Abstract
Obligate intracellular Chlamydophila pneumoniae induce apoptosis resistance in host cells to escape eradication by immune effector cells. Apoptosis resistance depends on the increased expression and stabilization of cellular inhibitor of apoptosis proteins (cIAPs) and X-linked inhibitor of apoptosis protein (XIAP). Here we investigated the role of XIAP in experimental pulmonary infection of mice with C. pneumoniae. XIAP knock out (KO) mice were sensitized for C. pneumoniae infection compared with wild type mice. XIAP was involved in lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and endotoxin shock. Hyper-secretion of tumor necrosis factor-α and lower NO in LPS-treated KO mouse macrophages revealed its regulatory role in inflammatory responses. Unexpectedly, activating stimuli like LPS, tumor necrosis factor-α, or interferon-γ very efficiently induced apoptotic cell death in KO macrophages but not in wild type macrophages. Cell survival transcription factor nuclear factor κB (NF-κB) p65 levels were reduced in lungs and pulmonary macrophages of infected KO mice. Furthermore, a reduced CD8 T cell population and their increased sensitivity for concanavalin A and chlamydial HSP60 stimulation revealed a defect in CD8 T cells in XIAP KO mice. These data demonstrated a role of XIAP for the integrity of both innate and cellular immune responses during C. pneumoniae infection.
Keywords: Apoptosis, Bacteria, Cell Death, Lung, Macrophage
Introduction
Chlamydophila pneumoniae, a Gram-negative obligate intracellular pathogen, is the cause of atypical pneumonia and has been associated with various inflammatory disorders like chronic obstructive pulmonary diseases, asthma, and chronic heart diseases (1). These bacteria replicate in a biphasic cycle in the host cell inside a membrane-bound vacuole called inclusion. So-called reticulate bodies, the replication-competent form, transform in the course of the cycle to the infectious, non-replicative elementary bodies that are released by bursting cells to start the next infection cycle. Infection with C. pneumoniae has recently been shown to confer resistance to various apoptosis inducers in epithelial cells (2–4). Inhibition of host cell apoptosis in response to infection is believed to be a mechanism by which these bacteria ensure completion of the developmental cycle and consequently efficient replication (5, 6). Because infected cells also resist external apoptosis-inducing stimuli, interfering with apoptosis may in addition play an important role in preventing the eradication of the infected cell by the host immune system.
Apoptosis inhibition by chlamydial infection has been attributed to mechanisms interfering with signaling events upstream and downstream of mitochondria (for review, see Refs. 5–7). We have previously demonstrated that anti-apoptotic inhibitors of apoptosis (IAPs),2 e.g. XIAP, cIAP-1, and cIAP-2, are either up-regulated and/or stabilized in cells infected with Chlamydia (4, 8, 9). Depletion of either of these IAPs sensitized Chlamydia-infected cells for apoptosis induction, suggesting their concerted action in maintaining apoptosis resistance in the infected cell (4, 8). Although these and overexpression experiments suggest a role of IAPs in the control of apoptosis (10, 11), the precise function of IAPs in vivo is still unknown. Biochemical analyses demonstrated that XIAP is the most potent inhibitor of apoptotic pathways as it directly inhibits the activation of caspases-3, -7, and -9 (12, 13). It was, therefore, surprising that XIAP knock-out (KO) mice do not exhibit defects typical of deregulated apoptosis signaling (14). There is accumulating evidence that Drosophila IAP functions in innate immune defense against Gram-negative bacteria rather than in apoptosis signaling (15–18). But also mammalian cIAP-1 and cIAP-2 have recently been demonstrated to play a role in TNF-α-mediated NF-κB activation (19, 20). Moreover, XIAP knock-out mice have been shown to be more sensitive to infection with Listeria monocytogenes due to defects in innate immune signaling (21).
To unravel the role of XIAP in chlamydial infection, we established pulmonary infection of XIAP KO mice. In contrast to WT mice, XIAP KO mice failed to control C. pneumoniae pulmonary infection. We identified macrophages from XIAP KO mice as the cells exhibiting compromised innate immune signaling and high sensitivity to undergo apoptosis.
EXPERIMENTAL PROCEDURES
Infection of Mice
XIAP KO mice (14) were purchased from Harlan and obtained from Colin Duckett, WT C57/Bl6 mice were purchased from The Jackson Laboratory. C. pneumoniae strain TWAR CDC/CWL-029 (ATCC VR1310) was routinely propagated and cultivated in HEp-2 cells as previously described (4). 8–11-week-old mice were infected using a non-invasive spray method (22). Briefly, mice were anesthetized by intraperitoneal injection of ketavet and Rompun and placed ventrally on a small box. A microsprayer (Model IA-1C, Penn-Century, Philadelphia, PA) was placed oropharyngeally into the trachea with the help of a laryngoscope and a binocular light microscope, and mice were infected by aerosolizing 25 μl of SPG buffer (250 mm sucrose, 3.8 mm KH2PO4, 7.2 mm K2HPO4, 0.5 mm l-glutamate, pH 7.4) with 5 × 106 bacteria into the airways. Control mice were kept on placebo and received the same amount of SPG buffer. The body weight of each mouse was documented over the course of infection.
Detection of C. pneumoniae by PCR
C. pneumoniae was detected in animal tissue by amplification of ompA gene by nested and quantitative real time PCR as described in Arno et al. (23). 1 μg of DNA from each lung sample was used to detect C. pneumoniae by nested PCR. The primers used for the amplification of ompA were HL-1 (5-GTTGTTCATGAAGGCCTACT-3), HM-1 (5-GTGTCATTCGCCAAGGTTAA-3), and HR-1 (5-TGCATAACCTACGGTGTGTT-3) (Invitrogen). PCR reaction was carried out in 25 μl containing 50 mm dNTPs, 1 unit of Red Taq polymerase, 5 mm Mg2+, 1 mm Tris-HCl, and 50 mm KCl, pH 8.3, and 0.5 μm each HL-1 and HR-1 primer pairs. 1 μl of the product from this reaction was nested for second reaction and amplified by using HR-1 and HM-1 primers. These two reactions yielded 437- and 229-bp products corresponding to the VD2 and VD4 fragment of ompA gene, respectively. The gapdh gene was used as positive control to validate the reaction. Quantification of bacterial load by quantitative real time PCR was achieved using 10 ng of the genomic DNA from each lung sample to amplify the ompA gene by the SYBR Green dye method under similar conditions. The fluorescence intensity of each sample amplified was measured, and the amount of ompA gene in each lung sample was quantified by using SDS software, Version 2.2.2 (Applied Biosystems, Darmstadt, Germany). All the values obtained for ompA gene were normalized to mouse gapdh as internal control.
Infectivity Assay
Infectivity of C. pneumoniae was monitored by exposing a HEp2 cell monolayer to lung homogenate of infected and control mice. Chlamydial inclusions formed in HEp2 cells were quantified by immunostaining and counted using automated microscopy and the Scan∧R-Analysis software provided with the microscope (Olympus) as previously described (4).
