Abstract
Malignant gliomas are the most common and deadly brain tumors. Nevertheless, survival for patients with glioblastoma, the most aggressive glioma, although individually variable, has improved from an average of 10 months to 14 months after diagnosis in the last 5 years due to improvements in the standard of care. Radiotherapy has been of key importance to the treatment of these lesions for decades, and the ability to focus the beam and tailor it to the irregular contours of brain tumors and minimize the dose to nearby critical structures with intensity-modulated or image-guided techniques has improved greatly. Temozolomide, an alkylating agent with simple oral administration and a favorable toxicity profile, is used in conjunction with and after radiotherapy. Newer surgical techniques, such as fluorescence-guided resection and neuroendoscopic approaches, have become important in the management of malignant gliomas. Furthermore, new discoveries are being made in basic and translational research, which are likely to improve this situation further in the next 10 years. These include agents that block 1 or more of the disordered tumor proliferation signaling pathways, and that overcome resistance to already existing treatments. Targeted therapies such as antiangiogenic therapy with antivascular endothelial growth factor antibodies (bevacizumab) are finding their way into clinical practice. Large-scale research efforts are ongoing to provide a comprehensive understanding of all the genetic alterations and gene expression changes underlying glioma formation. These have already refined the classification of glioblastoma into 4 distinct molecular entities that may lead to different treatment regimens. The role of cancer stem-like cells is another area of active investigation. There is definite hope that by 2020, new cocktails of drugs will be available to target the key molecular pathways involved in gliomas and reduce their mortality and morbidity, a positive development for patients, their families, and medical professionals alike.
Current Challenges in the Treatment of High–Grade Gliomas and the Promise of Personalized Medicine with Targeted Cancer Drugs
The life expectancy of patients with glioblastoma multiforme (GBM), the most malignant glioma (World Health Organization [WHO] grade IV), using the current standard of care is on average 14 months after diagnosis despite aggressive surgery, radiation, and chemotherapies. This puts the physicians in charge of delivering dismal news to the patients and families in a difficult position. Much skill is needed to progressively deliver this negative message with tact, and balance it with the known fact that outcome is individually variable and a small fraction of patients do much better than expected. Fortunately, there is optimism in that this situation is likely to change within the next 10 years. Tremendous new discoveries have been made in basic and translational research and there is unprecedented new knowledge that has been acquired in recent years. Summarizing these advances on various fronts is straightforward and the following comments help to put them in perspective. Radiotherapy has been of key importance to the treatment of these lesions for decades. Although the ionizing radiation itself has not changed, the ability to focus the beam and tailor it to the irregular contours of brain tumors and minimize the dose to nearby critical structures with intensity-modulated or image-guided techniques has improved greatly.1 The use of carmus-tine-impregnated biodegradable wafers (Gliadel; Eisai Inc, Woodcliff Lake, NJ) after surgical resection of newly diagnosed glioblastomas and recurrent malignant gliomas improves the time to disease progression and overall survival in selected patients.2,3 The use of temozolomide concurrently and after radiotherapy has clearly improved overall survival and has the advantage of wide applicability because of its relatively simple administration and favorable toxicity profile compared with older agents such as carmustine.4 Targeted therapies based on our understanding of the biology of these lesions are finding their way into clinical practice, as evidenced by the recent approval of bevacizumab, an antibody to vascular endothelial growth factor (VEGF), for the treatment of recurrent or progressive glioblastoma.5
Enthusiasm for the therapeutic advances mentioned above, although of value, must be tempered because these tumors reliably become resistant to and overcome the effect of these therapies. The tumor’s ability to repair radiation-induced injury accomplished by aberrant or amplified growth and survival signaling pathways are being appreciated.6 Although of value in extending tumor control and survival in selected cases, Gliadel is associated with more frequent episodes of wound infection, cerebral edema, and wound breakdown in patients compared with individuals not receiving this intervention.2 As a standard alkylating agent, temozolomide-induced injury is repaired by the DNA repair enzymes, including methylguanyl methyltransferase (MGMT). It is now commonly recognized that silencing of the MGMT gene promoter by methylation is associated with better tumor response to combination treatment with radiation and temozolomide.7 Bevacizumab has been observed to result in a conversion in the glioblastoma phenotype to a more invasive character over time, resulting in rapid decompensation at the time of treatment failure and withdrawal of this treatment.8,9
From a clinician’s standpoint, it is a simple fact that there will be heterogeneity of tumor response from one malignant glioma case to the next.10 Although simple demographics and functional status are certainly important in predicting responsiveness and outcome, the underlying molecular characteristics of the tumor are the true key to understanding this variability.11 Excitement over the recent success of bevacizumab in brain tumors and other cancer sites is likely to be tempered by the increasing recognition that there are underlying genetic variations in VEGF that clearly influence sensitivity or resistance to this treatment agent.12 The understanding and technology with which to identify these differences and take advantage of these data are now clearly at hand.13 Incorporation of these techniques into patient tumor analysis and treatment management allows for the provision of individualized tumor therapy, often referred to as personalized medicine. The information that follows provides details of our improved understanding of the biology of these tumors and offers insights into how that information may be used to individualize therapy. To offer a special perspective to the readers of this review, predictions of where the various facets of brain tumor therapy will be by 2020 are provided throughout the presentation.
Pathology and Molecular Genetics of Malignant Gliomas
A combined understanding of the genetic basis and pathology of gliomas provides insight into biologically based tumor classification. In turn, this information is the route by which the most effective therapy can be focused.
Pathology and Clinical Characteristics of Gliomas
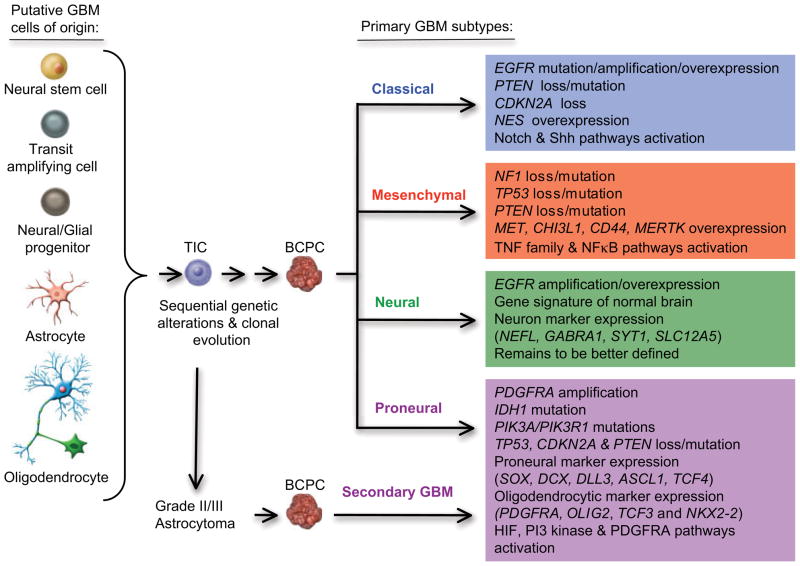
Gliomas represent a group of low–grade and high–grade brain tumors that originate from glia (from the Greek for “glue”), the brain tissue that was traditionally viewed as providing support functions to neural cells such as nutrients, oxygen, mechanical support, guidance in development, immune functions, and waste disposal. In reality, glial cells function as true partners to neurons and are involved in complex processes, including signal transduction and neurotransmission. The cell(s) of origin for the formation of gliomas is currently unknown (Fig. 1). One major theory postulates that neural stem cells or neural progenitors undergo transformation events when they are in a transit-amplifying phase during development.14,15 Other evidence points to the mutation-induced dedifferentiation of mature brain cells such as astrocytes and oligodendrocytes.16
FIGURE 1.
Sequential Genetic Changes Observed in the Pathogenesis of Different Subtypes of Glioblastoma. Some cells in the normal brain undergo genetic alterations, which leads to a population of tumor–initiating cells (TICs), which can then further accumulate genetic and epigenetic changes and become brain cancer–propagating cells (BCPC). The latter cells are responsible for the formation of glioblastoma. GBM indicates glioblastoma multiforme; EGFR, epidermal growth factor receptor; PTEN, phosphatase and tensin homolog; TNF, tumor necrosis factor; PDGFRA, platelet-derived growth factor receptor–A; IDH, isocitrate dehydrogenase; PI3K, phosphoinositol 3–kinase; HIF, hypoxia-inducible factor.
The major group of malignant gliomas in the brain are anaplastic astrocytomas (WHO grade III) and glioblastomas (WHO grade IV). Anaplastic astrocytomas are diffusely infiltrating neoplasms that demonstrate focal or dispersed anaplasia and an increased proliferation index compared with astrocytomas of a lesser grade (WHO grades I and II). The histological diagnosis is principally based on nuclear atypia and mitotic activity. Radiologically, these neoplasms present as masses with partial contrast enhancement due to limited disruption of the blood–brain barrier (BBB). The pathognomonic features that characterize glioblastoma at the tissue level are the presence of areas of vascular proliferation and/or necrosis.17 Most frequently, these tumors arise de novo, although approximately 10% have a prior clinical history of a lower grade astrocytoma, in which case they are also termed “secondary” glioblastoma. Radiologically, glioblastomas present with irregular contours and a peripheral zone with strong contrast enhancement around a darker, hypodense, necrotic area and with nonenhancing tumor extending outside the area of enhancement.
Genetics of Malignant Gliomas
Cancers originate as the result of hereditary or somatic alterations in genes that control critical biological processes. These mutational events, rarely visible through chromosomal analyses of the cell karyotype, are detected through sophisticated genetic analysis methods and typically trigger the activation of oncogenes or the silencing of tumor suppressor genes. The accumulation of such genetic damage over time permits the survival and progressive transformation of abnormal cell populations that eventually lead to the formation of a tumor. Genetic analyses over the past 30 years have defined the major mutational targets in the human genome that are associated with the formation of brain tumors (Fig. 1). Early studies had identified extra copies of chromosome 7 in malignant gliomas and in 1984, the primary oncogene targeted by these amplifications was found to be the EGFR gene, encoding the receptor for the epidermal growth factor (EGF).18 By 1989, further karyotypic and loss of heterozygosity analyses defined the location of tumor suppressor loci on chromosomes 9, 10, and 17.19 The TP53 tumor suppressor gene was identified as the main driver of chromosome 17 alterations in glioblastoma, and further studies indicated that p53 plays a critical role in monitoring the genome for DNA damage and can control cell cycle arrest to permit DNA repair or can trigger apoptosis to eliminate the damaged cell.20 Further critical discoveries came in 1993 and 1997, when the p16 cell cycle inhibitor and the phosphatase and tensin homolog (PTEN) phosphatase were identified as the tumor suppressors lost on chromosomes 19 and 10, respectively. p16 can slow down cell cycle progression, whereas PTEN is a negative regulator of the phosphoinositide 3–kinase (PI3K) pathway,21 a major signaling pathway that stimulates cellular proliferation in response to growth factor stimulation. The latest breakthrough came in 2008, when the genes encoding isocitrate dehydrogenase 1 (IDH1) (and to a lesser extent IDH2) were found to be mutated in lower grade gliomas and a subset of glioblastomas (those of the proneural type or having evolved from lower grade tumors).22 Interestingly, only 1 copy of the gene is mutated in the tumors, suggesting that the mutations do not result in a simple loss of function. The mutation is very specific and leads to a single amino acid change (arginine 132 usually becomes histidine) in the IDH1 active site, whereby the enzyme loses its ability to catalyze conversion of isocitrate to α-ketoglutarate. It was proposed that this might have an indirect oncogenic effect through the activation of the hypoxia-inducible factor pathway,23 a critical step in the metabolic adaptation of tumors to anaerobic growth and for the formation of new blood vessels through the angiogenic process.24 Others have found that the enzyme gains a new function: a conversion of substrate recognition (eg, the new ability to catalyze the nicotinamide adenine dinucleotide phosphate [NADPH]-dependent reduction of α-ketoglutarate to R(−)-2-hydroxyglut-arate [2HG]).25 Altogether, these findings enable the stratification of patients based on their molecular classification as a complement to traditional histology-based categorization.
Genetic discoveries in glioma spearheaded the use of similar technology for the discovery of new signaling pathways in medulloblastoma, meningioma, ependymoma, and other brain tumors.26
New Developments in the Classification of Human Glioblastoma
The diagnosis of brain tumors has been based on a complete clinicopathological assessment. This has been an extremely valuable approach and permits the distinction of different grades within categories of the same tumor type, such as astrocytomas, that have predictive value in determining clinical outcome. Nevertheless, it has become evident from genetic and patient outcome studies that subgroups might be present within each grade. Large-scale gene expression profile studies in glioblastoma have demonstrated that transcriptional profiles reflect the underlying tumor biology and can be used to predict tumor classification (eg, being a surrogate for pathological grading), patient outcome, and response to treatment.11 A further outcome of these investigations was to realize that each tumor is unique in its expression profile and therefore biology, suggesting that medicine needs to become more personalized. Although that is a distant goal, it was clear from these studies that it was already possible to cluster the profiles from glioblastoma patients into molecular subtypes defined by combinations of genes that were over- or underexpressed within each group. The exciting aspect of defining subgroups within a tumor entity is the possibility that patients within a given group may exhibit more homogenous responses to defined therapies and that by defining the common biology within each group, one may better select specific therapies tailored to the group and test them in clinical trials. This is a major advance because past results of clinical trials may have overlooked successful therapeutic agents because the populations of patients tested were too heterogeneous at the molecular level.
