508-CFTR channels activated by forskolin and chlorophenylthio-cAMP. The pipette solution contained 10 mm ATP. Addition of 50 μm genistein can further potentiate the channel activity. B, a continuous whole cell current trace of ΔF508-CFTR channels activated by forskolin and chlorophenylthio-cAMP. In this case, the ATP in the pipette solution was replaced by 100 μm P-dATP. Addition of 50 μm genistein did not further potentiate the activity of ΔF508-CFTR. C, summary of -fold increase in cAMP-stimulated currents in the presence of genistein (n = 3- 4). *, p < 0.005.
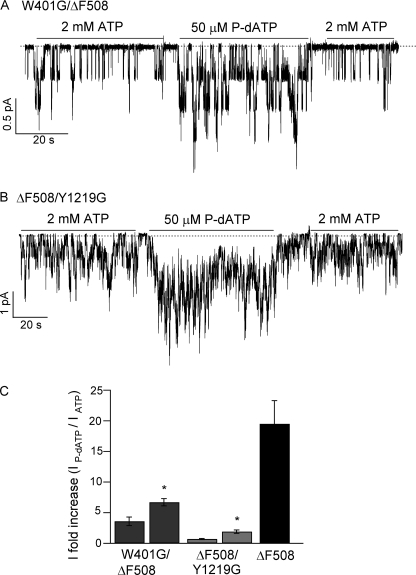
Although P-dATP greatly potentiated ΔF508 and G551D-CFTR channels, the effect of the analog on the behavior of the channel was different in both mutants. In the case of G551D-CFTR, P-dATP increased mainly the open time of the channels. The effect of P-dATP on ΔF508-CFTR was different, because it predominantly increased the opening rate of the channels, with a smaller increase in the open time. By introducing mutations that diminish the nucleotide binding affinity at both ABPs we found that for G551D channels P-dATP bound to ABP1 to increase the channel activity. Because P-dATP increased both the opening rate and the open time of ΔF508-CFTR, we predict that in this case both binding sites may be important. Indeed, introducing the mutation W401G (ABP1) or the corresponding mutation Y1219G (ABP2) in ΔF508-CFTR resulted in a reduction of the effect of 10 μm P-dATP (3.6 ± 0.7 and 0.7 ± 0.1 current -fold increase, respectively), suggesting that binding of P-dATP to both ABPs is involved in mediating the effect of P-dATP on ΔF508-CFTR channels (Fig. 8).
FIGURE 8.
P-dATP effect on W401G/ΔF508-CFTR and ΔF508/Y1219G-CFTR. Representative current traces of W401G/ΔF508 (A) and ΔF508/Y1219G (B) in the presence of 50 μm P-dATP. C, summary of the maximum current -fold increase in activity induced by 10 μm and 50 μm P-dATP in W401F/ΔF508 and ΔF508/Y1219G, and 10 μm P-dATP in ΔF508. *, p < 0.01 versus 10 μm P-dATP (n = 5–13).
DISCUSSION
In the current report, we show that the channel activity of G551D-CFTR and ΔF508-CFTR can be increased dramatically by a structurally defined ATP analog. Our result confirms our previous finding that the Po of G551D-CFTR is very small (14), because a 36-fold increase of the activity of G551D-CFTR can be accomplished with P-dATP (and thus reaching one-third of the maximal Po for WT-CFTR). We believe that a therapeutic goal can be achieved once a reagent with similar efficacy as P-dATP is developed (compare with Ref. 17). This novel ATP analog also effectively increases the Po of ΔF508-CFTR channels, mainly by increasing the opening rate, allowing for an accurate quantification of the gating defect associated with these mutant channels.