Cytokine Enzyme-linked Immunosorbent Assay
TNF-α concentrations in the lung homogenates of mice and macrophages culture supernatants were measured using enzyme-linked immunosorbent assay kit (BD Pharmingen). Briefly, polystyrene plates (Maxisorp) were coated with monoclonal anti-TNF capture antibody and incubated overnight at 4 °C. The plates were washed and blocked with PBS containing 10% fetal bovine serum for 1 h. Recombinant cytokine standards and samples were incubated for 2 h at 25 °C. After washing, plates were further incubated with biotinylated detection antibody for 1 h at 25 °C. Substrate solution was added, and plates were incubated for 10–30 min at 25 °C. The reaction was stopped, and light absorption was measured at 492 nm by a multiwell spectrometer, Spectra max 250 (Molecular Devices Munich, Germany). Cytokine concentrations in samples were calculated by SPF software using a recombinant cytokine standard curve. The titer of IL-2, IL-4, IL-6, IL-10, IL-12, IL-17, MCP-1, and IFNγ was quantified in the lung homogenate of mice at 20th post-infection by Luminex bead assay (Bio-Plex) system from Bio-Rad.
Isolation of Macrophage and Lymphocytes
To isolate macrophages, the lung were aseptically removed and digested for 1 h in RPMI medium containing 100 μg/ml collagenase (Roche Diagnostics). The digested lung tissues were then minced, and lung homogenates were prepared by pestle homogenization. The lung homogenates were filtered through a 40-μm cell strainer (BD Biosciences) to get rid of tissue debris and fatty tissues. Red blood cells were lysed by 0.2 n NH4Cl. Next, the CD11b+ macrophages in lung homogenate were separated and purified by a magnetic cell sorting-based positive selection method using CD11b+ magnetic micro beads (Miltenyi Biotech). The viable counts of these CD11b+ pulmonary macrophages were taken by trypan blue dye exclusion method, and various signaling parameters in these macrophages were analyzed further. Mouse peritoneal macrophages were isolated by a standard procedure (24). 106 cells per well were cultured in a final volume of 200 μl of RPMI 1640 medium in flat-bottom 96-well polystyrene microtiter plates (Corning) and incubated at 37 °C and 5% CO2 for 3 h, allowing the cells to adhere. Non-adherent cells were removed by washing with serum-free RPMI 1640 medium. Macrophages were identified by the presence of Mac-1 marker. Splenocytes were isolated from spleens of mice by the procedure described previously (25). The lymphocyte fraction was isolated by a reversal of the method applied for the isolation of macrophages. In this case, the non-adherent cell suspension (mainly lymphocytes and polymorphonuclear cells (PMNs) was used for the proliferation experiments.
Western Blotting
Mouse macrophages or lungs were lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 2 mm EDTA, 1% Nonidet P-40, and protease inhibitor mixture) and sonicated. The lysate was centrifuged at 14,000 rpm for 20 min at 4 °C to separate the particulate fraction. Protein concentration was determined by BCA kit (Peirce). Proteins (10 μg each lane) were separated on 8% SDS-PAGE and blotted on polyvinylidene difluoride membranes by wet electroblotting. Blots were blocked overnight at 4 °C with 5% nonfat dry milk in TBS-T (20 mm Tris base, 137 mm NaCl, and 0.1% Tween 20, pH 7.5) and then incubated overnight at 4 °C with mouse inducible NO synthase (iNOS; BD Pharmingen), NF-κB p65, or phospho-NF-κB p65 (Ser(P)-536; 93H1; Cell Signaling) followed by the horseradish peroxidase-conjugated secondary antibody. Blots were developed by ECL reagent (GE Healthcare), and β-actin was detected as a loading control for normalization.
Apoptosis Assay
Caspases-3 in XIAP KO macrophages were measured by a Caspase-Glo 3/7 assay kit® (Promega). Activity of caspase-3/7 was measured by cleavage of a proluminescent caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD. This substrate is cleaved to release aminoluciferin, a substrate of luciferase. One million macrophages in each experimental group were treated with various inflammatory mediators and cultured for different time periods. Cells were then scraped and incubated with Caspase-GloTM reagent for 1–2 h in opaque 96-well plates, and cleaved substrate was measured quantitatively by luminescence measurements.
Cell Viability (WST-1, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT), Lactate Dehydrogenase Release Assay)
Survival of peritoneal macrophages was analyzed by MTT dye reduction method. The macrophages were incubated with MTT (Sigma) dye (5 mg/ml) for 1 h at 37 °C in a CO2 incubator. The formazan (reduction product of dye by mitochondrial reductase) crystals formed were dissolved in DMSO and ethanol, and optical density was measured at 570 nm in a Spectra max 250 spectrometer. Metabolic activity of mouse peritoneal macrophages was determined by WST-1 assays (Roche Diagnostics). 10 μl of reagent were added to a 0.2-ml volume of cell culture and incubated for 1 h, and the absorbance was measured at 440 nm in a Spectra max 250 spectrometer. Cell death of macrophages was detected using the cytotoxicity detection kit (Roche Diagnostics). Macrophages from WT and XIAP KO were treated with lipopolysaccharide (LPS) for the indicated time intervals. After each incubation time period the cell culture plates were centrifuged (250 × g for 10 min, 25 °C) to pellet cell debris. The supernatants were carefully collected to avoid any contamination of cellular debris. 100 μl of culture supernatant was transferred to a 96-well plate, the same amount of freshly prepared lactate dehydrogenase reagent mixture was added for 1 h at 25 °C, and the plate was read at 490 nm in a multiwell spectrophotometer.
Immune Functions Assays
Proliferation induced by LPS and concanavalin A (ConA) treatment of mouse PMNs and T cells, respectively, was measured by the tritiated thymidine uptake method. Cells were pulsed with 0.5 μCi of tritiated thymidine (Amersham Biosciences) 72 h post-induction for 18 h and then harvested on glass fiber filter mats (PerkinElmer Life Sciences) using a cell harvester (PerkinElmer Life Sciences). Thymidine uptake was quantified in a liquid scintillation counter (PerkinElmer Life Sciences). Nitric oxide (NO) concentration was used as a standard marker of activated M-1 macrophage and analyzed quantitatively as nitrite (a stable form of NO) by Griess reagent assay. Briefly, equal volumes of the culture supernatants and Griess reagents (1% sulfanilamide, 0.1% N-(naphthyl)ethylenediaminedihydrochloride (Merck)) in a 1:1 ratio were mixed, and absorbance was measured at 550 nm by Spectra max spectrometer (Molecular Devices). The amount of nitrite produced was calculated using NaNO2 standard curves.
Infection of Mice with Salmonella typhimurium and Determination of Colony-forming Units (cfu) in the Spleen
Salmonella enterica typhimurium SL1344 was plated from frozen stock on LB agar (90 μg/ml streptomycin) and grown overnight at 37 °C. From the plate 10 colonies were inoculated into 5 ml of LB liquid medium containing 90 μg/ml streptomycin (LB-strep) and grown overnight (37 °C, 200 rpm). The next morning 5 ml of fresh LB-strep were inoculated 1:100 with overnight culture and grown to an optical density OD600 = 1.0 (late log phase). 2 ml of the culture were spun down (table centrifuge, 14,000 rpm), and supernatant was carefully removed and washed 3 times by resuspending the pellet in endotoxin-free PBS to remove endotoxin released by the Salmonella into the medium to avoid septic shock in mice. The suspension was adjusted to 500 cfu/100 μl, and 100 μl of this suspension injected into the tail-vein of WT and XIAP KO mice. Survival was monitored over 2 weeks, and cfu in the spleen was determined 5 days post-infection. To determine the cfu in the spleen, mice were sacrificed, the spleen was homogenized in 1 ml of PBS containing 1% Triton X-100 using the plunger of a 10-ml syringe, several dilutions were plated on LB-strep agar, and the cfu were calculated after overnight incubation (37 °C).