Further exciting developments in this area come from The Cancer Genome Atlas (TCGA), an unprecedented effort sponsored by the National Institutes of Health to understand cancer through the integration of expression profile data and genetic data. Glioblastoma is 1 of the tumors that received priority for analysis by the TCGA consortium.13 A total of 500 primary, untreated glioblastoma specimens from the collections of main brain tumor centers nationwide (the University of California at San Francisco, The University of Texas M. D. Anderson Cancer Center, Henry Ford Hospital, Emory University, etc) are being sent to a central repository at which they are quality controlled and then sent out to different genetic centers for analysis at the DNA (gene copy number, gene sequencing, and epigenetic methylation), mRNA (gene expression profile), and micro RNA (small RNAs that can regulate expression) levels. Although these cross-platform analyses are still ongoing, they have already uncovered new genetic alterations and provided preliminary evidence that glioblastoma can be subdivided in several subtypes (Fig. 1).27 The first subtype has a profile characteristic of highly proliferative cells and was labeled “classical.” The tumors in this group demonstrate uniform gains on chromosome 7, accompanied by losses on chromosome 10 (93%) and frequent focal losses on chromosome 9p21.3 (95%). These chromosomal events lead to amplification of the EGFR gene (in 50% of cases with gene rearrangements) and loss of the PTEN and CDKN2A gene loci. Alterations in the TP53, NF1, PDGFRA, or IDH1 genes are nearly absent. Classical GBM demonstrates responsiveness to the classical radiation and chemotherapies, likely because the p53 DNA damage response is intact in this group of patients. Such tumors may also be responsive to inhibitors of Mdm2, the negative regulator of p53. At the gene expression level, the classical subtype demonstrates elevated expression of the neural precursor and stem cell marker NES, and the Notch (NOTCH3, JAG1, and LFNG) and Sonic hedgehog (SMO, GAS1, and GLI2) signaling pathways.27 The second subtype is defined by an expression profile associated with mesenchyme and angiogenesis and overexpresses the CHI3L1/YKL40 and MET genes, as well as astrocytic markers CD44 and MERTK and genes in the TNF super family and NFκB pathways. This group, called “mesenchymal,” has frequent inactivation of the NF1 (37%), TP53 (32%), and PTEN (32%) genes. These tumors demonstrate response to aggressive chemoradiation therapies and might in addition be responsive to Ras, PI3K, and angiogenesis inhibitors. A third subtype, termed “proneural,” has an expression profile reminiscent of gene activation in neuronal development. This includes a high level of expression of oligodendrocytic (PDGFRA, OLIG2, TCF3, and NKX2-2) and proneural (SOX, DCX, DLL3, ASCL1, and TCF4) development genes. In this group, patients are younger, and overexpression or amplification/mutation of the gene encoding platelet–derived growth factor receptor–α (PDGFRA) and mutations in the IDH1 gene (30% of cases) are signature genetic alterations. Frequent mutations in TP53 (54%) and PIK3CA/PIK3R1 (19%) genes are also observed, whereas amplification of chromosome 7 and loss on chromosome 10 were significant (>50%) but less frequent findings than in the classical subtype. The finding of IDH1/2 gene mutations in lower grade gliomas also suggests that secondary glioblastoma might belong to this subtype.22,27 This subtype may be most responsive to inhibitors of the hypoxia-inducible factor (HIF), PI3K, and PDGFRA pathways. Survival in the proneural subtype was slightly better than in the other 3 tumor subtypes, yet these tumors were the least responsive to an aggressive course of classical therapies. The fourth subtype, called “neural,” is less well defined and has gene expression signatures that are most similar to those found in normal brain tissue, with activation of neuron markers such as NEFL, GABRA1, SYT1, and SLC12A5. These tumors demonstrate a low degree of infiltration by normal cells, excluding bias in the expression analyses; nevertheless, their expression signature is suggestive of cells with a differentiated phenotype. Comparison of the gene expression patterns of the 4 GBM subtypes with those of cultures of primary murine astrocytes, oligodendrocytes, neurons, and microglia suggests that the subtypes may reflect different cells of origin, a hypothesis that needs further substantiation.27 In sum, although this classification in subtypes may prove to be useful in defining the best molecular targets within each group, it is important to realize that all subgroups commonly demonstrate inactivation of the p53 and retinoblastoma (Rb) tumor suppressor pathways and activate the receptor tyrosine kinase pathways (Fig. 2).13
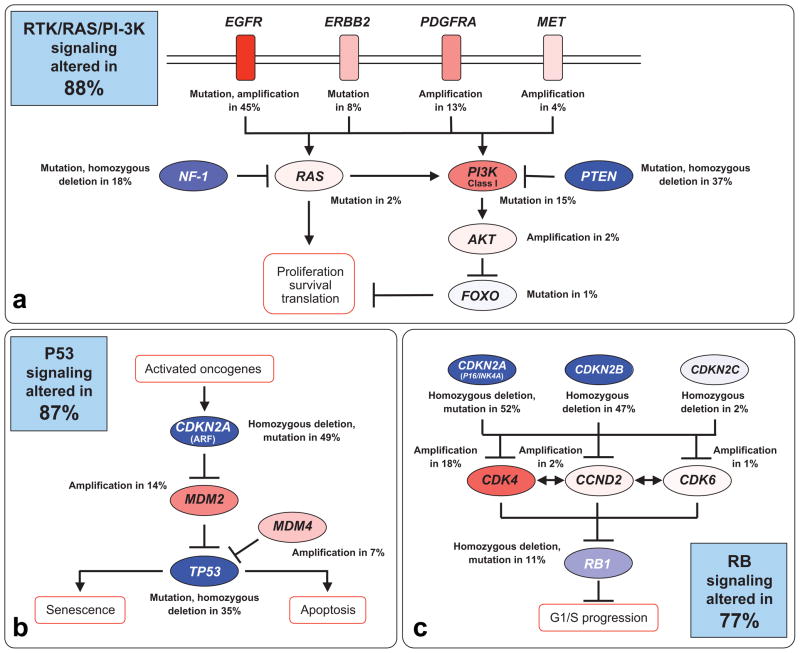
FIGURE 2.
Genetic Alterations in Glioblastoma Occur Frequently in 3 Cellular Signaling Pathways. DNA alterations and copy number changes in the following signaling pathways are indicated in (a) receptor tyrosine kinase (RTK), RAS, and phosphoinositol–3–kinase (PI3K); (b) p53 tumor suppressor; and (c) retinoblastoma (Rb) tumor suppressor. Activating genetic alterations are shown in red. Genetic alterations that lead to a loss of function are indicated in blue. In each pathway, the altered components, the type of alteration, and the percentage of tumors carrying each alteration are shown. Blue boxes contain the total percentages of glioblastomas with alterations in at least 1 known component gene of the designated pathway. EGFR indicates epidermal growth factor receptor; MET, mesenchymal- epithelial transition factor; PDGFRA, platelet-derived growth factor receptor–A; PTEN, phosphatase and tensin homolog; . Reprinted with permission from The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068.13
The TCGA has dramatically accelerated our comprehensive understanding of the gene mutations and expression profiles of GBM in a multidimensional fashion, thereby defining the key altered signaling pathways that need to be targeted for therapy. This effort has also been instrumental in narrowing down a problem of unknown magnitude to a limited set of genes and pathways on which to focus further studies and therapeutic targeting. Similar efforts are underway for other central nervous system (CNS) tumors so that by the year 2020, we expect to have a comprehensive understanding of the ontogeny of all brain tumors.
Application of Progress Made in Malignant Glioma Biology: Results of the First Targeted Molecular Therapies
Targeting of Intracellular Growth Signaling
The abnormalities in cellular signal transduction pathways identified in malignant gliomas (Fig. 2) have already led to a first generation of targeted molecular drugs to inhibit these pathways in the clinical setting (Table 1). Initial forays into this realm have not been universally successful, but the data and experience gained demonstrate the pertinence of the approach.28 An example of initial success is the humanized monoclonal antibody against VEGF, bevacizumab.29 On the basis of high radiographic response rates and a modest toxicity profile, the US Food and Drug Administration (FDA) has granted accelerated approval for the use of bevacizumab in the treatment of recurrent GBM. This section briefly summarizes the current status of the evolving field of targeted molecular therapies in adult malignant gliomas.
TABLE 1.
Selected Targeted Molecular Agents Currently in Clinical Development for High-Grade Glioma
| PRIMARY TARGET | AGENT | OTHER TARGETS | MECHANISM OF ACTION |
|---|---|---|---|
| EGFR | Gefitinib (ZD1839) | TKI | |
| Erlotinib (OSI-774) | TKI | ||
| Lapatinib (GW-572016) | HER-2 | TKI | |
| PF-00299804 | HER-2, HER-4 | TKI (irreversible) | |
| BIBW2992 | HER-2, HER-4 | TKI (irreversible) | |
| Cetuximab | Monoclonal antibody | ||
| Nimotuzumab | Monoclonal antibody | ||
| EGFRvlll | CDX110 | Vaccine | |
| Farnesyl transferase | Lonafarnib (SCH 66336) | FTI | |
| Tipifarnib (R115777) | FTI | ||
| FGFR | Brivanib (BMS-582664) | VEGFR2 | TKI |
| HDAC | Vorinostat (SAHA) | HDAC inhibitor | |
| Valproic acid | HDAC inhibitor | ||
| LBH589 | HDAC inhibitor | ||
| HGF/SF | AMG102 | Monoclonal antibody | |
| HSP-90 | 17-AAG | Blocks HSP-90 ATP binding | |
| Integrins αvβ3, αvβ5 | Cilengitide (EMD121974) | Synthetic RGD peptide | |
| c-Met | XL184 | VEGFR | TKI |
| mTOR | Sirolimus (rapamycin) | mTOR inhibitor | |
| Everolimus (RAD001) | mTOR inhibitor | ||
| Temsirolimus (CCI-779) | mTOR inhibitor | ||
| Ridaforolimus (AP23573) | mTOR inhibitor | ||
| PDGFR-α | IMC3G3 | Monoclonal antibody | |
| PDGFR-β | Imatinib | BCR/Abl, c-Kit | TKI |
| Dasatinib | Src, BCR/Abl, c-Kit, ephrin A2 | TKI | |
| Tandutinib (MLN518) | Flt3, c-Kit | TKI | |
| PI3K | XL765 | mTOR | STKI |
| PKC | Enzastaurin (LY31761) | STKI | |
| VEGF-A | Aflibercept (VEGF Trap) | VEGF-B, PlGF | Soluble decoy receptor |
| Bevacizumab | Monoclonal antibody | ||
| VEGFR-2 | Cediranib (AZD2171) | All VEGFR subtypes, PDGFR-β, c-Kit | TKI |
| CT-322 | All VEGFR subtypes | Adnectin | |
| Pazopanib | All VEGFR subtypes, PDGFR-α and β, c-Kit | TKI | |
| Sorafenib | VEGFR-3, B-Raf, PDGFR-β, c-Kit, Ras, p38α | TKI | |
| Sunitinib | PDGFR-β, Flt3, c-Kit | TKI | |
| Vandetanib (ZD6474) | EGFR | TKI | |
| XL-184 | c-Met, RET, c-Kit, Flt3, Tie-2 | TKI |
EGFR indicates epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; FGFR, fibroblast growth factor receptor; FTI, farnesyltransferase inhibitor; VEGFR2, vascular endothelial growth factor receptor 2; HDAC, histone deacetylase; SAHA, suberoylanilide hydroxamic acid; HGF/SF, hepatocyte growth factor/scatter factor; HSP-90, heat shock protein 90; 17-AAG indicates 17-allylamino-17-demethoxygeldanamycin; RGD, arginine-glycine-aspartate; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; STKI, serine-threonine kinase inhibitor; PKC, protein kinase C; VEGF, vascular endothelial growth factor; PlGF, placental growth factor.
Malignant Gliomas Display Aberrant Proliferation and Apoptosis Signaling
Growth factor pathways that stimulate cell proliferation are constitutively activated in malignant gliomas (Fig. 2). This can be achieved through overexpression or genetic amplification of growth factor receptor genes (EGFR, ERBB2, PDGFRA, MET, etc), as well as through gene mutations that lead to ligand-independent signaling as occurs for the epidermal growth factor receptor vIII (EGFRvIII), a mutant that sends constitutive growth signals. Alternatively, intracellular signaling pathways can be constitutively activated when the positive signaling molecules are mutated and signal constitutively, as occurs for PI3K pathway subunits (PIK3A, PIK3R1) or when the negative regulators of the pathway are lost through gene loss or mutation (eg, the PTEN tumor suppressor). Usually, such progrowth signaling is not enough to induce tumor formation and needs to be accompanied by loss of critical cell “switches” that normally monitor cell growth. The 2 main tumor suppressor pathways that accomplish this task are those of the p53 and Rb pathways, which directly monitor cell cycle entry and progression. p53 activates the transcription of p21, a molecule that blocks cell cycle progression in the G1 phase of the cell cycle by binding and inhibiting the function of the cyclin D family of proteins.20 These are the regulatory subunits of the cyclin/cyclin– dependent kinase complexes that regulate cell cycle entry and progression by inducing the phosphorylation and inactivation of Rb. The Rb protein can prevent cell entry into S-phase by inactivating the E2F family of transcription factors that are critical for the initiation of DNA replication. Although p53 and Rb can be the direct targets of mutations, the inactivation of these cell cycle control pathways can also be achieved indirectly by mutation or overexpression of other signaling molecules in the pathway (Fig. 2). Another contributor that directs cell proliferation in gliomas is c-Myc,30 a transcription factor that drives the expression of cell cycle promoters such as cyclins and cyclin-dependent kinases while blocking the transcription of cell cycle inhibitors (CKI).
