Abstract
Zinc is an essential trace element for human nutrition and is critical to the structure, stability, and function of many proteins. Zinc ions were shown to enhance activation of the intrinsic pathway of coagulation but down-regulate the extrinsic pathway of coagulation. The protein C pathway plays a key role in blood coagulation and inflammation. At present there is no information on whether zinc modulates the protein C pathway. In the present study we found that Zn2+ enhanced the binding of protein C/activated protein C (APC) to endothelial cell protein C receptor (EPCR) on endothelial cells. Binding kinetics revealed that Zn2+ increased the binding affinities of protein C/APC to EPCR. Equilibrium dialysis with 65Zn2+ revealed that Zn2+ bound to the Gla domain as well as sites outside of the Gla domain of protein C/APC. Intrinsic fluorescence measurements suggested that Zn2+ binding induces conformational changes in protein C/APC. Zn2+ binding to APC inhibited the amidolytic activity of APC, but the inhibition was reversed by Ca2+. Zn2+ increased the rate of APC generation on endothelial cells in the presence of physiological concentrations of Ca2+ but did not further enhance increased APC generation obtained in the presence of physiological concentrations of Mg2+ with Ca2+. Zn2+ had no effect on the anticoagulant activity of APC. Zn2+ enhanced APC-mediated activation of protease activated receptor 1 and p44/42 MAPK. Overall, our data show that Zn2+ binds to protein C/APC, which results in conformational changes in protein C/APC that favor their binding to EPCR.
Keywords: Blood Coagulation Factors, Endocytosis, Hemostasis, Thrombosis, Zinc, Endothelial Cell Protein C Receptor, Activated Protein C, Protein C, Protein C Activation, Zinc Binding
Introduction
Calcium is an obligatory cofactor in the initiation and regulation of blood coagulation. All vitamin K-dependent coagulation proteins have calcium-binding sites and thus presumably could interact to some degree with other divalent cations. A number of studies have reported that divalent metal ions other than Ca2+, such Mn2+, Mg2+, and Zn2+, bind to various coagulation factors and thereby modulate blood coagulation (1–11). Zinc deficiency has been associated with bleeding tendencies and defective platelet aggregation, suggesting an important role for zinc in hemostasis (11, 12). Zinc is an essential trace element, and the concentration of Zn2+ in plasma ranges between 10 and 25 μm in healthy individuals (13). In addition, platelets store, mainly in their cytoplasm and α-granules, up to 30 to 60 times (14) more Zn2+ than is present in plasma. These reserves could be released upon platelet activation (14).
Zinc ions were shown to enhance activation of the intrinsic pathway of coagulation by increasing the binding of high molecular weight kininogen and factor XII to negatively charged surfaces (15) or platelets (3, 16) and by accelerating the activation of factor XII and prekallikrein (2, 17, 18). In contrast, zinc ions have been found to inhibit the extrinsic pathway of coagulation by attenuating FVIIa3 and FVIIa/tissue factor activity (8, 9). Recent crystallography studies identified a pair of Zn2+-binding sites in the FVIIa protease domain (10). Except for Glu220, all of the side chains involved in both Zn1 and Zn2 coordination in FVIIa are unique to it and not present in other vitamin K-dependent clotting factors (10). Nonetheless, Zn2+ may bind to other vitamin K-dependent clotting factors as found recently with protein S (19).
Zinc is a multi-functional element and plays an important role in many biological functions primarily by binding to proteins at specific sites, thereby stabilizing the conformation and proper function of a particular protein (20–22). Zinc can also relocate calcium from its natural binding site and thus modify calcium-dependent processes (23). All of the vitamin K-dependent coagulation proteins have calcium-binding sites and could interact to some extent with both calcium and zinc (8–10, 19). The protein C pathway plays key roles in the regulation of blood coagulation and inflammation (24, 25). At present, there is no information on whether Zn2+ binds to protein C/APC and how this affects protein C/APC binding to EPCR and their subsequent functions. The data presented in this manuscript show that Zn2+ binds to protein C/APC, induces conformational changes in the protein, promotes protein C/APC binding to EPCR and thereby enhances the activation of protein C and APC-mediated cell signaling.
EXPERIMENTAL PROCEDURES
Reagents
Protein C, factor Va, factor Xa, prothrombin, and thrombin were obtained from either Enzyme Research Laboratories (South Bend, IN) or Hematologic Technologies Inc. (Essex Junction, VT). Recombinant APC (Xigris) was from Eli Lilly (Indianapolis, IN). The γ-carboxyglutamic acid domainless form of protein C (GD-protein C) was obtained from Enzyme Research Laboratories. GD-APC was prepared by digesting APC with chymotrypsin as described earlier (26). Recombinant hirudin was obtained from Calbiochem (La Jolla, CA). Chromogenic substrates for APC (S-2366) and thrombin (Chromozym TH) were obtained from Diapharma Group, Inc. (West Chester, Ohio) and Roche Applied Science, respectively. Mouse monoclonal anti-EPCR antibodies (JRK-1494 and JRK-1500) were kindly provided by Charles T. Esmon (Oklahoma Medical Research Foundation).
Cell Culture
Primary human umbilical vein endothelial cells (HUVEC) were purchased from Lonza (Walkersville, MD). Monolayers of HUVEC were grown to confluency at 37 °C and 5% CO2 in a humidified incubator in EBM-2 basal medium supplemented with 5% fetal bovine serum and growth supplements (Lonza). Endothelial cell passages between 3 and 8 were used in the present studies. Wild-type CHO-K1 cells (American Type Culture Collection, Manassas, VA) and CHO-K1 cells stably transfected with full-length human EPCR cDNA (CHO-EPCR) (27) were maintained in F12K medium containing 1% penicillin/streptomycin and 10% fetal bovine serum.
125I Labeling
Protein C and other ligands were labeled with 125I using IODO-GEN (Thermo Scientific Pierce Research Products, Rockford, IL)-coated polypropylene tubes and Na125I (PerkinElmer Life Sciences) according to the manufacturer's instructions and as described previously (27).
Measurement of 125I-Labeled Protein C/APC Binding to EPCR on Cell Surfaces and Their Internalization
To evaluate the effect of zinc ions on the binding of protein C and APC to EPCR, HUVEC or CHO-EPCR cells were first incubated with control vehicle or anti-EPCR antibodies (JRK-1494) in buffer A+ (10 mm HEPES, 0.15 m NaCl, 4 mm KCl, 11 mm d-glucose, pH 7.5 (buffer A) containing 1 mg/ml bovine serum albumin) at room temperature for 30 min. The monolayers were then chilled on ice followed by incubation for 3 h at 4 °C with 125I-protein C or 125I-APC (80 nm) in the presence of varying concentrations of ZnCl2 (0–100 μm) ± 5 mm CaCl2. At the end of the incubation period, the supernatant was removed from the wells, and the cells were washed four times with ice-cold buffer A containing 1 mg/ml bovine serum albumin and 5 mm CaCl2 (buffer B) to remove unbound radiolabeled ligand. The monolayers were then incubated with 0.1 m glycine, pH 2.3, for 5 min to elute the surface-bound radiolabeled ligand, and the eluted radioactivity was measured in a γ-counter. Nonspecific binding measured in the presence of anti-EPCR antibodies was subtracted from the total binding (binding observed in the absence of anti-EPCR antibodies) to calculate EPCR-specific binding. In general, nonspecific binding of protein C and APC is much lower (20% or less of the total binding) in CHO-EPCR cells compared with HUVEC (40–50%). For internalization studies, CHO-EPCR were incubated at 37 °C with 125I-APC (10 nm) in buffer A+ containing 25 μm Zn2+ or 5 mm Ca2+ alone or in combination. At varying time intervals, the surface-associated ligand was eluted as described above, and the cells were extracted with lysis buffer (0.1 m NaOH, 10 mm EDTA, 1% SDS); both eluted and solubilized radioactivity were measured to quantify cell surface-associated and internalized APC. To study the effect of zinc ions on EPCR mAb binding to EPCR, CHO-EPCR cells were incubated with varying concentrations of 125I-labeled EPCR mAb (JRK-1500; 0–100 nm) with 5 mm Ca2+ in the presence or absence of 25 μm ZnCl2 for 3 h at 4 °C. At the end of incubation, the bound radioactivity was eluted and measured as described above. Because very little nonspecific binding was noted with EPCR mAb, no attempts were made to measure the nonspecific binding.