FACS Analysis
T cells were isolated by mincing spleen tissue between frosted end slides. Red blood cells were lysed in 0.2 n NH4Cl, and the cell suspensions were filtered through a 40-μm cell strainer to get rid of fat tissue and debris. Both CD4 and CD8 cell were purified by positive selection using magnetic cell sorting according to the manufacturer's instructions (Miltenyi Biotech). Mouse CD4 and CD8 T cells were analyzed quantitatively by FACS. Cells were fixed with 4% paraformaldehyde for 15 min. After washing with PBS, cells were stained with fluorescein isothiocyanate-labeled mouse CD4- and Cy5-labeled CD8 antibody in FACS buffer (PBS, 0.1% bovine serum albumin, 0.2% mouse serum). Cells were washed and analyzed in a flow cytometer (FACSCalibur, BD Biosciences). Both CD4 and CD8 cells were gated, and the CD4/CD8 ratio was determined.
Statistical Analysis
The averages and S.D. as well as the t tests have been calculated using MS Excel. Significance is indicated with p < 0.01 (**) and p < 0.05 (*).
RESULTS
Sensitization of XIAP KO Mice to Chlamydial Lung Infection
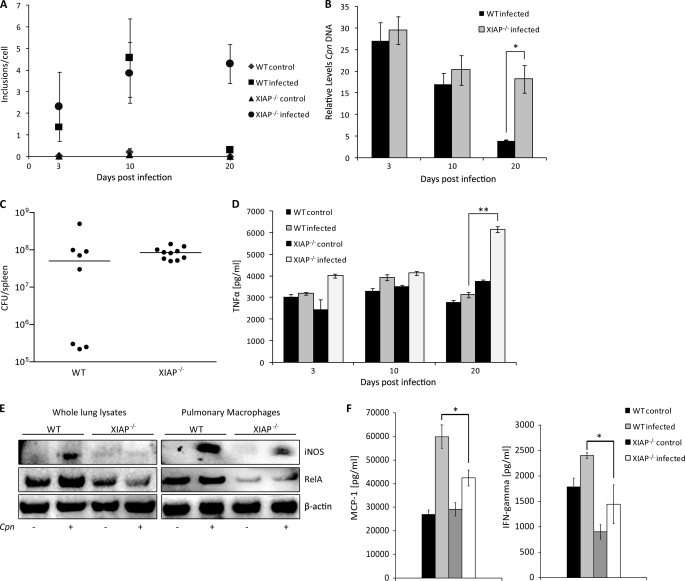
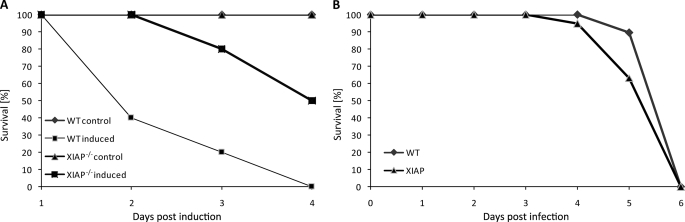
To test whether XIAP has a function in C. pneumoniae lung infection, we infected mice by direct non-invasive intratracheal application of defined bacterial suspensions. Both WT and XIAP KO mice were infected by this method and the bacterial load was measured by nested PCR (23) (Table 1). To determine whether C. pneumoniae isolated from the lungs of these animals were still infective, lung homogenates were prepared and used to infect HEp2 cells. Chlamydial inclusions were then quantified by counting using an automated microscope. These infectivity measurements revealed an increase in the bacterial load up to day 10 post-infection, which declined until day 20 in WT mice. No difference in the infection of WT and XIAP KO was measured at day 3 and 10 post-infection. However, the number of infective bacteria isolated from lungs of XIAP KO mice remained elevated even at day 20 post-infection (Fig. 1A), suggesting a defect in the immune response of KO mice. A similar tendency was observed if the bacterial load was determined by quantifying chlamydial DNA using real time PCR (Fig. 1B). To rule out that XIAP mice are generally more sensitive to infection with Gram-negative bacteria, the infection of WT and KO mice with S. typhimurium was tested. Neither bacterial survival nor the bacterial load in spleen was changed in XIAP KO mice compared with WT mice (Figs. 1C and 3B), ruling out a general sensitivity of XIAP KO mice for Gram-negative bacteria.
TABLE 1.
Detection of C. pneumoniae in the lungs of mice
Animals were infected and sacrificed at the indicated times, and DNA was prepared from their lungs. The DNA segment coding for the chlamydial ompA gene was amplified by nested PCR as described under “Experimental Procedures.” The PCR products were separated on agarose gels, which revealed the presence of 437- and 229-bp products corresponding to the VD2 and VD4 fragment of ompA gene, respectively. Index: +/+, 437/229 bp; ND, not detected.
| Experimental group | Day 3 post-infection | Day 10 post-infection | Day 20 post-infection |
|---|---|---|---|
| WT control | ND | ND | ND |
| WT infected | +/+ | +/+ | +/+ |
| XIAP KO control | ND | ND | ND |
| XIAP KO infected | +/+ | +/+ | −/+ |
FIGURE 1.
Sensitization of XIAP KO mice for C. pneumoniae lung infection. A, impaired resolution of C. pneumoniae infection from the lungs of XIAP deficient mice is shown. Five mice per group were infected with C. pneumoniae, and infectious bacteria were determined by lung infectivity assays using quantitative immunofluorescence microscopy. The infectivity was calculated as the number of chlamydial inclusions formed per infected HEp2 cell. The data are represented as the mean ± S.E. of the inclusions per cell from three independent experiments. B, five mice per group were infected with C. pneumoniae, and the chlamydial ompA gene was amplified from the lung tissue of each mouse. The relative amount of the ompA gene in each lung sample was calculated using glyceraldehyde-3-phosphate dehydrogenase as an internal control. *, p ≤ 0.05. C, shown is the bacterial load after intravenous infection with S. typhimurium. C57BL/6 and KO XIAP mice were infected with 500 cfu of S. enterica typhimurium SL1344 via tail vein. cfu per spleen were determined 5 days post-infection. Bacterial load is equivalent in both C57BL/6 and KO XIAP mice. D, increased TNF-α production in lungs of infected XIAP KO mice is shown. Levels of TNF-α were determined in lung homogenates from WT and XIAP-deficient mice by enzyme-linked immunosorbent assay at different time points post infection. Data represent the means ± S.E. from five independent infection experiments. ** indicates p ≤ 0.01. E, reduced expression of iNOS and RelA/p65 in infected XIAP KO mice is shown. Lungs were excised aseptically from WT and XIAP KO mice infected with C. pneumoniae (Cpn) 20 days. CD11b+ pulmonary macrophages were purified from the lungs of WT and XIAP KO mice as described under “Experimental Procedures.” Homogenized lungs and isolated macrophages were lysed in radioimmune precipitation assay buffer containing protease inhibitors. 20 μg of protein from either whole lung lysate or pulmonary macrophages was separated by PAGE and subjected to Western blot analysis as described under “Experimental Procedures.” β-Actin was detected as the loading control. F, reduced secretion of major macrophage stimulatory cytokines is shown. The lung homogenate from mice of different groups were prepared, and the titers of IFN and MCP-1 were quantified as described under “Experimental Procedures.” Data represent the means ± S.E. from three independent infection experiments. * indicates p ≤ 0.05.
FIGURE 3.