Excessive growth-stimulating signals emanating from growth factor receptors or overexpressed oncogenes such as Myc are sensed by the cell and will trigger a safeguard response that results in cell death through apoptosis. Apoptosis or programmed cell death is the outcome of a physiological response that leads to cell termination.31 It is the result of a precise signaling process that is initiated either at the cell surface (death receptor pathway) or intrinsically by intracellular signals such as extensive DNA damage. This process involves the activation of a series of proteins called caspases, which leads to the irreversible breakdown of cellular components and ultimately cell death. To overcome this limitation in their growth, tumors will typically also genetically inactivate proapoptotic pathways or activate the over-expression of genes that can promote cell survival. The inactivation of the p53 protein by mutation abrogates proapoptotic responses in the cell because this factor controls the transcription of both cell cycle arrest (p21CKI) and proapoptotic genes (Bax, Fas, etc).20 Genetic inactivation of caspases and other proteins directly involved in the apoptotic machinery have also been documented.32 Another mechanism through which the tumor can overcome apoptotic induction is the overexpression of factors that will prevent activation of the apoptotic cascade. One major antiapoptotic mediator that is overexpressed in gliomas is transcription factor NFκB.33 NFκB is well known as a mediator of immune and inflammatory responses, but it also activates the transcription of proteins that inhibit apoptosis such as members of the inhibitor of apoptosis (IAP) family (c-IAP, XIAP, and survivin). IAPs interact directly with activated caspases and can block their proapoptotic function. Furthermore, NFκB can induce the expression of Bcl-2 and Bcl-XL, which reside at the outer layer of the mitochondrial membrane and can prevent its permeabilization, a critical step in the activation of the apoptosome.
Although the tumor has developed strategies to overcome the physiological induction of apoptosis during its growth, in many cases the tumor cells remain sensitive to apoptosis induction by therapeutic agents. Classical chemotherapy and radiotherapy can induce apoptosis in the tumors, and new pro-apoptotic agents currently are being developed. As an example, there is great interest in tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a ligand for death receptors present at the surface of tumor cells.31
Clinical Targeting of Cell Surface Growth Factor Receptors
EGFR is one of the most attractive therapeutic targets in GBM.34 The EGFR gene is amplified and overexpressed in approximately 40% of primary GBMs, especially those of the “classical” subtype (Fig. 1). Nearly half of tumors with EGFR amplification also have a constitutively active EGFR mutant known as EGFRvIII, which has a large deletion in the extracellular domain and renders the receptor ligand independent for signaling. This deletion also engenders a unique codon, which is not found in the wild-type receptor, thereby creating a tumor-specific epitope that can be exploited for therapeutic targeting. Increased EGFR signaling drives tumor cell proliferation, invasiveness, motility, angiogenesis, and inhibition of apoptosis. Small-molecule EGFR inhibitors such as gefitinib and erlotinib (Table 1) are well tolerated in patients with malignant gliomas, but responses are infrequent and progression-free survival (PFS) is not prolonged.34 Neither the EGFR/HER-2 inhibitor lapatanib,35 nor the monoclonal antibody against EGFR, cetuximab,36 have proven to be effective.
Attempts to identify biomarkers to help predict response to EGFR inhibitors have yielded conflicting results. To the best of our knowledge, there is no convincing evidence of a correlation between EGFR expression in tumor tissue and response. Some studies found that tumors with EGFRvIII and intact PTEN37 and tumors with low phosphorylated Akt levels38 are more likely to respond to EGFR inhibitors. However, not all studies confirmed this initial observation.39
Recent phase 2 studies have combined EGFR inhibitors with temozolomide and radiotherapy for patients with newly diagnosed GBM. Conflicting results have been observed. Other current trials in patients with malignant glioma are evaluating irreversible EGFR inhibitors such as BIBW 2992 and PF-00299804, the dual EGFR and VEGF receptor (VEGFR) inhibitor vandetanib (ZD6474), and the humanized monoclonal antibody against EGFR, nimotuzumab. CDX-110, a peptide vaccine against the unique epitope of EGFRvIII, has a favorable toxicity profile and currently is being studied in combination with temozolomide in patients with newly diagnosed GBM.82 Finally, combinations of EGFR inhibitors with other targeted therapies, including inhibitors of mammalian target of rapamycin (mTOR) and VEGFR, are being evaluated (Table 1).
The PDGFR subtypes α and β and PDGF ligands A and B are also overexpressed in malignant gliomas, especially in the “proneural” subtype (Fig. 1).27,40 This creates autocrine or paracrine loops that promote tumor cell proliferation. The PDGFR inhibitor imatinib mesylate was reported to have significant antitumor activity both in vitro and in orthotopic glioma models.41 Unfortunately, the drug proved inactive in clinical trials, with rare responses and no prolongation of PFS.42 Studies with more potent PDGFR inhibitors and agents with improved BBB penetration such as tandutinib (MLN518) currently are underway.
Clinical Targeting of Intracellular Signaling Molecules
The PI3K/Akt pathway is a critical regulator of tumor cell metabolism, growth, proliferation, and survival. Ligand binding to receptor tyrosine kinases increases activity in the pathway, which ultimately activates mTOR. Downstream effectors of mTOR have an array of biological functions that promote hypoxic adaptation and protein translation. In malignant gliomas, PI3K/Akt/mTOR signaling is frequently increased because of receptor tyrosine kinase overactivity (EGFR, PDGFR, and mesenchymal-epithelial transition factor [MET]), mutated oncogenic PI3K subunits, and/or loss of PTEN tumor suppressor activity (Fig. 2).21 Several mTOR inhibitors are undergoing evaluation in malignant gliomas, including sirolimus (rapamycin), temsirolimus (CCI-779), everolimus (RAD001), and ridaforolimus (AP23573). To date, mTOR inhibitors have demonstrated only minimal single agent activity against malignant gliomas.43,44 A clinical trial of the dual PI3K/mTOR inhibitor, XL765, in combination with temozolomide for GBM is currently underway, but no preliminary data have been reported to date. Akt inhibitors are expected to enter clinical trials for malignant gliomas in the near future. Enzastaurin (LY317615) is a potent inhibitor of protein kinase C-β2 that also suppresses PI3K/Akt pathway signaling. After promising results in a phase 1 trial in patients with recurrent malignant gliomas,45 a phase 2 study was initiated. Unfortunately, the study was closed at interim analysis because of a lack of efficacy.
In addition to the PI3K/Akt/mTOR pathway, signal transduction from activated tyrosine kinases such as EGFR and PDGFR is mediated by the Ras/Raf/mitogen-activated protein kinase pathway. Activation of Ras requires localization to the intracellular surface of the cell membrane, a critical step that depends on farnesylation. Farnesyl transferase inhibitors (FTI) interfere with this process and have demonstrated promising activity in glioma models. Unfortunately, the FTI tipifarnib (R115777) did not demonstrate clear evidence of efficacy in a phase 2 trial in patients with recurrent malignant gliomas.46
Histone deacetylase (HDAC) inhibitors interfere with transcriptional regulation and can induce growth arrest, terminal differentiation, and apoptosis of tumor cells. The HDAC inhibitor vorinostat (sub-eroylanilide hydroxamic acid) proved effective in pre-clinical models but only modestly prolonged the 6-month PFS (PFS6) in a phase 2 trial in patients with recurrent GBM.47 Ongoing studies are investigating the combination of vorinostat with temozolomide and radiation. Other HDAC inhibitors currently in clinical trials for malignant gliomas include valproic acid and LBH589.
Resistance to Molecular Agents Targeting Tumor Growth Signaling Pathways
Results from the first generation of molecular agent trials targeted to proliferative pathways in malignant gliomas have been disappointing, with relatively rare radiographic responses and no significant prolongation of PFS reported. In an effort to improve on these initial results, an array of ongoing clinical trials combines novel molecular therapies with standard treatments such as radiation and chemotherapy.
One explanation for the initial failure of growth factor–targeted molecular drugs is that the targets critical for effective therapy have yet to be addressed. As a result of molecular profiling, network analysis, and correlative studies in clinical trials, novel targets that drive glioma growth and prevent tumor cell death are emerging rapidly. Among the promising therapeutic targets in early clinical development are MET, fibroblast growth factor receptor (FGFR), heat shock protein–90 (HSP–90), hypoxia-inducible factor 1α (HIF1α), cyclin-dependent kinases, and many others. In addition to the uncertainty about which targets require inhibition, there is an ongoing controversy regarding which cell types are most important. Glioma stem-like cells appear to initiate glioma formation and maintain the tumor mass. Inhibition of unique stem cell targets such as Notch and Sonic hedgehog may be required to overcome resistance to therapy.14,48
Another explanation for the failure of targeted molecular agent monotherapy comes from data demonstrating that multiple receptor tyrosine kinases are coactivated in GBM cell lines and primary cultures. In preclinical models, multiple kinase inhibition is required to reduce signaling through the PI3K/Akt/mTOR pathway and decrease glioma cell survival.49 These data provide a compelling rationale for the myriad of ongoing trials of single agents that inhibit multiple targets or multiple agents that inhibit complementary pathways. Examples of multitargeted drugs that are being evaluated in malignant gliomas are presented in Table 1.
Drugs that inhibit single targets can also be combined to achieve multiple target inhibition. Particular interest has focused on the combination of EGFR inhibitors and mTOR inhibitors. Results from a phase 2 study of erlotinib and sirolimus in patients with recurrent GBM were disappointing,50 but several other studies are currently ongoing. One important obstacle to combination therapy with targeted molecular drugs is additive toxicity, which may limit the doses that patients can tolerate. For example, combinations of EGFR inhibitors with mTOR inhibitors have been associated with a high incidence of dermatologic toxicity and mucositis.51
Another factor that may interfere with the efficacy of targeted molecular drugs in malignant gliomas is insufficient penetration into the tumor tissue due to a partially intact BBB or an active drug efflux transporter. Due to the difficulty in obtaining tumor tissue in the brain tumor patient population, few clinical trials have successfully measured drug levels in tumor tissue. However, neuro-oncologists are increasingly recognizing that it is critical to obtain and study tumor tissue whenever possible.37,52 Tissue studies permit assessments of drug penetration, degree of target inhibition in vivo, and resistance mechanisms. This information will facilitate the rational design of future trials and should increase the efficiency of clinical testing.
Targeting of Intercellular Signaling: Inhibition of Angiogenesis
As alluded to earlier, treatment directed toward new vessel growth in malignant gliomas has proven to be one of the most promising areas of targeted molecular therapy. To appreciate this, it is important to review the current understanding of angiogenesis in malignant gliomas and then examine the various approaches used to target these events with therapeutics.
Overview of Angiogenesis in Malignant Gliomas
Extensive experimental data support the concept that angiogenesis is required for malignant glioma growth.53,54 The process is driven primarily by tumor-secreted VEGF-A, but there are a large number of alternative secreted proangiogenic factors, including basic FGF (bFGF), angiopoietins, PDGF, interleukin-8 (IL-8), and hepatocyte growth factor/scatter factor (HGF/SF). Endothelial cells in the vicinity of the tumor express VEGFR2, which establishes a paracrine signaling loop that stimulates endothelial cell growth and proliferation. The level of VEGF production in a tumor increases with the degree of malignancy. In a study of surgical glioma specimens, high-grade tumors produced greater than 10-fold more VEGF compared with low-grade tumors.55 A subset of targeted molecular therapy for malignant gliomas includes agents that interfere with angiogenesis. The majority of the anti-angiogenic drugs that have been evaluated in clinical trials to date interfere with the VEGF pathway by directly blocking ligand or receptor. However, there is increasing interest in targeting proangiogenic molecules that function by alternative mechanisms.29
For example, the neuropilins are nontyrosine kinase receptors that are activated by VEGF binding and potentiate VEGFR signaling. Neuropilin-1 also facilitates HGF/SF signaling.56 The angiopoietins (Ang-1 and Ang-2) are involved in the stability and maintenance of the tumor vasculature. Binding of Ang-2 to its cognate receptor, Tie-2, serves to destabilize vessels, which is a requirement for angiogenesis to proceed.57 Ang-2 inhibitors are therefore of interest as therapeutic agents.58 Notch inhibitors may also prove effective. Notch receptors on tumor endothelial cells are activated by transmembrane jagged and delta-like ligands on the surfaces of neighboring cells. Inhibition of delta-like ligand 4 (Dll4) on endothelial cells in preclinical models promotes the growth of an abnormal neovasculature with reduced perfusion and tumor growth.59 Finally, tumor cells secrete chemokines that serve to recruit proangiogenic myeloid cells to the tumor. For this reason, inhibitors of specific chemotactic signaling may have therapeutic value.54
There is also evidence that antiangiogenic treatments may selectively target glioma stem-like cells.14,48 Recent data suggest that stem-like cells are highly resistant to treatment and proangiogenic. Glioma stem-like cells exist in a “vascular niche,” in the microenvironment created by tumor endothelium. As such, antiangiogenic treatments that disrupt tumor vasculature may preferentially target this subpopulation and help to overcome the marked treatment resistance of malignant gliomas.
