Abstract
The molecular mechanism underlying the post-Golgi transport of G protein-coupled receptors (GPCRs) remains poorly understood. Here we determine the role of Rab8 GTPase, which modulates vesicular protein transport between the trans-Golgi network (TGN) and the plasma membrane, in the cell surface targeting of α2B- and β2-adrenergic receptors (AR). Transient expression of GDP- and GTP-bound Rab8 mutants and short hairpin RNA-mediated knockdown of Rab8 more potently inhibited the cell surface expression of α2B-AR than β2-AR. The GDP-bound Rab8(T22N) mutant attenuated ERK1/2 activation by α2B-AR, but not β2-AR, and arrested α2B-AR in the TGN compartment. Co-immunoprecipitation revealed that both α2B-AR and β2-AR physically interacted with Rab8 and glutathione S-transferase fusion protein pulldown assays demonstrated that Rab8 interacted with the C termini of both receptors. Interestingly, mutation of the highly conserved membrane-proximal C terminus dileucine motif selectively blocked β2-AR interaction with Rab8, whereas mutation of residues Val431-Phe432-Asn433-Gln434, Pro447-Trp448, Gln450-Thr451, and Trp453 in the C terminus impaired α2B-AR interaction with Rab8. Furthermore, transport inhibition by Rab8(T22N) of a chimeric β2-AR carrying the α2B-AR C terminus was similar to α2B-AR. These data provide strong evidence indicating that Rab8 GTPase interacts with distinct motifs in the C termini of α2B-AR and β2-AR and differentially modulates their traffic from the TGN to the cell surface.
Keywords: G Proteins/Low Molecular Weight, G Proteins/Coupled Receptors (GPCR), Membrane/Trafficking, Protein/Export, Receptors/Structure-Function, Receptor Biosynthesis, Receptor Export Traffic
Introduction
G protein-coupled receptors (GPCRs)3 are the largest superfamily of cell surface receptors that regulate a variety of cell functions by response to a myriad of stimuli. The magnitude of ligand-elicited cellular response is at least in part dictated by the level of receptor expressed at the plasma membrane available for binding to the ligand and subsequently activating downstream effectors (1–3). The cell surface expression of GPCRs at a given time is determined by a number of elaborately regulated trafficking processes, including the export of newly synthesized receptors from the endoplasmic reticulum (ER) through the Golgi to the cell surface, the endocytosis of the cell surface receptors to endosomes upon agonist stimulation, the recycling of the internalized receptor from endosomes to the cell surface, and the transport to lysosome for degradation (4). However, compared with the extensive studies performed on the events of endocytosis, recycling, and degradation (1, 5–10), the molecular mechanisms governing GPCR cell surface transport and its role in regulating receptor function are relatively less well understood.
A number of studies have demonstrated that GPCR export from the ER, the first step in intracellular trafficking of GPCRs, is modulated by specific, conserved motifs located in the membrane-proximal C termini, multiple regulatory proteins that interact with GPCRs, and constitutive dimerization in the ER (4, 11–15). Several studies have also demonstrated that post-Golgi transport of GPCRs is a regulated process. For example, G protein-coupled olfactory and chemokine receptors are released from the ER, but accumulated in the Golgi (16, 17). The opsin mutant E150K is also accumulated in the cis and medial Golgi (18). We have recently identified the YS motif in the N termini, which regulates the exit of α2-adrenergic receptors (α2-ARs) from the Golgi (19).
Rab GTPases are the largest branch of the Ras-related GTPase superfamily, consisting of more than 60 members in mammals and 11 in yeast, and are involved in almost every step of vesicle-mediated protein transport. Each Rab GTPase has a distinct subcellular localization pattern that correlates with the compartments between which it coordinates transport (20). The function of Rab GTPases in coordinating vesicular transport is mediated through their GTP/GDP exchange cycle, which superimposes with Rab protein association with, and dissociation from, subcellular organelle membranes. The inactive, GDP-bound conformation of Rab GTPases is maintained in the cytosol through an association with GDP dissociation inhibitors, which function as chaperones and mediate Rab translocation from cytosol to membrane. Membrane-associated GDP-bound Rab-GDP dissociation inhibitor complexes undergo GDP exchange for GTP. GTP-bound Rab GTPases recruit their effectors, which mediate the migration, docking, and fusion of transport vesicles to acceptor membrane. GTP-bound Rab is then hydrolyzed to GDP-bound Rab for recycling back to donor membrane (20, 21). Therefore, GDP-bound Rab mutants, which are unable to undergo exchange for GTP, and GTP-bound Rab mutants, which prevent the recycling of Rab to be reused, function as negative regulators in protein transport.
Most studies on the roles of Rab GTPases in the intracellular trafficking of GPCRs have been focused on the events involved in the internalization, degradation, and transport between the ER and the Golgi of the receptors (22–24). In contrast, much less is known about the involvement of Rab GTPases in GPCR export from the Golgi to the plasma membrane. Our previous studies have demonstrated the roles of several Rab GTPases in regulating GPCR transport along the early secretory pathway (25–29). In this article, we expand these studies to determine the role of Rab8 GTPase in the cell surface targeting of α2B-AR and β2-AR and the possible mechanism involved. Rab8 GTPase has been demonstrated to regulate protein transport from the trans-Golgi network (TGN) to the apical/basolateral plasma membrane (30–37). Our data demonstrate for the first time that Rab8 differentially modulates the transport of two adrenergic receptors, which is likely determined by direct interactions of Rab8 with distinct motifs in the C termini of the receptors.
EXPERIMENTAL PROCEDURES
Materials
Antibodies against phospho-ERK1/2, green fluorescent protein (GFP), and α2B-AR were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against ERK1/2 were from Cell Signaling Technology. Anti-Rab8 antibodies were from BD Biosciences (San Jose, CA). Anti-p230 antibodies were from Transduction Laboratories (San Diego, CA). Alexa Fluor 594-labeled antibodies, penicillin/streptomycin, l-glutamine, and trypsin/EDTA were from Invitrogen. Isoproterenol (ISO) and UK14304 were obtained from Sigma. [3H]CGP12177 (specific activity = 51 Ci/mmol) and [3H]RX821002 (50 Ci/mmol) were purchased from Amersham Biosciences. All other materials were obtained as described elsewhere (25, 29).
Plasmid Construction
α2B-AR and β2-AR tagged with GFP at their C termini (α2B-AR-GFP and β2-AR-GFP, respectively) were generated as described previously (18). To generate the pcDNA3.1(-) vector containing three HA at the XbaI and XhoI restriction sites, two primers (forward primer: 5′-CTAGAATGTACCCATACGATGTTCCAGATTACGCTTACCCATACGATGTTCCAGATTACGCTTACCCATACGATGTTCCAGATTACGCTGATC-3′; reverse primer, 5′-TCGAGATCAGCGTAATCTGGAACATCGTATGGGTAAGCGTAATCTGGAACATCGTATGGGTAAGCGTAATCTGGAACATCGTATGGGTACATT-3′) encoding three YPYDVPDYA and containing XbaI and XhoI restriction sites were synthesized, annealed, and ligated into the pcDNA3.1(−) vector (Invitrogen), which was digested with XbaI and XhoI. To generate α2B-AR and β2-AR tagged with three HA at its N terminus, the full-length receptors were amplified by PCR. The PCR product was digested with XhoI and HindIII, purified, and ligated to 3HA-tagged pcDNA3.1(−) vector, which was digested with XhoI and HindIII. The GFP and HA epitopes have been used to label GPCRs, including α2B-AR and β2-AR, resulting in receptors with similar characteristics to the wild-type receptors (12, 29, 38, 39).
For generation of the construct β2-AR-ct in which the C-terminal 86 amino acid residues (Arg328–Leu413) was truncated from β2-AR, the full-length β2-AR was amplified by PCR (forward primer, 5′-GATCAAGCTTATGGGGCAACCCGGGAACGGCAGC-3′ and reverse primer, 5′-GATCGTCGACCAGCAGTAGATAAGGGGATTG-3′) in which the β2-AR-ct was in-frame with GFP, restricted with HindIII and SalI, and ligated into the pEGFP-N1 vector. For generation of chimeric receptor β2-ARα2B-ct in which the C terminus of β2-AR was substituted with that of α2B-AR, two complementary oligonucleotides coding the α2B-AR C terminus and carrying the sticky ends of SalI and BamHI were annealed and ligated into the β2-AR-ct-GFP in the pEGFP-N1 vector, which was cleaved with SalI and BamHI.
Rab8 and its GDP-bound mutant T22N tagged with the FLAG epitope at their N termini were kindly provided by Dr. Terrance E. Hebert (Department of Pharmacology, McGill University, Canada). Rab8 was also conjugated with GFP at its N terminus as described previously for Rab2 (29). The α2B-AR and β2-AR gene segments encoding the C termini of the receptors were amplified by PCR and subcloned into the BamHI and XhoI restriction sites of pGEX-4T-1 as described previously (7). Receptor mutants were generated using QuikChange site-directed mutagenesis (Agilent Technologies, La Jolla, CA). The structure of each construct used in the present study was verified by restriction mapping and nucleotide sequence analysis.