Resistance of XIAP-deficient mice for LPS-induced experimental endotoxin shock. A, both WT and XIAP KO mice were challenged with the LD100 of LPS (0.5 mg/kg body weight) intraperitoneally, and the survival of WT and XIAP KO mice was monitored over the period of 4 days. Shown is the representative result of one of three independent experiments. B, survival of mice after intravenous infection with S. typhimurium is shown. Survival of WT and XIAP KO was monitored for 1 week after infection. All mice were dead 6 days post-infection without any significant difference in WT or XIAP KO mice.
TNF-α is a cytokine with cytotoxic and antibacterial functions (26). It is produced in high amounts during chlamydial infection in vitro and in vivo (27–31). Because TNF-α blocks chlamydial growth on its own if applied to host cells before infection (32, 33), we reasoned that lung tissue of infected WT and KO animals may differ in the quantity of this cytokine. Contrary to our expectation, the TNF-α concentration in the lung of infected XIAP KO mice was higher at day 20 post-infection compared with WT mice (Fig. 1D). However, we could not detect any obvious inflammatory lesions in the lungs of infected XIAP KO mice (not shown). In conclusion, the reduced protection from chlamydial infection and the deregulated production of TNF-α suggested a defect of XIAP KO in innate and/or adaptive immune responses.
To further investigate the reason for the reduced competence of XIAP KO mice to control chlamydial infection, we tested the levels of iNOS, which has previously been shown to be crucial for defense against C. pneumoniae pulmonary infection in mice (34). Levels of iNOS were reduced in lung tissue and CD11b+ pulmonary macrophages from infected XIAP KO (Fig. 1E), suggesting a role of XIAP in the induction of iNOS. An important mechanism of iNOS regulation is transcriptional activation of the gene by a complex network of transcription factors including NF-κB (35). Pulmonary macrophages and, to a minor extent, also lung tissue of infected XIAP KO mice produced reduced levels of RelA/p65 (Fig. 1E), indicating the down-regulation of pro-inflammatory signaling cascades in these mice.
We then analyzed Th1/Th2 cytokines in the lungs of control and infected WT and XIAP mice at day 20 post-infection. We could not detect IL-2, IL-4, IL-6, and IL-17 in lungs of both WT and XIAP KO control and infected mice (not shown). No differences in IL-12 (Th1 cytokine) and IL-10 (Th2 cytokine) titers between lungs of infected WT and XIAP animals could be detected (not shown). In contrast, MCP-1 and IFNγ required for macrophage recruitment and activation, respectively, were significantly higher in the lungs of infected WT animals (Fig. 1F). In conclusion, the reduced protection from chlamydial infection and the deregulated production of the inflammatory cytokine TNF-α as well as the macrophage stimulatory cytokines MCP-1 and IFNγ suggested a defect of XIAP KO in innate and/or adaptive immune responses.
Sensitization of XIAP KO Macrophages to TNF-α Response
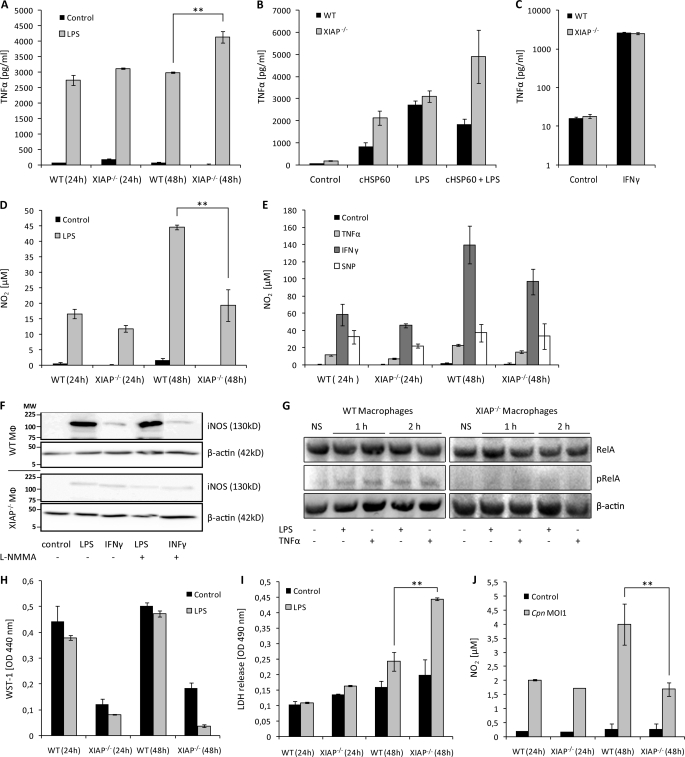
The increased concentration of TNF-α in the lungs of infected XIAP KO mice over WT mice in principle should have enforced resolution of the infection in XIAP KO mice. In contrast, bacteria survived better in XIAP KO mice. This prompted us to investigate the TNF-α response of WT and XIAP KO macrophages. WT and XIAP KO macrophages were stimulated with LPS or chlamydial HSP60 (cHSP60), a known inducer of macrophage activation (36), and the production of TNF-α was monitored. Our results revealed a time-dependent increase in TNF-α secretion in KO macrophages compared with WT macrophages in response to either LPS or cHSP60 stimulation (Fig. 2A). We observed a synergistic effect of LPS and cHSP60 co-stimulation in XIAP KO macrophages but not WT macrophages (Fig. 2B), suggesting an increased sensitization of XIAP KO macrophages for TNF-α secretion. TNF-α secretion in XIAP KO in response to IFN-γ stimulation, however, remained comparable with induced WT macrophages (Fig. 2C). These results together demonstrated the sensitization of KO macrophages for LPS- and cHSP60-induced TNF-α secretion.
FIGURE 2.
Dysregulated NO and TNF-α response in XIAP KO macrophages and mice. A, sensitization of XIAP KO macrophages for LPS-mediated TNF-α production is shown. Macrophages from WT and KO mice were cultured and treated with LPS. TNF-α concentrations were determined in culture supernatants at different post-treatment time points. Data are presented as the mean ± S.E. from three independent experiments. B, 106 peritoneal macrophages from WT and XIAP KO mice were stimulated with LPS and purified cHSP60 both separately and together. TNF-α generation was quantified at 24 h post-treatment in macrophage culture supernatant by enzyme-linked immunosorbent assay. C, XIAP is not involved in IFN-γ induced TNF-α production in macrophages. 104 peritoneal macrophages from WT and XIAP KO mice were stimulated with IFN-γ, and TNF-α generation was determined in culture supernatants at 24 h post-infection. Data represent the mean ± S.E. from three independent experiments. D, shown is poor induction of LPS-mediated NO2 in the XIAP KO macrophages. The isolated peritoneal macrophages from WT and XIAP KO mice were stimulated with LPS, and NO2 was determined in the culture supernatants at different post-treatment time points. Data represent the mean ± S.E. from three independent experiments. E, procedures were the same as in D, but macrophages were treated with inflammatory cytokines. NO2 was determined at 24 and 48 h post-treatment. Data represent the mean ± S.E. from three independent experiments. F, XIAP is required for induction of iNOS by LPS. Cells were incubated with LPS, IFN-γ, or left untreated (control), and iNOS expression was monitored by Western blot using anti- iNOS antibody (BD Pharmingen). β-Actin was detected as the loading control. Shown is one representative blot from three independent experiments with similar results. Lines indicate molecular weight markers (MW) in kDa. G, reduced stimulation of NF-κB RelA/p65 in pulmonary macrophages of XIAP KO mice is shown. CD11b+ pulmonary macrophages were purified from the lungs of WT and XIAP KO mice as described under “Experimental Procedures.” Purified macrophages were either left untreated (NS) or were treated for 1 or 2 h with TNF-α or LPS as indicated. Macrophages were lysed in radioimmune precipitation assay buffer and subjected to SDS-PAGE and Western blotting. RelA/p65 and phospho-RelA/p65 (Ser(P)-536) were detected as described under “Experimental Procedures.” Note that because of the reduced expression of RelA/p65 in XIAP KO macrophages (Fig. 1E), loading was adjusted to achieve similar levels of RelA/p65 in samples derived from WT and XIAP KO macrophages. H, poor stimulation of XIAP KO macrophages is shown. Both WT and XIAP KO CD11b+ peritoneal macrophages were treated with LPS, and metabolic activity was measured at the indicated time intervals. Data present mean of optical density (OD) ± S.E. from three independent experiments. I, shown is activation induced death of XIAP KO macrophages. The supernatants from the macrophages treated as in H were collected, and cytotoxicity of LPS treatment was detected by lactate dehydrogenase release at 490 nm in a spectrophotometer. Data are represented as the mean of lactate dehydrogenase release ± S.E. from three independent repeats. J, shown is reduced NO2 generation in XIAP KO-infected macrophages. WT and XIAP KO Mac-1+ macrophages (1 × 104) were infected with C. pneumoniae at multiplicity of infection 1(Cpn MOI1), and infection-induced NO2 was measured at indicated time intervals post-infection. Data present the concentration of NO2 generated ±S.E. from three independent experiments. **, p ≤ 0.01.
