Abstract
Ribonucleases of the T2 family are found in the genomes of protozoans, plants, bacteria, animals and viruses. A broad range of biological roles for these ribonucleases have been suggested including scavenging of nucleic acids, degradation of self-RNA, serving as extra- or intracellular cytotoxins, and modulating host immune responses. More recently, RNaseT2 family members have been implicated in human pathologies such as cancer and parasitic diseases. Interestingly, certain functions of RNaseT2 family members are independent of their nuclease activity suggesting that these proteins have additional functions. Moreover, humans lacking RNASET2 manifest a defect in neurological development, perhaps due to aberrant control of the immune system. Herein, we review the basic structure and function of RNaseT2 family members and their biological roles.
T2 Family Ribonucleases
Ribonucleases catalyze the cleavage of RNA, acting on single-stranded, double-stranded, or DNA–RNA hybrid substrates and are ubiquitous components of cells. A large amount of research has focused on specific ribonucleases that function in a variety of cellular processes including DNA synthesis, RNA processing, cytoplasmic or nuclear RNA degradation, RNAi, and antiviral defense. However, cells also produce a set of general RNases that are typically secreted or targeted to membrane-bound compartments such as the lysosome or vacuole. Such enzymes include members of the RNaseA, RNAseT1 and RNaseT2 families (Table 1). Interestingly, recent work suggests that RNaseA (reviewed in [1]) and RNaseT2 family members often play important roles in a variety of biological settings. In this review, we focus on ribonucleases of the T2 family, a specific subclass of endoribonucleases that cleave single-stranded RNA and exhibit diverse functions important to the biology of prokaryotes, eukaryotes, and human diseases.
TABLE 1.
The RNaseT2 family differs from other families of transferase-type ribonucleases.
| Family | Organismal Distribution | Optimum pH of Activity | Substrate Specificity | References |
|---|---|---|---|---|
| RNaseT2 | Broadly distributed across kingdoms | Acidic (pH4–5) | Little substrate specificity | [2, 3] |
| RNaseA | Primarily found in animals | Weakly acidic (pH6.5–7) or alkaline activity (pH7–8) | Pyrimidine base specificity | [2, 3] |
| RNaseT1 | Found in certain species of fungi and bacteria | Alkaline (pH7–8) | Guanylic-acid specificity | [2, 3] |
Properties of RNaseT2 Family Members
Ribonucleases of the T2 family are transferase type RNases and are classified by their similarity to the RNase T2 from Aspergillus oryzae [2, 3]. The two other major families of transferase type RNases are RNaseA and RNaseT1 protein families. All of these enzymes catalyze the cleavage of single-stranded RNA through a 2′,3′–cyclic phosphate intermediate, producing mono- or oligonucleotides with a terminal 3′-phosphate.
Three features distinguish T2 ribonucleases from the RNaseA and RNaseT1 protein families. First, T2 ribonucleases are more broadly distributed and are found in protozoans, plants, bacteria, animals and viruses [2]. By contrast, RNaseT1 enzymes exist only in bacterial and fungal organisms, and RNaseA family enzymes are highly represented in animals. Second, the optimal pH of activity of many T2 ribonucleases is between 4 and 5, contrasting with the alkaline (pH 7–8) or weakly acidic (pH 6.5–7) activities of enzymes of the RNaseT1 and RNaseA families [2, 3]. This acidic activity of T2 enzymes is consistent with their localization to the vacuole or lysosome and suggests that cleavage of self-RNAs within an acidic compartment could be one function of these ribonucleases. Third, T2 ribonucleases generally cleave at all four bases, whereas RNAseA and RNaseT1 family members tend to be specific for pyrimidines or guanosine, respectively [2, 3].
RNaseT2 family members are typically secreted from the cell, or localized to internal compartments such as the lysosome or vacuole [2, 3]. As these proteins enter the secretory pathway, they are generally glycosylated in eukaryotic cells [2, 3]. However, there are cases wherein RNaseT2 proteins enter the cytoplasm. For example, in budding yeast, the RNaseT2 protein, Rny1, is released from the vacuole to the cytosol during oxidative stress[4]. Moreover, in some cases, secreted T2 ribonucleases can be internalized by other cells in trans; this process is often cytotoxic to the target cell. Furthermore, release of T2 ribonucleases from internal membrane-bound compartments correlates with cleavage of some RNA substrates [4, 5]. Thus, compartmentalization of T2 ribonucleases and their subsequent release into the cytosol might play an important role in modulating their activity.
