Abstract
The Toll-IL-1 receptor (TIR) domain-containing adaptor molecule-1 (TICAM-1, also called TRIF) is a signaling adaptor for TLR3 and TLR4 that activates the transcription factors IRF-3, NF-κB, and AP-1, leading to induction of type I interferon and cytokines. The N-terminal region of TICAM-1 participates in IRF-3 activation, although the C-terminal region is involved in NF-κB activation. However, the mechanism by which TICAM-1 is activated and transmits signals is largely unknown. In this study, we identified Leu194 as a critical amino acid for TICAM-1-mediated IRF-3 activation. When Leu194 was substituted with Ala, the mutant TICAM-1 failed to recruit the IRF-3 kinase TBK1, resulting in lack of IRF-3 phosphorylation, although TRAF3 and NAP1 appeared to be recruited. The N-terminal 176 amino acids of TICAM-1 (N-terminal domain (NTD)) form a protease-resistant structural domain. A TICAM-1 mutant lacking the N-terminal 180 amino acids showed greater interferon-β promoter activation than wild-type TICAM-1. Furthermore, immunoprecipitation and protein-protein interaction analysis revealed that the NTD interacted with the N terminus of TICAM-1-TIR. These results suggest that the NTD folds into the TIR domain structure to maintain the naive conformation of TICAM-1. Upon stimulation of TLR3/4, TICAM-1 oligomerizes through the TIR domain and the C-terminal region, which may break the intramolecular association and induce a conformational change that allows TBK1 access to TICAM-1.
Keywords: Adaptor Proteins, Innate Immunity, Interferon, Signal Transduction, Toll-like Receptors (TLR)
Introduction
The innate immune system senses microbial infection using Toll-like receptors and cytoplasmic pattern-recognition receptors, which rapidly induce an antimicrobial response (1). Recent studies have demonstrated that these receptors also recognize molecular patterns associated with tissue damage and induce cytokine and chemokine production, which may lead to a sterile inflammation and progression of autoimmune diseases (2). Downstream of each pattern-recognition receptor is a signaling adaptor protein that determines the nature of response by activating distinct transcription factors (3). The Toll-IL-1 receptor (TIR)3 domain-containing adaptor molecule-1 (TICAM-1), which is also known as TIR domain-containing adaptor inducing IFN-β (TRIF), is a signaling adaptor for TLR3 and TLR4 that activates the transcription factors IRF-3, NF-κB, and AP-1, leading to induction of type I IFN and cytokines, as well as myeloid dendritic cell (mDC) maturation (4–7). The final response mediated by TICAM-1 depends on the cell type and the activating TLR3/4 ligand.
Poly(I-C) is a synthetic analog of viral double strand RNA that generates a unique maturation stage in mDCs via TLR3-TICAM-1-dependent gene expression, leading to activation of natural killer cells and cytotoxic T lymphocytes (8, 9). Monophosphoryl lipid A is a TLR4 ligand that activates mDCs to induce T cell immunity via TICAM-1 but not MyD88 (10), implying that TICAM-1 signaling is crucial for inducing effective cellular responses. In contrast, oxidized phospholipids generated by oxidative stress or virus infection activate cytokine production in lung macrophages through the TLR4-TICAM-1 pathway, and this is the key disease pathway in acute lung injury (11). Thus, specific stimuli have been discovered, but the mechanism by which TICAM-1 is activated by upstream pattern-recognition receptors and transmits downstream signals is largely unknown.
TICAM-1 is expressed at a low level in most tissues and cells and is diffusely localized in the cytoplasm of resting cells (4, 12). When endosomal TLR3 is activated by double strand RNA, TICAM-1 transiently colocalizes with TLR3 and then dissociates from the receptor and forms speckled structures that colocalize with downstream signaling molecules (12, 13). Upon lipopolysaccharide stimulation, TICAM-1 is activated by endosomal TICAM-2 (also called TRIF-related adaptor molecule), which associates with the internalized TLR4 (14). Forced expression of TICAM-1 leads to homo-oligomerization through the TIR domain and the C terminus, forming a complex called the TICAM-1 signalosome (15). The TIR domain of TICAM-1 is essential for binding to the TIR domain of TLR3 and to TICAM-2. The N-terminal region of TICAM-1 participates in IRF-3 activation by recruiting the IRF-3-activating kinases, TANK-binding kinase 1 (TBK1) and inhibitor of nuclear factor κB kinase ϵ (also called IKKι) (16–18). The C-terminal region of TICAM-1 is involved in NF-κB activation and inducing apoptosis by binding the RIP1 at the receptor-interacting protein homotypic interacting motif (RHIM) domain (19, 20). The tumor necrosis factor receptor-associated factor (TRAF) 3 and NF-κB-activating kinase-associated protein 1 (NAP1) engage in TICAM-1-mediated activation of IRF-3 (21–23). Thus, although molecules involved in the TICAM-1-mediated signaling have been identified, the molecular mechanism for TICAM-1 activation remains unknown.
Recently, the N-terminal 176 amino acids (aa) of TICAM-1 (NTD) were found to form a protease-resistant structure of eight α-helices (24). In this study, we analyzed the structure-function relationship of TICAM-1 with a series of TICAM-1 mutants and identified the critical amino acid for TICAM-1-mediated IRF-3 activation. Moreover, we offer a structural model of the resting form of TICAM-1, in which NTD folds into the TIR domain structure, preventing homodimerization and access of downstream signaling molecules to TICAM-1.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
HEK293 cells were maintained in Dulbecco's modified Eagle's medium low glucose (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (BIOSOURCE) and antibiotics. HEK293FT cells were maintained in Dulbecco's modified Eagle's medium high glucose supplemented with 0.1 mm nonessential amino acids, 10% heat-inactivated fetal calf serum, and antibiotics. HeLa cells were maintained in minimum essential media (Nissui, Tokyo, Japan) supplemented with 1% l-glutamine and 5% heat-inactivated fetal calf serum. Anti-FLAG M2 mAb, anti-HA pAb, 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI), and benzyloxycarbonyl-VAD-fluoromethyl ketone were from Sigma. Alexa Fluor®-conjugated secondary antibodies were from Invitrogen; anti-Myc mAb was from Neomarkers (Lab Vision Corp., Fremont, CA); anti-HA mAb was from Covance (Emeryville, CA); anti-TBK1 pAb was from Abcam (Cambridge, MA); anti-human IRF3 rabbit IgG was from IBL (Gunma, Japan); horseradish peroxidase-conjugated secondary Abs were from BIOSOURCE; and poly(I-C) was from GE Healthcare.