Confocal Microscopy
APC was labeled with AF546 (Invitrogen) following the instructions provided with the labeling kit. HUVEC cultured on glass coverslips were incubated with AF546-APC (50 nm) for 1 h at 37 °C in the presence or absence of Ca2+, Mg2+, or Zn2+. At the end of 1 h of incubation, the unbound ligand was removed, and the cells were fixed and processed for confocal microscopy. The cells were viewed with an Axio ObserverZ1 microscope using 63× (oil) plan-apochromate lens. The images were acquired from a field of view at 0.5-μm z axis increments using the LSM 510 Meta confocal system (Carl Zeiss). The laser wavelength settings were 543 ± 10 nm for excitation and 575 ± 10 nm for emission. All of the images were obtained using identical gain and off-set settings for the detector. The images were processed using LSM Zen 2007 (Zeiss) software and imported to Adobe Photoshop (versus 7.0) for the compilation of figures.
Measurement of Protein C Activation
Endothelial cells grown to confluence in a 96-well plate were incubated with protein C (80 nm), CaCl2 (5 mm), varying concentrations of ZnCl2 (0–100 μm), and thrombin (2 nm) in a total volume of 50 μl. At the end of 2 h of incubation at 37 °C, 25 μl of hirudin (4 units/ml) was added to the cells to neutralize thrombin. Ten minutes after adding hirudin, 25 μl of chromogenic substrate S-2366 (0.5 mm) was added to the well, and the change in absorbance at 405 nm (milli-optical density/min) was measured using a microplate reader (Molecular Devices, Palo Alto, CA). All of the concentrations listed were final concentrations. A standard curve generated with known concentrations of APC was used to determine the concentrations of APC generated in the reaction mixture. Under these experimental conditions, APC generation was dependent on protein C binding to EPCR and thrombin binding to thrombomodulin because preincubation of HUVEC with either anti-EPCR or anti-thrombomodulin antibodies (25 μg/ml) markedly attenuated APC generation. For Km and Vmax determinations, the activation assays were performed with increasing concentrations of protein C (0.05–2.0 μm) in the presence of 1.1 or 5 mm CaCl2 ± 25 μm ZnCl2 and/or 0.6 mm MgCl2 for 1 h at 37 °C. Other conditions for the assay were the same as above.
APC Amidolytic Activity Measurement
To examine the effect of Zn2+ on the amidolytic activity of APC, APC or GD-APC (10 nm) was incubated with varying concentrations of zinc ions (0 to 200 μm) ± Ca2+ (1.1 mm) for 5 min, and then the chromogenic substrate S-2366 was added to the reaction mixture. The rate of hydrolysis was monitored by measuring the change in the absorbance of the chromogenic substrate at 405 nm in a microplate reader.
Measurement of APC Anticoagulant Activity
APC (200 nm) was incubated with varying concentrations of Zn2+ (0–100 μm) for 20 min, and then APC at final concentration of 1 nm was added to HUVEC monolayers preincubated with FVa (2 nm) in a 96-well culture plate. After allowing APC to inactivate FVa for 30 min, the remaining FVa activity was measured in a prothrombinase assay by adding FXa (5 nm) and prothrombin (1.4 μm) to the monolayers and measuring the rate of thrombin generation in a chromogenic assay using Chromozym TH. Under these experimental conditions, APC inhibited the prothrombinase activity by 50%, and any changes in APC anticoagulant activity were readily detectable in FVa-dependent thrombin generation.
Measurement of PAR1 Activation
The AP-PAR1 reporter plasmid, in which alkaline phosphatase (AP) was fused to the N terminus of PAR1, was constructed as described earlier (28) and subcloned into an adenoviral vector. The high titer adenoviral particles were purified using ViraBindTM adenovirus purification kit (Cell Biolabs), and the viral titer was determined by QuickTiterTM adenovirus immunoassay kit (Cell Biolabs). HUVEC cultured in a 12-well plate were transduced with AP-PAR1 reporter adenoviral construct (20 multiplicity of infection/cell). In this assay, activation of AP-PAR1 releases the N-terminal peptide with the AP tag into the overlying conditioned medium, and thus the AP activity in the supernatant correlates with the extent of PAR1 activation. HUVEC monolayers expressing AP-PAR1 were treated with APC (80 nm) in the absence or presence of varying divalent cations. Aliquots (40 μl) were removed immediately after the addition of APC (0 time) and after the 45-min activation period. The aliquots were centrifuged for 5 min at 14,000 rpm to remove any cell debris, and AP activity in the supernatant (15 μl) was quantified using a chemiluminescence substrate from the BD Biosciences Great EscAPe SEAP detection kit.
p44/42 MAPK Activation
Confluent monolayers of HUVEC were serum-starved for 3 h and then treated with APC (20 nm) for 15 min in the presence or absence of Zn2+. To minimize potential interference of a direct effect of Zn2+ on p44/42 MAPK activation, Zn2+ was added to the cells during serum starvation, i.e. 3 h before the cells were exposed to APC. The cells were lysed with SDS-PAGE sample buffer, and equal amounts of protein were subjected to SDS-PAGE, transferred onto polyvinylidene difluoride membrane, and probed with phospho-specific and total p44/42 MAPK antibodies (Cell Signaling), and the blot was developed with chemiluminescence.
Equilibrium Dialysis
Zinc binding to APC and GD-APC was determined by equilibrium dialysis using 65Zn2+ (specific activity, 1.13 mCi/mmol; Oak Ridge National Laboratory, Oak Ridge, TN) and a 96-well equilibrium dialyzer (Harvard Apparatus, Holliston, MA). One hundred μl of protein (7.5 μm) in 50 mm Tris-HCl, pH 8.0, were added to one side of the dialysis cell; 100 μl of 50 mm Tris-HCl, pH 8.0, containing varying concentrations of 65Zn2+ (5–200 μm) were added to the other side of cell. The dialyzer was rotated continuously for 24 h at room temperature using a plate rotator. After 24 h, a 50-μl aliquot from each side of the cell was withdrawn and counted for radioactivity. The difference between 65Zn2+ in the protein chamber and that in the protein-free chamber was taken to be a measure of the amount of Zn2+ bound to the protein. Control experiments, where the protein was replaced with Tris buffer, gave equal amounts of 65Zn2+ radioactivity in both chambers, confirming that equilibrium between the two sides of the cell had been established. Control experiments also revealed no significant 65Zn2+ binding to the dialysis membrane.
Measurement of Intrinsic Fluorescence Quenching
Binding of Zn2+ or Ca2+ to protein C and protein C derivatives was evaluated by changes in the intrinsic fluorescence of proteins associated with the binding of these metal ions to the proteins. The fluorescence titration was carried out using a Spectroflurometer FP 6500 (Jason Inc., Easton, MD). The excitation and emission wavelengths were 295 and 345 nm, respectively. The titrations were carried out at room temperature in 50 mm Tris-HCl, pH 8.0 buffer by adding small aliquots (0.5–1.25 μl) of Ca2+ (0–5.0 mm) or Zn2+ (0–100 μm) to a 1 μm protein sample (250 μl) and measuring the emission spectra (λem, 300–400 nm) at an excitation maxima of 295 nm. The peak value at the emission wavelength of 345 nm was used to determine the extent of fluorescence quenching.
RESULTS
Zinc Modulates Binding of Protein C and APC to EPCR
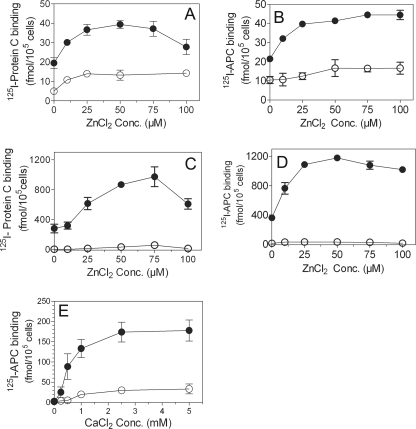
We investigated the effect of zinc ions on the interaction of protein C and APC with EPCR on cell surfaces as the first step in evaluating the potential role of Zn2+ in modulating protein C and APC functions. HUVEC were incubated with 125I-protein C and varying concentrations of ZnCl2 in the presence or absence of Ca2+, and the amount of the radioligand bound to EPCR was measured. As shown in Fig. 1A, Zn2+ enhanced protein C binding to EPCR by 2-fold in reaction mixtures that also contained saturating concentrations of Ca2+. Concentrations of zinc at levels comparable with those in plasma (∼20–25 μm) were sufficient to maximally enhance protein C binding to EPCR. At higher concentrations of zinc ions (100 μm), the enhancing effect of zinc ions was diminished. In the absence of Ca2+, only trace amounts of protein C were associated with the cells, and the addition of Zn2+ only slightly enhanced this base level of protein C binding. To determine whether zinc ions had a similar enhancing effect on APC binding to EPCR, we also evaluated APC binding to endothelial cells. As shown in Fig. 1B, zinc ions increased APC binding to endothelial cells in a dose-dependent manner. The data obtained with CHO-EPCR cells further confirmed the effect of zinc ions on protein C and APC binding to EPCR (Fig. 1, C and D). The effect of zinc ions on APC binding to EPCR appears to be more pronounced than their effect on protein C binding to EPCR. To investigate whether Zn2+ could induce protein C binding to EPCR independent of the Gla domain, we next examined the effect of Zn2+ on the binding of Gla domain-deleted protein C (GD-protein C) to EPCR on endothelial cells. GD-protein C bound poorly to EPCR, and Zn2+ was ineffective in promoting the binding (data not shown).