Cell Culture and Transient Transfection
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (25). NG108-15 neuroblastoma-glioma cells were cultured in DMEM containing 10% fetal bovine serum, 100 units/ml of penicillin, 100 μg/ml of streptomycin, 100 μm hypoxanthine, 0.4 μm aminopterin, and 16 μm thymidine as described previously (40). HL-1 cardiac myocytes were plated onto fibronectin-gelatin-coated plates or coverslips and cultured in Claycomb medium supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, 100 μg/ml of streptomycin, 0.1 mm norepinephrine, and 2 mm l-glutamine as described previously (26, 41). The human breast cancer MCF-7 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mm glutamine, 1 mm sodium pyruvate, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. Transient transfection of the cells was carried out using Lipofectamine 2000 (Invitrogen) or FuGENE 6 reagents (Roche Diagnostics) as described previously (25, 26). The transfection efficiency was estimated to be greater than 75% based on the GFP fluorescence.
Primary neuronal cultures were prepared from the thalamus of embryonic day 18 rat pups and grown on glass coverslips precoated with poly-l-lysine. After 10 days in vitro, GFP-Rab8 constructs were transfected into the neurons with the calcium phosphate precipitation method as described previously (42). Two days after transfection, the neurons were stained with antibodies recognizing α2B-AR at a dilution of 1:50 for 1 h at room temperature followed by incubation with Alexa 568-conjugated secondary antibodies for 1 h. Images were acquired using a confocal microscope (Zeiss 510 Meta) as described (43). Data were analyzed by NIH Image J software. Specifically, the α2B-AR images were thresholded to remove background. The amounts of the α2B-AR signal pixels in the dendrites (processes 3 μm away from cell body) were calculated and normalized over the same dendrite area containing the Rab8-GFP signal.
Radioligand Binding
Cell surface expression of α2B-AR and β2-AR was measured by ligand binding of intact live cells using [3H]RX821002 and [3H]CGP12177, respectively, as described previously (19, 29). Briefly, cells were cultured in 6-well dishes and transfected with 0.3 μg of the α2B-AR or β2-AR plasmid together with 1.8 μg of Rab8 or its mutant or empty vectors. After 6 h the cells were split into 12-well plates at a density of 4 × 105 cells/well and cultured for an additional 24 h. For measurement of α2B-AR expression at the cell surface, the cells were incubated with DMEM plus [3H]RX821002 at a concentration of 20 nm for 90 min at room temperature. The nonspecific binding was determined in the presence of rauwolscine (10 μm). For measurement of β2-AR expression at the cell surface, the cells were incubated with DMEM containing the ligand [3H]CGP12177 at a concentration of 20 nm for 90 min at room temperature. Nonspecific binding was determined by preincubation with alprenolol at a concentration of 20 μm for 30 min followed by incubation with [3H]CGP12177 (20 nm) in the presence of alprenolol (20 μm). The cells were washed twice with 1 ml of ice-cold phosphate-buffered saline (PBS), and the cell surface-bound ligands were extracted by 1 m NaOH treatment for 2 h. The radioactivity was counted by liquid scintillation spectrometry in 3.5 ml of Ecoscint A scintillation solution (National Diagnostics, Inc., Atlanta, GA).
shRNA-mediated Knockdown of Endogenous Rab8
Human Rab8-specific and scrambled short hairpin RNAs (shRNA) in the psiSTRIKE hMGFP vector were generously provided by Dr. Johan Peranen (Institute of Biotechnology, University of Helsinki, Finland) as described (44). HEK293 and MCF-7 cells were cultured on 6-well dishes and transfected with 2 μg of control or Rab8 shRNA with or without co-transfection with HA-tagged receptors. After transfection for 72 h, cell surface expression of the receptors was measured by intact cell ligand binding.
Measurement of ERK1/2 Activation
HEK293 cells were cultured in 6-well dishes and transfected with 0.5 μg of α2B-AR or β2-AR with or without co-transfection with 1.5 μg of Rab8(T22N). At 6–8 h after transfection, the cells were split into 6-well dishes and cultured for an additional 36 h. The cells were starved for at least 3 h and then stimulated with 1 μm UK14304 or ISO for 5 min. For measurement of ERK1/2 activation by endogenous α2B-AR in NG108 cells, the cells were cultured in 6-well plates and transfected with 2 μg of Rab8(T22N) with FuGENE 6 reagent in serum-free medium for 24 h. The cells were then cultured in fresh serum-free medium for 3 h and stimulated with 1 μm UK14304 for 15 min. Stimulation was terminated by addition of 1× SDS gel loading buffer. After solubilizing the cells, 20 μl of total cell lysates was separated by 12% SDS-PAGE. ERK1/2 activation was determined by measuring the levels of phosphorylation of ERK1/2 with phospho-specific ERK1/2 antibodies by immunoblotting (25).
Measurement of cAMP Production
cAMP production in response to stimulation with ISO was measured by using the cAMP enzyme immunoassay system (Biotrak, Amersham Biosciences, Piscataway, NJ) as described previously (45). HEK293 cells were cultured in 100-mm dishes and transfected with 3 μg of β2-AR. After 6 h, the cells were split into 12-well plates and cultured for 12 h. The cells were then starved for 24 h before stimulation with ISO at a concentration of 10 μm for 10 min. The reactions were stopped by aspirating the medium and then the cells were lysed using 200 μl of dodecyltrimethylammonium (2.5%). One hundred μl of cell lysate was transferred into microtiter plates and incubated with anti-cAMP antiserum, followed by incubation with cAMP peroxidase. After washing and addition of substrate, peroxidase activity was measured by spectrometry. cAMP concentrations were calculated based on the competition of cAMP in samples with a fixed quantity of peroxidase-labeled cAMP.
Immunofluorescence Microscopy
For fluorescence microscopic analysis of receptor subcellular localization, HEK293 cells were grown on coverslips pre-coated with poly-l-lysine in 6-well plates and transfected with 50 ng of GFP-tagged receptors with or without co-transfection together with 400 ng of Rab8(T22N). The cells were fixed with 4% paraformaldehyde, 4% sucrose mixture in PBS for 15 min. For co-localization studies involving immunostaining, HEK293 cells were permeabilized with PBS containing 0.2% Triton X-100 for 5 min, and blocked with 5% normal donkey serum for 1 h. The cells were then incubated with p230 antibodies for 1 h. After washing with PBS (3 × 5 min), the cells were incubated with Alexa Fluor 594-labeled secondary antibody (1:2000 dilution) for 1 h at room temperature. The coverslips were mounted, and fluorescence was detected with a Leica DMRA2 epifluorescent microscope as described previously (25). Images were deconvolved using SlideBook software and the nearest neighbors deconvolution algorithm (Intelligent Imaging Innovations, Denver, CO).
Co-immunoprecipitation of Receptors and Rab8 GTPase
HEK293 cells cultured on 100-mm dishes were transfected with 2 μg of HA-tagged receptor together with 2 μg of the pEGFP-C1 vector or GFP-tagged Rab8 GTPase in the pEGFP-C1 vector for 28 h. The cells were washed twice with PBS, harvested, and lysed with 500 μl of lysis buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS and Complete Mini protease inhibitor mixture. After gentle rotation for 1 h, samples were centrifuged for 15 min at 14,000 × g and the supernatant was incubated with 50 μl of protein G-Sepharose for 1 h at 4 °C to remove nonspecific bound proteins. Samples were then incubated with 3 μg of anti-HA antibodies overnight at 4 °C with gentle rotation followed by incubation with 50 μl of protein G-Sepharose 4B beads for 5 h. Resin was collected by centrifugation and washed three times each with 500 μl of lysis buffer without SDS. Immunoprecipitated receptors were eluted with 100 μl of 1× SDS gel loading buffer. Thirty μl from each sample was then separated by SDS-PAGE to probe for GFP-Rab8 in the immunoprecipitates by immunoblotting using GFP antibodies. In parallel, each sample was further diluted 5 times with 1× SDS gel loading buffer, separated by SDS-PAGE, and probed with anti-HA antibodies to determine the amount of the receptor in the immunoprecipitates (19).
Protein Interaction Assay
The GST fusion proteins were expressed in bacteria and purified using a glutathione affinity matrix as described previously (5, 7). Immobilized fusion proteins were either used immediately or stored at 4 °C for no longer than 3 days. Each batch of fusion protein used in experiments was first analyzed by Coomassie Blue staining following SDS-PAGE. Total HEK293 cell homogenates were incubated with ∼5 μg of GST fusion proteins tethered to the glutathione resin in 250 μl of binding buffer containing 20 mm Tris-HCl, pH 7.5, 70 mm NaCl, and 2% Nonidet P-40 for 2.5 h at 4 °C. The resin was washed three times with 0.5 ml of binding buffer, and the retained proteins were solubilized in SDS gel loading buffer and separated by SDS-PAGE. Rab8 GTPase bound to the GST fusion proteins was detected by immunoblotting using anti-Rab8 antibodies.