Plasmids
Complementary DNAs for human TICAM-1, RIP1, TRAF2, TRAF3, and TRAF6 were cloned in our laboratory by reverse transcription-PCR and ligated into the cloning site of the expression vectors, pEF-BOS and p3×FLAG-CMV-14 (C-terminal 3×FLAG tag) (15). The pCDNA3.1/NAP1-Myc and pCDNA3.1/TBK1-FLAG expression vectors were kindly provided by Dr. M. Nakanishi (Nagoya City University, Nagoya, Japan). N-terminal deletion mutants of TICAM-1 (Δ160, Δ170, Δ180, Δ190, Δ200, and Δ210) were made by PCR with Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA) using appropriate primers. pEF-BOS/TICAM-1-HA was used as a PCR template (33). Point mutations in TICAM-1 were generated by site-directed mutagenesis. Truncated TICAM-1 mutants TICAM-1-NTD (1–176 aa), TICAM-1-N (1–359 aa), TICAM-1-TIR (387–556 aa), and TICAM-1-C (534–712 aa) were generated by PCR using specific primers. An HA tag was inserted at the C terminus of each mutant.
Reporter Gene Assays
HEK293 cells (2 × 105 cells/well) cultured in 24-well plates were transfected with expression vectors for wild-type TICAM-1, TICAM-1 mutants, or empty vector, together with the reporter plasmid (100 ng/well) and an internal control vector, phRL-TK (Promega, Madison, WI) (5 ng/well) using FuGENE HD (Roche Diagnostics). The p-125 luciferase reporter contained the human IFN-β promoter (−125 to +19) and was provided by Dr. T. Taniguchi (University of Tokyo, Tokyo, Japan). A luciferase-linked NF-κB reporter gene and pAP-1-Luc reporter plasmid were from Stratagene. The total amount of DNA (500 ng/well) was kept constant by adding empty vector. After 24 h, cells were lysed in lysis buffer (Promega), and firefly and Renilla luciferase activities were determined using a Dual-Luciferase reporter assay kit (Promega). The firefly luciferase activity was normalized to the Renilla activity and expressed as the fold-stimulation relative to the activity of vector-transfected cells. All assays were performed in triplicate.
Confocal Microscopy
HeLa cells (1.0 × 105 cells/well) were plated onto micro cover glasses (Matsunami, Tokyo, Japan) in 12-well plates. The next day, cells were transfected with the indicated plasmids as above. For cells transfected with wild-type TICAM-1 or the L194A mutant, benzyloxycarbonyl-VAD-fluoromethyl ketone (20 μm) was added to the cells before transfection to inhibit apoptosis. 24 h after transfection, cells were fixed in acetone for 3 min and permeabilized with PBS containing 0.2% Triton X-100 for 10 min. Fixed cells were blocked in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and labeled with the indicated primary Abs (3.0 μg/ml) for 60 min at room temperature. For staining endogenous TBK1, cells were fixed with 4% paraformaldehyde for 10 min. Endogenous TBK1 was labeled with anti-TBK1 pAb (1:500). Alexa Fluor 488- or 594-conjugated secondary Abs (1:400) were used to visualize the primary Abs. Nuclei were stained with DAPI (2 μg/ml) in PBS for 10 min before mounting onto glass slides using PBS with 2.3% 1,4-diazabicyclo[2.2.2]octane and 50% glycerol. Cells were visualized at a 63× magnification with an LSM510 META microscope (Zeiss, Jena, Germany).
Immunoprecipitation and Immunoblotting
HEK293FT cells (5 × 105 cells/well) cultured in 6-well plates were transfected as above with the indicated plasmids. For wild-type TICAM-1 and the TICAM-1 mutants containing the RHIM domain, benzyloxycarbonyl-VAD-fluoromethyl ketone (20 μm) was added as described above. The total amount of DNA (2.0 μg/well) was kept constant with an empty vector. After 24 h, cells were lysed in lysis buffer (20 mm Tris-HCl, pH 7.5, containing 150 mm NaCl, 1% Nonidet P-40, 10 mm EDTA, 25 mm iodoacetamide, 2 mm phenylmethylsulfonyl fluoride, 5 mm Na3VO4 and a protease inhibitor mixture (Roche Diagnostics)). Lysates were pre-cleared with protein G-Sepharose (GE Healthcare) and incubated with 0.5 μg of anti-tag Abs or anti-TBK1 pAb (1:50). Immunocomplexes were recovered by incubation with protein G-Sepharose, washed five times with lysis buffer, and resuspended in denaturing buffer. Samples were analyzed by SDS-PAGE (7.5–12.5% gel) under reducing conditions followed by immunoblotting with anti-tag Abs. For immunoblotting, HEK293 cells cultured in 24-well plates were transfected with the indicated plasmids (100 ng). After 24 h, cells were lysed in lysis buffer. Lysates were clarified by centrifugation and subjected to SDS-PAGE (7.5% gel) followed by immunoblotting with anti-HA pAb.