FIGURE 1.
Zinc ions enhance Ca2+-mediated protein C and activated protein C binding to endothelial cell protein C receptor. Confluent monolayers of HUVEC (A and B) or CHO-EPCR cells (C and D) cultured in 24-well plates were treated with control vehicle or EPCR blocking mAb (10 μg/ml) for 30 min at room temperature. The cells were then chilled and incubated with 125I-protein C (80 nm, A and C) or 125I-APC (80 nm, B and D) and varying concentrations (0–100 μm) of ZnCl2 in the presence (●) or absence (○) of CaCl2 (5 mm) for 3 h at 4 °C. After the 3-h incubation, the supernatants were removed, the cells were washed, the bound 125I-protein C or 125I-APC was eluted with 0.1 m glycine, and the radioactivity in the eluate was counted. The data shown in the graph represent EPCR-specific binding, which was determined by subtracting the nonspecific binding (binding observed with cells pretreated with EPCR blocking mAb) from the total binding (binding observed with cells pretreated with control vehicle). E, CHO-EPCR cells, pretreated with control vehicle or EPCR blocking mAb, were incubated with 125I-APC (10 nm) and varying concentrations of CaCl2 in the presence (●) or absence (○) of 25 μm Zn2+. After 3 h of incubation at 4 °C, 125I-APC bound to EPCR was determined as described above. Conc., concentration.
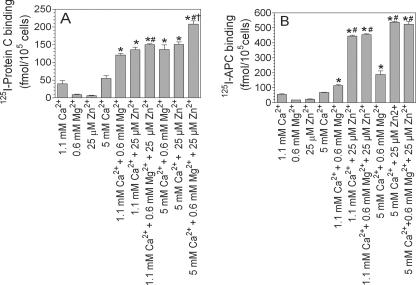
To examine how the Ca2+ concentration might influence the Zn2+-mediated protein C/APC binding to EPCR, we tested the effect of a fixed concentration of Zn2+ (25 μm) on APC binding to EPCR in the presence of varying concentrations of calcium ions. As shown in Fig. 1E, in the absence of Ca2+, zinc ions failed to mediate APC binding to EPCR. However, a Ca2+ concentration as low as 0.5 mm was sufficient to elicit a marked enhancing effect of Zn2+ on APC binding to EPCR. The zinc effect reached a maximum at 2.5 mm Ca2+. Even in the presence of saturating concentrations of Ca2+, Zn2+ significantly increased APC binding to EPCR. Earlier studies showed that physiological levels of Mg2+ enhanced APC binding to EPCR (29). Therefore, we next investigated the effect of Zn2+ ions on protein C and APC binding to EPCR in the absence and presence of a physiological concentration of Mg2+. As shown in Fig. 2A, Mg2+ enhanced the binding of protein C to CHO-EPCR cells by ∼3-fold. Zn2+ increased protein C binding to CHO-EPCR cells to the same extent. The presence of both Mg2+ and Zn2+ increased the binding of protein C to CHO-EPCR cells slightly but significantly (p < 0.01) over that observed in the presence of either Mg2+ or Zn2+ alone (Fig. 2A). The effect of Zn2+ is more pronounced on APC binding to EPCR. Mg2+ enhanced the binding of APC to CHO-EPCR cells by ∼2-fold (Fig. 2B). In the same experiment, physiological concentrations of Zn2+ enhanced the binding of APC to CHO-EPCR cells by ∼7-fold. The addition of a physiological concentration of Mg2+ did not further enhance APC binding to the cells. Similar enhancing effects of Zn2+ and Mg2+ on the binding of APC and protein C were also noted with HUVEC, but the amount of APC and protein C bound to HUVEC, as expected, was much lower than that observed with CHO-EPCR cells (data not shown).
FIGURE 2.
Effect of physiological concentrations of Zn2+ and Mg2+ on the binding of activated protein C and protein C to EPCR. CHO-EPCR cells pretreated with control vehicle or EPCR blocking mAb (10 μg/ml) for 30 min at room temperature were incubated with 125I-protein C (A) or 125I-APC (B) (80 nm) for 15 min at 37 °C in the presence of buffer containing 1.1 or 5 mm CaCl2 ± Zn2+ (25 μm) and/or Mg2+ (0.6 mm). The amount of APC and protein C bound to the cells was determined as described under “Experimental Procedures.” The data shown in the graph represent EPCR-specific binding (means ± S.E., n = 3–6 experiments). *, the value is significantly higher than the value obtained in the reaction mixture containing the corresponding concentration of Ca2+ alone (p < 0.01). #, the value significantly differs from the value obtained in the reaction mixture containing Mg2+ (p < 0.01). †, the value significantly differs for the value obtained in reaction mixture containing either Mg2+ or Zn2+ (p < 0.01).
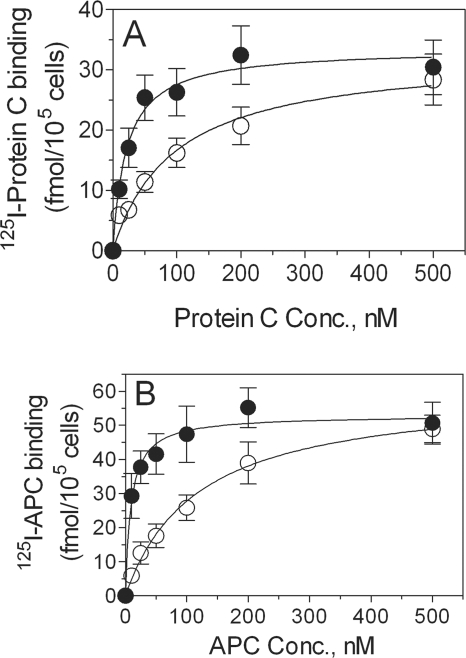
The effect of Zn2+ on the enhancement of protein C or APC binding to EPCR on cell surfaces could be the result of increased availability of EPCR receptors on cell surfaces upon exposure to zinc ions or of increased avidity of protein C and APC with bound Zn2+ to EPCR. To distinguish between these two possibilities, we first evaluated the effect of zinc ions on the binding affinity of protein C and APC to HUVEC and CHO-EPCR cells. Zinc ions markedly influenced the binding affinity of protein C and APC to EPCR (Fig. 3). In the absence of zinc ions, both protein C and APC bound relatively poorly to EPCR. The addition of zinc ions to the binding reaction mixture increased the binding affinity of both protein C and APC to EPCR by 5–10-fold. The effect of zinc on APC binding to EPCR, compared with that on protein C binding to EPCR, is more pronounced (Kd for APC in the absence of zinc ions, 117 ± 27 nm; Kd for APC in the presence of zinc ions, 9.3 ± 3.3 nm; Kd for protein C in the absence of zinc ions, 96 ± 26 nm; Kd for protein C in the presence of zinc ions, 21.4 ± 6.6 nm). The Bmax of protein C and APC binding to EPCR on endothelial cells did not differ significantly. Binding studies carried out with CHO-EPCR cells further confirmed that Zn2+ increases the affinity of protein C and APC binding to EPCR. Data analysis showed a 4- and 8-fold increase in the affinity of protein C and APC binding to EPCR, respectively, in the presence of zinc (data not shown). We next investigated the effect of zinc ions on EPCR mAb binding to EPCR. No detectable differences were observed in EPCR mAb binding to EPCR in the presence or absence of zinc ions. The binding curves are hyperbolic and overlapping and yielded essentially the same Kd and Bmax values (data not shown), suggesting that Zn2+ had no direct effect on EPCR on the cell surface.