Our previous studies showed that the high affinity ATP analog P-ATP enhanced G551D currents by ∼7-fold (15). Cai et al. (16) reported that the ATP analog dATP also increased G551D currents by ∼10-fold (∼8-fold in this study). Although the application of ATP analogs for CF treatment was not feasible, because they lack the specificity required for a clinically usable drug, the knowledge obtained from the use of those ATP analogs provides valuable mechanistic information useful for drug design. For example, in the current study we show that, by using the information obtained for P-ATP and dATP, we were able to synthesize an analog that is more effective. The newly synthesized analog, combining the modifications of P-ATP (an extra ring in the adenine ring) and dATP (a hydroxyl group missing at the ribose) increases the activity of G551D-CFTR channels by ∼36-fold and of ΔF508-CFTR by ∼20-fold. This magnitude of current enhancement is similar to the product of the -fold increases by P-ATP and dATP separately. Transition-state theory states that the equilibrium distribution of two states of a protein is proportional to exp(−ΔG/RT), where ΔG is the change in free energy between the two states. When two compounds (in our case, one compound with two different chemical modifications) work independently, one expects a summation of the free energy changes when two reagents are used simultaneously. Because of the logarithmic relationship between free energy changes and the equilibrium constant, this sort of mechanism predicts that the net result is a multiplication of individual effects. We thus conclude that the mechanisms by which the added phenyl ring and the removal of 2′-hydroxyl group of ATP affect the channel gating are energetically additive.
As shown in Figs. 3 and 6, the main effect of P-dATP on G551D channels is to increase the open time of the channels, whereas for ΔF508 channels it is the increase in the opening rate. The explanation for this difference lies in the different mechanisms by which each mutation disrupts channel gating. Although the exact mechanisms are not known, we will interpret our results in the context of what is known about CFTR gating and then speculate on possible mechanisms of action.
Effect of P-dATP on G551D-CFTR
We have previously shown that the G551D mutation abolishes the ATP-dependent opening of the channel (14), a process that is mainly controlled by ABP2 (2), the site where the G551D mutation is located. All our previous data indicate that ABP2 is not functional in G551D channels. We have shown that ATP fails to open the channel and that other nucleotides that act at ABP2 (ADP and AMP-PNP) have no effect on G551D-CFTR (14). The reason for this is not known, but because the Gly-551 residue is in the signature motif, which by itself does not bind ATP, nucleotide binding to the head of NBD1 is unlikely to be affected by the G551D mutation. Most likely both the negative charge and the bulky side chain of the aspartate do not allow the ATP molecule to properly interact with the signature sequence in ABP2 when two NBDs coalesce to form a dimer, hence impairing the opening of the channel.
Our new results are consistent with our previous findings. In this study we show that P-dATP binds to ABP1 to potentiate the activity of G551D-CFTR, and this potentiation is mainly due to a 14-fold increase in the open time of the channels. Similar results were obtained previously for P-ATP (15). The stabilization of the open channel conformation through nucleotide binding to ABP1 has already been demonstrated for WT (2) and G551D channels (15), but the exact mechanism remains unclear. Some structural and functional studies do provide some clues. Crystallographic studies of NBD dimers of ABC proteins suggest that ATP serves as a molecular glue that connects the two NBDs together (36, 38, 40). On the other hand, functional studies of CFTR gating suggest that the open state of the channel represents a dimeric configuration of the two NBDs of CFTR (1, 41). Although for WT-CFTR, ATP hydrolysis may provide the free energy to break NBD dimer apart, it is important to note that, for G551D channels, hydrolysis does not play a role in the closing of the channels (42). Thus, one can envision that the stronger the glue (i.e. tighter binding of the nucleotide), the more stable the open state. In support of this notion, the mutation W401G, which likely weakens nucleotide binding to ABP1 (2), decreases the potentiation effect of P-dATP in G551D channels (Fig. 4C). Not only does one see a rightward shift of the dose-response relationship, a decrease of the maximal effect of ATP analogs is also observed as the strength of the “glue” is weakened. However appealing this proposition appears, it is difficult to use the tight-binding argument to explain the prolonged open time of G551D channels in the presence of dATP, because there is no obvious reason for dATP to assume tighter binding in ABP1. Indeed, contrary to P-ATP or P-dATP, millimolar dATP is required to potentiate G551D-CFTR. Nevertheless, the fact that 3′-deoxy-ATP, compared with 2′-deoxy-ATP, has a much smaller effect on G551D channels points to a very specific interaction between the ribose of the ATP analog with the amino acids in the binding site. More studies are needed to elucidate the molecular nature of this particular interaction.