Assay for IRF-3 Activation
HEK293 cells (2 × 105 cells/well) cultured in 24-well plates were transfected with wild-type TICAM-1 or TICAM-1 L194A mutant (0.1 and 0.4 μg) using Lipofectamine 2000 reagent (Invitrogen). The total amount of DNA (0.8 μg/well) was kept constant with an empty vector. After 24 h, cells were lysed in lysis buffer (50 mm Tris-HCl, pH 8.0, containing 150 mm NaCl, 1% Nonidet P-40, 100 ng/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, and 5 mm Na3VO4). Lysates were clarified by centrifugation (15,000 rpm, 10 min) and subjected to native-PAGE (7.5% gel) as described previously (34). Immunoblotting was performed using rabbit anti-human IRF-3 antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG.
Protein-Protein Interaction Analysis
Protein-protein association in living cells was analyzed using the CoralHue Fluo-chase kit (MBL, Nagoya, Japan), which detects protein-protein interactions as fluorescent signals using the protein fragment complementation method. TICAM-1 NTD and TICAM-1-TIR cDNAs were subcloned into fusion protein expression plasmids according to the manufacturer's instructions. The TICAM-1 NTD gene was fused to the 3′-end of the divided monomeric Kusabira-Green (mKG) gene N- or C-terminal fragment (mKGN-NTD and mKGC-NTD), and the TICAM-1-TIR gene was fused to the 5′- or 3′-end of the mKG gene N- or C-terminal fragment (TIR-mKGN, TIR-mKGC, mKGN-TIR, and mKGC-TIR). HEK293FT cells (5 × 105 cells/well) cultured in 6-well plates were transfected with the indicated combinations of plasmids as above. After 24 h, the conditioned media were replaced with Dulbecco's PBS, and fluorescent living cells were visualized with fluorescent microscopy (Olympus).
RESULTS
TICAM-1 N Terminus from Gly191 to Pro200 Is Essential for IFN-β Promoter Activation
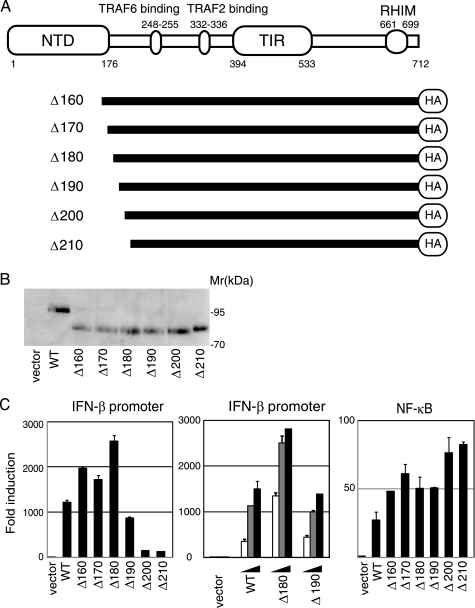
The N terminus of TICAM-1 contains TRAF6- and TRAF2-binding sites (Fig. 1A). Limited trypsin digestion of TICAM-1 (1–556 aa) for 4 h generates the N-terminal fragment TICAM-1(1–176), and 20 h of digestion yields the fragment TICAM-1(1–156) (24). To examine the role of the TICAM-1 N-terminal domain, we made a series of truncated constructs (Δ160–Δ210) and assayed NF-κB and IRF-3 activation by reporter assay. The protein expression levels of these mutants were approximately equivalent to wild type (Fig. 1B). TICAM-1-mediated IFN-β promoter activation was enhanced by deletion of the N-terminal 160, 170, or 180 aa, whereas the TICAM-1-mutant lacking the N-terminal 190 aa (Δ190) showed less activity than wild-type TICAM-1 (Fig. 1C, left and center panels). Interestingly, a TICAM-1 mutant lacking the N-terminal 200 or 210 aa (Δ200, Δ210) showed dramatic loss of activity. NF-κB activation ability, however, was enhanced in all the mutants (Fig. 1C, right panel). These results indicated that the N-terminal amino acids between Gly191 and Pro200 are essential for TICAM-1-mediated IFN-β promoter activation but not for NF-κB activation.
FIGURE 1.
TICAM-1 N-terminal region (191–200 aa) is essential for activation of the IFN-β promoter but not NF-κB. A, schematic structure of human TICAM-1/TRIF and TICAM-1 mutants. B, protein expression of truncated TICAM-1 mutants in HEK293 cells. HEK293 cells in 24-well plates were transfected with the expression plasmids for HA-tagged wild-type TICAM-1 or TICAM-1 mutants (100 ng). Protein expression levels were determined by immunoblotting using anti-HA pAb. C, functional analysis of TICAM-1 mutants. HEK293 cells were transfected with empty vector or expression plasmid for wild-type (WT) TICAM-1 or each TICAM-1 mutant (Δ160–Δ210) (100 ng) together with the IFN-β promoter reporter (left panel) or NF-κB reporter plasmid (right panel), and phRL-TK. In some experiments, HEK293 cells were transfected with increasing amounts of expression vectors (10, 50, and 100 ng) (center panel). Luciferase activity was measured 24 h after transfection. Representative data from a minimum of four separate experiments, each performed in triplicate, are shown.