Statistical Analysis
Differences were evaluated using Student's t test, and p < 0.05 was considered as statistically significant. Data are expressed as the mean ± S.E.
RESULTS
Differential Regulation of the Cell Surface Expression of α2B-AR and β2-AR by Rab8 GTPase
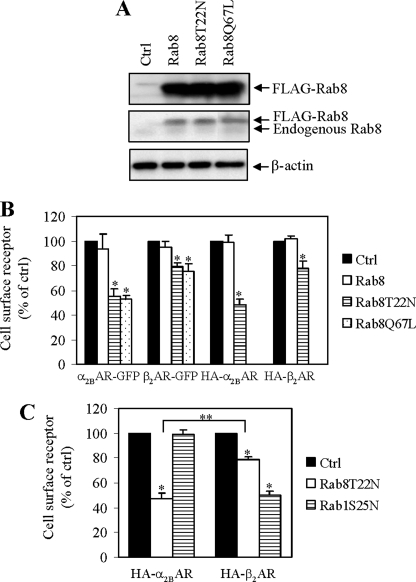
We first determined the effect of manipulating the function of Rab8 by transient expression of wild-type Rab8 and its GDP-bound Rab8(T22N) and GTP-bound Rab8(Q67L) mutants on cell surface expression of α2B-AR and β2-AR. Both receptors were tagged with either GFP at their C termini or three HA at their N termini. Rab8 was tagged with FLAG at its N terminus and expressed together with individual receptors in HEK293 cells. Rab8 expression was measured by immunoblotting using anti-FLAG and anti-Rab8 antibodies (Fig. 1A) and the cell surface expression of α2B-AR and β2-AR was quantified by ligand binding in intact live cells using the membrane-impermeable ligands [3H]RX821002 and [3H]CGP12177, respectively. Transient expression of wild-type Rab8 did not significantly alter the cell surface numbers of either receptor (Fig. 1B). In contrast, expression of Rab8(T22N) and Rab8(Q67L) inhibited the cell surface expression of α2B-AR and β2-AR, but at different magnitudes. Rab8(T22N) and Rab8(Q67L) inhibited the cell surface transport of α2B-AR by 45%, whereas the cell surface expression of β2-AR was moderately attenuated by 20% (Fig. 1B). Similar results were obtained with GFP- and HA-tagged receptors. The overall expression of these receptors was not significantly altered by Rab8 and its mutants as determined by measuring the total GFP signal (data not shown). As the total cell surface numbers of α2B-AR and β2-AR were similar (about 350 pmol/well), their differential inhibition by the Rab8 mutants was not due to the different cell surface expression levels of the receptors.
FIGURE 1.
Effect of transient expression of Rab8, Rab8(T22N), and Rab8(Q67L) on the cell surface expression of α2B-AR and β2-AR in HEK293 cells. A, Western blot analysis of Rab8 expression. HEK293 cells cultured on 6-well plates were transfected with the pcDNA vector (Ctrl) or FLAG-Rab8. Cell homogenates were separated by 12% SDS-PAGE and expression of Rab8 was detected by Western blotting using anti-FLAG (upper panel) and anti-Rab8 antibodies (middle panel). β-Actin expression is used as a control (lower panel). B, effect of transient expression of Rab8, Rab8(T22N), and Rab8(Q67L) on cell surface expression of α2B-AR and β2-AR. HEK293 cells were transfected with α2B-AR or β2-AR tagged with either GFP at their C termini or three HA at their N termini together with the pcDNA vector (Ctrl) or FLAG-Rab8. C, effect of Rab8(T22N) on the cell surface expression of α2B-AR and β2-AR when expressed in the same cell populations. HA-α2B-AR and HA-β2-AR were co-expressed together with either Rab8(T22N) or Rab1(S25N) in HEK293 cells. The expression of α2B-AR and β2-AR at the cell surface was determined by intact cell ligand binding using [3H]RX821002 and [3H]CGP12177, respectively, as described under “Experimental Procedures.” The data shown in B and C are percentages of the mean value obtained from cells transfected with the pcDNA vector and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus Ctrl and **, p < 0.05 between HA-α2B-AR and HA-β2-AR in the presence of Rab8(T22N).
To further define if Rab8 GTPase could differentially regulate the cell surface expression of α2B-AR and β2-AR, both receptors were co-expressed in the same cell populations with or without co-expression with Rab8(T22N) or Rab1(S25N) and the cell surface expression of α2B-AR and β2-AR was measured by intact cell ligand binding. We have previously demonstrated that β2-AR transport to the cell surface is mediated through a Rab1-dependent pathway, whereas cell surface transport of α2B-AR uses a non-conventional, Rab1-independent pathway (25). Consistent with our previous report, Rab1(S25N) selectively attenuated the cell surface number of β2-AR, but not α2B-AR. In contrast, Rab8(T22N) inhibited the cell surface transport of both receptors. The inhibitory effect on α2B-AR was significantly higher than that on β2-AR (Fig. 1C).
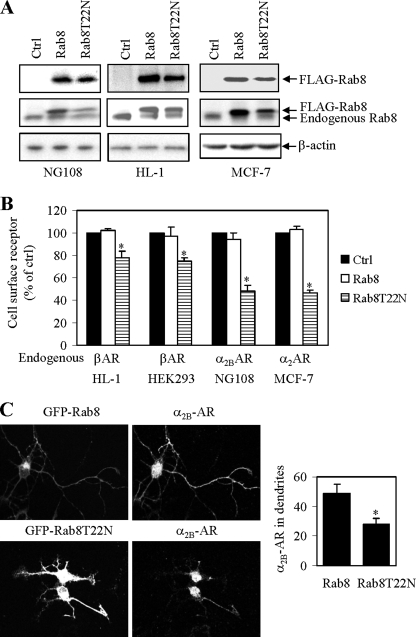
We then determined the effect of transient expression of Rab8 and Rab8(T22N) (Fig. 2A) on cell surface expression of endogenous receptors. NG108 and MCF-7 cells express endogenous α2B-AR, whereas HEK293 and HL-1 cells express endogenous β-AR. Similar to the results obtained in HEK293 cells transfected with exogenous receptors, Rab8(T22N) significantly inhibited the cell surface expression of endogenous α2-AR in NG108 and MCF-7 cells by about 50%, whereas the cell surface expression of endogenous β-AR in HEK293 and HL-1 cells was inhibited by about 20% (Fig. 2B). These data further suggest that Rab8 differentially modulates the export trafficking of α2B-AR and β2-AR.
FIGURE 2.
Effect of transient expression of Rab8 and Rab8(T22N) on cell surface expression of endogenous α2-AR and β2-AR. A, Western blot analysis of Rab8 expression. NG108, HL-1, and MCF-7 cells cultured on 6-well plates were transfected with the pcDNA vector (Ctrl) or FLAG-Rab8. Cell homogenates were separated by 12% SDS-PAGE and expression of Rab8 was detected by Western blotting using anti-FLAG (upper panels) and anti-Rab8 antibodies (middle panels). β-Actin expression is used as a control (lower panels). B, effect of transient expression of Rab8 on cell surface expression of endogenous α2-AR and β-AR. The cell surface expression of α2-AR in NG108 and MCF-7 cells and β-AR in HL-1 and HEK293 cells was determined by intact cell ligand binding using [3H]RX821002 and [3H]CGP12177, respectively, as described under “Experimental Procedures.” The data shown are percentages of the mean value obtained from cells transfected with the pcDNA vector and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus ctrl. C, effect of Rab8 on the transport of α2B-AR to the dendrites in the primary neurons. Left panel, representative images of α2B-AR antibody staining in neurons expressing GFP-Rab8. α2B-AR staining in the nuclei is likely nonspecific. Right panel, quantitative data. The data shown are percentages of the dendritic area that were stained with anti-α2B-AR antibodies and are presented as the mean ± S.E. (n = 9). *, p < 0.05 versus wild-type Rab8.
Our preceding data suggest that Rab8 more potently influences the cell surface targeting of α2B-AR than β2-AR in cell lines. We then asked the question if Rab8 could play a role in the transport of α2B-AR in the primary cultures of neurons isolated from the thalamus, which has been demonstrated to highly express endogenous α2B-AR (46). In the primary neurons, transient expression of Rab8(T22N) for 2 days significantly reduced α2B-AR expression in the dendrites by 44% as compared with that in neurons transfected with wild-type Rab8 (Fig. 2C).