Therapeutic Targeting of Angiogenesis VEGF Inhibitors
After bevacizumab was approved by the FDA for colon cancer, several neuro-oncology centers began to use it to treat patients with recurrent malignant glioma, often in combination with irinotecan. In the first published report, 19 of 29 patients (66%) treated with the combination achieved radiographic responses.60 Historical data from recurrent GBM trials demonstrated a response rate of only 5% to 8% with temozolomide therapy. Despite concerns about the risk of hemorrhage in brain tumor patients, only 1 patient was reported to have an intracerebral hemorrhage. A large number of retrospective series have now been published, with response rates of 25% to 74% reported, and PFS6 rates of 32% to 64%,5,61–66 which is superior to the 21% PFS6 rate reported for temozolomide.67 These reports demonstrated that bevacizumab therapy leads to rapid reductions in peritumoral edema, often permitting a decrease in dose or even cessation of corticosteroid use. These studies also indicated that bevacizumab treatment is well tolerated in most cases. The risk of intracranial hemorrhage is low. Common toxicities related to bevacizumab therapy in the malignant glioma population include hypertension, proteinuria, fatigue, thromboembolic events, and wound-healing complications.
The first phase 2 trial of bevacizumab and irinotecan was conducted in 35 patients with recurrent GBM and 33 patients with recurrent anaplastic glioma.68 The radiographic response rate of approximately 60% was consistent with retrospective data, as was the PFS6 rate (43% for GBM patients and 59% for anaplastic glioma patients, compared with 21%67 and 46%,69 respectively).
The accelerated FDA approval of bevacizumab for recurrent GBM was based on 2 subsequent phase 2 trials. The first trial randomly assigned 167 patients with recurrent GBM to bevacizumab therapy with or without irinotecan.70 Response rates were reported to be between 28% and 38%, and PFS6 rates ranged from 43% to 50%. As had been reported in previous studies, most patients reduced their corticosteroid doses by 50% or more due to the marked antipermeability effect of bevacizumab. Adverse events were infrequent, with 8 (4.9%) intracerebral hemorrhages reported, the majority of which were not life-threatening, and 23 (14.1%) thromboembolic complications noted.71 The other phase 2 trial evaluated by the FDA involved bevacizumab monotherapy in 48 heavily pretreated patients with recurrent GBM.72 The radiographic response rate was 35%, and the PFS6 rate was 29%. In addition to hemorrhagic and thromboembolic complications, common toxicities observed in these studies included hypertension, proteinuria, fatigue, and wound-healing complications.
Ongoing phase 3 studies are evaluating the combination of bevacizumab with temozolomide and radiotherapy. The results will be of great interest because of the uncertainty regarding the impact of bevacizumab on overall survival. Combinations of bevacizumab and other chemotherapeutics and targeted molecular drugs are also currently in clinical trials. Aflibercept (VEGF-Trap) is a soluble decoy VEGF receptor fused to an immunoglobulin constant region that binds VEGF-A, VEGF-B, and placental growth factor (PIGF). A phase 2 study in recurrent malignant gliomas is ongoing, as is a phase 1/2 study in combination with temozolomide and radiotherapy for patients with newly diagnosed GBM.
VEGFR Inhibitors
In addition to VEGF inhibitors, small molecule inhibitors of VEGFR have been tested in recurrent malignant gliomas. Cediranib (AZD2171) inhibits all known subtypes of VEGFR and was evaluated in a phase 2 trial of patients with recurrent GBM. Results were comparable to those reported for bevacizumab, with a response rate of 56% and a PFS6 rate of 26%.73 A striking steroid-sparing effect was observed. The drug was largely well tolerated, with hypertension, diarrhea, and fatigue as the most common adverse effects. Using dynamic contrast-enhanced magnetic resonance imaging (MRI) scans, the authors demonstrated that cediranib therapy reduced blood vessel size and permeability. These are the first clinical data to support the hypothesis that antiangiogenic therapy may transiently “normalize” the dilated, abnormally permeable tumor vasculature.53 The presumption that vascular normalization may improve chemotherapy delivery and reduce hypoxia provides a solid rationale for combining antiangiogenic therapies with chemotherapy and radiotherapy. A vascular normalization index has been proposed to predict survival after antiangiogenic therapy.74 Other VEGFR inhibitors of interest for malignant gliomas are listed in Table 1.
Other Antiangiogenic Approaches
In addition to VEGF or VEGFR inhibition, a variety of other approaches may have antiangiogenic activity. Because of its role in pericyte recruitment,75 inhibition of PDGFR may prove useful. Several trials of PDGFR and dually targeted VEGFR/PDGFR inhibitors are ongoing, as noted earlier. The integrins αvβ3 and αvβ5 are highly expressed by tumor endothelial cells, in which they interact with extracellular matrix (ECM) proteins to facilitate angiogenesis and invasion.76 Cilengitide (EMD121974) inhibits these integrins and appears promising in GBM patients with methylation of the MGMT gene promoter.77
Mechanisms of Resistance to Antiangiogenic Therapy
Although antiangiogenic therapies prolong PFS, further progression of disease is inevitable. Tumors that progress during antiangiogenic therapy cannot often be treated successfully thereafter, and most patients die of the disease within a few months. In the cediranib study, serum levels of the proangiogenic factors bFGF, stromal-derived factor 1α (SDF1α), and soluble VEGFR2 increased at the time of failure.73 These alternative proangiogenic pathways may drive angiogenesis in the setting of VEGFR inhibition. Other preclinical78 and MRI data5 suggest that anti-VEGF therapy may promote an infiltrative tumor growth pattern with co-option of existing cerebral blood vessels. Combining antiangiogenic therapy with anti-invasion therapy may therefore delay disease progression. Studies combining cediranib (pan-VEGFR inhibitor) with cilengitide (integrin inhibitor) and bevacizumab (neutralizing VEGF antibody) with dasatinib (PDGFRbeta inhibitor) currently are ongoing. Another potential mechanism of resistance to antiangiogenic therapies involves increased PDGF signaling. PDGF promotes stabilization of the neovasculature by recruiting pericytes and facilitating pericyte-endothelial cell interactions.75 Preclinical data suggest that dual VEGFR/PDGFR inhibition potentiates antiangiogenic efficacy and reduces resistance to therapy,79 and this approach is currently being evaluated in clinical trials.
Targeted Molecular Therapy: Summary and Future Outlook
Most human cancers, including high-grade gliomas, have abnormalities in cellular signal transduction pathways. Targeted molecular drugs that inhibit these pathways have potential therapeutic value (Table 1). It is important not to overstate this prospect because the majority of targeted molecular drugs that have been evaluated in malignant gliomas to date have been disappointing, with response rates of 10% to 15% or less and no prolongation of survival.28 In the case of EGFR inhibitors, for example, selected patients do respond, but reliable predictors of response have not been identified. An exception is the humanized monoclonal antibody against VEGF, bevacizumab.29 Antiangiogenic therapies that target VEGF or VEGFR produce striking radiographic responses in many patients, relieve symptoms, and prolong PFS. On the basis of high radiographic response rates and a modest toxicity profile, the FDA recently granted accelerated approval for bevacizumab in the treatment of recurrent GBM. Although its impact on overall survival has not been assessed, there is a concern that aggressive tumor growth after the failure of antiangiogenic treatment may diminish any survival benefit.
Our emerging knowledge of the molecular pathophysiology of malignant gliomas will improve therapeutic target selection in the future. Rigorous pre-clinical testing is needed to identify combinations of drugs and targets that are most likely to be effective and tolerable. Targets such as HSP-90 and HDAC are of particular interest as therapeutic targets because their function influences many other signaling molecules that may promote tumor cell growth and proliferation. Studies of resistance to antiangiogenic therapy are needed to optimize the use of bevacizumab and other VEGF or VEGFR inhibitors. Clinical trials that incorporate tumor tissue and molecular endpoints will help us to understand why certain drugs succeed or fail in individual tumors. Although the initial results have been disappointing, targeted molecular agents hold tremendous promise. We remain optimistic that the ultimate goal of identifying targeted molecular therapies with durable antitumor efficacy will be realized by 2020.
Toward Theragnostics of Malignant Gliomas: Discovery of Novel Biomarkers and Targets and Understanding the Biological Cause of Tumor Resistance to Therapy
Clearly, the current advances made in the treatment of malignant gliomas remain insufficient, and the tumor is either insensitive to the tested therapies or rapidly develops resistance. The new information gained regarding the origins of malignant gliomas must be applied to our understanding of the behavior of these lesions as they are subjected to therapy. Their genetic and metabolic characteristics affecting invasive ability, alteration of immune response, and the development of treatment resistance must be understood in detail to address them in a meaningful fashion. The advancing understanding of tumor stem-like cell biology is providing another route by which the resistant nature of these lesions can be addressed. An offshoot of this new understanding is the ability to diagnose and follow these tumors with biomarkers outside of the tumor itself, such as the cerebrospinal fluid (CSF) and blood.
The Invasive Phenotype of Malignant Gliomas Hampers Therapy
One of the distinguishing features of the astrocytic tumors is their diffuse infiltrative nature. Low- and high-grade astrocytomas grow in the brain as masses intermingled with normal brain cells, and the tumor borders are ill-defined as the tumor zone progressively transitions to normal tissue. It is this fact, more than any other, that is believed to underlie the surgical incurability of most of these tumors. This is in contrast to tumors that have metastasized to the brain, in which sharp tumor borders are observed. Individual groups of cells or single cells can detach from the primary tumor mass and travel significant distances, following migration routes that are defined by the underlying anatomical architecture of the brain and the related ECM. Typical dispersion routes include white matter tracks, along the basal lamina of brain blood vessels, or in between the glia limitans and the pia mater.80 The invasive process in the brain shares characteristics with that occurring in the stromal infiltration of non-neural carcinomas, namely cell detachment from the primary tumor mass, receptor-mediated adhesion to surrounding ECM, degradation of ECM to allow for cell passage, and active motility processes. It has also unique features because the neural parenchyma lacks many ECM elements found in other organs, including most basal lamina matrix (collagens, fibronectin, and type I laminin) and supporting stromal tissue.80 The ECM in the brain is largely comprised of the poly-saccharide hyaluronan and proteoglycan-based matrix, mostly hyaluronic acid-binding secreted chondroitin sulfate proteoglycans of the lectican family (aggrecan, brevican, neurocan, and versican). The unique brain parenchyma is believed to be refractory to axonal navigation and cell motility, thereby explaining the lack of dispersion of tumor cells that metastasize to the brain. Glioma cells may have evolved distinct mechanisms of invasion that are adapted to the unique composition and structure of brain ECM.80,81
The inherent capacity of glioma cells to deeply penetrate within normal brain structures poses a serious clinical challenge because these cells are widely believed to be responsible for tumor recurrence after surgery, radiation, and chemotherapy. Methods to track them in patients do not exist, and it is unclear to what extent existing therapies reach these cells and affect their survival. The challenges in targeting this specific tumor cell population also reside in the redundant nature of the signaling pathways that can promote a motility phenotype.81 Specific molecular targets exploiting the unique nature of the brain ECM, cell surface receptors, and signaling molecules activated in migrating cells are currently being investigated. These include the ECM protein tenascins C and R, phosphacan and brevican, the Src family of nonreceptor tyrosine kinases, the Rho family of small GTPases, glycogen synthase kinase 3, and integrins.81 The high level of plasticity of the promigratory cell signaling, the influence of the microenvironment, and the paucity of modeling systems that reflect the unique nature of the invasive process in the brain suggest that this will remain an area of intensive investigation for years to come.
The Role of the Immune System in Malignant Gliomas
One of the salient features of the brain is that it is considered an immune-privileged site, in that the brain is shielded to a certain extent from blood-originating immunoglobulins and circulating leukocytes. This initial concept was later revised to accommodate the immunosurveillance that does occur in the brain and the powerful immune responses that take place in the infected brain or under conditions of autoimmune disease. The current concept is that immunoediting occurs and, in the case of CNS neoplasms, can result in various outcomes, namely cancer elimination, an equilibrium, and tumor immune escape.82 Malignant gliomas clearly represent a scenario in which the tumor has overcome the immune system and even co-opts the leukocyte populations that invade the tumor. There is emerging evidence that glioblastoma releases secreted factors (VEGF, PlGF, SDF-1a, and angiopoietin-2) into the circulation, which in turn recruit various bone marrow progenitor cells to the tumor, including CD45-positive myeloid cells that play a supporting role in tumor angiogenesis.54 Tumor-infiltrating monocytes and macrophages can release proangiogenic factors, including VEGF, bFGF, IL-8, and others.83 Myeloid suppressor cells can infiltrate gliomas and develop immuno-suppressive functions against dendritic cell (DC) maturation and antitumor functions of T and natural killer (NK) cells. These findings demonstrate that malignant gliomas are systemic diseases and that their treatment needs to take into consideration the globality of tumor biological responses.
The highly infiltrative nature of malignant gliomas makes T-cell mediated immunity particularly attractive to track and destroy every disseminated tumor cell in the brain, while sparing the normal tissue.82 To elicit a potent T-cell response, naïve T cells have to be exposed to the appropriate antigen in the lymph nodes. This is usually accomplished by an antigen-presenting cell (typically a DC) that traffics to the draining lymph node. Although no lymphatic system has been identified in the brain and no resident DCs are present, recent evidence has convincingly shown that functional antigen drainage occurs from the tumor site to the cervical lymph nodes. The precise antigen-presenting cell involved is unknown, but resident microglial cells in the brain subjected to further differentiation are possible candidates. The signals that permit extravasation of amplified antigen–stimulated effector T cells into the brain are becoming better understood.82 Because our knowledge regarding the best way to activate endogenous T-cell responses against tumor antigens in the brain was initially limited, a widely used approach has been to perform adoptive immune transfer, that is, the local or systemic transfer of effector T cells after an in vitro priming process against a tumor antigen. This approach has shown success in some animal models, as well as in pilot clinical trials, but is challenging to make available for many patients. Novel approaches focus on eliciting an antitumor response directly in vivo, through direct injection of antigen (active immunotherapy or tumor vaccination) or by using DC-based vaccines, in which DCs are pulsed with antigen in vitro and implanted intradermally in vivo to stimulate a wide spectrum of immune effector mechanisms. Phase 1 trials are currently ongoing in which patients receive autologous DCs that are pulsed with their own acid-eluted tumor antigens; initial results have provided evidence of feasibility, safety, and bio-activity.82 Such approaches may lead to customized immunotherapy in the not-so-distant future, in which the patient’s own immune system is boosted to produce antitumor responses.