Leu194 Is Critical for TICAM-1-mediated IFN-β Promoter Activation
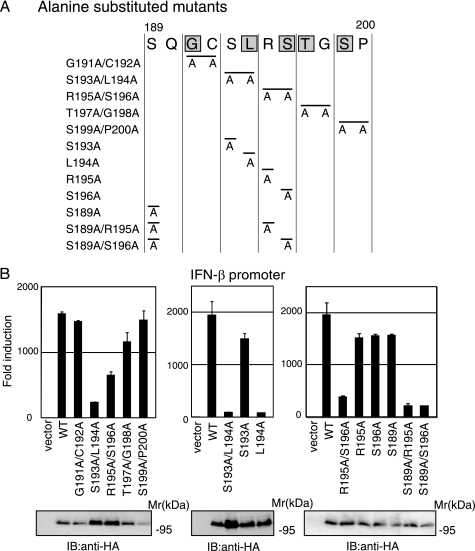
To identify residues crucial for TICAM-1-mediated IFN-β promoter activation, we performed alanine scanning of the region between Gly191 and Pro200 (Fig. 2A). Alanine-substituted mutants were expressed in HEK293 cells with an IFN-β reporter gene, and IFN-β promoter activation was assessed after 24 h. Western blot showed that the mutated proteins were expressed at levels similar to wild-type TICAM-1 in HEK293 cells (Fig. 2B, lower panels). Similar to the Δ200 mutant, the S193A/L194A mutant showed no activity, and the R195A/S196A mutant had partially diminished activity (Fig. 2B, left panel). Other mutations had no effect on the ability to activate the IFN-β promoter. Because substitution of Ser193 and Ser196 caused dysfunctional mutants, we made the additional single aa-substituted mutants S193A, L194A, R195A, S196A, and S189A and the combinations S189A/R195A and S189A/S196A, and we examined the role of these residues in TICAM-1 signaling (Fig. 2A). Remarkably, the substitution of Leu194 with Ala completely abolished IFN-β promoter-activation ability, whereas other single aa substitutions only slightly decreased activity. Both S189A/R195A and S189A/S196A mutants showed severely reduced activity (Fig. 2B, center and right panels). These results suggested that Leu194 is a critical amino acid for TICAM-1-mediated IFN-β promoter activation and that Ser189, Arg195, and Ser196 near Leu194 are associated with this promoter activation.
FIGURE 2.
Leu194 is critical for TICAM-1-mediated IFN-β promoter activation. A, amino acid sequence between Ser189 and Pro200 in TICAM-1. Gray indicates conserved residues between human and mouse. B, IFN-β promoter activation by alanine-substituted TICAM-1 mutants. HEK293 cells were transfected with empty vector or expression vectors for wild-type (WT) TICAM-1 or indicated alanine-substituted mutants, with the IFN-β promoter reporter and phRL-TK. Luciferase activity was measured 24 h after transfection. Representative data from a minimum of three separate experiments, each performed in triplicate, are shown. Lower panels, protein expression of alanine-substituted mutants in HEK293 cells. Cell lysates prepared in A were subjected to SDS-PAGE (7.5% gel) followed by immunoblotting (IB) with anti-HA pAb.
Leu194 Is Indispensable for TICAM-1-mediated IRF-3 Activation
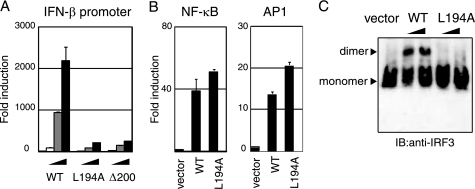
The L194A mutant did not activate the IFN-β promoter, even though its expression was comparable with wild-type TICAM-1 (Fig. 3A). The promoter of the human IFN-β gene possesses binding sites for IRF-3, NF-κB, and AP-1 (25), so we examined which activation pathways were affected by mutation of Leu194. As shown in Fig. 3B, substitution of Leu194 with Ala did not affect NF-κB- and AP-1-activation. In contrast, the ability to activate IRF-3 was abolished in the L194A mutant. Phosphorylation and dimer formation of IRF-3 were induced by the forced expression of wild-type TICAM-1 but not the L194A mutant in HEK293 cells (Fig. 3C), which suggested that Leu194 is indispensable for TICAM-1-mediated IRF-3 activation.
FIGURE 3.
Leu194 is critical for TICAM-1-mediated IRF-3 activation. A, L194A mutant lost IFN-β promoter-activating ability. HEK293 cells were transfected with increasing amounts of wild-type (WT) TICAM-1 or L194A mutant expression vector (1, 10, and 100 ng) with an IFN-β reporter and phRL-TK. Luciferase activity was measured 24 h after transfection. Representative data from a minimum of three separate experiments are shown. B, L194A mutant activates NF-κB and AP-1. HEK293 cells were transfected with wild-type TICAM-1 or L194A mutant expression vector (100 ng) together with NF-κB (left panel) or AP-1 (right panel) reporters. C, L194A lost IRF-3 activating ability. HEK293 cells were transfected with empty vector, wild-type TICAM-1 vector, or L194A vector (100 and 400 ng). After 24 h, lysates were prepared and subjected to native PAGE. Monomeric and dimeric forms of IRF-3 (arrowheads) were detected by Western blot. IB, immunoblot.
Leu194 Is a Critical Amino Acid for Recruitment of TBK1 to TICAM-1
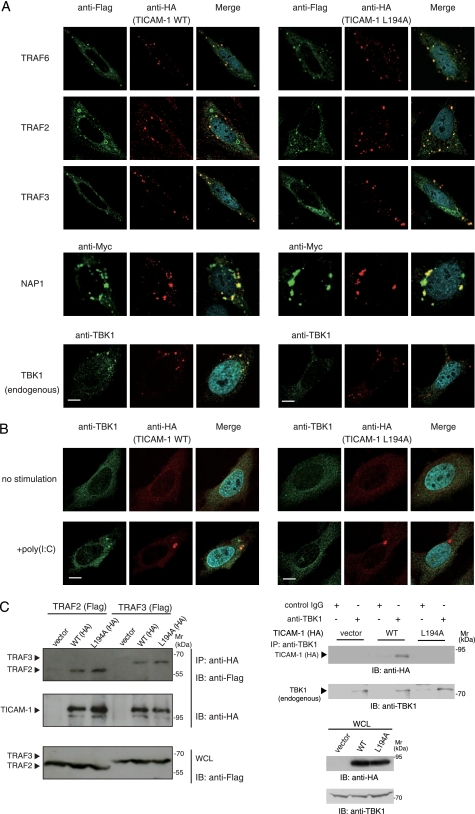
TRAF3 is an essential signaling molecule for TICAM-1-mediated IRF-3 activation (22, 23), and TRAF6 and TRAF2 directly bind to the N terminus of TICAM-1 through distinct binding sites (26). We analyzed the recruitment of TRAF family members to wild-type TICAM-1 or the L194A mutant by immunofluorescence staining and confocal microscopy. When HeLa cells were cotransfected with expression plasmids for HA-tagged wild-type or mutated TICAM-1, and FLAG-tagged TRAF6, TRAF2, or TRAF3, TRAF proteins colocalized with the L194A mutant as with the wild type (Fig. 4A). These results were confirmed by immunoprecipitation (Fig. 4C, left panel). We next examined whether Leu194 is required for recruitment of NAP1 and TBK1. NAP1, which is essential for TICAM-1-mediated IRF-3 activation and functions downstream of TRAF3, colocalized with both wild-type and the L194A mutant (Fig. 4A). However, the IRF-3 kinase TBK1 did not colocalize with the L194A mutant.