Structure and Catalysis by T2 ribonucleases
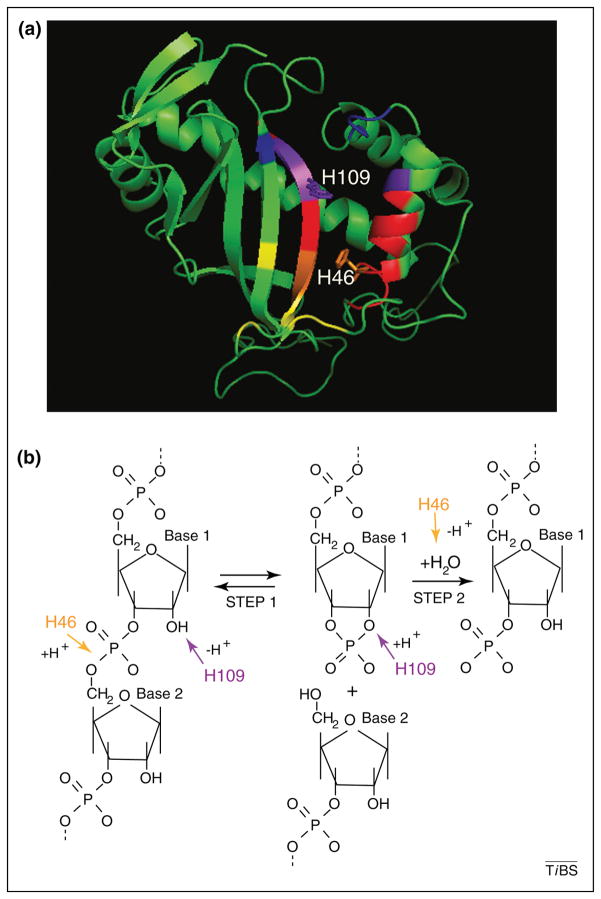
The structure and mechanism of RNA cleavage by T2 ribonucleases is well understood. A variety of crystal structures of T2 family members from bacteria, plants, and fungi have revealed a conserved α/β core structure as observed in the structure from the fungal ribonuclease, Rh (Rhizopus niveus; Figure 1A) [6–19]. Substrate binding regions have been identified by co-crystals of RNaseT2 family members with mono- or dinucleotides; two conserved regions, named B1 and B2 for sites 5′- and 3′- of the scissile bond, respectively, can be occupied by nucleotides and presumably function to position the phosphate bond to be cleaved in the active site of the enzyme [8, 9, 15, 17, 18].
Figure 1. Structure and catalytic mechanism for T2 family ribonucleases.
(a) 3D structure of fungal ribonuclease Rh (Rhizopus niveus) with conserved amino acid sequences (CASI and CASII) in dark red (I) and light red (II) [13]. Nucleotide binding sites (B1 and B2) are indicated by yellow (B1) and blue (B2) colors, and overlap with active site regions is indicated by orange (B1) and purple (B2) (regions mapped according to previous analyses [3, 17]). Histidines involved in acid-base catalysis are labeled [2, 3]. (b) Mechanism for transferase type RNase catalysis. Transphosphorylation and hydrolysis steps are shown. In the first step, one histidine acts as an acid (H46, as numbered for Rh) with the other acting as a base (H109, as numbered for Rh), generating a 2′–3′ cyclic phosphate intermediate. These histidines reverse roles in acid-base catalysis in the second step (hydrolysis) to produce oligo- or mononucleotides with a terminal 3′-phosphate. Composed from mechanism described in Kurihara et al., [13].
Catalysis is promoted by one to three histidine residues that are found in two blocks of conserved amino acids (designated CASI and CASII) located in the active site of the enzyme [20, 21]. In several proteins, altering these histidines through DNA mutation or chemical modification can inactivate the RNase activity both in vitro [22–28] and in vivo [4]. The ability to specifically inactivate the catalytic ability of these proteins provides a genetic mechanism to distinguish if functions of RNaseT2 proteins are dependent on their catalytic ability. Similar to other transferase ribonucleases, RNA cleavage by T2 enzymes occurs in two steps: transphosphorylation and hydrolysis (Figure 1B, [13]). Two histidines are required to perform acid-base catalysis at each of these steps, with these histidines reversing roles as acid and base at each step [3].
C-terminal extensions in certain T2 ribonucleases
Some RNaseT2 family members contain C-terminal extensions downstream of the conserved T2 region. For example, one of the envelope glycoproteins produced by several pestiviruses is a glycoprotein termed Erns, with an RNaseT2 domain and ribonuclease activity, as well as a 37kDa C-terminal extension [29, 30]. This Erns C-terminal extension functions to allow receptor-independent translocation across cellular membranes due to its high positive charge, which is similar to how some other cytotoxic RNases enter cells [31, 32]. Thus, the C-terminal region of pestivirus T2 ribonucleases could be a means of delivering the RNase to immune cells and altering their response to infection.
In another case, a C-terminal extension with regions of conservation exists in three T2 ribonucleases of Basidiomycetes fungi and in Rny1 of budding yeast [33, 34]. In the Basidiomycetes fungi, this region contains a conserved serine-threonine rich region, which resembles that of fungal glycanhydrolases, followed by a 10kDa C-terminus [33]. As fungal glycanhydrolases contain a substrate-binding domain downstream of their serine-threonine rich region, it has been hypothesized that the 10kDa C-terminal region might contain a substrate binding site [33].
