Abstract
3,4-Dichloropropionanilide (DCPA), or propanil, a post-emergent herbicide used on rice and wheat crops in the United States, is immunotoxic in vivo and in vitro. Although it has been documented that DCPA exerts differential effects on specific immune cell types and is toxic to the liver, the way in which DCPA modulates intracellular functions leading to these effects is less understood. In this study, Jurkat T cells and hepatocytes from C57Bl/6 mice were exposed to 100 μM DCPA for 1.5 h. Following incubation, subcellular fractions of each cell type were isolated. DCPA, when present, was removed from each cell fraction by liquid–liquid extraction. The extraction product was then analyzed for the presence of DCPA using liquid chromatography–tandem mass spectrometry (LC-MS/MS). The cellular uptake of DCPA was monitored by detection of the molecular ion and product ion of DCPA. The analyses demonstrate that DCPA, a lipophilic compound, localizes primarily in the cytosol of T cells and hepatocytes. These results indicate that DCPA is able to cross the plasma membrane and is accessible to intracellular immunomodulatory effectors.
Propanil (3,4-dichloropropionanilide, DCPA) is an herbicide used extensively on rice and wheat crops in the United States. Individuals involved in agriculture, in particular, are at risk for high-level exposure to DCPA. DCPA can be absorbed through the respiratory tract, the gastrointestinal tract, and the intact skin (Richards et al., 2001). There are well-known immunotoxic effects on various compartments of the immune system following in vivo DCPA exposure (reviewed by Salazar et al., 2008). DCPA exposure decreases contact hypersensitivity responses, reduces proliferative responses to T- and B-cell mitogens, and impedes mixed lymphocyte reactions (Barnett & Gandy, 1989). In addition to immunotoxicity, reports have demonstrated that DCPA is hepatotoxic to humans (De Silva & Bodinayake, 1997) and rodents (Santillo et al., 1995).
Although it has been documented that DCPA exerts differential effects on specific immune cell types and is hepatotoxic, to date, the subcellular localization from which DCPA modulates immune cells and hepatocytes has not been examined. The high lipophilicity of DCPA has led to the suggestion of a higher affinity of DCPA for membranes (Corsini et al., 2007). The aim of this study is to determine the localization of DCPA in T cells and hepatocytes following in vitro exposure. Following DCPA treatment, subcellular fractions of each cell type were isolated. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) provides a definitive detection method for DCPA subcellular localization. Our results demonstrate that DCPA, a lipophilic compound (Finizio, 1997), localizes primarily in the cytosol of both T cells and hepatocytes. This study improves our understanding of the cellular location in which DCPA exerts its toxic effects.
MATERIALS AND METHODS
DCPA Treatment
Experiments were performed using the human T-cell leukemia cell line, Jurkat clone E6-1, obtained from the ATCC (American Tissue Culture Collection, Manassas, VA). Jurkat cells were maintained in complete RPMI media (Mediatech, Inc., Herndon, VA). The cultures were kept at 37°C in 5% CO2. Cells in suspension were grown to obtain approximately 5 × 107 cells. Cells were stimulated with anti-CD3 (10 μg/ml) and anti-CD28 (2 μg/ml) (BD PharMingen, San Diego, CA) and at the same time exposed to 100 μM DCPA or ethanol (vehicle control) for 1.5 h at 37°C, 5% CO2.
Female C57Bl/6 mice at 8–10 wk of age were obtained from Hilltop Lab Animals, Inc. (Scottsdale, PA). Normal mouse hepatocytes were isolated using the method described by Muller et al. (1972). Overnight cultures of isolated primary hepatocytes were incubated with 100 μM DCPA or ethanol for 1.5 h at 37°C, 5% CO2.
Fractionation of T Cells and Hepatocytes
T cells were fractionated as described by Ramsby and Makowski (2005). Hepatocytes were fractionated as described by Graham (2000). The purity of each fraction was determined by Western blotting, in which cell-fraction-specific antibodies were used. Protein quantitation of each fraction was determined using the 2D Quant Kit (Amersham Biosciences).