Effect of shRNA-mediated Knockdown of Rab8 on the Cell Surface Expression of α2B-AR and β2-AR
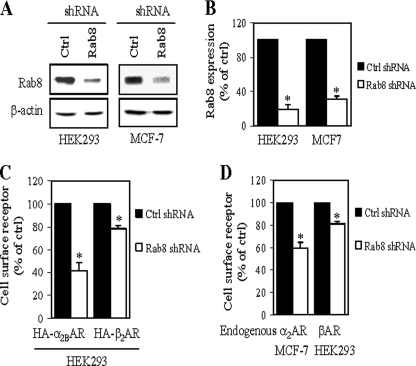
To further define the role of Rab8 in regulating the cell surface transport of α2B-AR and β2-AR, we determined the effect of shRNA-mediated knockdown of endogenous Rab8. Transient expression of shRNA targeting onto human Rab8 in HEK293 and MCF-7 cells markedly reduced the expression level of endogenous Rab8 as compared with cells transfected with control shRNA (Fig. 3, A and B). We then determined the effect of Rab8 shRNA on the cell surface expression of exogenously transfected HA-α2B-AR and HA-β2-AR and endogenous α2-AR and β-AR in HEK293 and MCF-7 cells. Similar to transient expression of the dominant-negative Rab8 mutants, down-regulation of endogenous Rab8 by shRNA transfection resulted in significant decreases in the cell surface expression of HA-α2B-AR and HA-β2-AR by 58 and 22%, respectively (Fig. 3C). The cell surface expression of endogenous α2-AR in MCF-7 cells and endogenous β-AR in HEK293 cells were attenuated by 40 and 19%, respectively, by Rab8 shRNA (Fig. 3D). These data further suggest that the normal function of Rab8 GTPase is required for the cell surface transport of both α2B-AR and β2-AR.
FIGURE 3.
Effect of shRNA-mediated knockdown of Rab8 on cell surface expression of α2-AR and β2-AR. A, reduction of Rab8 expression induced by shRNA targeting. HEK293 and MCF-7 cells cultured on 6-well dishes were transiently transfected with control shRNA (Ctrl) or Rab8 shRNA as described under “Experimental Procedures.” At 72 h after transfection, total homogenate proteins was separated by 12% SDS-PAGE, and expression of Rab8 was detected by Western blotting using anti-Rab8 antibodies. Representative blots of Rab8 (upper panels) and β-actin (lower panels) expression are shown. B, quantitative data of Rab8 expression. C, effect of shRNA-mediated knockdown of Rab8 on the cell surface expression of exogenously transfected HA-α2B-AR and HA-β2-AR in HEK293 cells. HEK293 cells were cotransfected with HA-α2-AR or HA-β2-AR together with control shRNA or Rab8 shRNA. D, effect of Rab8 shRNA on the cell surface expression of endogenous α2-AR in MCF-7 cells and β-AR in HEK293 cells. Cells were transfected with control or Rab8 shRNA. The cell surface expression of α2-AR and β-AR was determined by intact cell ligand binding using [3H]RX821002 and [3H]CGP12177, respectively, as described under “Experimental Procedures.” The data shown are percentages of the mean value obtained from cells transfected with control shRNA and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus control shRNA.
Effect of Rab8 on α2B-AR Signaling
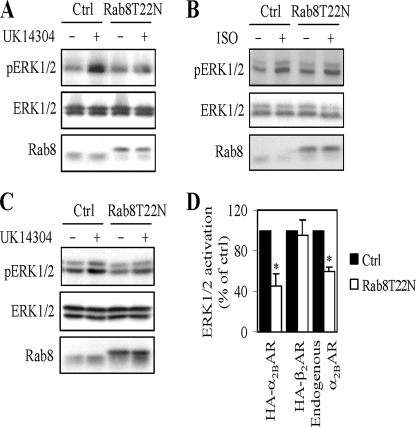
To further confirm that Rab8 GTPase modulated the transport of α2B-AR and β2-AR to the cell surface as measured by intact cell ligand binding and to define if Rab8 was capable of altering receptor signaling, we measured the effect of transient expression of Rab8(T22N) on ERK1/2 activation in response to stimulation with receptor agonists. ERK1/2 activation by UK14304 was reduced by 60% in HEK293 cells expressing α2B-AR and Rab8(T22N) compared with cells transfected receptor alone (Fig. 4, A and D). Furthermore, Rab8(T22N) expression also significantly attenuated ERK1/2 activation by endogenous α2B-AR in response to stimulation with UK14304 in NG108 cells (Fig. 4, C and D). In contrast, Rab8(T22N) did not significantly influence ERK1/2 activation by ISO in cells expressing β2-AR (Fig. 4, B and D). These data indicate that inhibition of Rab8 function by expressing its dominant-negative mutants is able to differentially modulate the cell surface expression and signaling of α2B-AR and β2-AR.
FIGURE 4.
Effect of Rab8 on the signaling of α2-AR and β2-AR. A and B, effect of Rab8 on ERK1/2 activation in HEK293 cells transfected with α2B-AR (A) and β2-AR (B). HEK293 cells were transfected with α2B-AR or β2-AR together the pcDNA vector (Ctrl) or Rab8(T22N) and stimulated with UK14304 (A) or ISO (B) at a concentration of 1 μm for 5 min. C, effect of Rab8 on ERK1/2 activation by endogenous α2B-AR in NG108 cells. NG108 cells were transfected with the pcDNA vector (Ctrl) or Rab8(T22N) and stimulated with UK14304 at a concentration of 1 μm for 15 min. ERK1/2 activation was determined by Western blot analysis using phospho-specific ERK1/2 antibodies. Upper panels, representative blots showing ERK1/2 activation; middle panels, total ERK1/2 expression; lower panels, Rab8 expression. D, quantitative data expressed as percentages of ERK1/2 activation obtained from cells transfected with control vector and stimulated with the agonist and presented as the mean ± S.E. of three experiments. *, p < 0.05 versus ctrl.
Rab8(T22N) Modulates Receptor Transport at the TGN
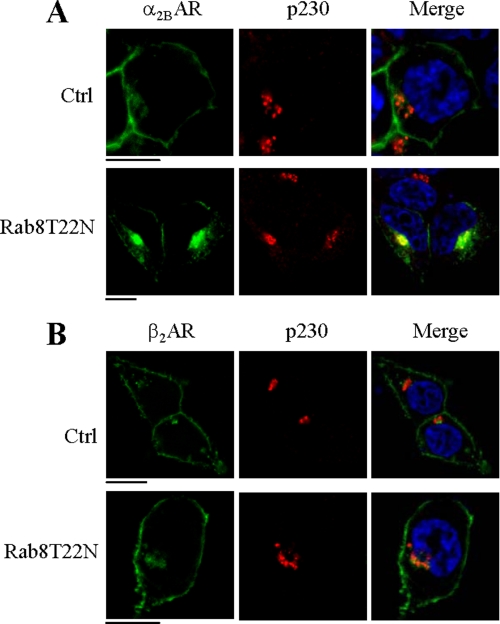
To define the intracellular compartment in which Rab8 regulates receptor transport, GFP-tagged α2B-AR and β2-AR was expressed together with Rab8(T22N) and the subcellular distribution of the receptors was visualized by co-localizing with different intracellular marker proteins. α2B-AR was strongly co-localized with the TGN marker p230 (Fig. 5A), but not with the ER marker calregulin (data not shown). In contrast, the majority of β2-AR was expressed at the cell surface in cells expressing Rab8(T22N) (Fig. 5B). These results are consistent with the differential influences of Rab8 on the cell surface expression of α2B-AR and β2-AR as measured by intact cell ligand binding. These data also suggest that Rab8(T22N) arrested α2B-AR in the TGN compartment, which is consistent with the Rab8 function in the TGN to plasma membrane traffic (31, 32).
FIGURE 5.
Effect of Rab8 on the subcellular distribution of α2B-AR and β2-AR. HEK293 cells cultured on coverslips were transfected with GFP-tagged α2B-AR or β2-AR together with the pcDNA vector (Ctrl) or Rab8(T22N). The cells were then stained with anti-p230 antibodies (1:200 dilution). Green, receptors; red, the TGN marker p230; blue, DNA staining by 4,6-diamidino-2-phenylindole (nucleus); yellow, co-localization of receptors with p230. The data are representative images of at least three independent experiments. Scale bars, 10 μm.
Association of α2B-AR and β2-AR with Rab8 GTPase
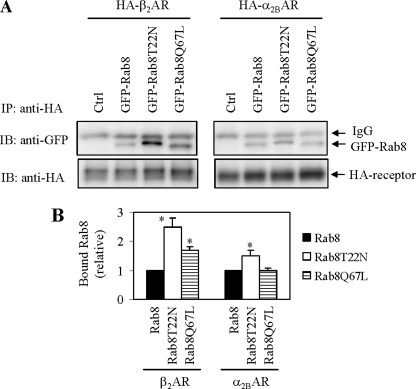
To elucidate the molecular mechanism underlying the regulation of AR export trafficking by Rab8 GTPase, we determined if both α2B-AR and β2-AR were capable of associating with Rab8 GTPase. HA-tagged α2B-AR or β2-AR were transiently expressed together with GFP-tagged Rab8, Rab8(T22N), or Rab8(Q67L) in HEK293 cells and the receptors were immunoprecipitated using anti-HA antibodies. Rab8 in the immunoprecipitates was determined by Western blotting using anti-GFP antibodies. Rab8 was found in the immunoprecipitates from cells expressing α2B-AR or β2-AR (Fig. 6A). Interestingly, the amounts of Rab8 in both receptor immunoprecipitates were higher in cells expressing Rab8(T22N) than in cells expressing Rab8 or Rab8(Q67L) (Fig. 6, A and B). These data suggest that both α2B-AR and β2-AR are able to interact with Rab8 GTPase, preferentially in its inactive, GDP-bound form.