Biological roles for T2 ribonucleases
T2 ribonucleases are ubiquitously represented in organisms across kingdoms and exhibit a conserved core structure. However, T2 ribonucleases have been suggested to perform a variety of functions in different organisms, including scavenging of nucleic acids, degradation of self-RNA, modulating host immune responses, and serving as extra- or intracellular cytotoxins. Interestingly, some of these roles appear to be independent of the ability to hydrolyze RNA.
Scavenging phosphates/nucleic acids
Scavenging of extracellular nucleic acids for nutrients is one possible function for T2 ribonucleases (Figure 2A). In plants, several T2 ribonucleases are induced during phosphate starvation and have been hypothesized to function in providing phosphates from nucleic acids [35–37]. Specifically, in tomato cells, Ribonuclease LE is one of several ribonucleases that are induced upon phosphate starvation [37], and the extracellular activity of this T2 ribonuclease is increased during these conditions [38]. Accordingly, a phosphodiesterase with increased synthesis and secretion during phosphate starvation was identified, supporting a model for scavenging of extracellular RNA substrates during phosphate starvation [39]. Moreover, plants can scavenge phosphates from nucleic acids as the inhibition of tomato cell growth due to phosphate starvation can be eliminated by providing yeast RNA in place of inorganic phosphate (KH2PO4) [39]; however, there is no direct evidence that this effect is due to RNase LE. In another example, an RNaseT2 protein secreted by the purine auxotroph Entamoeba histolytica might function in scavenging purines from this parasitic amoeba’s host [40].
Figure 2. Diverse biological roles for T2 family ribonucleases.
Although T2 ribonucleases exhibit a conserved core structure, these enzymes perform diverse functions. (a) Scavenging of extracellular or intracellular RNAs. Extracellular RNA. Plants secrete RNaseT2 proteins (red), such as tomato RNase LE, during phosphate starvation; the T2 proteins might scavenge extracellular RNA and recycle RNA components to the cell [37–39]. Intracellular RNA. During autophagy, protein aggregates are internalized by autophagosomes and taken up by the vacuole/lysosome [41, 43]. Intracellular RNA, as part of ribonucleoprotein complexes (RNPs; blue) such as P-granules [43], might be degraded in this manner by RNaseT2 proteins within the vacuole/lysosome. (b)Plant S-RNases and coordination of self-incompatibility. A model for compartmentalized control of S-RNase activity is depicted. S-RNases are secreted by pistil cells to the extracellular matrix (ECM) wherein they can be internalized to the cytosol of the pollen tube and have been shown to localize to a vacuole-like compartment [5, 59, 60, 75]. This compartment is maintained if pollen is compatible, but disintegration of the compartment occurs late in incompatible pollination, perhaps enabling the degradation of pollen RNAs and blocking pollen tube development [5]. Pistil factors required for decompartmentalization and the pollen protein SLF are not shown for simplicity. (c) Pestiviral envelope glycoprotein, Erns, and cytotoxicity. The Erns protein found in pestiviruses is secreted by its host’s infected cells and internalized by host immune cells in a receptor-independent manner through its positive charge (C-terminus) [31, 51]. There, it depletes host immune cells through both catalytic-dependent and –independent functions [54–56]. (d) The budding yeast RNaseT2 protein, Rny1, and the oxidative stress response. Rny1 localizes to the vacuole in unstressed cells, and oxidative stress causes its release into the cytoplasm where it cleaves tRNAs and rRNAs [4, 67]. Rny1 also possesses a catalytic-independent activity which regulates cellular viability during oxidative stress [4]. ER: endoplasmic reticulum
The presence of RNaseT2 proteins in the lysosome/vacuole seems likely to allow them to also function in the recycling of cytoplasmic RNAs that are delivered to the lysosome/vacuole during autophagy. One possible source of RNA substrates for autophagic degradation within an acidic compartment might be found in RNA granules. Aggregated proteins, for instance, can be removed by a selective form of autophagy involving specific receptors or bridging molecules that recognize ubiquitin and ubiquitin-like modifications on these cytoplasmic proteins [41], and some cytoplasmic aggregates of proteins also contain RNA, suggesting that these also might be targeted by autophagy. One such example is provided by P-granules, aggregates of proteins and RNA found in germline, but not somatic, cells during metazoan development [42]. Specifically, autophagy, selectively mediated by two P-granule components, SEPA-1 (suppressor of ectopic P granule in autophagy mutants-1) and PGL-3 (P-granule abnormality-3), interacting with an autophagy protein, LGG-1, is required for the degradation of certain P-granule components [43]. Hence, it is plausible to propose that mRNA interacting with these proteins might also be targeted by autophagic processes and degraded by T2 ribonucleases within acidic compartments. Taken together, one likely role of RNaseT2 proteins is to degrade either extracellular or intracellular RNAs to obtain nutrients.
A role in neural development: possible clearance of RNAs to modulate the immune response?