Another therapeutic approach using the immune system has been the delivery of therapeutic antibodies. Typically, a glioma antigen determinant is first identified at the cell surface of tumor cells, and a cytotoxin-conjugated or radioactively armed antibody is then developed against this determinant. Such antibodies have been prepared against several cell surface antigens (EGFRvIII, EphA2 receptor, IL–13Rα2, etc.), and clinical trials evaluating their efficacy are in process.84 Antibodies directed at factors essential for tumor growth have also been developed. Bevacizumab is an FDA-approved, fully humanized antibody that sequesters the angiogenic factor VEGF and does not need to cross the BBB for antitumor activity. An FDA registration trial is also ongoing with 81C6, a 131I-labeled antibody against tenascin C, an ECM glycoprotein that is overexpressed in malignant gliomas. Although these antibody approaches appear promising, they will need to be integrated in a multimodality treatment regimen, and cocktails of antibodies recognizing multiple antigenic determinants will have the best chances for success given the heterogeneity of the disease.84
The Molecular Basis of Resistance to Chemotherapy
Cytotoxic chemotherapy has historically had a minor role in malignant glioma therapy because of the marginal efficacy of adjuvant nitrosoureas in early studies. Meta-analyses subsequently confirmed that adjuvant chemotherapy is modestly effective, with a 6% to 10% increase noted in 1-year survival.85 In 2005, a large, randomized, phase 3 trial in newly diagnosed GBM patients compared radiotherapy alone with radiotherapy and concurrent daily temozolomide followed by adjuvant temozolomide.4 The radiation plus temozolomide group had a 2.5-month median survival benefit, and the proportion of 2-year survivors increased from 10.4% to 26.5%. The trial tumor specimens were evaluated for MGMT gene silencing.7 DNA methylation of the MGMT gene promoter region is an epigenetic modification that can reduce expression of the DNA repair enzyme, MGMT. These studies provided evidence that MGMT promoter methylation was associated with better outcome in the radiation/temozolomide group, suggesting that it may be a predictive marker for sensitivity to alkylating chemotherapy.7 Unfortunately, greater than 50% of patients with GBM retain MGMT expression and demonstrate less sensitivity to temozolomide, presumably because MGMT repairs the temozolomide-induced DNA damage, although this has not been formally demonstrated. Other studies showed that MGMT methylation is a predictive marker for better response to radiotherapy, independently of temozolomide treatment, suggesting that it may be a general surrogate marker of better therapeutic response in GBM.86 Investigational approaches to suppress MGMT activity include dose-intense temozolomide regimens, which may deplete the enzyme,87 and combination therapy with O6-benzylguanine or other MGMT inhibitors.88 Thus far, MGMT inhibitors have had limited efficacy, at least in part because of dose-limiting myelosuppression when combined with cytotoxic chemotherapy. In addition to MGMT, the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) may promote chemotherapy resistance in patients with malignant gliomas. PARP inhibitors such as BSI-201, ABT-888, and several others may be effective when combined with radiotherapy and cytotoxic chemotherapy.89
Brain Cancer–Propagating Cells and Implications for Malignant Glioma Therapy
Brain Cancer–Propagating Cells
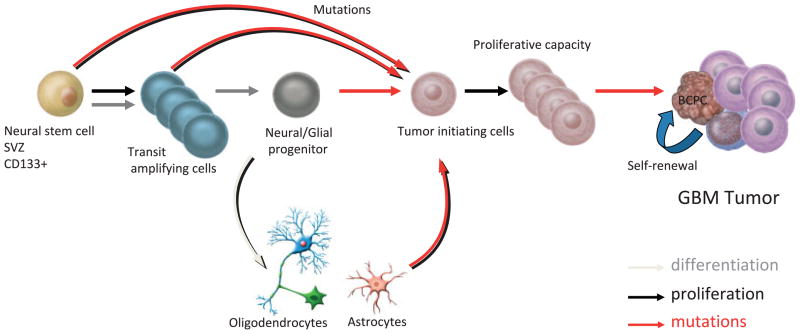
Recent evidence has suggested a subset of cancer cells, variably termed cancer-propagating cells (CPCs) or cancer stem-like cells, may underlie the growth of different types of cancer and be responsible for their resistance to therapy.15,48 CPCs have been isolated from different types of tumors, including primary brain tumors such as GBM, medulloblastoma, and ependymoma.14 These brain cancer-propagating cells (BCPCs) constitute a fraction of the total cell population within brain tumors but may be responsible for their growth. BCPCs are believed to originate from tumor-initiating cells, which are present in the early stages of tumor development and have not acquired full tumorigenic capacity (Fig. 3).
FIGURE 3.
Possible Lineage Relations for the Ontogeny and Production of Brain Cancer–Propagating Cells (BCPCs) and Generation of Glioblastoma Multiforme (GBM) Tumors. During normal central nervous system differentiation, neural stem cells undergo an amplification step to produce transit–amplifying progenitor cells (type C cells), which then differentiate into neural/glial progenitor cells. These progenitor cells retain the capacity to produce progeny along either neural or glial lineages (oligodendrocytes and/or astrocytes), but not both. Mutations generating GBM tumors can occur at all levels within this lineage and produce tumor–initiating cells (TICs). TICs are believed to be stem-like in behavior based on their ability to self-renew, proliferate, and generate BCPCs, differentiated tumor or cancer-like progenitor cells within the tumor mass. Dedifferentiation events may also take place to generate self-propagating cancer cells from astrocytes and oligodendrocytes. SVZ indicates subventricular zone. Adapted with kind permission of Springer Science+Business Media from figure 2 in Hadjipanayis CG, et al., Initiating cells in malignant gliomas: biology and implications for therapy. J Mol Med. 2009;87:363–374.15 © Springer
Neural Stem Cell Origin
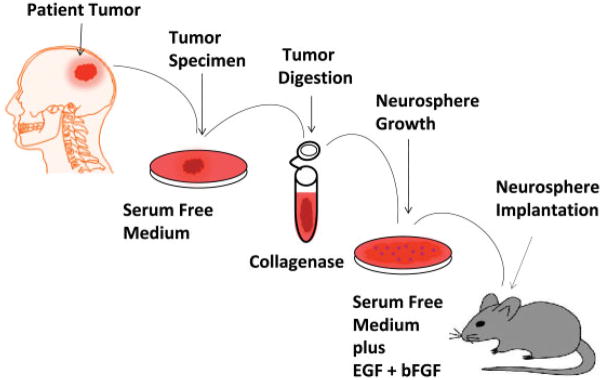
BCPCs share some characteristics with normal neural stem cells (NSCs), including the expression of NSC markers (eg, Nestin and CD133); the capacity for self-renewal and long-term proliferation; and the ability to differentiate into neurons, astrocytes, and oligodendrocytes. BCPCs are believed to originate from transformed NSC or progenitor cell populations in the brain or from de-differentiated mature brain cells that reacquire phenotypic and functional similarities of NSCs (Fig. 3).14 BCPCs may originate from similar locations in the brain in which NSCs are found, including around the ventricular system (subventricular zone or the subependymal zone, the lining of the lateral ventricles, and the cerebellar ventricular zone), the dentate gyrus, the hippocampus, and the subcortical white matter.14 The gold standard assay for the functional evaluation of both self-renewal and tumor propagation of BCPCs is the ability to serially propagate them in an undifferentiated state and have them form tumors in animals at the time of transplantation (Fig. 4).48 They are isolated from dissociated tumors after surgical resection and propagated as neurospheres in specific neurobasal medium supplemented with growth factors.
FIGURE 4.
Generation of Neurosphere Cultures and Propagation in Rodent Brains. EGF indicates epidermal growth factor; bFGF, basic fibroblast growth factor.
Resistance of BCPCs to Standard Therapies
Recent evidence supports the presence of BCPCs that contribute to tumor progression through preferential resistance to radiation and chemotherapy, and the promotion of tumor angiogenesis, invasion, and metastasis.14 BCPC populations have been found to have more efficient repair of damaged DNA in response to ionizing radiation in comparison with the bulk of more differentiated tumor cells.48 Primary brain tumor progression may also result from chemoresistant BCPCs. BCPCs have recently been shown to be resistant to the currently used chemotherapy agent, temozolomide. Elevated expression of transporters that pump out chemotherapeutic agents may be one important mechanism for chemoresistance.14,48
Targeting of BCPCs
Key signaling pathways (PI3K OLIG2, SHH, NOTCH, and Wnt) essential for the development and regulation of NSC have been shown to be active in BCPCs of GBMs, medulloblastomas, and ependymomas and need to be considered as candidate targets.14 Promoting the differentiation of BCPCs may be a new therapeutic mechanism for targeting BCPCs and primary brain tumors. Induction of differentiation by bone morphogenetic proteins (BMPs), specifically BMP4, can trigger a significant reduction in BCPCs in vitro and extend mouse survival and reduce tumor growth.14,48 Disruption of the vascular niche that has been shown to be important to providing a nurturing milieu to BCPCs may be therapeutic.14 The inhibition of blood vessel growth may be an effective method for eliminating GBM CPCs. The use of antiangiogenic drugs such as bevacizumab or cediranib may be able to target the vascular niche.29
Perspectives on Cancer Stem-Like Cells and Brain Tumors
It is unclear at this time whether the growth of primary brain tumors is driven exclusively by BCPCs, or all the cells of a tumor. Tumors are likely comprised of a heterogeneous group of cells, including differentiated tumor cells, BCPCs, and progenitor-like cancer cells. Targeting of each of these tumor cell types may be necessary for more effective treatment of primary brain tumors (Fig. 3).
New Clinical Guides on the Horizon: Biomarkers in the CSF and Blood of Glioma Patients
Entering the postgenomic era, proteomics will play a major role in the processes of understanding biological systems and identifying candidate drugs. The systematic proteomic analysis of CNS neoplasia has been limited because the technologies previously used did not permit the rapid analysis of multiple markers and samples, factors that have precluded its translation to the clinical setting.90 High-throughput proteomic methods have become available to functionally characterize proteins in complex biological materials. Sensitive, convenient, and cost-effective methods for detecting proteins will accelerate the understanding of disease process, the discovery of biomarkers, and the design of new drugs.
Many gliomas are highly malignant and hard to access for diagnostic and therapeutic purposes. Therefore, the development of methods that would complement biopsy through neurosurgery are highly desired. Until recently, serum has been the most common sample source for human biomarker research due to the ease of its clinical implementation and low cost. Serum is a complex medium containing proteins with concentrations that span 12 orders of magnitude. The identification of disease biomarkers within such intricate protein samples is challenging, especially because these markers are expected to be present in far lower concentrations than proteins that constitute the bulk of the mixture. Biomarkers can also bind platelets and blood cell populations, complicating their detection. Furthermore, due to the large volume of blood in circulation (5 L), any disease marker gets diluted many-fold versus its concentration at the production site. Limitations of serum biomarker studies are even greater when dealing with diseases of the brain and the nervous system, because they are relatively isolated from the remaining body due to the BBB and blood–CSF barrier.90 The presence of individual biomarkers (insulin–like growth factor binding protein 2 [IGFBP2], transforming growth factor–β1/2 [TGFβ1/2], and VEGF) and tumor-specific DNA, as well as endothelial progenitor cells in the plasma or serum of glioblastoma patients, have been correlated with patient outcome and demonstrate that the tumor can elicit systemic responses, warranting further proteomic probing of patient blood.
An increasing number of studies are also using CSF as a source of biomarkers for CNS diseases. The CSF bathes the brain and collects proteins secreted by brain tumors as the ependymal cells lining the ventricles are fenestrated and allow for protein passage (Fig. 5). We have shown that measuring alterations in the protein composition of the CSF can be a sensitive indicator of CNS pathology and permit the design of biomarker panels that can identify brain tumor type and grade.91 In addition, it can provide insight into the biology of the tumor92–97 and possibly help in developing personalized treatment plans for each patient. Given the respective advantages outlined earlier, the search for further biomarkers in both CSF and blood are warranted. Progress in the area of biomarker discovery will undoubtedly benefit from new technologies such as iTRAQ (isobaric tags for relative and absolute quantification), and the translation to the clinic will likely involve protein array platforms that are compatible with multiplexing capability and a low sample volume requirement.90 It is not unreasonable to foresee a time when this type of technology will largely replace surgical biopsies for diagnostic purposes.
