Summary
Sirolimus has been shown to have activity against human acute lymphoblastic leukaemia at serum levels used for immunosuppression. We hypothesized that the addition of sirolimus to a tacrolimus/methotrexate graft-versus-host disease (GVHD) prophylaxis regimen would decrease relapse after haematopoietic stem cell transplantation and initiated a phase I/II study to demonstrate safety, feasibility, and efficacy. The study cohort included 18 patients in high-risk (HR) first complete remission (CR1), 16 in HR CR2, 17 in intermediate risk (IR) CR2, and 12 in CR3+. The 2-year event-free survival (EFS) of the cohort was 66% (standard error 6·4). EFS of risk groups was 74%, 81%, 44% and 46% for CR1, IR CR2, HR CR2 and CR3+ patients respectively, and did not differ by stem cell source. Cumulative incidence of acute GVHD grade II–IV and III–IV was 38% and 21% respectively, while the cumulative incidence of chronic GVHD was 32%. Cumulative incidence of transplant-related mortality and relapse was 10% and 25% respectively. Significant toxicities included veno-occlusive disease [seven patients (11%)], transplant-associated microangiopathy (three patients), and idiopathic pneumonitis (one patient). In summary, sirolimus-based GVHD prophylaxis can be given safely in this population and early survival results are promising. A phase III trial to test whether sirolimus decreases relapse and improves outcome after transplantation for ALL is ongoing.
Keywords: acute lymphocytic leukaemia, paediatric allogeneic bone marrow transplantation, sirolimus, graft versus host disease prophylaxis, haematopoietic stem cell transplantation
Relapse remains the major obstacle to cure for children who undergo allogeneic hematopoietic stem cell transplant (HSCT) for acute lymphoblastic leukaemia (ALL). While transplant-related mortality (TRM) has decreased over the past decade (Pasquini et al, 2007), relapse rates have remained high (25–25%), and can exceed 50% in high risk disease (Bunin et al, 2002; Eapen et al, 2006). Achieving a low minimal residual disease (MRD) status prior to transplant and using total body irradiation (TBI)-based preparative regimens may decrease relapse to a degree (Bader et al, 2009), but new interventions that decrease relapse after transplant for ALL are needed.
Sirolimus is a naturally-occurring, potent immunosuppressant that has been used extensively in solid organ transplantation. The compound has structural similarity to tacrolimus, binds to FK-binding protein 12 as tacrolimus does, and is thus synergistic with tacrolimus, allowing lower doses of both medications to be used to achieve target levels of immune suppression (Cutler & Antin, 2004). Sirolimus is an attractive candidate to reduce relapse because it is a potent mammalian target of rapamycin (mTOR) pathway inhibitor and thus has anti-tumour properties documented in a number of cancer types (Rizell et al, 2008; Sillaber et al, 2008).
Both pre-clinical xenograft models and human clinical studies have shown promising results in lymphoid malignancies using this agent. Pronounced decreases in growth of human ALL-blasts treated with sirolimus in NOD-SCID mouse models have been reported (Brown et al, 2003; Teachey et al, 2006). Further studies performed using a combination of mTOR inhibitor plus methotrexate led to complete disappearance of ALL blasts in this model (Brown et al, 2003; Teachey et al, 2006, 2008). Armand et al (2008) described a clear improvement in the survival of adults with Non-Hodgkin lymphoma undergoing reduced intensity regimens when sirolimus was used as graft-versus-host disease (GVHD) prophylaxis compared to regimens not containing sirolimus.
Investigators from the Dana Farber Cancer Institute studied the use of sirolimus and tacrolimus with and without methotrexate for GVHD prophylaxis in adults undergoing hematopoietic cell transplantation (HCT) with goal of decreasing rates of GVHD (Antin et al, 2003; Cutler & Antin, 2004; Alyea et al, 2008). These early trials in adults showed lower rates of acute GVHD, but similar rates of chronic GVHD. This clinical experience using sirolimus to decrease GVHD, combined with the pre-clinical mouse model data described above, led us to hypothesize that the use of sirolimus after HCT for children with ALL would treat lymphoid blasts in a minimal residual disease state and lead to lower rates of relapse. We initiated a multi-institutional phase I/II trial to demonstrate safety and efficacy of the approach in children using different stem cell sources, especially unrelated cord blood (UCB), used frequently in paediatric transplantation. Early results from this trial have led to a phase III trial that is currently underway through the Children’s Oncology Group (COG).