XIAP Is Required for Inducible NO in Mac-1+/+ Macrophages
Inducible NO-free radicals are potential pro-inflammatory mediators secreted by activated macrophages during innate immune response (35). We, therefore, tested NO production in response to several stimuli in both WT and KO macrophages. LPS-induced NO generation was reduced in KO macrophages compared with WT in a time-dependent fashion (Fig. 2D). To test whether XIAP is involved in NO production induced by other stimuli, these macrophages were treated with various stimuli, and NO concentrations were quantified. Both WT and XIAP KO macrophages responded similarly to TNF-α, IFN-γ, and sodium nitroprusside, a NO donor (Fig. 2E). To understand the reason of the diminished production of NO in XIAP-deficient macrophages in response to LPS, we compared the expression level of iNOS in WT and XIAP KO macrophages. Western blot analysis revealed a LPS-specific defect in the induction of iNOS in XIAP KO macrophages since stimulation with IFN-γ induced similar levels of iNOS (Fig. 2F), ruling out a general defect in iNOS expression in KO macrophages. Because NF-κB RelA/p65 levels were strongly reduced in pulmonary macrophages of infected cells (Fig. 1E), we tested whether its activation was also affected in XIAP KO cells. Pulmonary macrophages were treated with the strong NF-κB activators LPS or TNF-α, and phosphorylation of RelA/p65 at serine 265, indicative of its stabilization and activation, (37) was monitored. XIAP KO macrophages responded weaker to these stimuli (Fig. 2G), indicating a reduced activation of NF-κB in these cells.
To test whether decreased NO production could be due to decreased viability of XIAP KO macrophages, the metabolic activity of these cells was measured. Metabolic activity of LPS-treated XIAP KO macrophages was nearly unchanged at 24 h post-treatments; however, metabolic activity dropped strongly at 48 h post-treatment (Fig. 2H). Likewise, lactate dehydrogenase release assays indicating membrane perforation also pointed to a toxic effect of long term LPS incubation (Fig. 2I), suggesting that the reduced production of NO by KO macrophages at least in part was due to a reduced viability.
To further define the role of XIAP in defeating C. pneumoniae infection, we compared the inducible NO concentrations between infected WT and KO macrophages. Infected WT and KO macrophages produced similar amounts of NO in their supernatant at 24 h post-infection. NO levels increased at 48 h post-infection in WT but not in KO macrophages (Fig. 2J), suggesting a defect in the NO production in KO macrophages also in response to infection.
Increased Resistance for Endotoxin Shock of XIAP KO Mice
NO is a major mediator of endotoxin shock by inducing vascular perfusion and hypotension. Because XIAP KO macrophages showed a reduced response for LPS-induced NO production, we predicted resistance of XIAP KO mice to endotoxin shock. To prove this hypothesis, we induced an endotoxin lethal shock in both WT and KO mice. Despite the hyper-inflammatory response of KO macrophages to LPS, XIAP KO mice survived the shock better than WT mice. At a dose of bacterial LPS (0.5 mg/kg body weight) resulting in 100% mortality in WT mice, 40% of the KO mice survived (Fig. 3A). This relative resistance could not be observed after lethal intravenous infection of mice with living S. typhimurium (Fig. 3B), suggesting that additional mechanisms besides exposure to endotoxin are the cause for lethality in infection with viable Salmonella.
Hypersensitivity of XIAP KO Macrophages
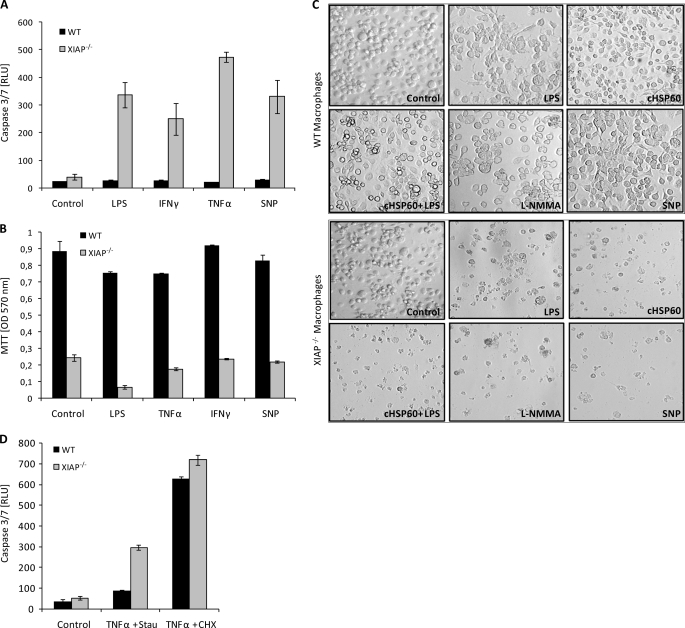
Among all IAPs, XIAP is the most potent direct inhibitor of different caspases including caspase-3, causing increased apoptosis inhibition upon over expression (11). Also, LPS-treated macrophages were clearly affected in their viability (Fig. 2, I and J). We, therefore, tested if macrophages from KO mice exhibited increased apoptosis by determining caspase-3 activity. Contrary to WT macrophages, XIAP KO macrophages were extremely sensitive for diverse stimuli, including LPS, TNF-α, IFN-γ, and excess NO visible by high levels of active caspase-3 and strongly reduced survival (Fig. 4, A–C) and increased detachment of KO cells (Fig. 4C). Interestingly, also inhibition of NO production by treatment with l-NG-monomethylarginine (l-NMMA), an inhibitor of nitrite-oxide synthases, caused detachment of KO macrophages (Fig. 4C). WT macrophages underwent apoptosis at a similar frequency as KO macrophages when treated with TNF-α and cycloheximide (Fig. 4D), ruling out a general defect in apoptosis signaling in WT cells.
FIGURE 4.