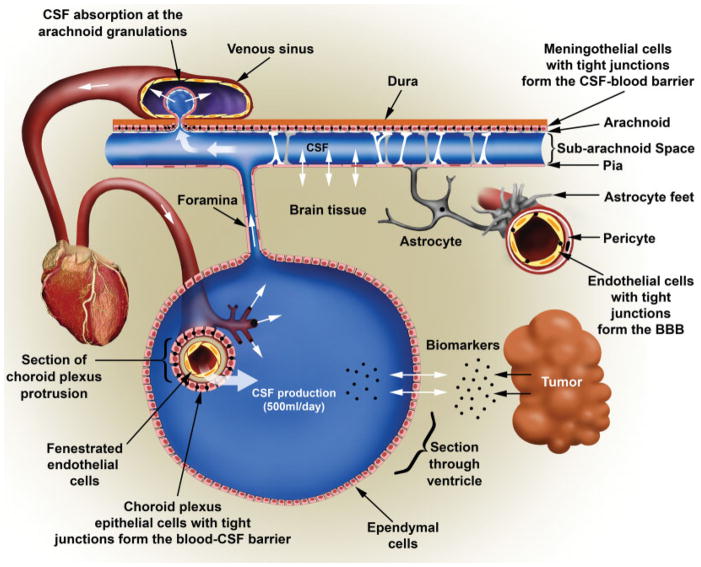
FIGURE 5.
Diagram Illustrating the Flow of Cerebrospinal Fluid (CSF) in the Brain and the Different Cellular Structures That Create the Blood–CSF Barrier. Incoming arterial blood flow from the heart connects to the choroid plexus, a cauliflower-shaped organ in which blood is “filtered” through a double cellular layer, endothelial cells lining the arterial capillary and the choroid plexus that are connected through tight junctions. This constitutes the first component of the blood–CSF barrier. Note that the endothelial cells in the choroid plexus are not connected by tight junctions. The CSF is produced in the choroid plexus and released into the ventricles, which are lined by ependymal cells in which exchanges between the normal and tumor brain tissue extracellular content and the CSF can occur. The hydrostatic pressure of incoming CSF creates CSF flow through foramens such as the median aperture at the skull base, and the CSF enters a second larger compartment called the subarachnoid space, which surrounds the brain. In this space, the CSF–blood barrier is established by the neurothelium, a layer of meningothelial cells that covers the arachnoid and is connected by tight junctions, constituting the second component of the CSF–blood barrier. The CSF is reabsorbed into the venous circulation through multiple arachnoid granulations, which are small, tufted protrusions that herniate through the dura mater and serve as 1-way, pressure-dependent valves. The positive hydrostatic pressure of the CSF moves fluid into the large superior sagittal sinus back into the venous circulation along with a limited number of proteins and other markers of the central nervous system environment. In addition, for comparison, a blood vessel irrigating the brain is shown on the right. The permeation of blood components into the brain parenchyma is restricted by the blood–brain barrier (BBB), which is constituted by endothelial cells with tight junctions, surrounded by pericytes and astrocytic feet. Drawing by Eric Jablonowski, Department of Radiology, Emory University. Reprinted with permission from Khwaja FW, Van Meir EG. Proteomic discovery of biomarkers in the cerebrospinal fluid of brain tumor patients. In: Van Meir EG, ed. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1st ed. New York: Humana Press (Springer); 2009:577–614.90
New Developments in the Surgery of Brain Tumors
Surgical intervention for malignant glioma remains key for the diagnosis and prevention of symptoms due to mass effect. Technical advances minimizing injury to surrounding normal tissue continue. Along with standard techniques, the advances may be useful in the delivery of molecular agents to the tumor in selected cases. Currently, the tissue obtained at the time of surgery is critical to allow for individualized analysis of the tumor’s molecular signature, which is needed to eventually design treatments with the many targeted therapies mentioned above.
Neuroendoscopy
The use of a fiber optic camera, known as an endoscope, for the diagnosis or treatment of conditions involving the CNS has been termed neuroendoscopy. Neuroendoscopy can provide a minimally invasive approach to CNS tumors for biopsy, resection, and the concurrent management of obstructive hydrocephalus associated with a tumor.98–100 Neuroendoscopic approaches use the natural CSF-filled ventricular cavities in the brain as a conduit for accessing tumors. Brilliant illumination from the tip of the neuroendoscope allows the neurosurgeon to navigate within the ventricular system in any direction and optimally visualize the tumor. Neuronavigation technology allows the surgeon to properly correlate the location of the endoscope with MRI of the brain.101
Typically, a neuroendoscope is used that measures approximately 3 to 6 mm in maximal diameter. The neuroendoscope includes a rigid fiber optic lens endoscope (measuring 2–3 mm in diameter) fitted into a working channel trocar with up to 3 working channels. The working channels in the neuroendoscope can be used for the placement of special instrumentation (measuring 1–2 mm in diameter) for the resection of brain tumors as well as performing endoscopic third ventriculostomies for the relief of obstructive hydrocephalus.102 The neuroendoscope enhances the surgeon’s view by increasing illumination and magnification. Because the diameter of the neuroendoscope is less than 1 cm, the exposure and trajectory to the CNS lesion with the neuroendoscope is achieved in a minimally invasive fashion. Most skin incisions measure approximately 2 cm in length, and the opening in the skull is less than 1 cm. No significant brain retraction is needed, and the unique visualization on a high-definition monitor afforded by the endoscope permits the proper understanding of anatomicopathological structures.
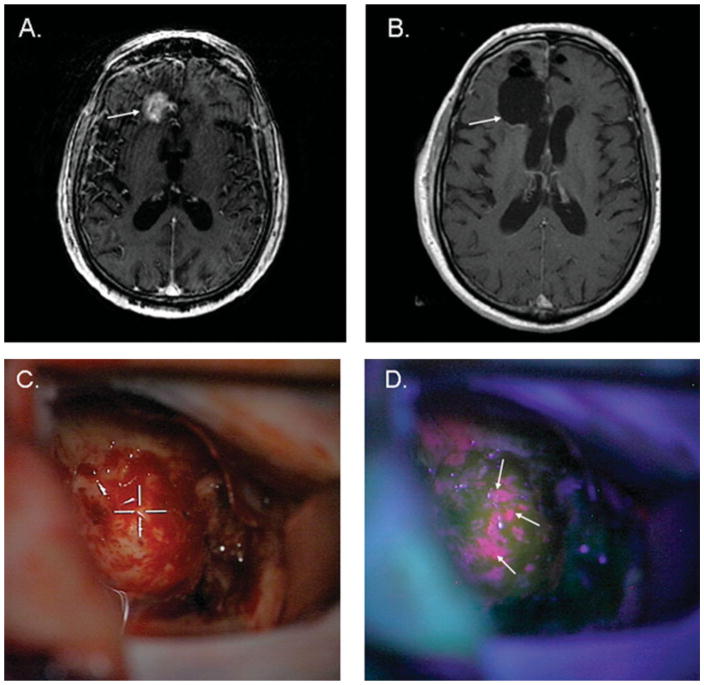
The application of neuroendoscopy to surgical neuro-oncology has become an invaluable supplement to traditional surgical approaches. The neuroendoscope can be used to remove intraventricular tumors and perform resections of tumors involving eloquent or deep midline portions of the brain due to the trajectory provided by the natural ventricular cavities in the brain (Fig. 6). Direct visualization of intraventricular and periventricular tumors can allow for more accurate sampling of tumors as well as improved hemostasis abilities in comparison with traditional stereotactic needle biopsy procedures. In addition, the ability to simultaneously manage CNS tumor-related hydrocephalus through removal of CSF pathway obstruction, the placement of a third ventriculostomy, or septostomy can prevent the placement of ventriculoperitoneal shunts in patients.
FIGURE 6.
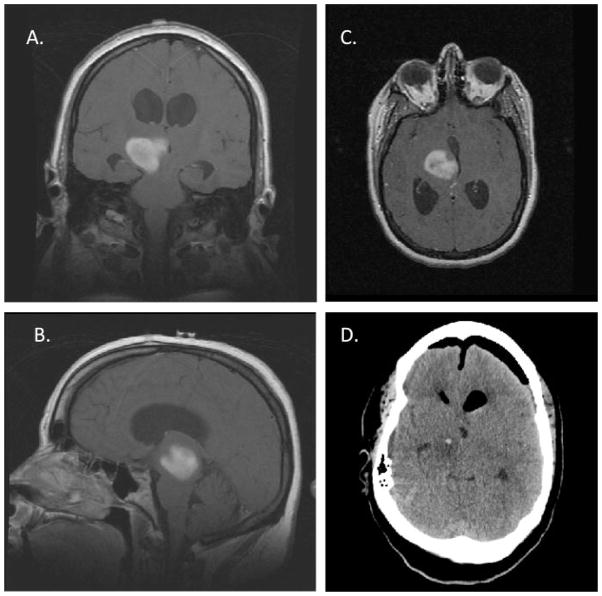
Imaging of a 23–Year–Old Female Who Underwent a Neuroendoscopic Biopsy of a Thalamic Malignant Glioma and an Endoscopic Third Ventriculostomy for Treatment of Her Obstructive Hydrocephalus. (A) Coronal, (B) sagittal, and (C) axial T1-weighted magnetic resonance imaging of the brain after gadolinium administration revealed contrast enhancement of a right thalamic mass (indicated by arrows) extending into the midbrain and causing obstructive hydrocephalus. (D) A postoperative computed tomography scan of the brain demonstrating ventricular decompression and an area of neuroendoscopic sampling of the tumor (indicated by arrow) was obtained for pathologic diagnosis.
Virtually any CNS lesion that is intraventricular or periventricular in location can be approached with the neuroendoscope for diagnostic and resection purposes. Examples of CNS tumors that can be approached with the neuroendoscope include malignant and nonmalignant gliomas, meningiomas, colloid cysts, dermoid/epidermoid tumors, sub-ependymal giant cell astrocytomas, pineal region tumors (including pineal cysts, pineocytomas, pineoblastomas, germ cell tumors [GCTs], and nongerminomatous GCTs), primary CNS lymphoma, central neurocytomas, and subependymomas.
Fluorescence–Guided Resection of Malignant Gliomas
Surgical resection remains an important therapeutic modality in the initial management of malignant gliomas. Despite limitations reported in the literature, mounting evidence suggests that more extensive surgical resection is associated with a longer life expectancy for patients with high-grade gliomas.103 One randomized trial performed in the elderly demonstrated a benefit from surgery in comparison with biopsy alone.104 However, there has never been a controlled, randomized study to determine whether simple debulking of tumors is as effective as maximal cytoreduction.
The influence of the degree of resection on survival in patients with GBM has recently been analyzed with a fluorescent tumor marker.105,106 The use of this marker for the intraoperative detection and fluorescence-guided resection of brain tumors has been shown to enhance the macroscopic total resection of malignant gliomas and maximize the survival benefits (PFS) associated with surgical removal. The technique involves the oral administration of the non-fluorescent prodrug, 5-aminolevulinic acid (ALA), which is metabolized to fluorescent protoporphyrin IX (PpIX) in tumor tissue through the heme biosynthesis pathway. The phenomenon of PpIX accumulation in WHO grade III and IV malignant gliomas may be explained by higher 5-ALA uptake into the tumor tissue or an altered pattern of expression or activity of enzymes (eg, ferrochelatase) involved in hemoglobin biosynthesis in tumor cells. Explanations for higher 5-ALA uptake include a disrupted BBB, increased neovascularization, and the overexpression of membrane transporters in glioma tissue. ALA-induced PpIX levels in normal brain tissue are very low, creating high intrinsic tumor-to-normal tissue contrast. The use of the operative microscope adapted for visualization of the fluorescent PpIX with “blue light” allows the neurosurgeon to visualize the tumor as “red” in color, thereby optimizing intraoperative tumor resection (Fig. 7). Visualization of the tumor by fluorescence may potentially be more reliable than the use of neuronavigation and standard microscopic “white light” visualization. Minimal side effects have been reported, which include skin photosensitivity after oral administration.
FIGURE 7.
Fluorescence-Guided Resection of a Glioblastoma Multiforme Tumor. (A) Preoperative axial T1-weighted magnetic resonance imaging (MRI) of the brain after gadolinium administration demonstrated a right frontal contrast–enhancing mass. (B) A postoperative axial T1-weighted MRI of the brain revealed macroscopic total resection of the right frontal mass using intraoperative fluorescence. (C) An intraoperative microscopic view (white light) of the tumor resection cavity is shown. (D) An intraoperative microscopic view of tumor fluorescence (indicated by arrows) using “blue light” is shown. Figures were kindly provided by Dr. David Roberts, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
A group in Germany has been performing the fluorescence-guided resection of malignant gliomas using the ALA compound (Gliolan; Photonamic Gmbh & Co. KG, Wedel, Germany) for approximately 10 years. A randomized, controlled, multi-center phase 3 trial reported a doubling of the PFS6 in patients with malignant gliomas who underwent fluorescence-guided resection by ALA administration.106 Interim analysis resulted in the early termination of the trial, with the primary endpoints being the number of patients without contrast-enhancing tumor on MRI (within 72 hours of surgery) and PFS6.
Currently, ALA is not approved by the FDA in the United States for surgical resection of brain tumors. Efforts are currently underway to potentially perform a controlled, randomized, multicenter trial in the United States using fluorescence-guided surgical resection of malignant gliomas to determine an effect on extent of resection.