As mentioned before, ATP fails to open G551D channels (14). The ATP analogs we studied here (dATP and P-dATP) also fail to effectively open G551D channels, because we only observe a moderate increase in the opening rate of the channels. In fact the mutation Y1219G in ABP2, which greatly decreases the apparent nucleotide-binding affinity at this site, barely diminishes the effect of P-dATP. This result indicates that this site is not responsible for most of the potentiation effect by P-dATP in G551D-CFTR. Nonetheless, we observed a small increase in the opening rate (∼4-fold for P-dATP). The exact mechanism for this increase is unclear, because the analogs we studied preserve the tri-phosphate moiety of ATP, which interacts with the region where the mutation is located. Based on the studies by Csanády et al. (43), who considered the energetics of the NBD dimerization process, we may speculate on a plausible answer. They showed that dimerization of the NBDs (thus channel opening) involves an increase in entropy that may be attributed to the removal of water molecules in the dimer interface. With an added hydrophobic phenyl ring and the removal of the hydrophilic hydroxyl group, P-dATP bound in the ATP-binding pockets may provide an energetic advantage for NBD dimerization. The presence of a more hydrophobic nucleotide may reduce the binding energy of the water molecules interacting with the NBDs, which are expelled upon dimerization, resulting in a smaller ΔG for this process. Thus, one can envision that, if the opening of G551D-CFTR also involves NBD dimerization, a more hydrophobic ligand in the binding site(s) may facilitate dimerization of the two NBDs. In support of this notion, the maximal opening rate of WT-CFTR with P-ATP is slightly higher than that of ATP (44, 45).
In this report we also showed that the potentiation effect of the ATP analogs (P-ATP, dATP, and P-dATP) on G551D-CFTR depends on the interaction of the nucleotide with the head of NBD1 and the signature sequence of NBD2, indicating that the analogs act at the NBD1-NBD2 interface. Although we do not know what happens at ABP2, our result suggests that some sort of NBD dimerization is involved in the gating of this mutant. More importantly, it opens the door for structure-based drug design using the NBD1-NBD2 interface as a molecular target.
Effect of P-dATP in ΔF508-CFTR
The dramatic effect of P-dATP on the G551D mutant compelled us to test its effect on other low Po mutants such as ΔF508-CFTR, the most common CF-causing mutation. The ΔF508-CFTR channels exhibit several physiological abnormalities; one of them is the low Po observed in intact cells due to a reduced opening rate (20, 22). It was suggested that this low opening rate was due to a very slow phosphorylation rate (30) and not to an intrinsic defect in the gating mechanism. In this study we show that P-dATP increases the opening rate of ΔF508-CFTR channels more than 20-fold in excised patches, whereas the open time increases by ∼3-fold. When patches activated with ATP were exposed to any of the ATP analogs studied, P-ATP, dATP, or P-dATP, more channel-opening steps were seen, indicating that ATP is not efficacious enough to yield an opening rate that is the same as that of WT-CFTR, even at saturating [ATP]. This finding is somehow puzzling, because Phe-508 is not believed to be located near the NBD dimer interface (46), where the ATP analogs bind. The effect of P-dATP on ΔF508 channels is mediated by nucleotide binding to both binding pockets, because mutations that lower the binding affinity at either ABP decrease the effect of P-dATP. The decrease in the effect of P-dATP in ΔF508 channels that contain the Y1219G mutation is easier to understand, because this mutation decreases the ATP-binding affinity at ABP2, the site that controls the ATP-dependent opening of the channel, by >50-fold. On the other hand, it was somewhat surprising to observe a reduction of the effect of P-dATP on W401G/ΔF508, because the W401G mutation alone does not affect much the effect of P-dATP on WT-CFTR (see supplemental information). This result may suggest that perhaps the ΔF508 mutation also disturbs the function of ABP1, an issue awaiting more extensive studies. Nevertheless, if that turns out to be the case, one needs to dwell on the possibility of dysfunction at the NBD dimer interface caused by the ΔF508 mutation.
We should note that Serohijos et al. (47) reported that interactions between the Phe-508 position and several amino acids in the fourth intracellular loop are presumably involved in mediating the signal transduction from the NBDs to the gate. Then, in this context, our findings suggest that binding of P-dATP at the NBD dimer interface allosterically affects post-dimerization events. Certainly more extensive studies are needed to elucidate the mechanism underlying the kinetic abnormalities caused by the ΔF508 mutation, but the observation that P-dATP can completely remedy this dysfunction does serve as a first step toward that ultimate goal.