Recent results have indicated that the human RNASET2 protein plays an important role in neurodevelopment, which might be related to effects on the immune system. Specifically, mutations in RNASET2 were identified as the causative lesion in an autosomal recessive disorder leading to cystic leukoencephalopathy [44]. Although how loss of RNASET2 causes this phenotype is not yet known, the specific neurological abnormalities are indistinguishable from those seen in infants infected intrauterinally with congenital cytomegalovirus (CMV) [44]. This raises the possibility that CMV infection might trigger a similar change in physiology as the loss of RNASET2. For example, CMV infection inactivates RNase L as a host evasion mechanism [45], and as suggested by Henneke et al. [44]., either RNase L inactivation or loss of RNASET2 could lead to a failure to degrade either extracelluar and/or intracellular single- stranded RNAs (ssRNAs), which then triggers the innate immune response with downstream consequences on development. An important area of research here will be the study of mice lacking RNASET2 to determine this enzyme’s contribution to mammalian immune function and neural development.
An RNaseT2 in schistosome infection: a role in modulating host immune response
A more direct connection of T2 ribonucleolytic activity to the immune response is illustrated by the manner in which a secreted RNaseT2 from the parasite Schistosoma mansoni (commonly referred to as blood flukes) affects host immune cells. During a human infection, the S. mansoni worm lays its eggs in its definitive mammalian host, and these eggs secrete a factor, termed omega-1, which is a known RNaseT2 family member. Omega-1 provokes a host immune response that aids in the egg’s excretion and transmittal to a molluscan intermediate host [46–48]. Recently, two groups showed that omega-1 is the major component in priming dendritic cells for Th2 polarization of CD4+ T cells during infection [49, 50]. This function appears to depend on the RNase activity of omega-1 as inactivation of the ribonuclease activity of soluble egg extracts or omega-1 inhibited their Th2-polarizing function [50]. This finding suggests that omega-1 might be taken up by dendritic cells and then alter their function; this idea is also supported by the observation that omega-1 can alter the cytoskeletal structure of dendritic cells [50].
RNaseT2 family members as cytotoxic agents
In several cases, RNaseT2 family members act as cytotoxic agents, both in trans on other cells or in cis, acting as an intracellular rather than a secreted protein. Interestingly, these cytotoxic roles can be both catalytic-dependent and catalytic-independent.
One example of RNaseT2 proteins inducing cytotoxicity, and modulating immune cell function, comes from analysis of certain pestiviruses, which include classical swine fever virus (CSFV) and bovine viral diarrhoea virus (BVDV) (Figure 2C). In these viruses one of the envelope glycoproteins, termed Erns, has an RNaseT2 domain and ribonuclease activity and is also secreted by infected cells [29, 30, 51]. In cell culture experiments, this enzyme produced cytotoxic effects against the lymphocytes, but not epithelial cells, of various host species [52] suggesting that virally infected cells secrete Erns to dampen the host immune response. Consistent with that possibility, mutations inactivating the RNase activity of Erns do not affect viral replication in cell culture [53–55] but do reduce viral infection in animals [55, 56]. Moreover, certain catalytic amino acid substitutions within the catalytic domain, but not all catalytically-dead versions, of the Erns protein prevent the reduction of host immune cell levels typically seen during CSFV infection [56]. Taken together, these observations argue that Erns functions both in both catalytic and catalytic-independent manners as a trans-acting cytotoxin to dampen the immune response during viral infection.
Another example of RNaseT2 family members as cytotoxic agents comes from the well-characterized S-RNases (Figure 2B). S-RNases are found in plants of the Solanaceae, Rosaceae and Scrophulariaciae families and function to maintain self-incompatibility in pollination [25, 57] and other pollen rejection mechanisms [58]. S-RNases are pistil proteins that are secreted into the extracellular matrix [59, 60] and enter incompatible and compatible pollen tubes [61]. Within incompatibly pollinated styles, pollen RNA is degraded, and self-fertilization is disrupted [62]. By contrast, compatible pollen RNA is protected from degradation by S-RNase [62].
S-RNase’s nuclease activity is required to exert cytotoxic effects on incompatible pollen tube development [25, 57, 63]. However, initial studies of heat-inactivated S-RNase demonstrated greater inhibition than the intact protein against pollen tubes [60], perhaps implicating S-RNases in a catalytic-independent function within other pollen rejection pathways.
The mechanism by which compatible pollen is protected from cytotoxicity is the subject of continuing research. The pollen protein SLF (S-Locus F-box) is required for maintaining self-incompatibility [64, 65], and S-RNase interacts with SLF, a putative ubiquitin ligase, which has been proposed to target the T2 enzyme for proteasomal degradation [66]. However, other pistil factors and S-RNase’s regulated compartmentalization within an acidic compartment might also play a role in controlling S-RNase’s cytotoxicity [5]. Additional research is necessary to ascertain how S-RNase activity is modulated to differentiate compatible and incompatible pollen.