Brain Tumor Surgery in 2020
Within the next 10 years, the incorporation of various nanotechnology platforms will likely advance the surgical management of malignant brain tumors. The multifunctional clinical nature of nanotechnology will provide for the targeting, imaging, and therapy of infiltrating brain tumor cells, including brain cancer stem-like cells, that escape surgical treatment and are responsible for tumor recurrence. Therapeutic nanoparticles (eg, magnetic nanoparticles) coated with various drugs and conjugated to brain tumor–specific antibodies or ligands will be delivered systemically or locally to brain tumors by convection-enhanced delivery. The ability to image nanoparticles by conventional methods, such as MRI, will provide precise information regarding therapeutic agent delivery and brain tumor targeting, as well as the ability for therapeutic follow-up monitoring. Local hyperthermia treatment of malignant brain tumors will also be possible with the same nanoparticles with the use of alternating magnetic fields that are safe for normal, noncancerous cells.107 Surgical resection will still be required to debulk malignant brain tumors and alleviate the mass effect on the surrounding brain.
New Developments in the Radiotherapy of Brain Tumors
Although the type of radiation used to treat malignant gliomas has not changed substantially for decades, the ability to minimize the radiation dose to surrounding normal tissue is improving significantly. Radiation resistance is an age-old problem in this group of tumors, but the same advances in understanding the genetics of these tumors that are guiding targeted molecular treatment are also providing clues to overcoming this important limitation.
Technical Advances in Radiotherapy
Radiotherapy has been a mainstay of therapy for patients with malignant gliomas. The value of radiation for the treatment of these brain tumors was firmly established with the landmark report that found that patients treated with radiation survived longer than those treated with best supportive care.108 The current radiotherapy standard for this disease is partial brain irradiation given over approximately 6 weeks. Prior to therapy, the regions at risk are mapped using a brain MRI (or computed tomography [CT] scan if MRI is contraindicated), and a conformal treatment plan (eg, using intensity-modulated radiotherapy) is generated that encompasses this target volume. Modern treatment techniques allow for significant sparing of surrounding normal structures/tissues while maintaining high doses to the defined treatment volumes.109 Due to the infiltrative nature of malignant gliomas, wide margins are typically used. The most common margins used that have largely been adopted by the Radiation Therapy Oncology Group (RTOG) for use on their protocols for malignant gliomas are comprised of expanding 2 cm around the T2/fluid–attenuated inversion recovery (FLAIR) signal abnormality for the initial volume (treated to 46 grays [Gy] at 2 Gy/day), followed by 2 cm around the enhancing tumor/tumor bed for the boost volume (treated to 14 Gy at 2 Gy/day to a total dose of 60 Gy).
Since the advent of clinical MRIs, these scans have been used to aid the radiation oncologist in defining tumor volume within the brain. Because tools to allow the registration/fusion of MRI and CT image sets did not become widely available until the past few years, target volumes were generally delineated on a planning CT image set with the MRI serving only as a guide for contouring these volumes. However, modern treatment planning software now has the capability of registering/fusing MRI and CT image sets, which significantly enhances the accuracy of target delineation. With this capability, the question is raised as to whether standard treatment margins can be reduced further, resulting in lower doses to surrounding brain and potentially less neurocognitive toxicities. Review of our institutional data at Emory University suggests that reducing margins for initial and boost volumes to 0.5 to 1.0 cm results in a targeted volume that is up to 70% less than the comparable volumes based on standard margins, with no apparent negative effects on local control.110
Another potential source of inaccuracies in the treatment of patients relates to the difficulty in daily repositioning the patient within the immobilization device in exactly the same way. Commercial onboard imaging (OBI) systems are now available from all the major linear accelerator manufacturers and can be used to not only check for but also correct patient positioning variations. Orthogonally paired, diagnostic-quality (kilovolt range) x-ray images of the patient are compared with the corresponding digitally reconstructed radiographs from the simulation scan to determine setup accuracy and permit isocenter positioning shifts as needed to correct for setup inaccuracies prior to each treatment. The real-time use of an OBI system is feasible from a clinical efficiency standpoint and can improve the accuracy of daily patient setup.111 This system is now increasingly used at many institutions to assist in the daily setup of brain tumor patients.
Although radiation is a critical part of the standard therapy for patients with newly diagnosed malignant gliomas, its use is more limited in the setting of recurrent disease. Retreatment with radiation poses a significant risk for the development of necrosis. However, in selected settings, reirradiation may be a useful adjunct to salvage therapy. Several factors may affect the decision to recommend reirradiation, including the availability of other salvage therapies; the patient’s performance status; the volume of tumor recurrence; the proximity of the recurrence to previously treated, high-dose regions; and the length of time since previous irradiation. General principles for reirradiating the brain include limiting treatment volumes and dose reduction. Although fractionated conformal radiation is most often used when the volume of tumor recurrence is moderate, for selected cases with small focal recurrences (measuring < 3–4 cm), stereotactic radiosurgery (SRS) may also be a viable option.112 Although SRS as a boost to a standard course of fractionated radiation was not found to be beneficial in patients with newly diagnosed malignant gliomas,113 its use in the recurrent disease setting produces responses and may lengthen the time to disease progression in selected patients.114,115
Although framed-based SRS is considered the standard, SRS can also be performed in a frameless manner. The main advantages of frameless SRS are improvement in patient comfort and the possibility of fractionating treatments while maintaining precision that is comparable to a frame-based procedure. Different frameless approaches have been developed.116–118 Our institution has chosen to combine an optical positioning system with cone-beam CT-based positioning to assure setup precision with this frameless procedure. An infrared optical positioning system is used for patient setup and monitoring of potential movement during the procedure.117 As a secondary positioning tool, a cone-beam CT scan is also obtained using the OBI system and registered with the planning CT scan to verify setup accuracy and to make further fine patient positioning adjustments, as needed.
In summary, radiotherapy remains an important part of the standard-of-care treatment for patients with malignant gliomas. Recent technical advances have enhanced our ability to map tumors and set up patients with greater accuracy, thereby improving our ability to focally treat these brain tumors. In fact, standard tumor margins can likely be decreased, further limiting possible toxicity to normal tissue. However, as we reach the technical limits of what is achievable with radiation, future advances in radiation oncology will likely involve the use of new targeted drugs that can modulate the response of these tumors to radiation.
Radiation Resistance
Although malignant gliomas are typically treated with radiotherapy, from a clinical standpoint they display significant radioresistance, with the great majority of treated tumors recurring within the high-dose region. Dose escalations up to 90 Gy have not proven to alter the course of this disease significantly.119,120 Agents capable of sensitizing tumor cells to the cytotoxic effects of radiation have also been explored. Patients with malignant gliomas who were treated with radiation and carmustine did not appear to gain additional survival benefit from the addition of misonidazole, a hypoxic cell radiosensitizer.121 Similarly, the use of bromodeoxyuridine, a halogenated pyrimidine that incorporates into the DNA to radiosensitize cells, did not further benefit patients with anaplastic astrocytomas who were treated with radiation and chemotherapy with vincristine, lomustine, and procarbazine.122 Despite these negative studies, a survival benefit was found for glioblastoma patients treated with temozolomide, an alkylating agent, in addition to radiation.4 In this trial, temozolomide was administered concurrently with radiation and afterward as adjuvant therapy. Temozolomide can radiosensitize glioma cells in vitro123 and thus, some of the benefits of this agent noted in that trial may be due to its radiosensitizing activity.
Given the significant radioresistance noted clinically with malignant gliomas, one would postulate that common molecular changes observed in these tumors contribute to this resistance. Two of the most common abnormalities noted in malignant gliomas are amplification/mutation of EGFR and loss of PTEN, resulting in PI3K activation. Engineered glioma cells with decreased EGFR signaling result in greater radiosensitivity124 and expression of a mutant, constitutively active EGFR was found to produce radioresistance, primarily through activation of the PI3K pathway.125 Several specific PI3K inhibitors are currently in clinical development and it will be of interest to examine whether these agents radiosensitize glioma cells in experimental systems as occurs for temozolomide. Those that do will be worthy of further testing as drug candidates administered with radiation in the upfront treatment of patients with malignant gliomas.
Significant potential exists for enhancing overall therapy by modulating the response of malignant gliomas to radiation. Although early attempts using nonspecific radiosensitizers were largely unsuccessful, the results reported with temozolomide establish a possible precedent that radiosensitization can improve clinical outcomes. We expect that with the discovery and increased use of new molecularly targeted drugs, clinically useful agents that enhance the effects of radiation will eventually become a reality for patients with this disease.
Brain Radiotherapy in 2020
The field of radiation oncology has always been one that is driven by technology. The next 10 years should be no different, with a host of technical advances that will impact radiotherapy for brain tumors. These advances will range from new and improved tumor imaging modalities to tools to improve the delivery of radiation treatments.
Advances in neuroimaging are likely to come from several different fronts. T1 postcontrast and T2 (or FLAIR) MRI sequences are routinely used in radiation oncology to define brain tumor extent. In the near future, complementary MRI techniques currently in development will find increased clinical use including ones capable of quantifying cerebral blood flow and volume, diffusion of water (to provide information regarding cellular density and white matter tractography), and leakage of gadolinium within tumors using dynamic contrast-enhanced imaging.126 Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has gained increased acceptance in oncology but is still problematic for neuroimaging due to high background signals in brain. Many newer PET tracers do not have this problem and are currently at various stages of development. These tracers, including 11C-methionine,127 18F-1-amino-3-fluorocyclobutane-1-carboxylic acid (leucine analogue that demonstrates increased uptake within tumors),128 18F-fluorothymidine (a thymidine analogue that demonstrates increased uptake in tumor cells undergoing DNA synthesis),129 and 18F-fluoromisonidazole (a nitroimidazole that demonstrates increased uptake within hypoxic cells),130 have been shown to be potentially useful for imaging malignant gliomas.
Many advances in radiation delivery will be made over the next 10 years, and it is beyond the scope of this review to speculate about all the different directions this may take. However, proton therapy is one area in particular that is likely to gain greater prominence in the treatment of malignant gliomas if its availability can be significantly expanded. Although proton beams have significant physical characteristic advantages (namely, no exit dose beyond a certain depth penetration in tissue depending on its energy), it requires a large particle accelerator such as a cyclotron, which is extremely expensive to build. Despite the opening of several new proton centers in this country over the past few years, this technology remains a limited resource that is unlikely to be used prominently for patients with a diagnosis that is not curable the great majority of the time. However, this situation can potentially change if the cost of proton acceleration is significantly reduced. Accelerating protons with lasers to produce a clinically useful beam is actively being pursued as a viable means of bringing proton therapy to the “masses.”131 If this and other similar ideas are ultimately developed, they have the potential to revolutionize radiotherapy by reducing the cost and physical plant requirements for proton therapy and potentially making it widely available.
Depending on the speed at which these and other advances are developed and implemented within the clinic, radiotherapy for malignant gliomas will look very different in 2020. All patients are likely to undergo an increased battery of neuroimaging examinations that will give us much more information regarding the tumor and allow us to tailor therapy more precisely for each individual patient depending on these tumor characteristics. In addition, if the cost of proton therapy is reduced to a level approaching high-energy x-ray therapy, then it may eventually supplant x-rays as the primary radiation modality used.
Outlook for Brain Tumor Therapy: Vision 2020
The science available to support our understanding of brain tumor development, growth, and therapy is burgeoning, as evidenced by this review. The fruition of imaging advances, targeted therapy, and molecular tissue analysis techniques leads one to envision a greatly improved environment for brain tumor management in the next decade. For example, physicians will be able to diagnose a cerebral neoplasm accurately and effectively by imaging alone, a specific set of pathological markers, and possibly with the added information from biomarkers present in the CSF or circulating tumor DNA. This same imaging technology will serve as guidance for optimizing the design for surgical resection, radiation ports, and subsequent assessment of therapy. Following tumor debulking, neuropathologists will provide a diagnosis assisted by algorithm-based quantification of specific histological features on digitized tumor sections. In selected patients in whom surgery is not otherwise indicated, stereotactic needle biopsy may be undertaken to provide tissue for molecular analysis. More often, simple blood draws to assess for circulating DNA could be used to obtain this information.132 With the lesion’s molecular data in hand, the treating physicians can create a therapeutically unique regimen, using multiple treatment agents specific for the lesion and the individual (personalized medicine). Treatment-unique imaging will be available to assess the success of treatment delivery, efficacy, and toxicity early (ideally after only 1 to 2 doses), so as to maximize the use of beneficial agents and to minimize time spent on treatment that is ineffective. Of course, the goal is to see malignant cerebral neoplasms treated in a curative fashion by 2020. However, not attaining that goal would not reflect defeat. It would still be of great value to patients to convert this disease to a chronic condition that is treated with nontoxic, tumor-directed agents and followed by disease-specific and treatment-specific imaging or regular serologic or CSF analysis of circulating nucleic acid or protein markers. Scientific progress in the understanding of the molecular mechanisms at the root of the disease has been tremendous, and the accelerated development of new drugs will likely achieve dramatic improvements in therapeutic outcomes in the next 10 years.
Footnotes
DISCLOSURES: Supported by National Institutes of Health (NIH) grants CA86335 and CA116804, the Goldhirsh Foundation, the Brain Tumor Funders Collaborative, the Southeastern Brain Tumor Foundation (to E.G.V.M.), NIH NS053454, the American Brain Tumor Association, the Southeastern Brain Tumor Foundation, the Georgia Cancer Coalition Distinguished Cancer Clinicians and Scientists Program, and the Dana Foundation (to C.G.H.). Dr. Norden has received honoraria from Genentech for serving on the advisory board and from Schering-Plough for serving on the speaker’s bureau. Dr. Wen has received research support from Amgen, Genentech, Exelixis, Schering, Novartis, AstraZeneca, Boehringer, and Merck. The authors report no other conflicts of interest.