Materials and methods
Patients
Between August 2003 and August 2008 a total of 63 consecutive patients at four paediatric centres in the United States, Children’s Hospital of Philadelphia, Methodist Children’s Hospital of South Texas (San Antonio), Primary Children’s Medical Center (University of Utah School of Medicine, Salt Lake City), and Children’s Hospital of Pittsburgh, were enrolled on a prospective trial aimed at assessing the toxicity and efficacy of the administration of post-transplant sirolimus to recipients of allogeneic bone marrow (BM), peripheral blood stem cells (PBSC), and UCB transplantation for very high risk paediatric ALL. The trial was approved by the institutional review boards at each institution and was monitored centrally by the Children’s Hospital of Philadelphia Data Safety Monitoring Committee. Informed consent was obtained from the guardians and assent or consent from patients, if applicable, in accordance with the Declaration of Helsinki.
Patients were required to have ALL in first (CR1), second (CR2), or third (CR3) morphological remission prior to transplant. First remission patients had to either have primary induction failure (PIF, failure to achieve M1 marrow status after induction), extended MRD+ after consolidation by flow cytometry (COG poor risk) (Borowitz et al, 2008), secondary ALL, or very high risk cytogenetics {t(9;22) or t(4;11) with slow early response [M2 or M3 at day +14 after induction, expected 5-year event-free survival (EFS) of 10% with chemotherapy approaches (Schultz et al, 2007)]}. Median time from initial diagnosis to transplant for patients in CR1 was 151 d (range 90–272 d).
Patients were required to have cardiac fractional shortening ≥27%, creatinine clearance ≥60 ml/(min 1·73 m2), bilirubin <25·6 µmol/l, transaminases <3 × normal and no active infection at the time of transplant.
Treatment protocol
Related and unrelated BM or PBSC donors were allowed to have no more than a single antigen/allele mismatch at HLA A, B, C, or DRB1. BM alone was allowed for related donors (RD) and preferred for unrelated donors (URD), but PBSC from URD was allowed. Cord blood units had to be at least a 4/6 match at HLA A, B and DRB1 with high resolution typing of the DRB1 allele. Minimum pre-thaw cord blood cell dose was 3 × 107 total nucleated cell (TNC)/kg recipient body weight. Multiple UCB infusions were not allowed.
The preparative regimen consisted of fractionated TBI 200 cGy bid × 3 d (total dose 1200 cGy), thiotepa 5 mg/(kg d) for 2 d (total dose 10 mg/kg), followed by cyclophosphamide 60 mg/(kg d) for 2 d (total dose 120 mg/kg). GVHD prophylaxis consisted of tacrolimus starting on day −2, given as a continuous infusion (starting dose 0·03 mg/(kg d), target level 5–10 ng/ml) and methotrexate given IV at a dose of 5 mg/m2 on days +1, +3 and +6 after transplant for all patients, with an additional dose on day +11 for those receiving unrelated donor BM or PBSC. There were three different time-points at which sirolimus administration was initiated during the course of the trial (see Treatment Cohorts in the Results section). The first three patients started sirolimus after engraftment. Once feasibility of oral administration was established after engraftment, the initiation of sirolimus therapy was moved to day −3 with a loading dose as described by Antin et al (2003). Toxicity was noted after four patients using this approach, and the protocol was amended to start sirolimus at day 0 with no loading dose. Fifty-six patients were enrolled starting sirolimus on day 0. Sirolimus was given orally with a starting dose of 2·5 mg/m2/d (maximum dose 4 mg), with target trough levels of 3–12 ng/mg. The loading dose given for the four patients starting on day −3 was 7 mg/m2 (12 mg max). In the absence of GVHD, tacrolimus was tapered between day +42 and 96 for matched sibling donor recipients and between day +100 and 180 for mismatched related donor, unrelated donor and unrelated cord blood recipients. Sirolimus was tapered over 4 weeks starting 6 months after transplant.
Supportive care measures, such as use of growth factors or infection prophylaxis, were according to institutional practise.