Another T2 ribonuclease which exhibits a catalytic-independent function in cytotoxic responses is Rny1 (Figure 2D). During oxidative stress, Rny1 is released from the vacuole and cleaves cytoplasmic tRNA and rRNA [4, 67]. Rny1 plays some role in modulating cell viability as strains lacking Rny1 are sensitive to some triggers of cell death [34], and over-expression of Rny1 decreases cell growth and leads to hypersensitivity to oxidative stress [4]. Strikingly, the Rny1-dependent effects on cell viability are independent of its catalytic activity as mutations which inactivate the nuclease function affect cell viability similarly to the wild-type protein [4]. Rny1 is also secreted [34] but does not appear to be able to be taken up by other yeast cells, thus suggesting its effects in this case only occur within the cell where it is produced [4]. Hence, increased levels of Rny1 are cytotoxic to the cell during oxidative stress, independent of its catalytic activity.
Human RNASET2: possible roles in tumor progression and growth control
Evidence for a conserved, catalytic-independent function for T2 ribonucleases is provided by studies of RNASET2, the only identified T2 ribonuclease in humans [68, 69]. RNASET2 is implicated as a tumor suppressor as its expression is reduced in various ovarian tumors and cancer cell lines [69]; however some groups have argued this effect is not significant [70]. Moreover, introduction of RNASET2 into cells inhibits clonogenicity of ovarian cancer cells in culture [69] and reduces metastatic potential [71], tumorigenesis [69] and tumor volume [72] in mouse xenograft models. Inactivation of the T2 enzyme through mutation or denaturation failed to block tested anti-carcinogenic effects [71, 72], consistent with a cleavage-independent role for RNASET2 in tumor suppression. To date, mutations within the RNASET2 coding region have not been identified in cancers [69, 70], and Henneke et al. [44] noted that human subjects harboring mutations at RNASET2 locus did not exhibit an increased susceptibility to cancer; however, their young age (less than 20 years) could preclude this phenotype. Thus, although it is clear that RNASET2 can affect cell transformation in a cleavage-independent manner, additional work will be required to determine how and if this activity contributes to tumor formation in mammals.
Possible Cleavage-Independent Functions of RNASET2 and ACTIBIND
An unresolved issue is the mechanism by which RNaseT2 proteins can affect cell function independent of their catalytic ability. One possibility is that these proteins can bind to and modulate the cytoskeleton, which is suggested by the analysis of a T2 ribonuclease from the mold, Aspergillus niger, termed ACTIBIND. ACTIBIND, as well as some other RNaseT2 family members, can bind actin in vitro [72, 73]. Interestingly, ACTIBIND inhibits the clonogenicity and invasiveness of various tumors in culture and in mouse models [73, 74]. Both ACTIBIND and the catalytically inactive form of the ribonuclease reduced aberrant crypt formation (an early marker of colon cancer development) when they were locally administered to mouse colons along with dimethylhydrazine, which promotes colon cancer development [73]. Consistent with this being an effect on the cytoskeleton, ACTIBIND disrupts the fine actin networks at the interior of colon cancer cells [73]. Thus, the interaction of RNaseT2 proteins with the actin cytoskeleton provides one possible mechanism for RNase-independent cytotoxicity. However, until a specific mutation disrupting the T2 ribonuclease-actin interaction can be identified and shown to affect the cytotoxicity, this remains an intriguing hypothesis.
Concluding remarks
RNaseT2 family members function in a surprising diversity of biological roles. Some of their functions rely on their activity as nucleases, and it will be important to determine if there is any specificity in the RNAs cleaved by T2 enzymes in cells. The use of CLIP (cross-linking and immunoprecipitation) in conjunction with high-throughput sequencing might enable the identification of novel substrates of T2 ribonucleolytic cleavage. By contrast, other functions appear to be independent of these enzymes’ nuclease activity, and understanding these nuclease-independent functions could reveal new, specific roles for RNaseT2 family members. The identification and characterization of novel protein–protein interactions might indicate how these enzymes function in a catalytic-independent manner. Moreover, mutational analysis of T2 enzymes might reveal the regions necessary for nuclease-independent functions. Finally, it will be of interest to understand how RNaseT2 proteins enter cells, either from extracellular pools, or from membrane-bound compartments within the cytoplasm; such studies might reveal novel mechanisms by which proteins cross membranes.