FIGURE 4.
Leu194 is indispensable for recruitment of TBK1 to TICAM-1. A, confocal images show HeLa cells coexpressing HA-tagged wild-type (WT) TICAM-1 (left panels) or L194A mutant (right panels) and FLAG-tagged TRAF6, TRAF2, and TRAF3 or Myc-tagged NAP1. HeLa cells, transfected with the expression plasmids for HA-tagged wild-type TICAM-1 or L194A mutant (50 ng) and the indicated FLAG- or Myc-tagged signaling molecules (300 ng), were stained with anti-FLAG or anti-Myc mAb and anti-HA pAb, followed by Alexa Fluor 488-labeled goat anti-mouse Ab and Alexa Fluor 568-labeled goat anti-rabbit Ab. Colocalization of TICAM-1 with endogenous TBK1 was detected using anti-TBK1 pAb and Alexa Fluor 488-labeled goat anti-rabbit Ab. Red, wild-type and L194A mutant; green, TRAF6, TRAF2, TRAF3, NAP1, and TBK1; blue, DAPI-stained nuclei. Bar, 10 μm. B, recruitment of endogenous TBK1 to TICAM-1 by poly(I-C) stimulation. HeLa cells were transfected with the expression vector for HA-tagged wild-type TICAM-1 or L194A mutant (0.1 ng). Twenty four hours after transfection, cells were stimulated with buffer alone or 10 μg/ml poly(I-C) for 30 min. Fixed cells were labeled with anti-HA mAb and anti-TBK1 pAb, followed by Alexa Fluor 568-labeled goat anti-mouse IgG and Alexa Fluor 488-labeled goat anti-rabbit IgG. C, endogenous TBK1 physically associates with wild-type TICAM-1 but not the L194A mutant. Left panels, HEK293FT cells were transfected with empty vector or expression vectors for HA-tagged wild-type (WT) TICAM-1 or L194A mutant together with FLAG-tagged TRAF2, TRAF3. After 24 h, cells were lysed, and TICAM-1 was immunoprecipitated using an anti-HA pAb. Right panels, cells were transfected with empty vector or expression vector for HA-tagged wild-type TICAM-1 or L194A mutant. After 24 h, cells were lysed, and endogenous TBK1 was immunoprecipitated (IP) using an anti-TBK1 pAb. Rabbit IgG was used as a control Ab. The immunoprecipitants were resolved on SDS-PAGE (7.5% gel) under reducing conditions followed by immunoblotting (IB) with anti-tag mAb or anti-TBK1 pAb. Whole cell lysates (WCL) were subjected to immunoblotting with anti-FLAG mAb, anti-HA pAb, or anti-TBK1 pAb to detect protein expression (IB). Molecular weight markers are on the right.
The lack of association between the L194A mutant and endogenous TBK1 was also observed in TLR3-mediated activation. Wild-type and the L194A mutant diffusely localized in cytoplasm when expressed at a low level (Fig. 4B). After poly(I-C) stimulation, wild-type TICAM-1 formed a speckle-like signalosome where endogenous TBK1 colocalized (Fig. 4B, left panels). In contrast, the L194A mutant did not recruit TBK1 to its signalosome (Fig. 4B, right panels). We analyzed physical association of TBK1 with wild-type or the L194A mutant by immunoprecipitation. Overexpressed HA-tagged wild-type TICAM-1 was coimmunoprecipitated with endogenous TBK1, although the L194A mutant was not (Fig. 4C, right panels). These results indicate that Leu194 is a key amino acid for recruitment of TBK1.
TICAM-1 NTD Interacts with the TICAM-1-TIR Domain
We previously showed that recruitment of NAP1 and TBK1 to TICAM-1 requires homo-oligomerization through the TIR domain and the C terminus and RIP1 binding to the RHIM domain (15). Upon TLR3/4 ligand stimulation, homo-oligomerization is triggered by binding to dimerized TLR3 or TICAM-2, and overexpression of TICAM-1 induces homo-oligomerization (15). How TICAM-1 molecules are prevented from auto-activation in the resting state remains unresolved. TICAM-1 contains two structural domains, the NTD and the TIR domain, and Leu194 and the TRAF2/6-binding sites are between these domains. We hypothesized that these interacting sites are covered by the NTD to prevent downstream signaling molecules from accessing TICAM-1.