FIGURE 3.
Zinc ions increase the binding affinity of protein C and activated protein C to EPCR. HUVEC, preincubated with control vehicle or EPCR blocking mAb, were incubated at 4 °C for 3 h with varying concentrations of 125I-protein C (A) or 125I-APC (B) in the presence (●) or absence (○) of 25 μm Zn2+. All of the reaction mixtures contained 5 mm CaCl2. At the end of 3 h, the amount of 125I-protein C or 125I-APC associated with the cell surface EPCR was determined as described under “Experimental Procedures.” Conc., concentration.
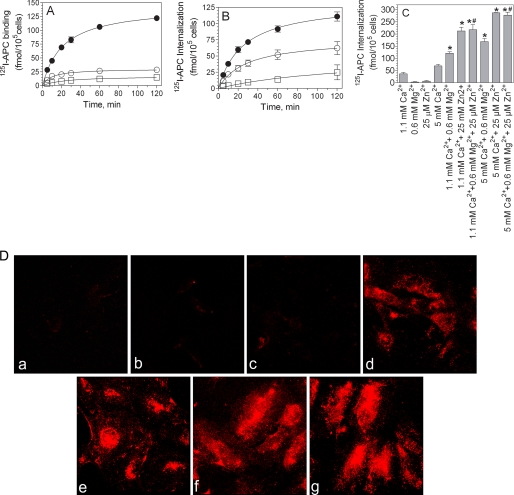
We next examined whether the Zn2+-mediated increase in APC and protein C binding to EPCR on cell surfaces leads to increased internalization of the ligand. CHO-EPCR cells were incubated with 125I-APC (10 nm) in the presence or absence of 25 μm Zn2+ and/or 5 mm Ca2+ at 37 °C for varying time periods. As expected, APC bound to CHO-EPCR in a time-dependent and hyperbolic fashion. 4–5-fold more APC was bound to CHO-EPCR cells when both Ca2+ and Zn2+ were present compared with the amount of APC bound to the cells in the presence of calcium ions alone (Fig. 4A). APC internalization reflected the APC binding properties, i.e. internalization of APC was increased in reaction mixtures containing both Ca2+ and Zn2+ in comparison with the reaction mixture that contained either Ca2+ or Zn2+ alone (Fig. 4B). Although Mg2+ also enhanced the internalization of APC, it was significantly lower than that noted with Zn2+ (Fig. 4C). The presence of Mg2+ with Zn2+ did not further increase the internalization of APC (Fig. 4C). Similar findings were noted with protein C internalization (data not shown). The extent of Zn2+-mediated increase in APC internalization correlates well with the amount of APC bound to EPCR in the presence of Zn2+ (compare Fig. 4C with Fig. 2B), suggesting that the increased internalization of protein C/APC in the presence of Zn2+ stems from the increased association of protein C/APC to EPCR in the presence of Zn2+ and is not the result of an increase in the rate of internalization of the bound ligand. Additional radioligand binding studies performed with HUVEC confirmed the data obtained with CHO-EPCR cells, i.e. significantly more APC was internalized in the presence of Zn2+ and Ca2+ compared with Ca2+ alone (data not shown). The differences in the extent of APC internalization in HUVEC in the presence or absence of Zn2+ was clearly demonstrable by fluorescence confocal microscopy of HUVEC exposed to APC tagged with a fluorescent dye (Fig. 4D).
FIGURE 4.
Effect of zinc ions on activated protein C binding and internalization. CHO-EPCR cells were incubated at 37 °C with 125I-APC (10 nm) in a buffer containing ZnCl2, 25 μm (□), CaCl2, 5 mm (○), or both ZnCl2 and CaCl2 (●). At varying times, the amount of 125I-APC bound to the cell surface (A) or internalized (B) was determined as described under “Experimental Procedures.” C, CHO-EPCR cells were incubated with 125I-APC (80 nm) for 15 min at 37 °C in the presence of one or more divalent cations, Ca2+, Mg2+, or Zn2+, as indicated on the x axis of the figure. The amount of 125I-APC internalized was measured as described under “Experimental Procedures.” The results are presented as the means ± S.E. of three or more experiments. *, the values are significantly higher compared with the values obtained in the reaction mixture containing Ca2+ alone (p < 0.05). #, the values are significantly higher (p < 0.05) compared with the values obtained in the reaction mixture containing Ca2+ and Mg2+. D, HUVEC were incubated with AF546-APC (50 nm) for 1 h at 37 °C in the presence of one or more divalent cations. The monolayers were fixed, and the fluorescence of AF546-APC was analyzed by confocal microscopy. The images shown are the composite images of all z-stacks. Treatments were as follows: panel a, no salt; panel b, 25 μm Zn2+; panel c, 0.6 mm Mg2+; panel d, 1.1 mm Ca2+; panel e, 1.1 mm Ca2+ + 0.6 mm Mg2+; panel f, 1.1 mm Ca2+ + 25 μm Zn2+; panel g, 1.1 mm Ca2+ + 0.6 mm Mg2+ + 25 μm Zn2+.
Zinc Binding to APC and GD-APC
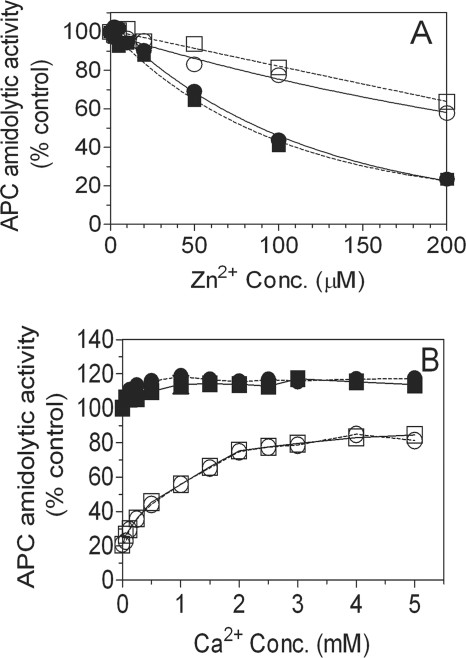
To characterize the binding of zinc ions to APC, we first evaluated the effect of Zn2+ on the amidolytic activities of APC and GD-APC. As shown in Fig. 5A, zinc ions, in the absence of Ca2+, inhibited the amidolytic activity of APC with half-maximum at 100 μm. However, in the presence of Ca2+, the inhibitory effect of Zn2+ was attenuated significantly. Zinc ions also inhibited the amidolytic activity of GD-APC in an almost identical fashion. Moreover, as observed with APC, the presence of physiological concentrations of Ca2+ substantially reduced the inhibitory effect of Zn2+ on the amidolytic activity of GD-APC. Ca2+ reversed the Zn2+-mediated inhibition of the amidolytic activities of APC and GD-APC with an identical concentration dependence profile (Fig. 5B). In contrast to Ca2+, the presence of Mg2+ (0.6 mm) had no influence on Zn2+ inhibition of the amidolytic activity of protein C (data not shown).
FIGURE 5.
Inhibition of activated protein C amidolytic activity by zinc ions. A, APC (circles) or GD- APC (squares) (10 nm) were incubated for 5 min with varying concentrations of zinc ions (0–200 μm) in the absence (filled symbols) or presence (open symbols) of the physiological concentration of Ca2+ (1.1 mm). The amidolytic activity of APC was then determined using chromogenic substrate S-2366. B, APC (○) or GD-APC (□) (10 nm) was incubated with 100 μm ZnCl2 in the presence of varying concentrations of CaCl2 for 10 min, and the amidolytic activity of APC was then determined using S-2366. The amidolytic activity of APC measured in the absence of Ca2+ and Zn2+ was taken as 100%. The effect of varying concentrations of Ca2+ with no Zn2+ on APC (●) and GD-APC (■) amidolytic activity was also measured. Conc., concentration.