Acknowledgments
We thank all members of the Parker lab for their valuable feedback and useful discussions in the preparation of this manuscript, and we especially express our appreciation to former member, Dr. Debrah Thompson, for her input. We express our gratitude to Anne Webb for her aid in composing the figures on biological roles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol. 2008;83:1079–1087. doi: 10.1189/jlb.1107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande RA, Shankar V. Ribonucleases from T2 family. Crit Rev Microbiol. 2002;28:79–122. doi: 10.1080/1040-840291046704. [DOI] [PubMed] [Google Scholar]
- 3.Irie M. Structure-function relationships of acid ribonucleases: lysosomal, vacuolar, and periplasmic enzymes. Pharmacol Ther. 1999;81:77–89. doi: 10.1016/s0163-7258(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DM, Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J Cell Biol. 2009;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldraij A, et al. Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature. 2006;439:805–810. doi: 10.1038/nature04491. [DOI] [PubMed] [Google Scholar]
- 6.de Leeuw M, et al. Binding assay and preliminary X-ray crystallographic analysis of ACTIBIND, a protein with anticarcinogenic and antiangiogenic activities. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:716–719. doi: 10.1107/S1744309107034483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ida K, et al. The 1.55 A resolution structure of Nicotiana alata S(F11)-RNase associated with gametophytic self-incompatibility. J Mol Biol. 2001;314:103–112. doi: 10.1006/jmbi.2001.5127. [DOI] [PubMed] [Google Scholar]
- 8.Kawano S, et al. Guanine binding site of the Nicotiana glutinosa ribonuclease NW revealed by X-ray crystallography. Biochemistry. 2002;41:15195–15202. doi: 10.1021/bi026247l. [DOI] [PubMed] [Google Scholar]
- 9.Kawano S, et al. Crystal structures of the Nicotiana glutinosa ribonuclease NT in complex with nucleoside monophosphates. J Biochem. 2006;140:375–381. doi: 10.1093/jb/mvj164. [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, et al. Amino acids conserved at the C-terminal half of the ribonuclease T2 family contribute to protein stability of the enzymes. Biosci Biotechnol Biochem. 2004;68:1748–1757. doi: 10.1271/bbb.68.1748. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, et al. Primary structure of a base non-specific and adenylic acid preferential ribonuclease from the fruit bodies of Lentinus edodes. Biosci Biotechnol Biochem. 1992;56:2003–2010. doi: 10.1271/bbb.56.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara H, et al. Crystal and molecular structure of RNase Rh, a new class of microbial ribonuclease from Rhizopus niveus. FEBS Lett. 1992;306:189–192. doi: 10.1016/0014-5793(92)80997-u. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara H, et al. The crystal structure of ribonuclease Rh from Rhizopus niveus at 2.0 A resolution. J Mol Biol. 1996;255:310–320. doi: 10.1006/jmbi.1996.0025. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura T, et al. Crystal structure at 1.5-A resolution of Pyrus pyrifolia pistil ribonuclease responsible for gametophytic self-incompatibility. J Biol Chem. 2001;276:45261–45269. doi: 10.1074/jbc.M107617200. [DOI] [PubMed] [Google Scholar]
- 15.Numata T, et al. Crystal structures of the ribonuclease MC1 mutants N71T and N71S in complex with 5′-GMP: structural basis for alterations in substrate specificity. Biochemistry. 2003;42:5270–5278. doi: 10.1021/bi034103g. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan S, et al. Overexpression, biophysical characterization, and crystallization of ribonuclease I from Escherichia coli, a broad-specificity enzyme in the RNase T2 family. Arch Biochem Biophys. 2001;390:42–50. doi: 10.1006/abbi.2001.2359. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez SM, et al. Nonspecific base recognition mediated by water bridges and hydrophobic stacking in ribonuclease I from Escherichia coli. Protein Sci. 2008;17:681–690. doi: 10.1110/ps.073420708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki A, et al. Crystal structures of the ribonuclease MC1 from bitter gourd seeds, complexed with 2′-UMP or 3′-UMP, reveal structural basis for uridine specificity. Biochem Biophys Res Commun. 2000;275:572–576. doi: 10.1006/bbrc.2000.3318. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka N, et al. Crystal structure of a plant ribonuclease, RNase LE. J Mol Biol. 2000;298:859–873. doi: 10.1006/jmbi.2000.3707. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa S, et al. Two histidine residues are essential for ribonuclease T1 activity as is the case for ribonuclease A. Biochemistry. 1987;26:8620–8624. doi: 10.1021/bi00400a019. [DOI] [PubMed] [Google Scholar]
- 21.Roberts GC, et al. The mechanism of action of ribonuclease. Proc Natl Acad Sci U S A. 1969;62:1151–1158. doi: 10.1073/pnas.62.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irie M, et al. Site of alkylation of the major ribonuclease from Aspergillus saitoi with iodoacetate. J Biochem. 1986;99:627–633. doi: 10.1093/oxfordjournals.jbchem.a135521. [DOI] [PubMed] [Google Scholar]