FIGURE 6.
Co-immunoprecipitation of α2B-AR and β2-AR with Rab8 GTPase. A, HEK293 cells were transiently transfected with HA-α2B-AR (right panel) or HA-β2-AR (left panel) together with the pEGFP-C1 vector (Ctrl) or GFP-Rab8. The cells were solubilized and the receptors were immunoprecipitated with anti-HA antibodies as described under “Experimental Procedures.” The immunoprecipitate was separated by SDS-PAGE and the level of Rab8 in the HA immunoprecipitate was determined by Western blotting using anti-GFP antibodies (upper panels) and the immunoprecipitated receptor was revealed using anti-HA antibodies (lower panels). B, quantitative data presented as the mean ± S.E. of three experiments. *, p < 0.05 versus Rab8. IB, immunoblot.
Interaction of the C Termini of α2B-AR and β2-AR with Rab8 GTPase
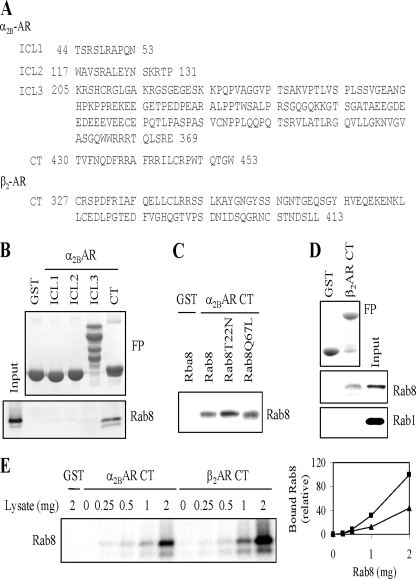
To identify the motif in α2B-AR that mediates receptor interaction with Rab8, the intracellular domains of α2B-AR including the first (ICL1, 10 residues), second (ICL2, 15 residues), and third (ICL3, 165 residues) intracellular loops and the C terminus (CT, 24 residues) (Fig. 7A) were generated as GST fusion proteins and their abilities to interact with Rab8 were determined in a GST fusion protein pulldown assay. The GST fusion proteins containing the C terminus, but not ICL1, ICL2, and ICL3 fusion proteins, were capable of interacting with Rab8 (Fig. 7B). The interaction of the GST-C-terminal fusion protein with GDP-bound Rab8(T22N) was stronger than Rab8 and Rab8(Q67L) (Fig. 7C), consistent with the co-immunoprecipitation results using the intact receptors.
FIGURE 7.
Rab8 interaction with the C termini of α2B-AR and β2-AR. A, sequences of the first (ICL1), second (ICL12), and third (ICL3) intracellular loops and the C terminus of α2B-AR and the C terminus of β2-AR generated as GST fusion proteins. B, Rab8 GTPase interaction with different intracellular domains of α2B-AR generated as GST fusion proteins. GFP-tagged Rab8 was expressed in HEK293 cells and total cell homogenates were incubated with GST fusion proteins as described under “Experimental Procedures.” Rab8 interaction with the fusion proteins was revealed by immunoblotting using anti-GFP antibodies. Upper panel, Coomassie Blue staining of purified GST fusion proteins; lower panel, GFP-Rab8 tethered to the GST fusion proteins. C, the α2B-AR C terminus interaction with Rab8, GDP-bound mutant Rab8(T22N), and GTP-bound mutant Rab8(Q67L). GFP-tagged Rab8 and its mutants were expressed in HEK293 cells and cell extracts were incubated with the GST-α2B-AR C terminus fusion protein. D, the interactions of Rab8 and Rab1 GTPases with the β2-AR C terminus. GFP-tagged Rab8 and Rab1 were expressed in HEK293 cells and total cell extracts were incubated with the GST fusion protein encoding the β2-AR C terminus and the interactions of Rab1 and Rab8 with the fusion protein was determined as in B. Upper panel, Coomassie Blue staining of purified GST fusion proteins; middle panel, GFP-Rab8 tethered to the GST fusion protein; lower panel, GFP-Rab1 bound to the GST fusion protein. E, comparison of Rab8 binding to the C termini of α2B-AR and β2-AR. GST-α2BAR CT and GST-β2AR CT were incubated with increasing concentrations of Rab8. Left panel, a representative blot; right panel, quantitative data showing Rab8 interaction with the α2B-AR C termini (triangles) and the β2-AR C termini (squares). Similar results were obtained in three experiments.
We then determined if the β2-AR C terminus was also able to interact with Rab8 GTPase. Similar to the α2B-AR C terminus, the β2-AR C terminus consisting of 87 residues interacted with Rab8 in the GST fusion protein pulldown assay (Fig. 7D). In contrast to Rab8, Rab1 GTPase did not interact with the β2-AR C terminus fusion protein (Fig. 7D). These data indicate that the α2B-AR and β2-AR C termini are able to interact with Rab8 GTPase.
We next sought to determine relative binding affinities of the C termini of α2B-AR and β2-AR for Rab8. GST fusion proteins containing the α2B-AR or β2-AR C terminus were incubated with increasing concentrations of total cell homogenates prepared from cells transfected with GFP-Rab8 (from 0 to 2 mg). At lower concentrations of Rab8, both fusion proteins did not clearly bind Rab8. At higher concentrations of Rab8, the β2-AR C terminus consistently bound more Rab8 than the α2B-AR C terminus (Fig. 7E).
Identification of the Rab8-binding Sites in the C Termini of α2B-AR and β2-AR
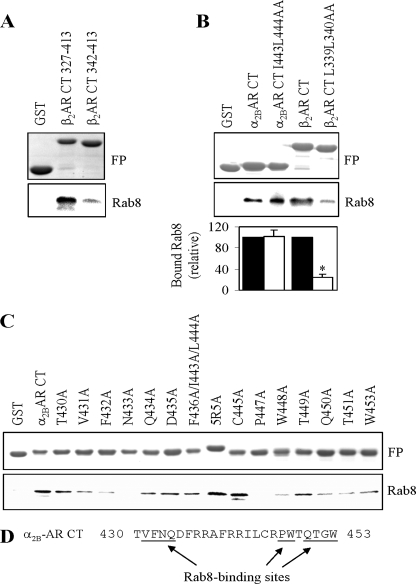
To identify specific residues in the C termini interacting with Rab8, we first focused on the β2-AR C terminus and determined if the membrane-proximal α-helix region (Cys327–Cys341) is essential for interaction with Rab8. Rab8 interaction with the β2-AR C-terminal fusion protein containing the region Leu342–Leu413 (lacking the region Cys327–Cys341) was markedly reduced as compared with the whole C-terminal fusion protein (Fig. 8A).
FIGURE 8.
Identification of Rab8 binding motifs in the C termini of α2B-AR and β2-AR. A, effect of removing the membrane-proximal C-terminal α-helix region on the interaction of the C terminus of β2-AR with Rab8 GTPase. B, effect of mutating the LL motif on the interaction of the α2B-AR and β2-AR C termini with Rab8. Bottom panel, quantitative data presented as the mean ± S.E. of three experiments. *, p < 0.05 versus β2-AR CT. C, site-directed alanine scanning mutagenesis to identify Rab8 binding sites in the C terminus of α2B-AR. The β2-AR C terminus and its truncated form lacking the region Cys327–Cys341 (A), the α2B-AR and β2-AR termini and their mutants in which the LL motif were mutated to alanines (B), and the α2B-AR C-terminal mutants in which each residue was individually or in combination mutated to alanines (C) were generated as GST fusion proteins and their abilities to interact with GFP-Rab8 GTPase were determined as described in the legend of Fig. 7. Similar results were obtained in at least three independent experiments. In A–C, upper panels, Coomassie Blue staining of GST fusion proteins; lower panels, GFP-Rab8 bound to the GST fusion proteins revealed by immunoblotting using anti-GFP antibodies. D, a summary of the Rab8-binding sites in the C terminus of α2B-AR as demonstrated in C.
We then determined the effect of mutating the dileucine (Leu339–Leu340) motif to alanines on the interaction with Rab8. Similar to the deletion of the LL-containing region Cys327–Cys341, mutation of the LL motif in the β2-AR C terminus dramatically reduced the interaction with Rab8 (Fig. 8B). Surprisingly, mutation of the Ile443–Leu444 motif in the α2B-AR C terminus did not influence Rab8 interaction (Fig. 8B). These data demonstrate that the LL motif specifically mediates the interaction of β2-AR, but not α2B-AR, with Rab8 GTPase.