Mass Spectrometric Analysis of Cell Fractions
Volumes of all fractions were adjusted using phosphate-buffered saline (PBS) to achieve a protein concentration of 0.5 μg/μl. DCPA was extracted from cell fractions using a liquid–liquid extraction procedure, with acetaminophen as an internal standard. A 5-point standard curve of a DCPA concentration range of 5–500 ng/ml was used to quantify DCPA in cell fractions. LC-MS/MS was used to determine DCPA localization in cells.
RESULTS AND DISCUSSION
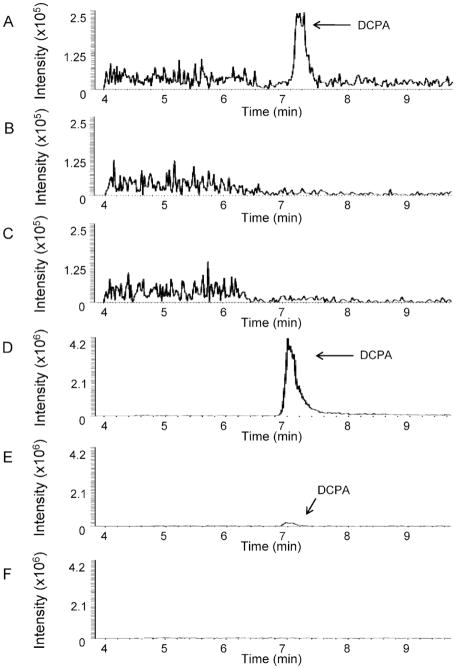
Analysis of fractionated T cells following stimulation and treatment with 100 μM DCPA for 1.5 h demonstrated that DCPA localizes in the cytosol (Figure 1A and Table 1). DCPA was not detected in the membrane/organelle fraction (Figure 1B) or in the nuclear/cytoskeletal fraction (Figure 1C). Therefore, DCPA, a lipophilic molecule, passes through the plasma membrane and resides in the cytosol of T cells following 1.5 h of exposure. Analysis of fractionated hepatocytes following treatment with DCPA for 1.5 h demonstrated that the molecule localizes mostly in the cytosol (Figure 1D) (Table 1). DCPA was also detected in the light mitochondrial fraction (Figure 1E), but the level was minimal relative to the level detected in the cytosol (Table 1). There was no DCPA detected in the peroxisomal fraction of hepatocytes (Figure 1F). Therefore, DCPA localizes predominatly in the cytosol of hepatocytes following 1.5 h of exposure.
FIGURE 1.
Chromatograms of DCPA localization in T cells and hepatocytes. Jurkat T cells and mouse hepatocytes were exposed to 100 μM DCPA for 1.5 h. Cells were fractionated using fractionation/homogenization and centrifugation. LC-MS/MS was used to determine DCPA location in cell fractions. Acetaminophen was used as an internal standard. Protein concentration of each fraction was 0.5 μg/μl. (A) T-cell cytosol fraction, (B) T-cell membrane/organelle fraction, (C) T-cell nuclear/cytoskeletal fraction, (D) hepatocyte cytosol, (E) hepatocyte light mitochondrial fraction, and (F) hepatocyte peroxisome fraction. Purity of each fraction was verified through Western blot. All values are means of three separate experiments.
TABLE 1.
DCPA Concentration in T-Cell and Hepatocyte Subcellular Fractions
| T cell | DCPA concentration (ng/ml)a | Hepatocyte | DCPA concentration (ng/ml)a |
|---|---|---|---|
| Cytosol | 26.3 ± 2.0 | Cytosol | 120 ± 5.8 |
| Membrane/organelle | 0b | Light mitochondrial | 25.3 ± 3.1 |
| Nuclear/cytoskeletal | 0 | Peroxisomal | 0 |
Mean ± SEM, n = 3.
below the level of detection.
The fivefold greater DCPA concentration observed in the hepatocyte cytosol compared to that in the T-cell cytosol is likely attributed to differences in cell size and cell composition (Table 1). Hepatocytes are among the largest of cell types, with an average diameter of 18–22 μm. In addition, they display an eosinophilic cytosplasm which contains numerous mitochondria. T cells are smaller than hepatocytes, with an average diameter of 7–12 μm. The nucleus is the major constituent of T cells; thus, there is little eosinophilic cytoplasm. The finding that DCPA is only detected in the cytosol of T cells, coupled with the size of this constituent, suggests that DCPA’s mechanism of action has specificity.