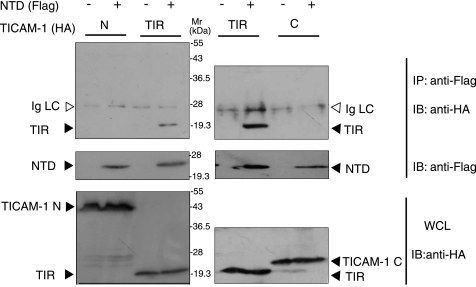
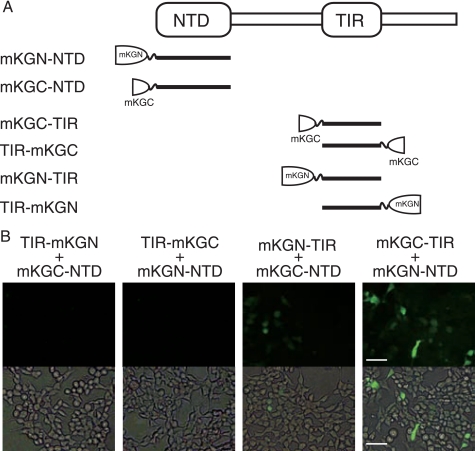
To test this hypothesis, we investigated the physical association of the NTD with truncated TICAM-1 fragments. FLAG-tagged NTD was coexpressed with HA-tagged TICAM-1-N, TICAM-1-TIR, or TICAM-1-C in HEK293FT cells, and coimmunoprecipitation was performed using anti-tag Abs. Notably, NTD coimmunoprecipitated with TICAM-1-TIR but not with TICAM-1-N or TICAM-1-C (Fig. 5), suggesting an intramolecular association between the NTD and the TIR domain. To further assess direct interaction between the NTD and the TIR domain, protein-protein interaction analysis was performed using a protein fragment complementation method. We made six constructs fusing the N- or C-terminal fragment of the monomeric Kusabira-Green protein to the N terminus of the NTD (mKGN-NTD, mKGC-NTD), the N terminus of the TIR domain (mKGN-TIR, mKGC-TIR), or the C terminus of the TIR domain (TIR-mKGN, TIR-mKGC) (Fig. 6A). Fluorescence was detected when expressed fused proteins interacted, restoring the mKG protein from the mKG fragments. HEK293FT cells were transfected with combinations of expression plasmids, and 24 h after transfection, strong fluorescent signals were detected in cells coexpressing mKGC-TIR and mKGN-NTD, and faint signals were detected in the cells coexpressing mKGN-TIR and mKGC-NTD (Fig. 6B). In contrast, cells coexpressing TIR-mKGN or TIR-mKGC and mKG-NTD did not fluoresce (Fig. 6B). These results strongly suggested that the NTD interacts with the N-terminal TICAM-1-TIR domain.
FIGURE 5.
NTD physically associates with TICAM-1-TIR. HEK293FT cells were transfected with FLAG-tagged NTD and HA-tagged TICAM-1-N (1–359 aa), TIR domain (387–566 aa), or TICAM-1-C (567–712 aa). After 24 h, cells were lysed, and NTD was immunoprecipitated (IP) using an anti-FLAG mAb. The immunoprecipitants were resolved on SDS-PAGE (12.5% gel) under reducing conditions followed by immunoblotting (IB) with anti-HA pAb or anti-FLAG mAb. Whole cell lysates (WCL) were subjected to immunoblotting with anti-HA pAb to detect protein expression. Open arrowheads indicate immunoglobulin light chain (Ig LC). Molecular weight markers are on the right.
FIGURE 6.
NTD interacts with the N-terminal TICAM-1-TIR. A, NTD or TICAM-1-TIR was fused to mKG fragments. B, fluorescence images show HEK293FT cells coexpressing NTD and TICAM-1-TIR, fused to mKG fragments at the N or C terminus. HEK293FT cells were transfected with the indicated combinations of expression plasmids for truncated TICAM-1 mutants fused to mKG fragments (each 500 ng). Twenty four hours after transfection, conditioned media were replaced with Dulbecco's PBS. Cells were visualized by fluorescence microscopy. Signal was detected in cells coexpressing mKGC-TIR and mKGN-NTD, and faint fluorescence was observed in cells coexpressing mKGN-TIR and mKGC-NTD. Bar, 100 μm.
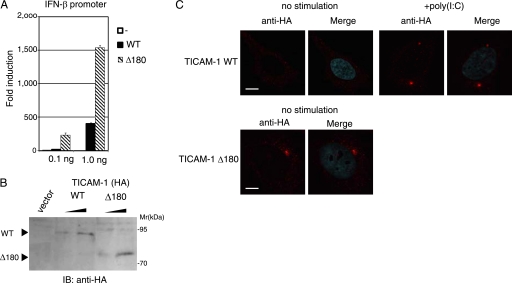
A TICAM-1 mutant lacking the NTD (Δ180) had high potential to activate the IFN-β promoter compared with wild-type TICAM-1 (Fig. 1C). This augmented activity of Δ180 mutant was more clearly shown when wild-type or Δ180 mutant was expressed at a low level (Fig. 7A). In HEK293 cells transfected with 0.1 ng of wild-type- or the L194A-expressing plasmid, wild-type TICAM-1 was inactive, whereas Δ180 mutant exerted the ability to activate the IFN-β promoter, although their protein expression levels were almost equivalent (Fig. 7B). Under these conditions, wild-type TICAM-1 diffusely localized in cytoplasm (Fig. 7C). In contrast, Δ180 mutant formed a speckle-like signalosome in unstimulated cells as seen in poly(I-C)-stimulated wild-type TICAM-1 (Fig. 7C), suggesting that deletion of the NTD facilitates the homo-oligomerization of Δ180 mutant through the TIR domain.
FIGURE 7.
Δ180 mutant had high potential to activate the IFN-β promoter compared with wild-type TICAM-1. A, IFN-β promoter activation by the Δ180 mutant. HEK293 cells were transfected with empty vector or expression plasmid for wild-type (WT) TICAM-1 or Δ180 mutant (0.1 and 1.0 ng) together with the IFN-β promoter reporter. Luciferase activity was measured 24 h after transfection. Representative data from a minimum of four separate experiments, each performed in triplicate, are shown. B, protein expression of wild-type and the Δ180 mutant in HEK293 cell lysates. IB, immunoblot. C, confocal images of HeLa cells expressing a low level of HA-tagged wild-type TICAM-1 (upper panels) or Δ180 mutant (lower panels). Cells were transfected with the expression plasmid for wild-type TICAM-1 or Δ180 mutant (0.1 ng). After 24 h, cells were stimulated with buffer alone or 10 μg/ml poly(I-C) for 30 min, and fixed cells were labeled with anti-HA pAb and Alexa Fluor 568-labeled secondary Ab. The Δ180 mutant formed a speckle-like signalosome in unstimulated cells. Red, wild-type and Δ180 mutant; blue, DAPI-stained nuclei. Bar, 10 μm.