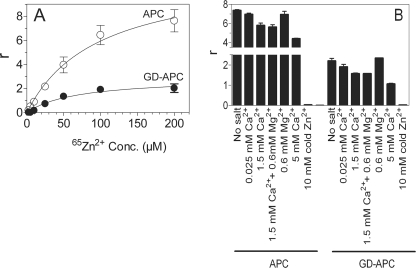
Next, we measured Zn2+ binding to APC and GD-APC by equilibrium dialysis. As shown in Fig. 6A, Zn2+ bound to both APC and GD-APC in a dose-dependent manner. Approximately 3–4-fold more Zn2+ bound to APC compared with GD-APC. Analysis of binding curves by curve fitting (GraphPad, PRISM 4.0) revealed that Zn2+ binds to both APC and GD-APC with relatively high affinity (Kd, 70–80 μm). Determination of the total number of binding sites from the Bmax obtained from the curve fitting predicted the presence of two zinc-binding sites in GD-APC and ∼8–10 sites in the full-length APC. These data suggest that zinc binds to both the Gla domain and region(s) outside of the Gla domain. 65Zn2+ binding to GD-APC was decreased by ∼50% in the presence of saturating concentrations of Ca2+ (5 mm) (Fig. 6B). Ca2+ also competed with Zn2+ binding to the full-length APC, but to a lesser extent. Although part of this reduction reflects the competition between Ca2+ and Zn2+ for sites in the protease domain, it does not fully account for all of the inhibition, which suggests that at least a few of the Zn2+-binding sites in the Gla domain must also be common binding sites for both Ca2+ and Zn2+. The presence of physiological concentrations of Mg2+ neither enhances nor decreases the partial replacement of 65Zn2+ binding to APC observed in the presence of Ca2+ (Fig. 6B).
FIGURE 6.
65Zn2+ binding measurements by equilibrium dialysis. A, APC (○) or GD-APC (●) (7.5 μm, 100 μl) were subjected to equilibrium dialysis against varying concentrations of 65Zn2+ (2.5–200 μm). B, displacement of Zn2+ by Ca2+ or Mg2+. APC or GD-APC (7.5 μm) were subjected to equilibrium dialysis against 65Zn2+ (100 μm) ± varying concentrations of Ca2+ or physiological concentrations of Ca2+ and Mg2+. To demonstrate the specificity of Zn2+ binding to APC and GD-APC, 100-fold molar excess of unlabeled Zn2+ was used in the displacement. r on the y axis denotes mol of Zn2+ bound to mol protein. Conc., concentration.
Zinc Induces Conformational Changes in Protein C and APC
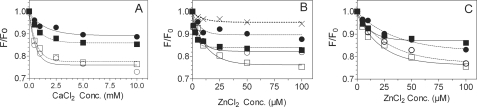
Changes in the intrinsic fluorescence reflect metal-induced conformational changes in protein C (30–32). To investigate whether zinc binding to the Gla domain and/or the protease domain induces conformational changes in the protein, we measured the quenching of intrinsic fluorescence intensity of protein C, GD-protein C, APC, and GD-APC in the presence of increasing concentrations of calcium or zinc ions. Both protein C and APC underwent a saturable Ca2+-dependent fluorescence quenching of ∼25% (Fig. 7A). The fluorescence quenching profiles of protein C and APC were very similar. Ca2+-dependent fluorescence quenching was also observed with GD-protein C and GD-APC; however, the extent of fluorescence quenching was substantially lower compared with protein C and APC (Fig. 7A). When we examined the effect of increasing concentrations of zinc ions on the intrinsic fluorescence of protein C and its derivatives, we observed an effect similar to that seen with Ca2+. Zn2+ caused a substantial decrease in the intrinsic fluorescence of protein C, APC, and their Gla domain-deleted derivatives (Fig. 7B). However, in contrast to that observed with Ca2+ binding, the extent of the decrease in fluorescence emission and the profile of fluorescence quenching differed between protein C and APC upon zinc titration. Protein C underwent a 15% decrease in the fluorescence emission, whereas APC underwent an approximately 25% decrease. Differences in Zn2+ binding to protein C and APC were also evident with their Gla domain-deleted derivatives. We also investigated whether zinc ions induce further conformational changes in Ca2+-bound protein C and APC. For these studies, first protein C and APC were preincubated with 5 mm CaCl2 for 5 min, and then fluorescence quenching was measured following the addition of varying concentrations of zinc ions. As shown in Fig. 7C, zinc ions induced further fluorescence quenching, indicating that Zn2+ binding to protein C/APC induces additional conformational changes to protein C/APC saturated with Ca2+. Similarly, Zn2+ also induced fluorescence quenching of protein C/APC in the presence physiological amounts of both Ca2+ and Mg2+ (data not shown), suggesting that Zn2+-induced conformational changes in the protein were different from those induced by Mg2+.
FIGURE 7.
The influence of Zn2+ on the intrinsic fluorescence of protein C, activated protein C, and their Gla domain-less derivatives. A and B, protein C (○), APC (□), GD-protein C (●), GD-APC (■), and factor X (as a control; ×) (1 μm) were titrated against varying concentrations of Ca2+ (A) or Zn2+ (B), and the intrinsic fluorescence was determined as described under “Experimental Procedures.” F and F0 correspond to the emission intensity with or without divalent cation present, respectively. C, same as in B except that the proteins were saturated with Ca2+ (5 mm) before they were titrated against Zn2+. In a separate experiment where we examined the reversibility of intrinsic fluorescence quenching by EDTA, EDTA was found to reverse Ca2+- and Zn2+-mediated intrinsic fluorescence quenching by ∼90%. Conc., concentration.
Effect of Zinc on Protein C Activation
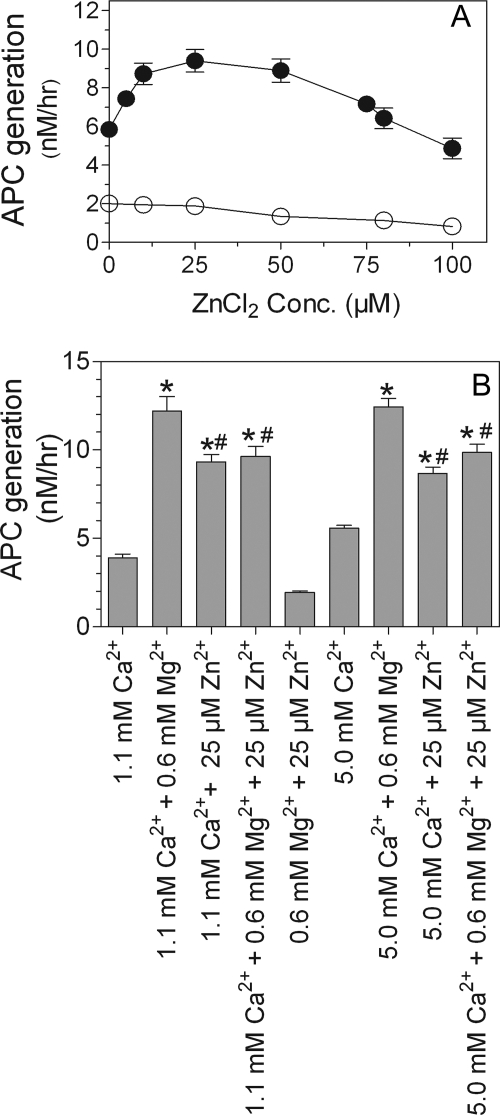
To investigate whether the observed increase in protein C binding to EPCR in the presence of zinc ions leads to increased protein C activation, we measured EPCR-dependent protein C activation in HUVEC in the presence of varying concentrations of ZnCl2 (0.0–0.1 mm) ± CaCl2 (5 mm). Zinc ions enhanced the rate of thrombin-mediated protein C activation in the presence of Ca2+. A physiological concentration of Zn2+ (∼25 μm) was sufficient to increase protein C activation by ∼2-fold (Fig. 8A). The presence of Ca2+ was essential for Zn2+ to enhance protein C activation because Zn2+ alone failed to support protein C activation. At higher concentrations, Zn2+ slightly reduced protein C activation. However, this could reflect Zn2+-mediated inhibition of APC amidolytic activity rather than the direct inhibition of protein C activation. The zinc effect was observed both at the physiological concentration of Ca2+ (1.1 mm) as well as at a saturating concentration of Ca2+ (5 mm). However, the zinc effect on protein C activation was evident only in the absence of Mg2+. Physiological concentrations of Mg2+ enhanced protein C activation by 3-fold, and Zn2+ did not further increase the rate of protein C activation (Fig. 8B, bottom panel). In fact, Zn2+ slightly reduced Mg2+-enhanced protein C activation. Further analysis of Zn2+-dependent protein C activation on endothelial cells revealed that the presence of physiological amounts of either Mg2+ or Zn2+ lowered the apparent Km of protein C activation (Table 1). The presence of both Mg2+ and Zn2+ together with Ca2+ did not further lower the Km (Table 1). Overall these data suggest that although Zn2+ is capable of enhancing APC generation on HUVEC, it may not have any significant effect on APC generation in the presence of physiological concentrations of Ca2+ and Mg2+. In additional studies, we investigated the effect of Zn2+ on EPCR-independent but thrombin-thrombomodulin-dependent protein C activation utilizing recombinant soluble thrombomodulin (provided by Ray Rezaie, St. Louis University, St. Louis, MO). The data of these studies revealed that Zn2+ had no effect on thrombin-thrombomodulin-mediated protein C activation (data not shown).