Statistical methods
Neutrophil engraftment was defined as an achievement of an absolute neutrophil count of ≥0·5 × 109/l sustained for three consecutive laboratory measurements on different days. Platelet engraftment was defined as an achievement of a platelet count recovery of ≥20 × 109/l sustained for three consecutive laboratory measurements on different days with no platelet transfusions in the previous 7 d. A severity grade for acute GVHD was calculated according to the reported stages of skin, liver and intestinal involvement using the Glucksberg grading system (Glucksberg et al, 1974). Treatment-related mortality was defined as death in continuous complete remission. Death from any cause was considered an event for overall survival (OS). Events for EFS included rejection (donor chimerism <5%), relapse, or death in remission.
Univariate probabilities of OS were calculated using the Kaplan–Meier estimator; the log-rank test was used for univariate comparisons of survival (Kaplan & Meier, 1958). Probabilities of acute and chronic GVHD, relapse, and transplant-related mortality were calculated using the cumulative incidence function estimator with a subsequent transplant as a censoring event (Gooley et al, 1999; Klein & Moeschberger, 2003). For neutrophil and platelet engraftment, and acute and chronic GVHD, death without an event was a competing risk. For TRM, relapse was the competing risk; for relapse, TRM was the competing risk. The Statistical Package for the Social Sciences (spss) software, version 14.0, was used for the Kaplan–Meier analysis and r version 2.8.0 (©2008 The R Foundation for Statistical Computing, Vienna, Austria) was used for the competing risks analysis of engraftment, relapse, transplant-related mortality, and chronic GVHD.
Stopping rules were in place for excessive day +100 mortality (>25%) or severe acute GVHD (grade III–IV >30%). Veno-occlusive (VOD) disease was diagnosed by Seattle Criteria and graded for severity as outlined by McDonald et al (1993). Transplant-associated microangiopathy (TAM, also called transplant associated haemolytic uremic syndrome) was defined as outlined by the Blood and Marrow Transplant Clinical Trials Network consensus group (Ho et al, 2005). After demonstrating the safety and feasibility of administering oral sirolimus at day 0 in an initial cohort of 20 patients, enrollment was extended to obtain phase II efficacy data in ALL risk group subsets.
Results
Patient/donor characteristics
Median patient age was 9 (range 1–22) years (Table I). Just over half of the analysis cohort were patients in CR2, split between Berlin–Frankfürt–Münster (BFM) ‘high risk’ early relapsed patients (relapse <36 months from diagnosis) and BFM ‘intermediate risk relapse,’ who either relapsed late in the BM or very early (relapse <18 m from diagnosis) isolated to extramedullary areas (IEM), mostly in the central nervous system (Borgmann et al, 2003). Eighteen patients (29%) were in CR1 and 12 patients (19%) were in CR3. Most of the patients had pre-B cell disease (81%). Notably, T-cell BM relapses were all BFM high risk, with most occurring <18 m from diagnosis.
Table I.
Patient/disease characteristics and stem cell sources.
| n | 63 |
| Median age, years (range) | 9 (1–22) |
| Gender: female/male | 25/38 |
| Diagnoses | Number of recipients |
| Immunophenotypes | |
| Pre-B cell (1 patient infant MLL) | 51 |
| T-cell | 12 |
| High risk CR1 | |
| Primary induction failure (PIF) | 10 [3 also t(9;11)] |
| t(9;11) | 7 (3 also PIF) |
| Other [secondary ALL 1, | 4 |
| patient; t(4;11) plus M2 at day 14, | |
| 1 patient, high MRD+ after | |
| consolidation 2 patients] | |
| High risk CR2 | |
| BM relapse <18 months (5 T-cell, 1 MLL) | 8 |
| BM relapse 18–36 months (3 T-cell) | 8 |
| Intermediate risk CR2 | |
| BM relapse ≥36 months (all B-cell) | 12 |
| Isolated-CNS relapse <18 | 5 |
| months (2 T-cell, 3 B-cell) | |
| All patients beyond CR2 | |
| CR3 (11 patients), CR4 (1 patient) | 12 |
| Stem cell source/HLA matching | |
| Related donor-bone marrow | |
| Fully matched sibling | 26 |
| 5/6 HLA matched sibling | 1 |
| 6/6 HLA matched parent | 1 |
| Unrelated donor-bone marrow/PBSC | |
| 10/10 HLA match | 3 |
| 9/10 HLA match | 2 |
| Unrelated donor-cord blood | |
| 6/6 HLA match | 1 |
| 5/6 HLA match | 19 |
| 4/6 HLA match | 9 |
| 3/6 HLA match | 1 |
A significant percentage of patients enrolled received unrelated cord blood (UCB) grafts (n = 30, 48%), with a small percentage receiving either unrelated BM or PBSC (n = 5, 8%), and the remainder related donor BM (n = 28, 44%). The UCB units given were predominantly 5/6 HLA antigen matched (63%, HLA A, B and DRB1), while 30% of UCB units were 4/6, 3% were 3/6 and 3% were 6/6 HLA antigen matched. One of the related donors was a single antigen mismatched sibling and a second was a 6/6 phenotypic matched relative (mother to daughter). Of the unrelated BM/PBSC donors, by high-resolution typing at HLA A, B, C, DRB1 and DQB1 loci, two had single allele mismatches, while three were fully matched. Actual infused UCB doses varied from 2·3–15·2 × 107 TNC/kg recipient weight (median 4·6 × 107 TNC/kg). BM/PBSC doses varied from 0·8–7·6 × 108 TNC/kg recipient weight (median 3·3 × 108 TNC/kg).