To further search for the Rab8 binding motif in α2B-AR, each residue in the α2B-AR C terminus was mutated to alanine individually or in combination and the effect on the interaction with Rab8 was tested by GST fusion protein pulldown assays. Mutation of Thr430, Asp435, Phe436/Ile443/Leu444, five Arg (Arg437/Arg438/Arg441/Arg442/Arg446), and Thr449 did not influence Rab8 binding. In contrast, mutation of Val431, Phe432, Gln434, Trp448, Gln450, Thr451, and Trp453 clearly inhibited and mutation of Asn433 and Pro447 almost abolished Rab8 binding (Fig. 8C). These data suggest that multiple Rab8 binding sites exist in the membrane proximal region and distal end of the α2B-AR C terminus (Fig. 8D).
Effect of the α2B-AR C Terminus on the β2-AR Responsiveness to Rab8(T22N)
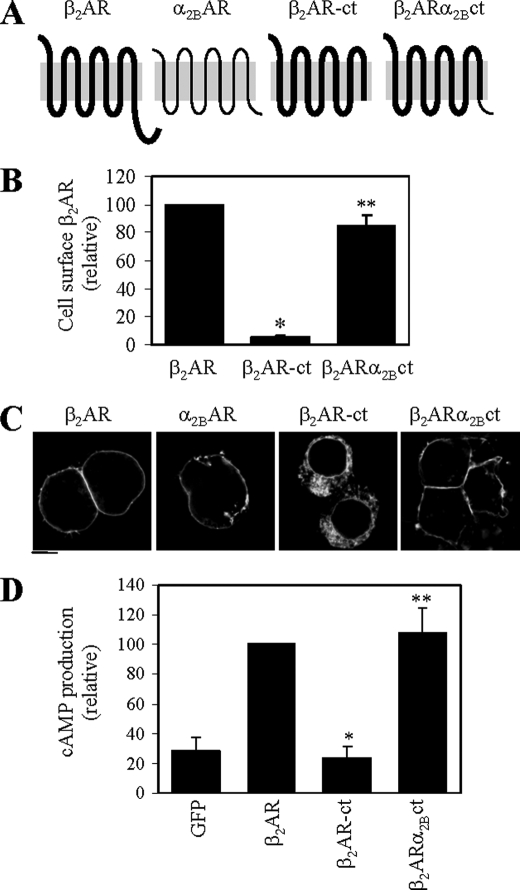
Our preceding data have demonstrated that Rab8 differentially modulates the cell surface expression of α2B-AR and β2-AR and interacts with distinct motifs in the C termini of the receptors. To test if the differential regulation of α2B-AR and β2-AR by Rab8 is determined by the C termini of the receptors, we measured the effect of substituting the β2-AR C terminus with the α2B-AR C terminus on the β2-AR response to Rab8(T22N). We generated a chimeric receptor, β2-ARα2B-ct in which the β2-AR C terminus was replaced by the α2B-AR C terminus (Fig. 9A). Truncation of the C terminus from β2-AR (β2-AR-ct) dramatically blocked receptor transport to the cell surface as measured by intact cell ligand binding (Fig. 9B) and subcellular localization (Fig. 9C). In contrast to β2-AR-ct, the chimeric receptor β2-ARα2B-ct retained its abilities to transport to the cell surface (Fig. 9, B and C), which are comparable with its wild-type counterpart. Consistently, cAMP production in response to ISO stimulation was almost completely inhibited in cells expressing β2-AR-ct and was about normal in cells expressing β2-ARα2B-ct as compared with cells expressing β2-AR (Fig. 9D).
FIGURE 9.
Characterization of the chimeric receptor β2-ARα2B-ct. A, a diagram showing generation of the chimeric adrenergic receptor β2-ARα2B-ct, in which the C terminus of β2-AR was substituted with that of α2B-AR. β2-AR-ct is a β2-AR mutant in which the C-terminal 87 residues was deleted. B, cell surface expression of β2-AR, β2-AR-ct, and β2-ARα2B-ct. Receptors were transiently expressed in HEK293 cells and their cell surface expression was measured by intact cell ligand binding using the radioligand [3H]CGP12177 as described in the legend of Fig. 1. The data shown are percentages of the mean value obtained from cells transfected with β2-AR and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus cells transfected with β2-AR; **, p < 0.05 versus cells transfected with β2-AR-ct. C, the subcellular distribution of β2-AR, β2-AR-ct, and β2-ARα2B-ct. HEK293 cells cultured on coverslips were transfected with GFP-conjugated receptors and the subcellular distribution of the receptors was revealed by fluorescence microscopy detecting GFP as described under “Experimental Procedures.” D, cAMP production in cells expressing β2-AR, β2-AR-ct, or β2-ARα2B-ct. HEK293 cells were cultured in 10-cm plates and transfected with 3 μg of β2-AR, β2-AR-ct, or β2-ARα2B-ct and then stimulated with ISO (10−5 m) for 10 min. cAMP concentrations were determined by using the cAMP enzyme immunoassay system as described under “Experimental Procedures.” The data shown are percentages of the mean value obtained from cells transfected with β2-AR and stimulated with ISO and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus cells transfected with β2-AR and stimulated with ISO; **, p < 0.05 versus cells transfected with β2-AR-ct and stimulated with ISO.
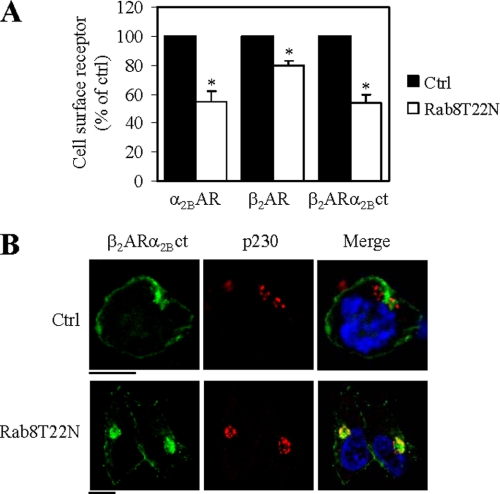
We then determined the effect of Rab8(T22N) on the cell surface expression of the chimeric receptor. Measurement of the cell surface receptor expression revealed that, in contrast to β2-AR, β2-ARα2B-ct transport to the cell surface was inhibited by 45% (Fig. 10A). Such an inhibitory effect of Rab8(T22N) on the transport of β2-ARα2B-ct was very much similar to that on α2B-AR (Fig. 10A). The chimeric receptor β2-ARα2B-ct was partially co-localized with p230 in cells expressing Rab8(T22N) (Fig. 10B). These data suggest that the C termini of α2B-AR and β2-AR play an important role in defining the sensitivity of the receptors in response to the inhibition of Rab8 function.
FIGURE 10.
Effect of the α2B-AR C terminus on β2-AR responsiveness to Rab8(T22N) inhibition. A, effect of transient expression of Rab8(T22N) on the cell surface expression of β2-ARα2B-ct. HEK293 cells were transfected with α2B-AR, β2-AR, or β2-ARα2B-ct together with the pcDNA vector (control) or FLAG-Rab8(T22N). The cell surface expression of the receptors was determined by intact cell ligand binding. The data shown are percentages of the mean value obtained from cells transfected with individual receptors and the pcDNA vector and are presented as the mean ± S.E. of three experiments. *, p < 0.05 versus Ctrl. B, effect of Rab8(T22N) on the subcellular distribution of β2-ARα2B-ct. HEK293 cells cultured on coverslips were transfected with GFP-tagged β2-ARα2B-ct together with the pcDNA vector (ctrl) or Rab8(T22N). The cells were then stained with anti-p230 antibodies (1:200 dilution). Green, receptors; red, the TGN marker p230; blue, DNA staining by 4,6-diamidino-2-phenylindole (nucleus); yellow, co-localization of receptors with p230. The data are representative images of at least three independent experiments. Scale bars, 10 μm.