FIGURE 8.
Zn2+ promotes protein C activation. A, endothelial cells were incubated with protein C (80 nm) and thrombin (2 nm) with varying concentrations (0–100 μm) of ZnCl2 in the presence (●) or absence (○) of CaCl2 (5 mm) at 37 °C for 2 h. At the end of 2 h, thrombin in the reaction mixture was inhibited by adding hirudin (4 units/ml), and the amount of APC generated was measured in an amidolytic activity assay using chromogenic substrate S-2366 (500 μm). B, endothelial cells were incubated with protein C (80 nm) and thrombin (2 nm) for 1 h at 37 °C in the presence of one or more divalent cations as listed on the x axis. The amount of APC generated was measured as described above. The results are presented as the means ± S.E. of three or more experiments. *, the values are statistically significantly higher compared with the values obtained in the reaction mixture containing only Ca2+ (p < 0.01). #, the values are statistically significantly lower compared with the values obtained in the reaction mixture containing Ca2+ and Mg2+ (p < 0.05).
TABLE 1.
Effect of Zn2+ on protein C activation
The values shown represent the means ± S.E. (n = 3–5).
| Divalent cations (Concentration) | Km | Vmax |
|---|---|---|
| μm | nm/h | |
| Ca2+(1.1 mm) | 0.70 ± 0.06 | 13.4 ± 0.48 |
| Ca2+ (1.1 mm) + Zn2+ (25 μm) | 0.38 ± 0.03a | 10.9 ± 0.33 |
| Ca2+ (1.1 mm) + Mg2+ (0.6 mm) | 0.18 ± 0.01a | 12.4 ± 0.22 |
| Ca2+ (1.1 mm) + Zn2+ (25 μm) + Mg2+ (0.6 mm) | 0.32 ± 0.03a,b | 10.5 ± 0.31 |
| Ca2+ (5 mm) | 0.43 ± 0.03 | 10.8 ± 0.25 |
| Ca2+ (5 mm) + Zn2+ (25 μm) | 0.20 ± 0.02a | 8.6 ± 0.29 |
| Ca2+ (5 mm) + Mg2+ (0.6 mm) | 0.15 ± 0.02a | 9.5 ± 0.32 |
| Ca2+ (5 mm) + Zn2+ (25 μm) + Mg2+ (0.6 mm) | 0.22 ± 0.03a,b | 7.7 ± 0.29 |
a The values significantly differ (p < 0.01) from the value obtained in the presence of corresponding concentration of Ca2+ alone.
b The values do not significantly differ from the value obtained in the presence of Ca2+ and Zn2+.
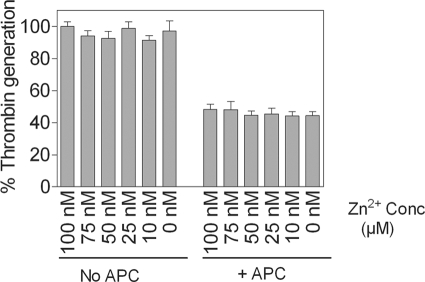
Zinc Does Not Alter the Anticoagulant Activity of APC
To investigate whether Zn2+ binding to APC alters the anticoagulant activity of APC, we examined the effect of varying concentrations of Zn2+ on APC inactivation of FVa. FVa activity was measured by its ability to support FXa-catalyzed activation of prothrombin on the endothelial cell surface. As shown in Fig. 9, APC inhibited FVa-dependent prothrombinase activity in HUVEC. Incubation of APC with Zn2+ had no effect on APC inactivation of Va.
FIGURE 9.
Zn2+ does not inhibit APC anticoagulant activity. APC (200 nm) was incubated in the presence of varying concentrations of Zn2+ (0–100 μm) for 20 min, and then 2 nm of APC from the reaction mixture was added to HUVEC monolayers incubated with FVa (1 nm) for 5 min. APC was allowed to inactivate FVa for 30 min, and the remaining Va activity was measured by adding FXa (5 nm) and prothrombin (1.4 μm) and measuring the amount of thrombin generated in a chromogenic assay. The rate of thrombin generation measured in the absence of APC and Zn2+ was taken as 100%. The data are presented as the means ± S.E. of five or six independent experiments. Conc, concentration.
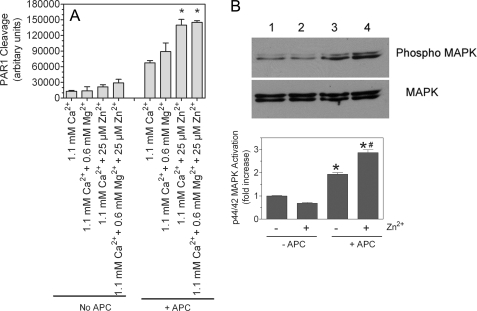
Zinc Enhances APC-induced PAR1 Cleavage and p44/42 MAPK Activation
APC upon binding to EPCR was shown to activate PAR1 (33). To investigate whether the enhanced binding of APC to EPCR in presence of zinc ions would enhance APC-mediated PAR1 activation and subsequent cell signaling, we measured the effect of Zn2+ on APC cleavage of PAR1 and APC-induced p44/42 MAPK activation in HUVEC. As shown in Fig. 10A, the presence of physiological concentrations of zinc ions significantly enhanced APC cleavage of PAR1. Mg2+ also slightly enhanced APC cleavage of PAR1, but the increase was not statistically significant. Inclusion of Mg2+ with Zn2+ did not further enhance APC cleavage of PAR1. Consistent with PAR1 cleavage data, the presence of physiological concentrations of zinc significantly enhanced APC-induced p44/42 MAPK activation (Fig. 10B). The observed increase in APC-mediated activation of PAR1 and p44/42 MAPK in the presence of Zn2+ stems from the increased number of EPCR-APC complexes formed on cell surfaces in the presence of Zn2+ rather than enhanced catalytic function of APC.
FIGURE 10.
Effect of physiological concentrations of Zn2+ and Mg2+ on PAR1 cleavage of APC and downstream p44/42 MAPK activation. A, HUVEC monolayers were transduced with AP-PAR1 adenovirus (20 multiplicity of infection/cell) and allowed to grow for 48 h. HUVEC monolayers expressing AP-PAR1 were treated with APC (80 nm) in the presence and absence of physiological concentrations of Ca2+, Mg2+, or Zn2+ alone or in combination. A small aliquot of the supernatant was removed immediately after adding APC and after 45 min, and AP activity in the supernatant was measured as described under “Experimental Procedures.” The basal readings obtained at 0 min were subtracted from the readings obtained at 45 min. The data shown in the graph represent the means ± S.E. (n = 3). *, the value is significantly higher than the value obtained in the reaction mixture containing the corresponding concentration of Ca2+ alone (p < 0.005). B, confluent monolayers of HUVEC were serum-starved in the presence and absence of 25 μm Zn2+ for 3 h in serum-free medium, which contains near physiological concentrations of Ca2+ (1.8 mm) and Mg2+ (0.8 mm). Thereafter, APC (20 nm) was added to the cells for 15 min. The cell lysates were subjected to SDS-PAGE and immunoblotted with MAPK antibodies. The band intensities were calculated with Quantity One software of four independent experiments and presented as the means ± S.E. (n = 4). *, the value is significantly higher (p < 0.05) compared with the corresponding value obtained in the absence of APC. #, APC-mediated p44/42 activation is significantly higher (p < 0.05) in the presence of Zn2+ compared with in its absence.
DISCUSSION
Zinc is essential for growth, development, and the transmission of the genetic message (13, 35). Earlier studies have suggested that zinc ions may also play a role in hemostasis by modulating the activity of plasma clotting factors, platelet aggregation, and platelet interaction with endothelial cells (11). The current study describes the influence of zinc ions on the protein C pathway. Our data show that zinc ions promote the binding of protein C and activated protein C to their receptor, EPCR. Zn2+-mediated enhancement of protein C/APC binding to EPCR results in a net increase in internalization of the ligands. The enhanced binding of APC to EPCR on the endothelial cell surface in the presence of zinc ions may also enhance APC-mediated PAR1 activation.