DISCUSSION
Activation of the transcription factor IRF-3 is a key downstream event in the signaling cascade of TICAM-1, resulting in induction of antiviral genes, including IFN-β. Direct binding of TICAM-1 to the IRF-3 activating kinase TBK1 is necessary for IRF-3 phosphorylation. Here, we identified Leu194 as essential in TICAM-1 for recruiting TBK1. Although Leu194 was critical for TBK1 binding, Ser189, Arg195, and Ser196 may stabilize the interaction.
TICAM-1 has two structural domains, the NTD and the TIR domain. Results from trypsin digestion of the TICAM-1 (1–566 aa) suggest the region between the NTD and the TIR domain forms a loose structure that might recruit downstream signaling molecules (24). Because the crucial amino acids for TRAF2, TRAF6, and TBK1 binding reside in this region, naive TICAM-1 may have a closed conformation that covers these sites. Indeed, using the protein-protein association analysis, we clearly showed that the NTD interacted with the N-terminal TIR domain. These observations suggest that the NTD folds into the TIR domain, preventing downstream signaling molecules from accessing their binding sites. Upon stimulation of TLR3/4, or TICAM-1 overexpression, TICAM-1 oligomerizes through the TIR domain and the C-terminal region (15). This may break the intramolecular association and induce a conformational change that allows downstream signaling molecules to their binding sites.
Deletion of the NTD augmented the TICAM-1 activity (Fig. 7). The association sites of TRAF2/6 and TBK1 are likely to be available in the Δ180 mutant. This would facilitate recruitment of the IRF-3 kinase complex. The Δ190 mutant showed reduced IFN-β promoter activation compared with the Δ180 mutant, probably because the absence of Ser189 stabilized TBK1 binding to TICAM-1 (Fig. 1C). Thus, the NTD may act as a repression domain within TICAM-1. Remarkably, exogenous NTD did not affect activation of IRF-3 or NF-κB by wild-type TICAM-1, the Δ180 mutant, or by TLR3-TICAM-1 activated with poly(I-C) (supplemental Fig. 1). We propose that exogenous NTD failed to interact with naive TICAM-1 because the TIR domain was occupied intramolecularly with NTD. Once TICAM-1 was recruited to TLR3, and oligomerized, the TRAF2/6 and TBK1 association sites appeared and signaling occurred quickly. When expressed with mutant Δ180, exogenous NTD interacted with the TIR domain of Δ180, whose binding site is distant from the TBK1 association sites, and did not affect TBK1 recruitment.
RIP1 binding to the C-terminal RHIM domain is necessary for TICAM-1 to mediate NF-κB activation (18), and the L194A mutant recruited RIP1 and activated NF-κB (Fig. 3B and supplemental Fig. 2). How NF-κB activation is controlled when TICAM-1 is in the resting form is unknown, but we surmise that the RHIM domain is unexposed in the resting state and that homodimerization opens the RHIM domain for RIP1 binding. Pro434 in the BB loop of the TICAM-1-TIR domain is critical for TICAM-1 homodimerization, but the C-terminal homodimerization determinant remains unidentified (15). Further analysis is required to elucidate the mechanism of TICAM-1 activation.
TICAM-1 acts as a platform that accumulates signaling molecules to the TICAM-1 signalosome and triggers diversified cellular responses. TICAM-1-dependent gene expression directs mDCs to activate natural killer cells and cytotoxic T lymphocytes, which are both the most effective for antiviral response, and are an anti-cancer immune response (8, 9). Indeed, TLR3/4 ligands are strong candidates for adjuvant anti-cancer and infectious disease immunotherapy (10, 27–29). Interestingly, the RIG-I-like receptor-mediated signaling pathway shares most of its downstream signaling molecules with the TICAM-1 pathway, but it plays a distinct role in antivirus immunity by producing type I IFNs (30). Although TICAM-1-mediated signaling is initiated from the endosome, RIG-I-like receptor-mediated signaling occurs at the mitochondrial outer membrane (31). Hence, compartmentalization of signal platforms might be significant for activating distinct transcription factors, resulting in different cellular responses.
In certain RNA viral infections, TLR3-TICAM-1-dependent inflammatory cytokine and chemokine production affects virally induced pathology and host survival (32). In addition, biased activation of the TLR4-TICAM-1 pathway in lung macrophages by oxidized phospholipids triggers acute lung injury through cytokine production (11). Therefore, control of TICAM-1 activation is a novel therapeutic target for acute lung injury. The development of an inhibitory molecule that blocks homo-oligomerization of TICAM-1 might be a straightforward approach for controlling excessive activation of TICAM-1 after viral infection.
Supplementary Material
Acknowledgments
We are grateful to M. Sasai, K. Funami, A. Watanabe, H. Takaki, H. Shime, and T. Ebihara for invaluable discussions. Thanks are also due to T. Taniguchi (University of Tokyo) and M. Nakanishi (Nagoya City University, Nagoya, Japan) for providing the plasmids and to K. Arimoto (Kyoto University, Kyoto, Japan) and K. Shimotohno (Chiba Institute of Technology, Chiba, Japan) for providing reagents.