DISCUSSION
The molecular mechanism underlying the transport of nascent GPCRs from the Golgi to the functional destination, the plasma membrane, remains poorly understood. This issue is addressed in this article by determining the functional role of Rab8 GTPase, which has been demonstrated to modulate vesicular protein transport from the TGN to the apical/basolateral plasma membrane, in the cell surface targeting of α2B-AR and β2-AR. We first demonstrated that expression of Rab8 mutants and Rab8 shRNA differentially modulated the cell surface expression of α2B-AR and β2-AR. The Rab8 mutants and Rab8 shRNA more potently inhibited the transport of exogenously expressed α2B-AR than β2-AR in HEK293 cells. α2B-AR was strongly colocalized with the TGN marker p230 in cells expressing the GDP-bound mutant Rab8(T22N), suggesting that Rab8 GTPase likely controls GPCR movement at the TGN level, which is consistent with the Rab8 function in vesicle-mediated transport from the TGN (30–36). Expression of Rab8(T22N) also more strongly inhibited the cell surface expression of endogenous α2B-AR in NG108 and MCF-7 cells than endogenous β-AR in HL-1 and HEK293 cells. Moreover, expression of the Rab8(T22N) mutant clearly attenuated the transport of α2B-AR to the dendrites in the primary cultures of neurons. In addition, expression of Rab8(T22N) significantly attenuated ERK1/2 activation by α2B-AR, but not β2-AR, paralleling the effects of Rab8 on the transport of these receptors to the cell surface. Therefore, it is likely that the influences of Rab8(T22N) on receptor-mediated ERK1/2 activation were due at least in part to its effects on the receptor transport to the cell surface. However, we cannot exclude that altering Rab8 function may also modulate the intracellular trafficking of other signaling molecules involved in receptor signaling systems, which may also contribute to disruption of the normal signaling of the receptors. Nevertheless, our data demonstrated that the cell surface targeting of different GPCRs may have different sensitivities in response to the inhibition of Rab8 function, which will ultimately influence receptor signal propagation.
We have previously demonstrated that inhibition of Rab1 function similarly blocks the transport of α1A-AR, α1B-AR, β1-AR, β2-AR, and angiotensin II type 1 receptor (AT1R), without influencing the transport of α2B-AR (25–27). Similar to Rab1, inhibition of Rab6 function, which modulates retrograde protein transport from the Golgi to the ER, blocks the transport of β2-AR and AT1R, but not α2B-AR (29), demonstrating that α2B-AR transport along the early secretory pathway uses an unconventional pathway independent of Rab1 and Rab6. Here we have demonstrated that Rab8 differentially modulates the post-Golgi transport of distinct GPCRs. These data further indicate that different Rab GTPases may selectively coordinate GPCR transport at different intracellular compartments.
To explore the molecular mechanism underlying the function of Rab8 GTPase in regulating GPCR post-Golgi transport, we determined if α2B-AR and β2-AR could directly associate with Rab8 by coimmunoprecipitation. It has been shown the transport machinery of Rab GTPases may directly associate with cargo proteins. For example, Rab4 and Rab11 GTPases interact with the C termini of AT1R, β2-AR, and the thromboxane A2 receptor, which is involved in the recycling of internalized receptors from endosomes back to the plasma membrane (47–49). Rab3 modulates intracellular localization of polymeric immunoglobulin receptor via directly interacting with the receptor (50). Our data demonstrate that both α2B-AR and β2-AR are able to form a complex with Rab8 GTPase, suggesting that the cargo GPCRs may directly interact with Rab8 GTPase to coordinate their transport from the TGN to the plasma membrane. Interestingly, similar to the interaction of β2-AR and thromboxane A2 receptor with Rab11 (47, 49), α2B-AR and β2-AR preferentially associate with the inactive, GDP-bound form of Rab8. Therefore, the receptors unlikely function as the downstream effectors of Rab8 GTPase, as it has been well demonstrated that the downstream effectors strongly interact with the GTP-bound Rab GTPase mutants. It is possible that the receptors may function as anchoring proteins for Rab8 GTPase localization to the TGN by providing docking sites for inactive, GDP-bound Rab8. The receptors may also function as guanine nucleotide exchange factors to facilitate the exchange of GDP for GTP and promote activation of Rab8 GTPase. This possibility is supported by the fact that some cargo proteins can activate transport machinery to modulate their transport. For example, AT1R is able to interact with and activate Rab5 GTPase, which is involved in the regulation of receptor internalization (48).
The most interesting data presented here are that α2B-AR and β2-AR use distinct motifs in the C termini to interact with Rab8 GTPase and modulate their post-Golgi transport. We first demonstrated that the α2B-AR C terminus, but not three intracellular loops, interacted with Rab8 GTPase in GST fusion protein pulldown assays. Similar to the α2B-AR C terminus, the β2-AR C terminus also interacted with Rab8. We further demonstrated that mutation of the LL motif located in the membrane-proximal C-terminal region markedly and specifically reduced Rab8 interaction with the C terminus of β2-AR, but not α2B-AR, suggesting that the LL motif selectively mediates β2-AR interaction with Rab8. It has been well documented that the LL motif functions as sorting signals at the TGN for basolateral cell surface transport and at the plasma membrane for endocytosis in clathrin-coated vesicles through interacting directly with the clathrin adaptor protein complex (51–53). The LL motif is highly conserved in the membrane-proximal C-terminal portion among the family A GPCRs (54) and we and others have demonstrated that the LL motif regulates export trafficking of a number of GPCRs (54, 55). However, the molecular mechanism underlying the function of the LL motif in GPCR transport remains unknown. It has been suggested that the LL motif is involved in the folding of GPCRs, as mutation of the LL motif disrupts receptor ligand binding (55). Our data presented in this article have demonstrated a novel function of the LL motif to mediate receptor interaction with Rab8 GTPase, which is likely to modulate receptor transport at the TGN level. These data also suggest that a single LL motif may modulate export trafficking of newly synthesized GPCRs at multiple organelles. In addition to regulating ER export, the LL motif may also coordinate nascent GPCR exit from the Golgi.
In contrast to β2-AR using the LL motif to interact with Rab8, α2B-AR uses multiple sites located in the membrane-proximal (VFNQ) and distal (PW and QTGW) C terminus to interact with Rab8 GTPase. In particular, residues Asn433 and Pro447 likely play a crucial role in mediating α2B-AR interaction with Rab8 GTPase as mutation of either one almost abolished Rab8 interaction in GST fusion protein pulldown assays. These data suggest that different GPCRs (i.e. α2B-AR and β2-AR) may provide distinct docking sites for Rab8 GTPase to coordinate their export from the TGN.
To define if differential modulation of α2B-AR and β2-AR transport by Rab8(T22N) is determined by the receptor C termini, we determined the effect of Rab8(T22N) on the transport of the chimeric receptor β2-ARα2B-ct in which the β2-AR C terminus was substituted with the α2B-AR C terminus. The cell surface transport of β2-ARα2B-ct was inhibited at a level similar to α2B-AR, but greater than β2-AR. These data suggest that the C termini may possess the structural determinants that modulate receptor response to Rab8(T22N). As we have shown that Rab8 GTPase interacts with distinct motifs in the C termini of α2B-AR and β2-AR, different responsiveness of α2B-AR and β2-AR to the inhibition of Rab8 function is likely determined by their differential interactions with Rab8 GTPase. Interestingly, β2-AR and α2B-AR interaction with Rab8 and their responsiveness to Rab8 inhibition appear to be opposite. The simplest explanation could be that in cells that express Rab8 dominant mutants or shRNA and have less functional Rab8, β2-AR, which has higher affinity for Rab8, is still able to bind Rab8 to maintain its transport to the cell surface. In contrast, under the same condition, α2B-AR, which has lower affinity for Rab8, might not be able to associate enough Rab8 required for its normal export. However, whether or not differential regulation of distinct receptors by Rab8 GTPase is indeed dictated by their differential interactions with Rab8 needs further investigation.
There are several possibilities regarding the differential regulation of α2B-AR and β2-AR transport by Rab8 GTPase. It is possible that transport from the TGN to the cell surface of α2B-AR and β2-AR is mediated through distinct pathways, which have different requirements for Rab8. Consistent with this possibility, we have demonstrated that α2B-AR and β2-AR use different routes to move from the ER to the Golgi (25). Furthermore, a recent study has demonstrated that vesicular stomatitis virus glycoprotein and Na+-K+-ATPase follow different pathways to the basolateral cell surface in polarized cells (37). It is also possible that post-Golgi transport of α2B-AR and β2-AR may be mediated through different transport vesicles that are differentially modulated by Rab8 GTPase. Consistent with this possibility, a number of proteins are involved in sorting of cargo proteins at the TGN to different destinations, such as the plasma membrane and the early and late endosomes. For example, adaptor proteins and GGAs (Golgi-localized γ-ear-containing ADP-ribosylation factor-binding proteins) directly interact with different LL-based motifs in cargo proteins to direct the sorting at the TGN (53). The post-translational modification of α2B-AR and β2-AR may also play a role in their differential regulation by Rab8 GTPase. Specifically, β2-AR is a glycosylated receptor that has three putative N-linked glycosylation sites at positions 4, 176, and 188, whereas α2B-AR does not contain glycosylation signals. Glycosylation of the receptors occurs during their transport to the Golgi to achieve a fully maturated conformation competent for export from the TGN. Different glycosylation statuses of α2B-AR and β2-AR may influence the characteristics of receptor export from the TGN and regulation by Rab8 GTPase.
Acknowledgments
We thank Dr. Hailong Hou for preparing primary neuronal cultures. We are grateful to Drs. Stephen M. Lanier, John D. Hildebrandt, Terrance E. Hebert, Steven M. Hill, and Johan Peranen for sharing reagents.
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM076167 (to G. W.) and R01NS060879 (to H. X.).