- 23.Kawata Y, et al. Identification of two essential histidine residues of ribonuclease T2 from Aspergillus oryzae. Eur J Biochem. 1990;187:255–262. doi: 10.1111/j.1432-1033.1990.tb15303.x. [DOI] [PubMed] [Google Scholar]
- 24.Parry S, et al. Identification of active-site histidine residues of a self-incompatibility ribonuclease from a wild tomato. Plant Physiol. 1997;115:1421–1429. doi: 10.1104/pp.115.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, et al. Ribonuclease activity of Petunia inflata S proteins is essential for rejection of self-pollen. Plant Cell. 1994;6:1021–1028. doi: 10.1105/tpc.6.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohgi K, et al. Evidence that three histidine residues of a base non-specific and adenylic acid preferential ribonuclease from Rhizopus niveus are involved in the catalytic function. J Biochem. 1992;112:132–138. doi: 10.1093/oxfordjournals.jbchem.a123852. [DOI] [PubMed] [Google Scholar]
- 27.Numata T, et al. Expression and mutational analysis of amino acid residues involved in catalytic activity in a ribonuclease MC1 from the seeds of bitter gourd. Biosci Biotechnol Biochem. 2000;64:603–605. doi: 10.1271/bbb.64.603. [DOI] [PubMed] [Google Scholar]
- 28.Royo J, et al. Loss of a histidine residue at the active site of S-locus ribonuclease is associated with self-compatibility in Lycopersicon peruvianum. Proc Natl Acad Sci U S A. 1994;91:6511–6514. doi: 10.1073/pnas.91.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulst MM, et al. Glycoprotein E2 of classical swine fever virus: expression in insect cells and identification as a ribonuclease. Virology. 1994;200:558–565. doi: 10.1006/viro.1994.1218. [DOI] [PubMed] [Google Scholar]
- 30.Schneider R, et al. Identification of a structural glycoprotein of an RNA virus as a ribonuclease. Science. 1993;261:1169–1171. doi: 10.1126/science.8356450. [DOI] [PubMed] [Google Scholar]
- 31.Langedijk JP. Translocation activity of C-terminal domain of pestivirus Erns and ribotoxin L3 loop. J Biol Chem. 2002;277:5308–5314. doi: 10.1074/jbc.M104147200. [DOI] [PubMed] [Google Scholar]
- 32.Makarov AA, Ilinskaya ON. Cytotoxic ribonucleases: molecular weapons and their targets. FEBS Lett. 2003;540:15–20. doi: 10.1016/s0014-5793(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, et al. A new type of RNase T2 ribonuclease in two Basidiomycetes fungi, Lentinus edodes and Irpex lacteus. Biosci Biotechnol Biochem. 2003;67:2307–2310. doi: 10.1271/bbb.67.2307. [DOI] [PubMed] [Google Scholar]
- 34.MacIntosh GC, et al. Characterization of Rny1, the Saccharomyces cerevisiae member of the T2 RNase family of RNases: unexpected functions for ancient enzymes? Proc Natl Acad Sci U S A. 2001;98:1018–1023. doi: 10.1073/pnas.98.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bariola PA, et al. The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J. 1994;6:673–685. doi: 10.1046/j.1365-313x.1994.6050673.x. [DOI] [PubMed] [Google Scholar]
- 36.Taylor CB, et al. RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc Natl Acad Sci U S A. 1993;90:5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loffler A, et al. Phosphate-Regulated Induction of Intracellular Ribonucleases in Cultured Tomato (Lycopersicon esculentum) Cells. Plant Physiol. 1992;98:1472–1478. doi: 10.1104/pp.98.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurnberger T, et al. Induction of an Extracellular Ribonuclease in Cultured Tomato Cells upon Phosphate Starvation. Plant Physiol. 1990;92:970–976. doi: 10.1104/pp.92.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abel S, et al. Induction of an extracellular cyclic nucleotide phosphodiesterase as an accessory ribonucleolytic activity during phosphate starvation of cultured tomato cells. Plant Physiol. 2000;122:543–552. doi: 10.1104/pp.122.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGugan GC, Jr, et al. Identification and biochemical characterization of unique secretory nucleases of the human enteric pathogen, Entamoeba histolytica. J Biol Chem. 2007;282:31789–31802. doi: 10.1074/jbc.M705975200. [DOI] [PubMed] [Google Scholar]
- 41.Kirkin V, et al. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Henneke M, et al. RNASET2-deficient cystic leukoencephalopathy resembles congenital cytomegalovirus brain infection. Nat Genet. 2009;41:773–775. doi: 10.1038/ng.398. [DOI] [PubMed] [Google Scholar]
- 45.Child SJ, et al. Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus. J Virol. 2002;76:4912–4918. doi: 10.1128/JVI.76.10.4912-4918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doenhoff MJ, et al. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75:41–53. doi: 10.1016/0035-9203(81)90012-2. [DOI] [PubMed] [Google Scholar]
- 47.Murare HM, et al. Schistosoma mansoni: control of hepatotoxicity and egg excretion by immune serum in infected immunosuppressed mice is schistosome species-specific, but not S. mansoni strain-specific. Exp Parasitol. 1992;75:329–339. doi: 10.1016/0014-4894(92)90218-y. [DOI] [PubMed] [Google Scholar]