Increased apoptosis in XIAP KO CD11b+ macrophages. A, macrophages (106) were treated with pro-inflammatory mediators as indicated, and apoptosis was evaluated quantitatively using Caspase-Glo® luminescent assay. Data are represented as the mean of luminescence in relative light units (RLU) ± S.E. from two independent experiments. B, the survival of macrophages treated as in A was measured by standard MTT assay by measuring formazan formation at 570 nm in a spectrometer. C, control, WT, and XIAP KO macrophages were treated with LPS, cHSP60, sodium nitroprusside (SNP), and l-NG-monomethylarginine (l-NMMA) and then analyzed by phase contrast microscopy. D, macrophages (106) were treated with TNF-α (50 ng/ml) together with staurosporine (Stau; 10 ng/ml) or cycloheximide (CHX; 10 ng/ml), and apoptosis was measured by Caspase-Glo® luminescent assay.
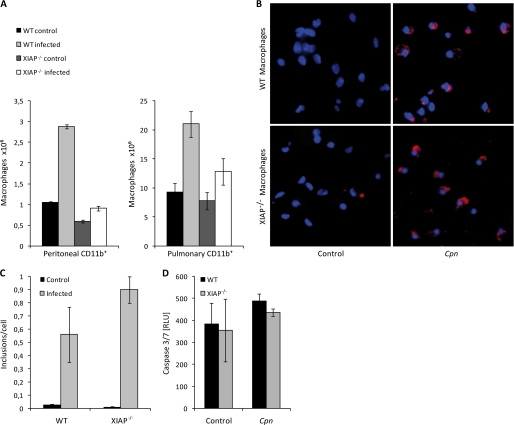
Considering the sensitive response of KO macrophages for caspase-3 induction and detachment, an obvious reason for the attenuated response toward C. pneumoniae may depend on the depletion of macrophages by infection. We, therefore, quantified CD11b+ pulmonary and peritoneal macrophages from non-infected and infected WT and KO animals. Interestingly, the numbers of macrophages increased in the lungs and the peritoneum of WT animals, whereas this increase was not observed in KO animals (Fig. 5A), indicating a general defect in the macrophage response in infected KO animals. Depletion of macrophages may have occurred by a direct toxic effect of replicating bacteria in macrophages and possibly by increased TNF-α concentrations in response to infection. However, the infection of WT and KO macrophages by C. pneumoniae was similar (Fig. 5, B and C); an increased infection susceptibility as reason for the depletion of KO macrophages is, therefore, unlikely. Moreover, infection of WT and KO macrophages with C. pneumoniae did not affect levels of active caspase-3 above that of mock-infected cells (Fig. 5D), ruling out a direct induction of apoptosis by infection under these conditions.
FIGURE 5.
Depletion of macrophages in infected XIAP KO mice. A, shown is depletion of mouse peritoneal macrophages in infected XIAP KO mice. Peritoneal macrophages were isolated from different experimental groups, and their viability was tested by trypan blue staining. Data represent the mean of the number of viable macrophages ± S.E. from three independent experiments. B, infection of macrophages by C. pneumoniae is shown. Peritoneal macrophages from wild type and KO mice were infected with C. pneumoniae at a multiplicity of infection of 1. 72 h post-infection, the macrophages were fixed with chilled absolute methanol and stained with anti C. pneumoniae (Cpn) antibody (red) and Hoechst to stain for nuclei (blue). Shown are representative micrographs of infected macrophages. C, shown is quantification of the experiment shown in B. D, C. pneumoniae infection does not activate caspase-3 in XIAP KO Mac-1+ peritoneal macrophages. 104 macrophages from WT and XIAP KO mice were infected and co-stimulated with TNF-α and IFN-γ as indicated. Caspase-3 activity was quantified 24 h post-infection by luminescence assay. Data are represented as the mean ± S.E. from three independent experiments.
Loss of Immunological Response in XIAP KO Immune Cells
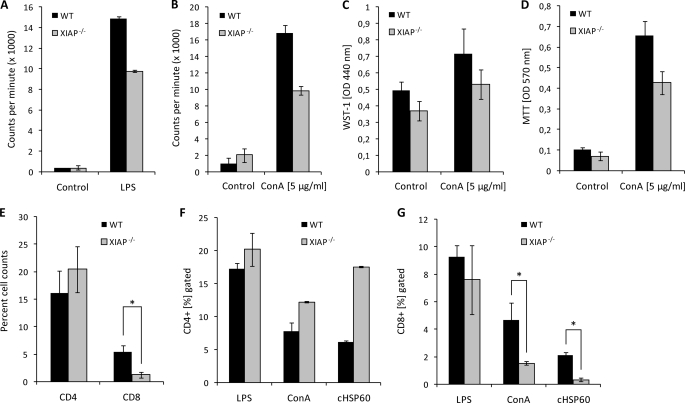
To get more insight into the immunoregulatory role of XIAP in cellular immune responses, XIAP KO PMNs and T cells were analyzed for their capacity to respond to stimulatory signals. Both PMNs and T cells were stimulated with their cognate antigen, e.g. LPS and ConA, respectively, and their proliferation was quantified by measuring the uptake of [3H]thymidine. These experiments revealed compromised proliferation of XIAP KO PMNs and T cells in comparison to WT cells (Fig. 6, A and B), indicating a role of XIAP also in the activation-induced proliferation of peripheral blood mononuclear cells and T cells.
FIGURE 6.
Impaired proliferation of XIAP KO immune cells. A and B, 106 PMNs (A) and splenic T cells (B) from both WT and XIAP KO mice were stimulated with LPS (5 μg/ml) and ConA (2.5 μg/ml), respectively, and the stimulatory potential was measured by [3H]thymidine incorporation. Data represent the mean of counts per min ±S.E. from three independent experiments. C, the stimulation of T cells in response to ConA treatment was analyzed by WST-1 assays, and the survival of T cells was measured by MTT assays as described under “Experimental Procedures” (D). E, both CD4 and CD8 T cells were purified by magnetic cell sorting from the spleen of WT and XIAP KO mice. Viable cells were quantified by trypan blue dye exclusion. Both CD4 (F) and CD8 (G) T cells were stimulated with LPS, ConA, and purified chlamydial cHSP60 for 48 h and analyzed by FACS as described under “Experimental Procedures.”
Because T cells have been shown to play an important role in immunity against C. pneumoniae infection (34), a defect in the T cell proliferation or survival in addition to the strongly affected macrophage response could explain the severe defect of KO mice to control chlamydial infection. Viability and survival of wild type T cell populations were higher upon stimulation with ConA compared with KO T cells, consistent with reduced activation and proliferation (Fig. 6, C and D). Quantitative FACS analysis revealed no significant difference in the population of CD4 T cells between WT and XIAP KO splenocytes; however, the CD8 T cell population was strongly reduced in the latter (Fig. 6E). Whereas the relative number of CD4 T cells was higher in KO versus wild type mice upon stimulation with ConA and cHSP60, the CD8 population was strongly reduced in KO mice under these conditions (Fig. 6, F and G), suggesting a reduced population of these effector T cells as reason for the reduced immune response against lung infection in XIAP KO mice.
DISCUSSION
C. pneumoniae infection induces the expression and increases stability of IAPs, which confers resistance of infected cells to apoptosis induced by different stimuli (4). Based on these observations, we assumed that inhibiting apoptosis of infected cells ensures bacterial survival as induction of apoptosis has been suggested as a potential strategy to activate anti-bacterial response required for the resolution of bacterial infections. Interfering with infection-induced anti-apoptotic signaling, e.g. by affecting IAP protein expression (4), should have improved the resolution of bacterial infection. Contrary to our expectation, XIAP-deficient mice were found sensitized for C. pneumoniae pulmonary infection, suggesting XIAP as important part of the anti-bacterial host response. These data are in line with recent findings demonstrating a role of XIAP in the control of L. monocytogenes infection (21). We could, however, not detect any difference between WT and KO mice in the control of Salmonella infection (Fig. 1B), indicating a specific role XIAP in certain bacterial infections.