The Gla domain of protein C is responsible for much of the binding energy and specificity of the protein C-EPCR interaction. This binding is dependent on the divalent metal ion Ca2+ (36). The data presented in this manuscript show that the presence of physiological concentrations of Zn2+ (25 μm) markedly increases the Ca2+-dependent protein C and APC binding to EPCR. Zn2+, however, does not replace Ca2+ as a mandatory cofactor for protein C/APC binding to EPCR. The presence of physiological amounts of Mg2+ had no effect on Zn2+-mediated enhancement of protein C and APC binding to EPCR. The kinetic analysis of protein C and APC binding to EPCR suggests that Zn2+ promotes protein C/APC binding to EPCR by increasing the binding affinity (i.e. lowering Kd) of protein C/APC for its receptor. The Zn2+-mediated increase in protein C and APC binding to EPCR led to increased endocytosis of protein C/APC, suggesting that Zn2+ may facilitate the clearance of protein C/APC.
Zn2+ modulation of Gla domain-mediated protein C/APC binding to EPCR and the Gla domain-independent APC amidolytic activity suggest that Zn2+, like Ca2+, may bind to the Gla domain as well as the protease domain of protein C. Data from the equilibrium binding studies performed with 65Zn2+ provide strong support for this hypothesis. These data show that Zn2+ binds to both GD-APC and APC but that the amount of Zn2+ bound to full-length APC was 3–4-fold higher than the amount bound to GD-APC. Kinetic analysis of equilibrium binding studies suggests that two Zn2+ atoms bind to APC outside the Gla domain with relatively high affinity (∼70 μm). At least one of the Zn2+-binding sites may overlap with the Ca2+-binding site because the Zn2+ binding to GD-APC was inhibited by ∼50% in the presence of saturating concentrations of Ca2+. These data were consistent with earlier findings suggesting that Zn2+ probably binds to a high affinity Ca2+-binding site in GD-protein C (37). However, at present there is no direct evidence that Zn2+ and Ca2+ may have overlapping sites on the protease domain. It is possible that the two metal sites are energetically linked, and thus Ca2+ binding to the protease domain may allosterically regulate Zn2+ binding to the protease domain as observed with sodium binding (38). Irrespective of the mechanism by which Ca2+ reduces Zn2+ binding to the protease domain, these data suggest that at physiological concentrations of Ca2+, only one Zn2+ atom may bind to the protease domain of APC. The substantially increased Zn2+ binding to the full-length APC compared with GD-APC suggests that the N terminus of the Gla domain of protein C contains multiple Zn2+-binding sites. Interestingly, Zn2+ bound to full-length APC and GD-APC with a similar affinity, suggesting that the Gla domain, as well the protease domain, may contain relatively high affinity binding sites for Zn2+. A majority of the Zn2+-binding sites in the Gla domain appear to be distinct from the Ca2+-binding sites, because less than 40% of Zn2+ was replaced by Ca2+. The intrinsic fluorescence quenching of both GD-APC and APC upon the addition of Zn2+ suggests that Zn2+ binding induces conformational changes in protein C/APC. Overall our observations suggest that Zn2+ binds to protein C/APC in both the Gla and the protease domains. Zn2+ binding to the Gla domain of protein C/APC stabilizes the conformation of the Gla domain, thereby favoring its binding to EPCR, whereas Zn2+ binding to the protease domain destabilizes the catalytic triad and thereby inhibits the amidolytic activity of APC. However, the occupancy of the protease domain by Ca2+ protects against the destabilizing effect of Zn2+.
The locations of zinc coordination sites in protein C/APC are uncertain at this time. An examination of the protein C/APC primary structure revealed no consensus canonical zinc binding sequences homologous to other known zinc-binding proteins. The most common amino acids found in coordination sites for Zn2+ are His, Glu, Asp, and Cys (39). Although the protease domain of APC contains multiple His, Glu, Asp, and Cys residues, they are not present in any significant abundance compared with other amino acids. Recent crystallography studies of FVIIa revealed that the Zn1 site involves the side chains of His216, Glu220, and Ser222, and the Zn2 site involves the side chains of His257, Asp219, and Lys161 (10). Interestingly, none of these residues, except for Glu220, are conserved in APC. Therefore, it seems unlikely that Zn2+ binds to APC at the Ca2+ loop as it does in FVIIa. However, as observed with FVIIa, Ca2+ partly reverses the inhibitory effect of Zn2+ on APC amidolytic activity, raising the possibility that the Zn2+- and Ca2+-binding sites in the protease domain are either overlapping or energetically linked. Analysis of the protein C sequence with a recently developed program (PREDZINC) that predicts zinc-binding sites from amino acid sequences by combining a support vector machine (SVM) and homology-based predictions (40) identified one potential Zn2+-binding site in the protease domain involving Cys141, His246, Cys331, and Asp359 (analysis was performed by Nanjiang Shu, Structural Chemistry, Stockholm University, Stockholm, Sweden). Scanning the crystal structure of GD-APC from the Protein Data Bank for zinc-binding sites using the recently developed FEATURE algorithm yielded a cluster of hits in the protease domain. Analysis of the cluster for potential coordinating residues revealed that Zn2+-binding sites may involve the side chains of Asp254, His212, Cys211, and Cys96.
Although we expected Zn2+ to bind to the Gla domain as it modulated the Gla domain-dependent protein C/APC binding to EPCR, it was surprising for us to find that the Gla domain of protein C contains as many as six to eight Zn2+-binding sites. Although this observation is unexpected, it is unlikely that this is an experimental artifact because the data were reproduced with two different batches of APC. Moreover, consistent with earlier studies (8, 10), we noted only two Zn2+ atoms binding to full-length factor VIIa. Furthermore, 100-fold molar excess of unlabeled zinc completely abrogated 65Zn2+ binding to APC, which confirms the specificity of the observed Zn2+ binding. Residues 1–41 of the Gla domain of protein C, a fragment that was removed from APC by selective proteolysis with chymotrypsin, contain one His residue (His10) and two Cys residues (Cys17 and Cys22), the two predominant residues that participate in zinc coordination. Both Cys17 and Cys22 are predicted as zinc-binding sites in protein C by the PREDZINC program (40). One could speculate that other residues outside of the N-terminal Gla domain region (residues 1–41) may contribute to Zn2+ binding. It is possible that the removal of amino acids 1–41 may change the conformation of the protein and abolish the Zn2+ binding to the region outside of the N terminus of the Gla domain. It may be pertinent to note here that PREDZINC identified several residues outside of the N-terminal Gla region as putative zinc-binding residues with high binding score (>0.45). They include His44, Cys50, Cys63, Cys89, Cys98, and Cys105. FEATURE also recognized one or more of these residues as potential coordinating residues for zinc binding. Although most of the zinc-binding sites are coordinated by three or four amino acid residues, in rare cases zinc atoms could be coordinated by only one or two amino acid residues (41). In light of multiple Zn2+ binding to the Gla domain of protein C, it is likely that zinc binding to the Gla domain may be coordinated by a single zinc-binding residue. Further studies are needed to identify zinc-binding sites in protein C.
The full physiological significance of Zn2+ binding to protein C/APC remains to be elucidated. Our present data using the endothelial cell model system show that Zn2+-mediated increase in protein C binding to EPCR results in increased APC generation. However, it is difficult to judge the physiological significance of Zn2+ effect on APC generation because physiological concentrations of Mg2+ also enhanced APC generation, and Zn2+ did not further enhance APC generation in the presence of Mg2+. Although Zn2+ inhibits the amidolytic activity of APC, the physiological concentrations of Zn2+ will have no significant effect on the anticoagulant function of APC, because high concentrations of zinc ions were required to inhibit the amidolytic activity of APC, and this inhibition was attenuated by the presence of physiological levels of Ca2+. Consistent with this, we found that Zn2+ had no effect on the anticoagulant activity of APC. Recent studies suggest that APC bound to EPCR activates PAR1-mediated cell signaling, and this may be responsible for some of the nonhemostatic functions of EPCR (28, 33, 34, 42). Zn2+ binding to APC could promote APC-induced cell signaling because it increases the formation of EPCR-APC complexes on cell surfaces, which results in increased activation of PAR1.