- 48.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 49.Everts B, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinfelder S, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rumenapf T, et al. Processing of the envelope glycoproteins of pestiviruses. J Virol. 1993;67:3288–3294. doi: 10.1128/jvi.67.6.3288-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruschke CJ, et al. Glycoprotein Erns of pestiviruses induces apoptosis in lymphocytes of several species. J Virol. 1997;71:6692–6696. doi: 10.1128/jvi.71.9.6692-6696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hulst MM, et al. Inactivation of the RNase activity of glycoprotein E(rns) of classical swine fever virus results in a cytopathogenic virus. J Virol. 1998;72:151–157. doi: 10.1128/jvi.72.1.151-157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer C, et al. Recovery of virulent and RNase-negative attenuated type 2 bovine viral diarrhea viruses from infectious cDNA clones. J Virol. 2002;76:8494–8503. doi: 10.1128/JVI.76.16.8494-8503.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyers G, et al. Mutations abrogating the RNase activity in glycoprotein E(rns) of the pestivirus classical swine fever virus lead to virus attenuation. J Virol. 1999;73:10224–10235. doi: 10.1128/jvi.73.12.10224-10235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Freyburg M, et al. Comparison of the effects of RNase-negative and wild-type classical swine fever virus on peripheral blood cells of infected pigs. J Gen Virol. 2004;85:1899–1908. doi: 10.1099/vir.0.79988-0. [DOI] [PubMed] [Google Scholar]
- 57.McCubbin AG, et al. A Mutant S3 RNase of Petunia inflata Lacking RNase Activity Has an Allele-Specific Dominant Negative Effect on Self-Incompatibility Interactions. Plant Cell. 1997;9:85–95. doi: 10.1105/tpc.9.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murfett J, et al. S RNase and interspecific pollen rejection in the genus Nicotiana: Multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell. 1996;8:943–958. doi: 10.1105/tpc.8.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson MA, et al. Cloning of Cdna for a Stylar Glycoprotein Associated with Expression of Self-Incompatibility in Nicotiana-Alata. Nature. 1986;321:38–44. [Google Scholar]
- 60.Jahnen W, et al. Identification, isolation, and N-terminal sequencing of style glycoproteins associated with self-incompatibility in Nicotiana alata. Plant Cell. 1989;1:493–499. doi: 10.1105/tpc.1.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luu DT, et al. S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature. 2000;407:649–651. doi: 10.1038/35036623. [DOI] [PubMed] [Google Scholar]
- 62.McClure BA. Self-incompatibility in Nicotiana alata involves degradation of pollen rRNA. Nature. 1990;347:757–760. [Google Scholar]
- 63.McClure BA, Franklin-Tong V. Gametophytic self-incompatibility: understanding the cellular mechanisms involved in “self” pollen tube inhibition. Planta. 2006;224:233–245. doi: 10.1007/s00425-006-0284-2. [DOI] [PubMed] [Google Scholar]
- 64.Sijacic P, et al. Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature. 2004;429:302–305. doi: 10.1038/nature02523. [DOI] [PubMed] [Google Scholar]
- 65.Ushijima K, et al. The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and P. mume. Plant J. 2004;39:573–586. doi: 10.1111/j.1365-313X.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- 66.Qiao H, et al. The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell. 2004;16:582–595. doi: 10.1105/tpc.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson DM, et al. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campomenosi P, et al. Characterization of RNASET2, the first human member of the Rh/T2/S family of glycoproteins. Arch Biochem Biophys. 2006;449:17–26. doi: 10.1016/j.abb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 69.Acquati F, et al. Cloning and characterization of a senescence inducing and class II tumor suppressor gene in ovarian carcinoma at chromosome region 6q27. Oncogene. 2001;20:980–988. doi: 10.1038/sj.onc.1204178. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, et al. Physical and transcript map of the region between D6S264 and D6S149 on chromosome 6q27, the minimal region of allele loss in sporadic epithelial ovarian cancer. Oncogene. 2002;21:387–399. doi: 10.1038/sj.onc.1205067. [DOI] [PubMed] [Google Scholar]
- 71.Acquati F, et al. Tumor and metastasis suppression by the human RNASET2 gene. Int J Oncol. 2005;26:1159–1168. [PubMed] [Google Scholar]
- 72.Smirnoff P, et al. A recombinant human RNASET2 glycoprotein with antitumorigenic and antiangiogenic characteristics: expression, purification, and characterization. Cancer. 2006;107:2760–2769. doi: 10.1002/cncr.22327. [DOI] [PubMed] [Google Scholar]
- 73.Roiz L, et al. ACTIBIND, an actin-binding fungal T2-RNase with antiangiogenic and anticarcinogenic characteristics. Cancer. 2006;106:2295–2308. doi: 10.1002/cncr.21878. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz B, et al. ACTIBIND, a T2 RNase, competes with angiogenin and inhibits human melanoma growth, angiogenesis, and metastasis. Cancer Res. 2007;67:5258–5266. doi: 10.1158/0008-5472.CAN-07-0129. [DOI] [PubMed] [Google Scholar]
- 75.Gray JE, et al. Action of the Style Product of the Self-Incompatibility Gene of Nicotiana alata (S-RNase) on in Vitro-Grown Pollen Tubes. Plant Cell. 1991;3:271–283. doi: 10.1105/tpc.3.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]