TNF-α is a major cytokine of innate immunity that mediates inflammatory responses against infection and has been shown to limit C. pneumoniae replication (32, 33). We observed higher TNF-α titers in the lung of infected XIAP mice, which in principle should help to clear chlamydial infection. The fact that these mice had higher bacterial loads in their lungs already suggested a defect in TNF-α-mediated defense against Chlamydia infection. MCP-1 is yet another critical cytokine that is required for the recruitment of macrophages to the site of infection. During bacterial infection, MCP-1 (also known as CCL2) has been shown to activate T cells, monocytes, and basophiles and to recruit monocytes/macrophages to sites of inflammation (38). In line with these observations, we identified the macrophage as a cell type clearly affected in XIAP mice. First, LPS-induced secretion of TNF-α was increased in these cells, whereas that of NO was decreased. The same response of KO macrophages to IFN-γ was similar as in WT macrophages, indicating a defect in selective signaling pathways induced by endotoxin. This assumption is supported by the relative resistance of KO mice for endotoxin-induced shock. Furthermore, the report of Bauler et al., (21) who found reduced TNF-α secreted by XIAP KO macrophages infected with Listeria compared with the WT macrophages, indicates that LPS and Listeria infection affects TNF-α signaling via different pathways in these macrophages.
The observation of a clear defect in iNOS expression in pulmonary macrophages from infected XIAP KO mice was further substantiated by ex vivo experiments, demonstrating low iNOS induction by LPS or TNF-α treatment and C. pneumoniae infection. Our data and previous work (34) strongly suggests that the XIAP-mediated induction of iNOS in response to these stimuli is an important parameter to resolve C. pneumoniae pulmonary infection. Transcriptional regulation of iNOS is very complex, and more than 15 different consensus binding sites for transcription factors have been identified in the promoter region of the iNOS gene (39). One of the factors regulating iNOS transcription is NF-κB, which is not only controlled by cIAP-1 and cIAP-2 (19) but also by XIAP. Mouse embryonic fibroblasts from XIAP KO mice have a severe defect in NF-κB signaling (40). Also, nuclear translocation of the NF-κB p50 subunit is strongly affected in macrophages also derived from XIAP KO mice (21). We demonstrated the reduced expression and activation of NF-κB RelA/p65 in XIAP KO peritoneal macrophages, strongly supporting the notion of a general role of XIAP in these pro-inflammatory and pro-survival pathways. It is, therefore, very likely that XIAP has an important function in selective signaling pathways of the innate immune response in macrophages. This assumption is also reflected by reduced NO concentrations in the supernatant of infected KO macrophages compared with WT macrophages. An interesting parallel is given by the recently published phenotype of Nod−/− and Rip2−/− mice, which also fail to control C. pneumoniae lung infection (41). Macrophages from these mice exhibit reduced NO production in response to Chlamydia infection.
The impaired LPS-induced innate and cellular immune response of XIAP KO mouse macrophages is in line with several recent reports from Drosophila attributing IAP proteins a role in the innate immune defense against Gram-negative bacteria rather than in apoptosis signaling (15–18). The strong sensitization of KO macrophages for the induction of apoptosis by diverse stimuli, however, points to a second important, thus far, undiscovered function of XIAP in the immune defense; that is, the control of survival of the stimulated macrophage. The anti-apoptotic function of XIAP may well be part of its role in proinflammatory innate signaling as the activation of NF-κB, the central transcription factor involved in pro-inflammatory and anti-apoptotic gene activation, is at least partially controlled by XIAP (21). cIAP-1 and cIAP-2 have also been shown to be part of the TNF-α-induced NF-κB signaling pathway (19). It is, thus, possible that these IAPs function together in selective signaling pathways of the innate immune response in macrophages. Functional and biochemical evidence has been provided for the existence of heteromeric high molecular weight IAP complexes (8, 42). Such complexes consisting of cIAP-1, cIAP-2, and XIAP previously described in epithelial cells and Jurkat T cells (8) may be part of the innate proinflammatory signaling cascades in macrophages. A concerted action would also explain why mice with a single knock-out in either of these genes have similar phenotypes. For example, cIAP-1 (43), cIAP-2 (44), and XIAP (this work) knock-out mice exhibit a marked increased tolerance for endotoxin-induced shock. In addition, and in contrast to other IAP family members, XIAP has a strong caspase-inhibiting activity (13, 45). Although the XIAP KO mice are without major defect related to a malfunction of apoptotic signaling (14), we find that the macrophages of these mice are highly sensitized for apoptosis induced by divers triggers like LPS and pro-inflammatory cytokines. In mammals, XIAP may, thus, function to connect pro-inflammatory and anti-apoptotic signaling of phagocytes with a direct control of their survival in the course of an immune response against Gram-negative bacteria.
Apart from the dysregulated innate immune response of XIAP KO macrophages, compromised stimulation of T cells in response to LPS and ConA treatment demonstrated an additional defect in the cellular immune response in these mice. Further analysis of CD4 and CD8 T cell populations revealed an imbalance in XIAP KO splenocytes. An interesting observation was the reduced CD8 T cell population in XIAP KO lymphocytes after ConA/cHSP60 stimulation, as particularly CD8 T cells independent of CD4 T cells are important for immunity against C. pneumoniae (46). In summary, these data demonstrate that besides a role in apoptosis regulation, XIAP is also an essential component of innate and adaptive cellular immunity controlling C. pneumoniae infection.
Acknowledgments
We thank Kirstin Hoffmann and Alexander Klein for excellent technical support. We also thank Colin Duckett (Departments of Pathology and Internal Medicine, The University of Michigan Medical School) for providing XIAP knockout mice and Clarissa Prazeres da Costa (Institute for Medical Microbiology, Immunology, and Hygiene, Technical University Munich) for providing purified HSP60 from C. pneumoniae.
This work was supported by Sixth Research Framework Program Grant LSHB-CT-2004-005276 (to T. R.).
- IAP
- inhibitor of apoptosis protein
- cIAP
- cellular IAP
- XIAP
- X-linked IAP
- WT
- wild type
- KO
- XIAP knock out
- ConA
- concanavalin A
- PMN
- polymorphonuclear cell
- LPS
- lipopolysaccharide
- cfu
- colony forming units
- iNOS
- inducible nitric-oxide synthase
- TNF
- tumor necrosis factor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- cHSP60
- Chlamydia heat shock protein 60
- PBS
- phosphate-buffered saline
- IL
- interleukin
- FACS
- fluorescence-activated cell sorter
- IFN
- interferon
- MCP
- monocyte chemoattractant protein-1.