We hypothesized that DCPA exerts its effects on immune cells and hepatocytes by interfering with cell activation and cell signaling processes at the cell membrane, due to DCPA being a highly hydrophobic, lipophilic compound. Our finding that DCPA is not in the membrane does not eliminate the possibility that DCPA is affecting membrane proteins or channels as it migrates into the cytosol. An earlier finding in our laboratory demonstrated that DCPA exposure does not disrupt the motional properties of lipid hydrocarbon chains in the bilayer, but does alter distribution of lipids in distinct motional environments in the membrane (Brundage et al., 2003). The present study provides an explanation for the limited effect observed in hydrocarbon chain mobility. The finding that DCPA is able to move through the membrane of T cells and hepatocytes and localize in the cytosol will aid in the understanding of DCPA’s mechanism of toxicity to cellular functions.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Barnett JB, Gandy J. Effect of acute propanil exposure on the immune response of C57Bl/6 mice. Fundam Appl Toxicol. 1989;12:757–764. doi: 10.1016/0272-0590(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Brundage KM, Barnett JB, Mahaney JE. The amide class herbicide 3,4-dichloropropionanilide (DCPA) alters the mobility of hydrocarbon chains in T-lymphocyte but not macrophage membranes. J Toxicol Environ Health A. 2003;66:2253–2265. doi: 10.1080/713854000. [DOI] [PubMed] [Google Scholar]
- Corsini E, Codeca I, Mangiaratti S, Birindelli S, Minoia C, Turci R, Viviani B, Facchi A, Vitelli N, Lucchi L, Galli CL, Marinovich M, Colosio C. Immunomodulatory effects of the herbicide propanil on cytokine production in humans: In vivo and in vitro exposure. Toxicol Appl Pharmacol. 2007;222:202–210. doi: 10.1016/j.taap.2007.04.017. [DOI] [PubMed] [Google Scholar]
- De Silva WA, Bodinayake CK. Propanil poisoning. Ceylon Med J. 1997;42:81–84. [PubMed] [Google Scholar]
- Finizio A, Vighi M, Sandroni D. Determination of n-octanol/water partition coefficient (Kow) of pesticide critical review and comparison of methods. Chemosphere. 1997;34:131–161. [Google Scholar]
- Graham JM. Isolation of peroxisomes from tissues and cells by differential and density gradient centrifugation. Curr Protocols Cell Biol. 2001;3:3.5. doi: 10.1002/0471143030.cb0305s06. [DOI] [PubMed] [Google Scholar]
- Muller M, Schreiber M, Kartenbeck J, Schreiber G. Preparation of single-cell suspensions from normal liver, regenerating liver, and Morris hepatomas 9121 and 5123tc. Cancer Res. 1972;32:2568–2576. [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS. The proteomics protocols handbook. Totowa, NJ: Humana Press; 2005. Differential detergent fractionation of eukaryotic cells; pp. 37–48. [Google Scholar]
- Richards SM, McClure GY, Lavy TL, Mattice JD, Keller RJ, Gandy J. Propanil (3,4-dichloropropionanilide) particulate concentrations within and near the residences of families living adjacent to aerially sprayed rice fields. Arch Environ Contam Toxicol. 2001;41:112–116. doi: 10.1007/s002440010227. [DOI] [PubMed] [Google Scholar]
- Salazar KD, Ustyugova IV, Brundage KM, Barnett JB, Schafer R. A review of the immunotoxicity of the pesticide 3,4-dichloropropionanilide. J Toxicol Environ Health B. 2008;11:630–645. doi: 10.1080/10937400701724386. [DOI] [PubMed] [Google Scholar]
- Santillo M, Rippa C, Morte RD, Villani GRD, Santangelo F, Staiano N, Mondola P. Enhancement of tissue lipoperoxidation in propanil-treated rats. Toxicol Lett. 1995;78:215–218. doi: 10.1016/0378-4274(95)03257-l. [DOI] [PubMed] [Google Scholar]