Finally, our results also bear some pharmacological implications for the treatment of cystic fibrosis. The magnitude of the current increase by P-dATP is perhaps the largest increase reported for G551D-CFTR channels so far. P-dATP also helps overcome the ΔF508-CFTR gating defect. Although ATP analogs are not suitable to be used as drugs, our finding could be important for in silico drug development. Conceptually, it can be used as a proof of principle: by combining elements that can potentiate the channel activity independently, the increase in chloride transport necessary to reach a therapeutic target is attainable. Practically, the location where P-dATP binds to increase the activity of the channel could serve as a molecular target for designing therapeutic reagents to improve the function of CF-associated mutant channels.
Supplementary Material
Acknowledgment
We thank Dr. Shengyou Huang for providing the CFTR NBD dimer model figure.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK55835, R01HL53455 (to T.-C. H.), and K01DK075408 (to S. G. B.). This work was also supported by the Cystic Fibrosis Foundation (Research Grant BOMPAD06G0 to S. G. B.). This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program (Grant C06 RR-016489-01) from the National Center for Research Resources, NIH.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- CFTR
- cystic fibrosis transmembrane conductance regulator
- ABC
- ATP-binding cassette
- NBD
- nucleotide-binding domain
- PKA
- protein kinase A
- ABP
- ATP-binding pocket
- CF
- cystic fibrosis
- WT
- wild type
- P-ATP
- N6-(2-phenylethyl)-ATP
- dATP
- 2′-deoxy-ATP
- P-dATP
- N6-(2-phenylethyl)-2′-deoxy-ATP
- CHO
- Chinese hamster ovary
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
REFERENCES
- 1.Mense M., Vergani P., White D. M., Altberg G., Nairn A. C., Gadsby D. C. (2006) EMBO J. 25, 4728–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Z., Wang X., Liu H. Y., Zou X., Li M., Hwang T. C. (2006) J. Gen. Physiol. 128, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powe A. C., Jr., Al-Nakkash L., Li M., Hwang T. C. (2002) J. Physiol. 539, 333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bompadre S. G., Cho J. H., Wang X., Zou X., Sohma Y., Li M., Hwang T. C. (2005) J. Gen. Physiol. 125, 377–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksandrov L., Aleksandrov A. A., Chang X. B., Riordan J. R. (2002) J. Biol. Chem. 277, 15419–15425 [DOI] [PubMed] [Google Scholar]
- 6.Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. (1989) Science 245, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 7.Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. (1983) Science 221, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 8.Welsh M. J., Liedtke C. M. (1986) Nature 332, 467–470 [DOI] [PubMed] [Google Scholar]
- 9.Gray M. A., Greenwell J. R., Argent B. E. (1988) J. Membr. Biol. 105, 131–142 [DOI] [PubMed] [Google Scholar]
- 10.Quinton P. M. (1986) Am. J. Physiol. 251, C649–652 [DOI] [PubMed] [Google Scholar]
- 11.Cutting G. R., Kasch L. M., Rosenstein B. J., Zielenski J., Tsui L. C., Antonarakis S. E., Kazazian H. H., Jr. (1990) Nature 346, 366–369 [DOI] [PubMed] [Google Scholar]
- 12.Chang X. B., Tabcharani J. A., Hou Y. X., Jensen T. J., Kartner N., Alon N., Hanrahan J. W., Riordan J. R. (1993) J. Biol. Chem. 268, 11304–11311 [PubMed] [Google Scholar]
- 13.Welsh M. J., Smith A. E. (1993) Cell 73, 1251–1254 [DOI] [PubMed] [Google Scholar]
- 14.Bompadre S. G., Sohma Y., Li M., Hwang T. C. (2007) J. Gen. Physiol. 129, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bompadre S. G., Li M., Hwang T. C. (2008) J. Biol. Chem. 283, 5364–5369 [DOI] [PubMed] [Google Scholar]
- 16.Cai Z., Taddei A., Sheppard D. N. (2006) J. Biol. Chem. 281, 1970–1977 [DOI] [PubMed] [Google Scholar]
- 17.Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng S. H., Gregory R. J., Marshall J., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Cell 65 [DOI] [PubMed] [Google Scholar]