REFERENCES
- 1.Hahn D. L., Azenabor A. A., Beatty W. L., Byrne G. I. (2002) Front. Biosci. 7, e66–76 [DOI] [PubMed] [Google Scholar]
- 2.Rajalingam K., Al-Younes H., Müller A., Meyer T. F., Szczepek A. J., Rudel T. (2001) Infect Immun. 69, 7880–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer S. F., Schwarz C., Vier J., Häcker G. (2001) Infect. Immun. 69, 7121–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paland N., Rajalingam K., Machuy N., Szczepek A., Wehrl W., Rudel T. (2006) Cell Microbiol. 8, 1643–1655 [DOI] [PubMed] [Google Scholar]
- 5.Byrne G. I., Ojcius D. M. (2004) Nat. Rev. Microbiol. 2, 802–808 [DOI] [PubMed] [Google Scholar]
- 6.Häcker G., Kirschnek S., Fischer S. F. (2006) Med. Microbiol. Immunol. 195, 11–19 [DOI] [PubMed] [Google Scholar]
- 7.Sharma M., Rudel T. (2009) FEMS Immunol. Med. Microbiol. 55, 154–161 [DOI] [PubMed] [Google Scholar]
- 8.Rajalingam K., Sharma M., Paland N., Hurwitz R., Thieck O., Oswald M., Machuy N., Rudel T. (2006) PLoS Pathog. 2, e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajalingam K., Sharma M., Lohmann C., Oswald M., Thieck O., Froelich C. J., Rudel T. (2008) PLoS One 3, e3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux Q. L., Roy N., Stennicke H. R., Van Arsdale T., Zhou Q., Srinivasula S. M., Alnemri E. S., Salvesen G. S., Reed J. C. (1998) EMBO J. 17, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckelman B. P., Salvesen G. S., Scott F. L. (2006) EMBO Rep. 7, 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiozaki E. N., Chai J., Rigotti D. J., Riedl S. J., Li P., Srinivasula S. M., Alnemri E. S., Fairman R., Shi Y. (2003) Mol. Cell 11, 519–527 [DOI] [PubMed] [Google Scholar]
- 13.Scott F. L., Denault J. B., Riedl S. J., Shin H., Renatus M., Salvesen G. S. (2005) EMBO J. 24, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlin H., Reffey S. B., Duckett C. S., Lindsten T., Thompson C. B. (2001) Mol. Cell. Biol. 21, 3604–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leulier F., Lhocine N., Lemaitre B., Meier P. (2006) Mol. Cell. Biol. 26, 7821–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleino A., Valanne S., Ulvila J., Kallio J., Myllymäki H., Enwald H., Stöven S., Poidevin M., Ueda R., Hultmark D., Lemaitre B., Rämet M. (2005) EMBO J. 24, 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gesellchen V., Kuttenkeuler D., Steckel M., Pelte N., Boutros M. (2005) EMBO Rep. 6, 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh J. R., Foe I., Muro I., Chen C. H., Seol J. H., Yoo S. J., Guo M., Park J. M., Hay B. A. (2007) J. Biol. Chem. 282, 2056–2068 [DOI] [PubMed] [Google Scholar]
- 19.Mahoney D. J., Cheung H. H., Mrad R. L., Plenchette S., Simard C., Enwere E., Arora V., Mak T. W., Lacasse E. C., Waring J., Korneluk R. G. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11778–11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varfolomeev E., Goncharov T., Fedorova A. V., Dynek J. N., Zobel K., Deshayes K., Fairbrother W. J., Vucic D. (2008) J. Biol. Chem. 283, 24295–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauler L. D., Duckett C. S., O'Riordan M. X. (2008) PLoS Pathog. 4, e1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bivas-Benita M., Zwier R., Junginger H. E., Borchard G. (2005) Eur. J. Pharm. Biopharm. 61, 214–218 [DOI] [PubMed] [Google Scholar]
- 23.Arno G., Kaski J. C., Smith D. A., Akiyu J., Zal B., Millar B. C., Moore J. E., Baboonian C. (2005) Br. J. Biomed. Sci. 62, 155–160 [DOI] [PubMed] [Google Scholar]
- 24.Edelson P. J., Cohn Z. A. (1976) J. Exp. Med. 144, 1581–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ly I. A., Mishell R. I. (1974) J. Immunol. Methods 5, 239–247 [DOI] [PubMed] [Google Scholar]
- 26.Fiers W. (1991) FEBS Lett. 285, 199–212 [DOI] [PubMed] [Google Scholar]
- 27.Conway D. J., Holland M. J., Bailey R. L., Campbell A. E., Mahdi O. S., Jennings R., Mbena E., Mabey D. C. (1997) Infect. Immun. 65, 1003–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinemann M., Susa M., Simnacher U., Marre R., Essig A. (1996) Infect. Immun. 64, 4872–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kragsbjerg P., Vikerfors T., Holmberg H. (1998) Respiration 65, 299–303 [DOI] [PubMed] [Google Scholar]
- 30.Netea M. G., Kullberg B. J., Galama J. M., Stalenhoef A. F., Dinarello C. A., Van der Meer J. W. (2002) Eur. J. Immunol. 32, 1188–1195 [DOI] [PubMed] [Google Scholar]
- 31.Zhao H., Li H. (2004) J. Huazhong. Univ. Sci. Technolog. Med. Sci. 24, 630–632 [DOI] [PubMed] [Google Scholar]
- 32.Holtmann H., Shemer-Avni Y., Wessel K., Sarov I., Wallach D. (1990) Infect. Immun. 58, 3168–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paland N., Böhme L., Gurumurthy R. K., Mäurer A., Szczepek A. J., Rudel T. (2008) J. Biol. Chem. 283, 6438–6448 [DOI] [PubMed] [Google Scholar]
- 34.Rottenberg M. E., Gigliotti Rothfuchs A. C., Gigliotti D., Svanholm C., Bandholtz L., Wigzell H. (1999) J. Immunol. 162, 2829–2836 [PubMed] [Google Scholar]
- 35.Bogdan C. (2001) Nat. Immunol. 2, 907–916 [DOI] [PubMed] [Google Scholar]
- 36.Kol A., Lichtman A. H., Finberg R. W., Libby P., Kurt-Jones E. A. (2000) J. Immunol. 164, 13–17 [DOI] [PubMed] [Google Scholar]
- 37.Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. (1999) J. Biol. Chem. 274, 30353–30356 [DOI] [PubMed] [Google Scholar]
- 38.Serbina N. V., Jia T., Hohl T. M., Pamer E. G. (2008) Annu. Rev. Immunol. 26, 421–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinert H., Schwarz P. M., Förstermann U. (2003) Biol. Chem. 384, 1343–1364 [DOI] [PubMed] [Google Scholar]
- 40.Winsauer G., Resch U., Hofer-Warbinek R., Schichl Y. M., de Martin R. (2008) Cell. Signal. 20, 2107–2112 [DOI] [PubMed] [Google Scholar]
- 41.Shimada K., Chen S., Dempsey P. W., Sorrentino R., Alsabeh R., Slepenkin A. V., Peterson E., Doherty T. M., Underhill D., Crother T. R., Arditi M. (2009) PLoS Pathog. 5, e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dohi T., Okada K., Xia F., Wilford C. E., Samuel T., Welsh K., Marusawa H., Zou H., Armstrong R., Matsuzawa S., Salvesen G. S., Reed J. C., Altieri D. C. (2004) J. Biol. Chem. 279, 34087–34090 [DOI] [PubMed] [Google Scholar]
- 43.Prakash H., Becker D., Böhme L., Albert L., Witzenrath M., Rosseau S., Meyer T. F., Rudel T. (2009) PLoS One 4, e6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conte D., Holcik M., Lefebvre C. A., Lacasse E., Picketts D. J., Wright K. E., Korneluk R. G. (2006) Mol. Cell. Biol. 26, 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckelman B. P., Salvesen G. S. (2006) J. Biol. Chem. 281, 3254–3260 [DOI] [PubMed] [Google Scholar]
- 46.Tvinnereim A., Wizel B. (2007) J. Immunol. 179, 3947–3957 [DOI] [PubMed] [Google Scholar]