References
- 1.Stieber VW, Mehta MP. Advances in radiation therapy for brain tumors. Neurol Clin. 2007;25:1005–1033. doi: 10.1016/j.ncl.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 6.Noda SE, El-Jawahri A, Patel D, Lauten-schlaeger T, Siedow M, Chakravarti A. Molecular advances of brain tumors in radiation oncology. Semin Radiat Oncol. 2009;19:171–178. doi: 10.1016/j.semradonc.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 8.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuettenberg J, Grobholz R, Seiz M, et al. Recurrence pattern in glioblastoma multiforme patients treated with anti-angiogenic chemotherapy. J Cancer Res Clin Oncol. 2009;135:1239–1244. doi: 10.1007/s00432-009-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulman EP, Guerrero M, Aldape K. Transcription profiling of brain tumors: tumor biology and treatment stratification. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 529–552. [Google Scholar]
- 12.Schneider BP, Radovich M, Miller KD. The role of vascular endothelial growth factor genetic variability in cancer. Clin Cancer Res. 2009;15:5297–5302. doi: 10.1158/1078-0432.CCR-08-2576. [DOI] [PubMed] [Google Scholar]
- 13.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjipanayis CG, Van Meir EG. Brain cancer propagating cells: biology, genetics and targeted therapies. Trends Mol Med. 2009;14:519–530. doi: 10.1016/j.molmed.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjipanayis CG, Van Meir EG. Tumor initiating cells in malignant gliomas: biology and implications for therapy. J Mol Med. 2009;87:363–374. doi: 10.1007/s00109-009-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 17.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- 18.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 19.James CD, Carlbom E, Nordenskjold M, Collins VP, Cavenee WK. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989;86:2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin S, Van Meir EG. p53 pathway alterations in brain tumors. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 283–314. [Google Scholar]
- 21.Stokoe D, Furnari FB. The PTEN/PI3 kinase pathway in human glioma. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 315–357. [Google Scholar]
- 22.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belozerov VE, Van Meir EG. Inhibitors of hypoxia-inducible factor-1 signaling. Curr Opin Investig Drugs. 2006;7:1067–1076. [PubMed] [Google Scholar]
- 25.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra A, Feuerstein BG. Discovery of genetic markers for brain tumors by comparative genomic hybridization. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. [Google Scholar]
- 27.Verhaak RGW, Hoadley KA, Purdom E, et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 29.Gerstner ER, Batchelor TT. Clinical agents for the targeting of brain tumor vasculature. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 731–728. [Google Scholar]
- 30.Zheng H, Ying H, Yan H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellail AC, Mulligan P, Hao C. Targeting of TRAIL apoptotic pathways for glioblastoma therapies. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 977–1010. [Google Scholar]
- 32.Li YC, Tzeng CC, Song JH, et al. Genomic alterations in human malignant glioma cells associate with the cell resistance to the combination treatment with tumor necrosis factor-related apoptosis-inducing ligand and chemotherapy. Clin Cancer Res. 2006;12:2716–2729. doi: 10.1158/1078-0432.CCR-05-1980. [DOI] [PubMed] [Google Scholar]
- 33.Laver T, Nozell S, Benveniste EN. The NF-kB signaling pathway in GBMs: implications for apoptotic and inflammatory responses and exploitation for therapy. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 1011–1036. [Google Scholar]
- 34.Hwang Y, Latha K, Gururaj A, Rojas M, Bogler O. Aberrant EGFR signaling in glioma. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 441–459. [Google Scholar]
- 35.Thiessen B, Stewart C, Tsao M, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 36.Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20:1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 37.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 38.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 39.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assanah M, Lopez KA, Bruce JN, Canoll P. Modeling gliomas using PDGF-expressing retroviruses. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 3–27. [Google Scholar]
- 41.Kilic T, Alberta JA, Zdunek PR, et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60:5143–5150. [PubMed] [Google Scholar]
- 42.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 43.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 44.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 45.Kreisl TN, Kim L, Moore K, et al. A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res. 2009;15:3617–3623. doi: 10.1158/1078-0432.CCR-08-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 47.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27:2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atkinson JM, Gilbertson RJ, Rich JN. Brain cancer stem cells as targets of novel therapies. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 1157–1175. [Google Scholar]
- 49.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 50.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96:219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robins HI, Wen PY, Chang SM, et al. Phase I study of erlotinib and CCI-779 (temsirolimus) for patients with recurrent malignant gliomas (NABTC 04-02) J Clin Oncol. 2007;25:2057. [Google Scholar]
- 52.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 54.Bergers G. Bone marrow-derived cells in GBM neovascularization. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 749–773. [Google Scholar]
- 55.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Hu B, Guo P, Bar-Joseph I, et al. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26:5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 58.Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 59.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 60.Stark Vance V. Bevacizumab (Avastin®) and CPT-11 (Camptosar®) in the treatment of relapsed malignant glioma. Neurooncol. 2005;7:369. [Google Scholar]
- 61.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 62.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poulsen HS, Grunnet K, Sorensen M, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48:52–58. doi: 10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]
- 64.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 65.Guiu S, Taillibert S, Chinot O, et al. Bevacizumab/irinotecan. An active treatment for recurrent high grade gliomas: preliminary results of an ANOCEF multi-center study. Rev Neurol (Paris) 2008;164:588–594. doi: 10.1016/j.neurol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 67.Yung W, Albright R, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. Abstract. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 69.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 70.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 71.United States Food and Drug Administration. FDA Briefing Document. Oncology Drug Advisory Committee Meeting. Washington, DC: United States Food and Drug Administration; 2009. [Google Scholar]
- 72.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stupp R, Goldbrunner R, Neyns B, et al. Mature results of a phase I/IIa trial of the integrin inhibitor cilengitide (EMD121974) added to standard concomitant and adjuvant temozolomide and radiotherapy for newly diagnosed glioblastoma. Neurooncol. 2007;9:517. [Google Scholar]
- 78.Lamszus K, Kunkel P, Westphal M. Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl. 2003;88:169–177. doi: 10.1007/978-3-7091-6090-9_23. [DOI] [PubMed] [Google Scholar]
- 79.Erber R, Thurnher A, Katsen A, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 80.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Viapiano MS, Lawler SE. Glioma invasion: mechanisms and targeting opportunities. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 1219–1252. [Google Scholar]
- 82.Walker PR, Prins RM, Dietrich P-Y, Liau LM. Harnessing T-cell immunity to target brain tumors. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 1165–1217. [Google Scholar]
- 83.Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayriss JE, Kuan C-T, Boulton ST, Reardon DA, Bigner DD. Molecular targets for antibody-mediated immunotherapy of malignant glioma. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 865–898. [Google Scholar]
- 85.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 86.Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, Bekele BN, Aldape KD. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Broniscer A, Gururangan S, MacDonald TJ, et al. Phase I trial of single-dose temozolomide and continuous administration of O6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin Cancer Res. 2007;13:6712–6718. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 89.Donawho CK, Luo Y, Penning TD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 90.Khwaja FW, Van Meir EG. Proteomic discovery of biomarkers in the cerebrospinal fluid of brain tumor patients. In: Van Meir EG, editor. CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. 1. New York: Humana Press (Springer); 2009. pp. 577–614. [Google Scholar]
- 91.Khwaja FW, Reed MS, Olson JJ, et al. Proteomic identification of biomarkers in the cerebrospinal fluid (CSF) of astrocytoma patients. J Proteome Res. 2007;6:559–570. doi: 10.1021/pr060240z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Meir EG, Sawamura Y, Diserens A-C, Hamou M-F, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 93.Van Meir E, Ceska M, Effenberger F, et al. Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer Res. 1992;52:4297–4305. [PubMed] [Google Scholar]
- 94.Kuratsu J, Yoshizato K, Yoshimura T, Leonard EJ, Takeshima H, Ushio Y. Quantitative study of monocyte chemoattrac-tant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993;85:1836–1839. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- 95.Frei K, Piani D, Malipiero UV, Van Meir EG, de Tribolet N, Fontana A. Granulocyte–macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells: despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992;148:3140–3146. [PubMed] [Google Scholar]
- 96.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 97.Khwaja F, Duke-Cohan JS, Brat DJ, Van Meir EG. Attractin is elevated in the cerebrospinal fluid (CSF) of patients with malignant astrocytoma and mediates glioma cell migration. Clin Cancer Res. 2006;12:6331–6336. doi: 10.1158/1078-0432.CCR-06-1296. [DOI] [PubMed] [Google Scholar]
- 98.Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intra-ventricular lesions. Neurosurgery. 2008;62(suppl 2):575–597. doi: 10.1227/01.neu.0000316262.74843.dd. [DOI] [PubMed] [Google Scholar]
- 99.Souweidane MM, Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105:271–278. doi: 10.3171/jns.2006.105.2.271. [DOI] [PubMed] [Google Scholar]
- 100.Greenlee JD, Teo C, Ghahreman A, Kwok B. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62:51–55. doi: 10.1227/01.neu.0000317373.00018.6f. [DOI] [PubMed] [Google Scholar]
- 101.Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A. Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery. 1999;44:795–804. doi: 10.1097/00006123-199904000-00062. [DOI] [PubMed] [Google Scholar]
- 102.Lekovic GP, Gonzalez LF, Feiz-Erfan I, Rekate HL. Endoscopic resection of hypothalamic hamartoma using a novel variable aspiration tissue resector. Neurosurgery. 2006;58:ONS166–ONS169. doi: 10.1227/01.NEU.0000193512.87279.69. [DOI] [PubMed] [Google Scholar]
- 103.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 104.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145:5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 105.Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008;55:20–26. [PubMed] [Google Scholar]
- 106.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminole-vulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 107.Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–1929. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 109.Chan MF, Schupak K, Burman C, Chui CS, Ling CC. Comparison of intensity-modulated radiotherapy with three-dimensional conformal radiation therapy planning for glioblastoma multiforme. Med Dosim. 2003;28:261–265. doi: 10.1016/j.meddos.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 110.McDonald MW, Shu HG, Curran WJ, Crocker IR. Pattern of failure after limited margin radiotherapy and temozolomide in glioblastoma. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.10.048. In Press. [DOI] [PubMed] [Google Scholar]
- 111.Lawson JD, Fox T, Elder E, et al. Early clinical experience with kilovoltage image-guided radiation therapy for interfraction motion management. Med Dosim. 2008;33:268–274. doi: 10.1016/j.meddos.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63:47–55. doi: 10.1016/j.ijrobp.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 113.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Larson DA, Prados M, Lamborn KR, et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54:1397–1404. doi: 10.1016/s0360-3016(02)03743-4. [DOI] [PubMed] [Google Scholar]
- 115.Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM) Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 116.Adler JR, Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 117.Meeks SL, Tome WA, Willoughby TR, et al. Optically guided patient positioning techniques. Semin Radiat Oncol. 2005;15:192–201. doi: 10.1016/j.semradonc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 118.Wurm RE, Erbel S, Schwenkert I, et al. Novalis frameless image-guided noninvasive radiosurgery: initial experience. Neurosurgery. 2008;62(suppl 5):A11–A17. doi: 10.1227/01.neu.0000325932.34154.82. [DOI] [PubMed] [Google Scholar]
- 119.Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251–260. doi: 10.3171/jns.1999.91.2.0251. [DOI] [PubMed] [Google Scholar]
- 120.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 121.Nelson DF, Diener-West M, Weinstein AS, et al. A randomized comparison of misonidazole sensitized radiotherapy plus BCNU and radiotherapy plus BCNU for treatment of malignant glioma after surgery: final report of an RTOG study. Int J Radiat Oncol Biol Phys. 1986;12:1793–1800. doi: 10.1016/0360-3016(86)90321-4. [DOI] [PubMed] [Google Scholar]
- 122.Prados MD, Seiferheld W, Sandler HM, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58:1147–1152. doi: 10.1016/j.ijrobp.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 123.van Nifterik KA, van den Berg J, Stalpers LJ, et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–1253. doi: 10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 124.O’Rourke DM, Kao GD, Singh N, et al. Conversion of a radioresistant phenotype to a more sensitive one by disabling erbB receptor signaling in human cancer cells. Proc Natl Acad Sci U S A. 1998;95:10842–10847. doi: 10.1073/pnas.95.18.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes ra-dioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 126.Gerstner ER, Sorensen AG, Jain RK, Batchelor TT. Advances in neuroimaging techniques for the evaluation of tumor growth, vascular permeability, and angiogenesis in gliomas. Curr Opin Neurol. 2008;21:728–735. doi: 10.1097/WCO.0b013e328318402a. [DOI] [PubMed] [Google Scholar]
- 127.Ogawa T, Shishido F, Kanno I, et al. Cerebral glioma: evaluation with methionine PET. Radiology. 1993;186:45–53. doi: 10.1148/radiology.186.1.8380108. [DOI] [PubMed] [Google Scholar]
- 128.Shoup TM, Olson J, Hoffman JM, et al. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40:331–338. [PubMed] [Google Scholar]
- 129.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46:945–952. [PubMed] [Google Scholar]
- 130.Cher LM, Murone C, Lawrentschuk N, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–418. [PubMed] [Google Scholar]
- 131.Martin M. Laser accelerated radiotherapy: is it on its way to the clinic? J Natl Cancer Inst. 2009;101:450–451. doi: 10.1093/jnci/djp071. [DOI] [PubMed] [Google Scholar]
- 132.Weaver KD, Pierce L, Herman JG, Grossman SA. Real-time polymerase chain reaction technique determines absolute copy number of plasma methylated MGMT gene promoter copies in newly diagnosed malignant glioma patients. J Clin Oncol. 2008;26 Abstract 22073. [Google Scholar]