- 19.Ward C. L., Omura S., Kopito R. R. (1995) Cell 83, 121–127 [DOI] [PubMed] [Google Scholar]
- 20.Hwang T. C., Wang F., Yang I. C., Reenstra W. W. (1997) Am. J. Physiol. 273, C988–C998 [DOI] [PubMed] [Google Scholar]
- 21.Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. (1991) Nature 354, 526–528 [DOI] [PubMed] [Google Scholar]
- 22.Haws C. M., Neponuceno I. B., Krouse M. E., Wakelee H., Law T., Xia Y., Nguyen H., Wine J. J. (1996) Am. J. Physiol. 270, C1544–C1555 [DOI] [PubMed] [Google Scholar]
- 23.Lukacs G. L., Chang X. B., Bear C. E., Kartner N., Mohamed A., Riordan J. R., Grinstein S. (1993) J. Biol. Chem. 268, 21592–21598 [PubMed] [Google Scholar]
- 24.Gentzsch M., Chang X. B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E., Riordan J. R. (2004) Mol. Biol. Cell 15, 2684–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denning G. M., Ostedgaard L. S., Welsh M. J. (1992) J. Cell Biol. 118, 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral M. D., Kunzelmann K. (2007) Trends Pharmacol. Sci. 28, 334–341 [DOI] [PubMed] [Google Scholar]
- 27.Ostedgaard L. S., Rogers C. S., Dong Q., Randak C. O., Vermeer D. W., Rokhlina T., Karp P. H., Welsh M. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15370–15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. (1992) Nature 358, 761–764 [DOI] [PubMed] [Google Scholar]
- 29.Schultz B. D., Frizzell R. A., Bridges R. J. (1999) J. Membrane Biol. 170, 51–66 [DOI] [PubMed] [Google Scholar]
- 30.Wang F., Zeltwanger S., Hu S., Hwang T. C. (2000) J. Physiol. 524, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Ramjeesingh M., Reyes E., Jensen T., Chang X., Rommens J. M., Bear C. E. (1993) Nat. Genet. 3, 311–316 [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Bernard K., Li G., Kirk K. L. (2007) J. Biol. Chem. 282, 4533–4544 [DOI] [PubMed] [Google Scholar]
- 33.Csanády L. (2000) Biophys. J. 78, 785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Hu S., Hwang T. C. (2001) J. Physiol. 532, 435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleksandrov A. A., Aleksandrov L., Riordan J. R. (2002) FEBS Lett. 518, 183–188 [DOI] [PubMed] [Google Scholar]
- 36.Smith P. C., Karpowich N., Millen L., Moody J. E., Rosen J., Thomas P. J., Hunt J. F. (2002) Mol. Cell 10, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu G., Westbrooks J. M., Davidson A. L., Chen J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17969–17974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Lu G., Lin J., Davidson A. L., Quiocho F. A. (2003) Mol. Cell 12, 651–661 [DOI] [PubMed] [Google Scholar]
- 39.Huang S. Y., Bolser D., Liu H. Y., Hwang T. C., Zou X. (2009) J. Mol. Graphics Model. 27, 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaitseva J., Jenewein S., Jumpertz T., Holland I. B., Schmitt L. (2005) EMBO J. 24, 1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vergani P., Lockless S. W., Nairn A. C., Gadsby D. C. (2005) Nature 433, 876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramjeesingh M., Ugwu F., Stratford F. L., Huan L. J., Li C., Bear C. E. (2008) Biochem. J. 412, 315–321 [DOI] [PubMed] [Google Scholar]
- 43.Csanády L., Nairn A. C., Gadsby D. C. (2006) J. Gen. Physiol. 128, 523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z., Wang X., Li M., Sohma Y., Zou X., Hwang T. C. (2005) J. Physiol. 569, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai M. F., Li M., Hwang T. C. (2010) J. Gen. Physiol. 135, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis H. A., Zhao X., Wang C., Sauder J. M., Rooney I., Noland B. W., Lorimer D., Kearins M. C., Conners K., Condon B., Maloney P. C., Guggino W. B., Hunt J. F., Emtage S. (2005) J. Biol. Chem. 280, 1346–1353 [DOI] [PubMed] [Google Scholar]
- 47.Serohijos A. W., Hegedus T., Aleksandrov A. A., He L., Cui L., Dokholyan N. V., Riordan J. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3256–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.