Treatment cohorts/early toxicity
The first three patients enrolled started sirolimus therapy just after engraftment on days +21, +22 and +24. After these three patients were noted to do well, the initiation date of sirolimus was moved to day −3 with a loading dose, similar to the approach published by the Dana Farber Group (Antin et al, 2003). Of the four patients receiving sirolimus starting on day 3 before transplant (during conditioning with cyclophosphamide), one patient in CR4, receiving a 3/6 HLA matched UCB, and a second patient with very early relapse in CR2, receiving a 5/6 HLA matched UCB, experienced overwhelming early toxicity [multisystem organ failure (MSOF), infection and subsequent death]. Based upon descriptions of synergistic effects of cyclophosphamide with mTOR inhibitors (Cejka et al, 2008) along with well-described transplant toxicities associated with cyclophosphamide (McDonald et al, 2003), the protocol was amended to avoid overlap with cyclophosphamide by starting sirolimus at day 0 with no loading dose. Early, significant toxicity did not occur with subsequent patients, and another 56 patients accrued to the study.
Causes of mortality and sirolimus-related toxicities
Table II reviews significant toxicities noted during the trial, describing all non-relapse deaths along with an attribution of possible involvement of sirolimus. In addition, Table II includes all patients with significant toxicities (grade 3 or higher) with an attribution of possibly, probably, or definitely linked to sirolimus administration. All patients were able to successfully start sirolimus, and only the patients listed in Table II stopped the medication. Three further patients had sirolimus held temporarily, one for 1 week for high levels and temporary renal dysfunction, a second for low counts and pneumonia, and the third for delayed engraftment. These three patients successfully went back on sirolimus and completed their planned 6-month course and taper. Finally, 10 of 35 (29%) of URD or UCB recipients were scored as having engraftment syndrome (fever, skin rash, fluid retention ± hypoxia/pulmonary oedema) and treated with methyl-prednisilone. Of note, 8 of 10 with engraftment syndrome were eventually scored as acute GVHD because of other evidence of aGVHD or flare of GVHD symptoms during their steroid taper.
Table II.
Transplant related mortality and other significant toxicities.