This work was supported in part by grants-in-aid from the Ministry of Education, Science, and Culture, the Ministry of Health, Labor, and Welfare of Japan, The NorthTec Foundation, The Akiyama Life Science Foundation, Sapporo Biocluster “Bio-S” (Knowledge Cluster Initiative of Ministry of Education, Culture, Sports, Science and Technology), and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, Ministry of Education, Culture, Sports, Science and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- TIR
- Toll-IL-1 receptor
- DAPI
- 4′,6-diamidine-2′-phenylindole dihydrochloride
- mDC
- myeloid dendritic cell
- NTD
- N-terminal domain (1–176 aa)
- RHIM
- RIP homotypic interacting motif
- TRAF
- tumor necrosis factor receptor-associated factor
- TRIF
- TIR domain-containing adaptor-inducing IFN-β
- aa
- amino acid
- PBS
- phosphate-buffered saline
- HA
- hemagglutinin
- IFN
- interferon
- Ab
- antibody
- mAb
- monoclonal antibody
- pAb
- polyclonal antibody.
REFERENCES
- 1.Akira S., Uematsu S., Takeuchi O. (2006) Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 2.Baccala R., Hoebe K., Kono D. H., Beutler B., Theofilopoulos A. N. (2007) Nat. Med. 13, 543–551 [DOI] [PubMed] [Google Scholar]
- 3.O'Neill L. A., Bowie A. G. (2007) Nat. Rev. Immunol. 7, 353–364 [DOI] [PubMed] [Google Scholar]
- 4.Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. (2003) Nat. Immunol. 4, 161–167 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Science 301, 640–643 [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald K. A., Rowe D. C., Barnes B. J., Caffrey D. R., Visintin A., Latz E., Monks B., Pitha P. M., Golenbock D. T. (2003) J. Exp. Med. 198, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshiumi H., Sasai M., Shida K., Fujita T., Matsumoto M., Seya T. (2003) J. Biol. Chem. 278, 49751–49762 [DOI] [PubMed] [Google Scholar]
- 8.Schulz O., Diebold S. S., Chen M., Näslund T. I., Nolte M. A., Alexopoulou L., Azuma Y. T., Flavell R. A., Liljeström P., Reis e., Sousa C. R. (2005) Nature 433, 887–892 [DOI] [PubMed] [Google Scholar]
- 9.Akazawa T., Ebihara T., Okuno M., Okuda Y., Shingai M., Tsujimura K., Takahashi T., Ikawa M., Okabe M., Inoue N., Okamoto-Tanaka M., Ishizaki H., Miyoshi J., Matsumoto M., Seya T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mata-Haro V., Cekic C., Martin M., Chilton P. M., Casella C. R., Mitchell T. C. (2007) Science 316, 1628–1632 [DOI] [PubMed] [Google Scholar]
- 11.Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C. J., Penninger J. M. (2008) Cell 133, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funami K., Sasai M., Ohba Y., Oshiumi H., Seya T., Matsumoto M. (2007) J. Immunol. 179, 6867–6872 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M., Funami K., Tanabe M., Oshiumi H., Shingai M., Seto Y., Yamamoto A., Seya T. (2003) J. Immunol. 171, 3154–3162 [DOI] [PubMed] [Google Scholar]
- 14.Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) Nat. Immunol. 9, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funami K., Sasai M., Oshiumi H., Seya T., Matsumoto M. (2008) J. Biol. Chem. 283, 18283–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S., Sugiyama M., Yamamoto M., Watanabe Y., Kawai T., Takeda K., Akira S. (2003) J. Immunol. 171, 4304–4310 [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 18.Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. (2003) Science 300, 1148–1151 [DOI] [PubMed] [Google Scholar]
- 19.Meylan E., Burns K., Hofmann K., Blancheteau V., Martinon F., Kelliher M., Tschopp J. (2004) Nat. Immunol. 5, 503–507 [DOI] [PubMed] [Google Scholar]
- 20.Kaiser W. J., Offermann M. K. (2005) J. Immunol. 174, 4942–4952 [DOI] [PubMed] [Google Scholar]
- 21.Sasai M., Oshiumi H., Matsumoto M., Inoue N., Fujita F., Nakanishi M., Seya T. (2005) J. Immunol. 174, 27–30 [DOI] [PubMed] [Google Scholar]
- 22.Häcker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L. C., Wang G. G., Kamps M. P., Raz E., Wagner H., Häcker G., Mann M., Karin M. (2006) Nature 439, 204–207 [DOI] [PubMed] [Google Scholar]
- 23.Oganesyan G., Saha S. K., Guo B., He J. Q., Shahangian A., Zarnegar B., Perry A., Cheng G. (2006) Nature 439, 208–211 [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi M., Sakakibara H., Kumeta H., Takahashi K., Enokizono A., Ishii A., Matsumoto M., Seya T., Inagaki F. (2008) Biochem. Mol. Biol. Abstr. 31, 533 [Google Scholar]
- 25.Honda K., Taniguchi T. (2006) Nat. Rev. Immunol. 6, 644–658 [DOI] [PubMed] [Google Scholar]
- 26.Sasai M., Tatematsu M., Oshiumi H., Funami K., Matsumoto M., Hatakeyama S., Seya T. (2010) Mol. Immunol. 47, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 27.Salem M. L., Kadima A. N., Cole D. J., Gillanders W. E. (2005) J. Immunother. 28, 220–228 [DOI] [PubMed] [Google Scholar]
- 28.Seya T., Matsumoto M. (2009) Cancer Immunol. Immunother. 58, 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoneyama M., Fujita T. (2009) Immunol. Rev. 227, 54–65 [DOI] [PubMed] [Google Scholar]
- 31.Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto M., Seya T. (2008) Adv. Drug Deliv. Rev. 60, 805–812 [DOI] [PubMed] [Google Scholar]
- 33.Funami K., Matsumoto M., Oshiumi H., Akazawa T., Yamamoto A., Seya T. (2004) Int. Immunol. 16, 1143–1154 [DOI] [PubMed] [Google Scholar]
- 34.Iwamura T., Yoneyama M., Yamaguchi K., Suhara W., Mori W., Shiota K., Okabe Y., Namiki H., Fujita T. (2001) Genes Cells 6, 375–388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.