- GPCR
- G protein-coupled receptor
- AR
- adrenergic receptor
- AT1R
- angiotensin II type 1 receptor
- ER
- endoplasmic reticulum
- TGN
- trans-Golgi network
- HA
- hemagglutinin
- GFP
- green fluorescent protein
- ERK1/2
- extracellular signal-regulated kinase 1 and 2
- PBS
- phosphate-buffered saline
- DMEM
- Dulbecco's modified Eagle's medium
- GST
- glutathione S-transfer
- ISO
- isoproterenol
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Hanyaloglu A. C., von Zastrow M. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 4.Dong C., Filipeanu C. M., Duvernay M. T., Wu G. (2007) Biochim. Biophys. Acta 1768, 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu G., Krupnick J. G., Benovic J. L., Lanier S. M. (1997) J. Biol. Chem. 272, 17836–17842 [DOI] [PubMed] [Google Scholar]
- 6.Moore C. A., Milano S. K., Benovic J. L. (2007) Annu. Rev. Physiol. 69, 451–482 [DOI] [PubMed] [Google Scholar]
- 7.Wu G., Benovic J. L., Hildebrandt J. D., Lanier S. M. (1998) J. Biol. Chem. 273, 7197–7200 [DOI] [PubMed] [Google Scholar]
- 8.Tan C. M., Brady A. E., Nickols H. H., Wang Q., Limbird L. E. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 559–609 [DOI] [PubMed] [Google Scholar]
- 9.Marchese A., Chen C., Kim Y. M., Benovic J. L. (2003) Trends Biochem. Sci. 28, 369–376 [DOI] [PubMed] [Google Scholar]
- 10.Xia Z., Gray J. A., Compton-Toth B. A., Roth B. L. (2003) J. Biol. Chem. 278, 21901–21908 [DOI] [PubMed] [Google Scholar]
- 11.Carrel D., Hamon M., Darmon M. (2006) J. Cell Sci. 119, 4276–4284 [DOI] [PubMed] [Google Scholar]
- 12.Duvernay M. T., Dong C., Zhang X., Robitaille M., Hébert T. E., Wu G. (2009) Traffic 10, 552–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvernay M. T., Dong C., Zhang X., Zhou F., Nichols C. D., Wu G. (2009) Mol. Pharmacol. 75, 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bermak J. C., Li M., Bullock C., Zhou Q. Y. (2001) Nat. Cell Biol. 3, 492–498 [DOI] [PubMed] [Google Scholar]
- 15.Robert J., Clauser E., Petit P. X., Ventura M. A. (2005) J. Biol. Chem. 280, 2300–2308 [DOI] [PubMed] [Google Scholar]
- 16.Venkatesan S., Petrovic A., Van Ryk D. I., Locati M., Weissman D., Murphy P. M. (2002) J. Biol. Chem. 277, 2287–2301 [DOI] [PubMed] [Google Scholar]
- 17.Gimelbrant A. A., Haley S. L., McClintock T. S. (2001) J. Biol. Chem. 276, 7285–7290 [DOI] [PubMed] [Google Scholar]
- 18.Zhu L., Imanishi Y., Filipek S., Alekseev A., Jastrzebska B., Sun W., Saperstein D. A., Palczewski K. (2006) J. Biol. Chem. 281, 22289–22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong C., Wu G. (2006) J. Biol. Chem. 281, 38543–38554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez O., Goud B. (1998) Biochim. Biophys. Acta 1404, 101–112 [DOI] [PubMed] [Google Scholar]
- 21.Grosshans B. L., Ortiz D., Novick P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seachrist J. L., Anborgh P. H., Ferguson S. S. (2000) J. Biol. Chem. 275, 27221–27228 [DOI] [PubMed] [Google Scholar]
- 23.Fan G. H., Lapierre L. A., Goldenring J. R., Richmond A. (2003) Blood 101, 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murph M. M., Scaccia L. A., Volpicelli L. A., Radhakrishna H. (2003) J. Cell Sci. 116, 1969–1980 [DOI] [PubMed] [Google Scholar]
- 25.Wu G., Zhao G., He Y. (2003) J. Biol. Chem. 278, 47062–47069 [DOI] [PubMed] [Google Scholar]
- 26.Filipeanu C. M., Zhou F., Claycomb W. C., Wu G. (2004) J. Biol. Chem. 279, 41077–41084 [DOI] [PubMed] [Google Scholar]
- 27.Filipeanu C. M., Zhou F., Fugetta E. K., Wu G. (2006) Mol. Pharmacol. 69, 1571–1578 [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Wang G., Dupré D. J., Feng Y., Robitaille M., Lazartigues E., Feng Y. H., Hébert T. E., Wu G. (2009) J. Pharmacol. Exp. Ther. 330, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C., Wu G. (2007) Cell Signal. 19, 2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber L. A., de Hoop M. J., Dupree P., Zerial M., Simons K., Dotti C. (1993) J. Cell Biol. 123, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber L. A., Pimplikar S., Parton R. G., Virta H., Zerial M., Simons K. (1993) J. Cell Biol. 123, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., Harada R., Harada A. (2007) Nature 448, 366–369 [DOI] [PubMed] [Google Scholar]
- 33.Moritz O. L., Tam B. M., Hurd L. L., Peränen J., Deretic D., Papermaster D. S. (2001) Mol. Biol. Cell 12, 2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ang A. L., Fölsch H., Koivisto U. M., Pypaert M., Mellman I. (2003) J. Cell Biol. 163, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachury M. V., Loktev A. V., Zhang Q., Westlake C. J., Peränen J., Merdes A., Slusarski D. C., Scheller R. H., Bazan J. F., Sheffield V. C., Jackson P. K. (2007) Cell 129, 1201–1213 [DOI] [PubMed] [Google Scholar]
- 36.Au J. S., Puri C., Ihrke G., Kendrick-Jones J., Buss F. (2007) J. Cell Biol. 177, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr G. A., Hull M., Mellman I., Caplan M. J. (2009) J. Cell Biol. 186, 269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barak L. S., Ferguson S. S., Zhang J., Martenson C., Meyer T., Caron M. G. (1997) Mol. Pharmacol. 51, 177–184 [DOI] [PubMed] [Google Scholar]
- 39.Kallal L., Gagnon A. W., Penn R. B., Benovic J. L. (1998) J. Biol. Chem. 273, 322–328 [DOI] [PubMed] [Google Scholar]
- 40.Zhou F., Filipeanu C. M., Duvernay M. T., Wu G. (2006) Cell Signal. 18, 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claycomb W. C., Lanson N. A., Jr., Stallworth B. S., Egeland D. B., Delcarpio J. B., Bahinski A., Izzo N. J., Jr. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia H., von Zastrow M., Malenka R. C. (2002) J. Biol. Chem. 277, 47765–47769 [DOI] [PubMed] [Google Scholar]
- 43.Hu X. D., Huang Q., Yang X., Xia H. (2007) J. Neurosci. 27, 4674–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linder M. D., Uronen R. L., Hölttä-Vuori M., van der Sluijs P., Peränen J., Ikonen E. (2007) Mol. Biol. Cell 18, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filipeanu C. M., Zhou F., Lam M. L., Kerut K. E., Claycomb W. C., Wu G. (2006) J. Biol. Chem. 281, 11097–11103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheinin M., Lomasney J. W., Hayden-Hixson D. M., Schambra U. B., Caron M. G., Lefkowitz R. J., Fremeau R. T., Jr. (1994) Brain Res. Mol. Brain Res. 21, 133–149 [DOI] [PubMed] [Google Scholar]
- 47.Hamelin E., Thériault C., Laroche G., Parent J. L. (2005) J. Biol. Chem. 280, 36195–36205 [DOI] [PubMed] [Google Scholar]
- 48.Seachrist J. L., Laporte S. A., Dale L. B., Babwah A. V., Caron M. G., Anborgh P. H., Ferguson S. S. (2002) J. Biol. Chem. 277, 679–685 [DOI] [PubMed] [Google Scholar]
- 49.Parent A., Hamelin E., Germain P., Parent J. L. (2009) Biochem. J. 418, 163–172 [DOI] [PubMed] [Google Scholar]
- 50.van Ijzendoorn S. C., Tuvim M. J., Weimbs T., Dickey B. F., Mostov K. E. (2002) Dev. Cell 2, 219–228 [DOI] [PubMed] [Google Scholar]
- 51.Heilker R., Manning-Krieg U., Zuber J. F., Spiess M. (1996) EMBO J. 15, 2893–2899 [PMC free article] [PubMed] [Google Scholar]
- 52.Hunziker W., Fumey C. (1994) EMBO J. 13, 2963–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 54.Duvernay M. T., Zhou F., Wu G. (2004) J. Biol. Chem. 279, 30741–30750 [DOI] [PubMed] [Google Scholar]
- 55.Schülein R., Hermosilla R., Oksche A., Dehe M., Wiesner B., Krause G., Rosenthal W. (1998) Mol. Pharmacol. 54, 525–535 [DOI] [PubMed] [Google Scholar]