Overall our present data show that Zn2+ binds to protein C/APC and induces conformational changes in the protein. Zn2+ binding to protein C/APC increases their affinity for EPCR, which in turn may result in increased APC generation and APC-mediated cell signaling. These observations suggest that zinc ions may play an important and physiologically relevant role in modulating the protein C/APC pathway. Further studies are needed to map the specific Zn2+-binding sites in protein C/APC and to elucidate the specific physiological functions mediated by the interaction of Zn2+ with protein C and APC.
Acknowledgments
We thank C. T. Esmon (Oklahoma Medical Research Foundation) for providing EPCR mAb, A. Rezaie (St. Louis University) for providing soluble thrombomodulin, P. Neuenschwander (The University of Texas Health Science Center at Tyler) for helping in preparation of Gla domain-deleted APC, N. Shu (Stockholm University) for predicting potential zinc-binding sites in protein C, and Russ Altman and Jessica Ebert (Stanford University) for helpful discussion on the use of FEATURES web interface.
This work was supported, in whole or in part, by National Institutes of Health Grants HL58869 (to L. V. M. R.) and HL65500 (to U. R. P.).
- FVIIa
- activated factor VII
- APC
- activated protein C
- GD
- Gla domain-deleted
- EPCR
- endothelial cell protein C receptor
- PAR
- protease-activated receptor
- HUVEC
- human umbilical vein endothelial cell(s)
- AP
- alkaline phosphatase
- mAb
- monoclonal antibody
- CHO
- Chinese hamster ovary.
REFERENCES
- 1.Byrne R., Amphlett G. W., Castellino F. J. (1980) J. Biol. Chem. 255, 1430–1435 [PubMed] [Google Scholar]
- 2.Shore J. D., Day D. E., Bock P. E., Olson S. T. (1987) Biochemistry 26, 2250–2258 [DOI] [PubMed] [Google Scholar]
- 3.Greengard J. S., Heeb M. J., Ersdal E., Walsh P. N., Griffin J. H. (1986) Biochemistry 25, 3884–3890 [DOI] [PubMed] [Google Scholar]
- 4.Church W. R., Boulanger L. L., Messier T. L., Mann K. G. (1989) J. Biol. Chem. 264, 17882–17887 [PubMed] [Google Scholar]
- 5.Butenas S., Lawson J. H., Kalafatis M., Mann K. G. (1994) Biochemistry 33, 3449–3456 [DOI] [PubMed] [Google Scholar]
- 6.Sekiya F., Yoshida M., Yamashita T., Morita T. (1996) J. Biol. Chem. 271, 8541–8544 [DOI] [PubMed] [Google Scholar]
- 7.Liaw P. C., Neuenschwander P. F., Smirnov M. D., Esmon C. T. (2000) J. Biol. Chem. 275, 5447–5452 [DOI] [PubMed] [Google Scholar]
- 8.Petersen L. C., Olsen O. H., Nielsen L. S., Freskgård P. O., Persson E. (2000) Protein Sci. 9, 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen A. H., Lund-Hansen T., Komiyama Y., Petersen L. C., Oestergard P. B., Kisiel W. (1991) Thromb. Haemost. 65, 528–534 [PubMed] [Google Scholar]
- 10.Bajaj S. P., Schmidt A. E., Agah S., Bajaj M. S., Padmanabhan K. (2006) J. Biol. Chem. 281, 24873–24888 [DOI] [PubMed] [Google Scholar]
- 11.Tubek S., Grzanka P., Tubek I. (2008) Biol. Trace Elem. Res. 121, 1–8 [DOI] [PubMed] [Google Scholar]
- 12.Gordon P. R., Woodruff C. W., Anderson H. L., O'Dell B. L. (1982) Am. J. Clin. Nutr. 35, 113–119 [DOI] [PubMed] [Google Scholar]
- 13.Vallee B. L., Falchuk K. H. (1993) Physiol. Rev. 73, 79–118 [DOI] [PubMed] [Google Scholar]
- 14.Gorodetsky R., Mou X., Blankenfeld A., Marx G. (1993) Am. J. Hematol. 42, 278–283 [DOI] [PubMed] [Google Scholar]
- 15.Bernardo M. M., Day D. E., Olson S. T., Shore J. D. (1993) J. Biol. Chem. 268, 12468–12476 [PubMed] [Google Scholar]
- 16.Greengard J. S., Griffin J. H. (1984) Biochemistry 23, 6863–6869 [DOI] [PubMed] [Google Scholar]
- 17.Shimada T., Kato H., Iwanaga S. (1987) J. Biochem. 102, 913–921 [DOI] [PubMed] [Google Scholar]
- 18.Schousboe I. (1993) Blood Coagul. Fibrinolysis 4, 671–678 [PubMed] [Google Scholar]
- 19.Heeb M. J., Prashun D., Griffin J. H., Bouma B. N. (2009) FASEB J. 23, 2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman J. E. (1992) Annu. Rev. Biochem. 61, 897–946 [DOI] [PubMed] [Google Scholar]
- 21.Berg J. M., Shi Y. (1996) Science 271, 1081–1085 [DOI] [PubMed] [Google Scholar]
- 22.Brewer G. J., Hill G. M., Prasad A. S., Cossack Z. T. (1983) Prog. Clin. Biol. Res. 129, 35–51 [PubMed] [Google Scholar]
- 23.Csermely P., Sándor P., Radics L., Somogyi J. (1989) Biochem. Biophys. Res. Commun. 165, 838–844 [DOI] [PubMed] [Google Scholar]
- 24.Esmon C. T. (2003) J. Thromb. Haemost. 1, 1343–1348 [DOI] [PubMed] [Google Scholar]
- 25.Esmon C. T. (2004) Crit. Care Med. 32, S298–S301 [DOI] [PubMed] [Google Scholar]
- 26.Esmon N. L., DeBault L. E., Esmon C. T. (1983) J. Biol. Chem. 258, 5548–5553 [PubMed] [Google Scholar]
- 27.Ghosh S., Pendurthi U. R., Steinoe A., Esmon C. T., Rao L. V. (2007) J. Biol. Chem. 282, 11849–11857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludeman M. J., Kataoka H., Srinivasan Y., Esmon N. L., Esmon C. T., Coughlin S. R. (2005) J. Biol. Chem. 280, 13122–13128 [DOI] [PubMed] [Google Scholar]
- 29.Fukudome K., Kurosawa S., Stearns-Kurosawa D. J., He X., Rezaie A. R., Esmon C. T. (1996) J. Biol. Chem. 271, 17491–17498 [DOI] [PubMed] [Google Scholar]
- 30.Rezaie A. R., Esmon C. T. (1992) J. Biol. Chem. 267, 26104–26109 [PubMed] [Google Scholar]
- 31.Rezaie A. R., Esmon C. T. (1995) Biochemistry 34, 12221–12226 [DOI] [PubMed] [Google Scholar]
- 32.Yang L., Prasad S., Di Cera E., Rezaie A. R. (2004) J. Biol. Chem. 279, 38519–38524 [DOI] [PubMed] [Google Scholar]
- 33.Riewald M., Petrovan R. J., Donner A., Mueller B. M., Ruf W. (2002) Science 296, 1880–1882 [DOI] [PubMed] [Google Scholar]
- 34.Feistritzer C., Riewald M. (2005) Blood 105, 3178–3184 [DOI] [PubMed] [Google Scholar]
- 35.Falchuk K. H. (1993) Prog. Clin. Biol. Res. 380, 91–111 [PubMed] [Google Scholar]
- 36.Regan L. M., Mollica J. S., Rezaie A. R., Esmon C. T. (1997) J. Biol. Chem. 272, 26279–26284 [DOI] [PubMed] [Google Scholar]
- 37.Johnson A. E., Esmon N. L., Laue T. M., Esmon C. T. (1983) J. Biol. Chem. 258, 5554–5560 [PubMed] [Google Scholar]
- 38.He X., Rezaie A. R. (1999) J. Biol. Chem. 274, 4970–4976 [DOI] [PubMed] [Google Scholar]
- 39.Auld D. S. (2001) Biometals 14, 271–313 [DOI] [PubMed] [Google Scholar]
- 40.Shu N., Zhou T., Hovmöller S. (2008) Bioinformatics 24, 775–782 [DOI] [PubMed] [Google Scholar]
- 41.Vallee B. L., Auld D. S. (1990) Biochemistry 29, 5647–5659 [DOI] [PubMed] [Google Scholar]
- 42.Cheng T., Liu D., Griffin J. H., Fernández J. A., Castellino F., Rosen E. D., Fukudome K., Zlokovic B. V. (2003) Nat. Med. 9, 338–342 [DOI] [PubMed] [Google Scholar]