| Age, years (gender) |
ALL risk group |
Stem cell source |
Description and timing of events | Contribution of sirolimus | Outcome |
|---|---|---|---|---|---|
| 9(M) | IRCR2 | 6/6 MSD | Pneumonia, cGVHD | Not related | Died day +789 |
| 11(M) | HRCR2 | 5/6 UCB | Started sirolimus day −3, sepsis, aspergillosis, severe VOD day +14, TAM, MSOF |
Possibly related | Died day +50 |
| 7(M) | CR4 | 3/6 UCB | Started sirolimus day −3, gram− sepsis – sirolimus stopped day +4, resp failure day +9, second gram− sepsis day +20, severe VOD day +25, MSOF, ARDS |
Possibly related | Died day +29 |
| 15(M) | CR1 | 4/6 UCB | Graft failure, second transplant CB day +59. Developed cGVHD |
Possibly related to graft failure, not related to cGVHD death |
Died day +365 of pneumonia |
| 12(F) | CR3 | 5/6 UCB | Late autoimmune haemolytic anaemia/cGVHD | Not related, not on sirolimus | Died day +365 |
| 18(M) | CR3 | 4/6 UCB | Respiratory failure day +22 with influenza, BK cystitis, adenovirus and rotavirus. Developed severe VOD, Ascites, MSOF, and pulmonary haemorrhage |
Possibly related | Died day +33 |
| 16(M) | CR3 | 5/6 UCB | Early reverible TAM without significant renal impairment, cGVHD |
Possibly related to TAM, resolved fully after stopping sirolimus. Not related to cGVHD/death |
Died day +431 |
| 1(F) | HRCR2 | 6/6 UCB | Pseudomonas sepsis, severe VOD, MSOF, pulmonary haemorrhage |
Possibly related | Died day +39 |
| 20(F) | HRCR2 | 10/10 URD | Moderate VOD (max bilirubin 42·75 µmol/l) | Possibly related, resolved completely, sirolimus not held |
Died of relapse day +239 |
| 9(M) | HRCR2 | 6/6 MSD | Moderate VOD (max bilirubin 44·46 µmol/l) | Possibly related, resolved completely after stopping sirolimus |
Died of relapse day +100 |
| 6(M) | IRCR2 | 6/6 MSD | Moderate VOD (max bili 25·65 µmol/l) | Possibly related, resolved completely after stopping sirolimus |
Alive and well, f/u day +732 |
| 18(F) | IRCR2 | 6/6 MSD | TAM, BK+ cystitis, renal failure (not requiring dialysis) |
Probably related, resolved completely after stopping sirolimus |
Alive and well, f/u day +1020 |
| 10(F) | CR3 | 5/6 UCB | Interstitial pneumonitis, BK cystitis | Possibly related, resolved completely, sirolimus not held |
Alive and well, f/u day +1315 |
| 10(M) | CR1 | 9/10 URD | CTC grade 4 pleural and grade 3 pericardial effusions, grade 3 muscle weakness and neuropathy |
Probably related (effusions), possibly related (weakness and neuropathy), rapid resolution of effusions after stopping sirolimus |
Alive and well, f/u day +998 |
| 4(M) | HRCR2 | 5/6 UCB | Late grade 4 thrombocytopenia, leucopenia and grade 3 anaemia |
Probably related, resolved completely after stopping sirolimus |
Alive and well, f/u day +851 |
IR, intermediate risk; HR, high risk; CR, complete remission; MSD, matched sibling donor; UCB, unrelated cord blood; URD, unrelated donor; cGVHD, chronic graft-versus-host disease; VOD, veno-occlusive disease; TAM, transplant-associated microangiopathy; MSOF, multi-system organ failure; ARDS, acute respiratory distress syndrome; CTC, common toxicity criteria; f/u, follow-up.
Toxicities listed in Table II include seven cases of VOD (11%), four noted to be severe and three moderate. Two of the cases of VOD occurred in the patients described in the section on early toxicity noted when sirolimus was started on day −3. The VOD in the third patient on the table occurred after two gram-negative bacterial infections and an extended intensive care course. In that patient, sirolimus was only given for 1 week and stopped 3 weeks prior to the onset of VOD symptoms. Of patients starting sirolimus on or after day 0, the VOD rate was 8% (5/59). TAM occurred in three patients (5%) and completely resolved in two. In the third patient, TAM occurred in the context of gram-negative sepsis, MSOF, and the patient expired. A final notable toxicity was pleural and pericardial effusions, which resolved within days of stopping sirolimus.
Engraftment and graft versus host disease
The median time to neutrophil engraftment for recipients of BM and PBSC compared to UCB was 20 (range 13–31) vs. 24 d (range 11–118) respectively, with a cumulative incidence of engraftment for RD and URD BM/PBSC recipients of 100% [95% confidence interval (CI) 94–100%] and 92% (95% CI 78–100%) for UCB recipients. The median time to platelet engraftment for recipients of BM and PBSC compared to UCB was 27 (range 11–118) vs. 87 d (range 28–217) respectively, with a cumulative incidence of platelet engraftment for RD and URD BM/PBSC recipients of 100% (95% CI 94–100%) and 80% (95% CI 63–96%) for UCB recipients.
The cumulative incidence of grade II–IV and III–IV acute GVHD at 180 d was 38% (95% CI 26–50%) and 21% (95% CI 11–31%) respectively. The cumulative incidence of chronic GHVD at 2 years was 32% (95% CI 20–43%), while the cumulative incidence of chronic extensive GVHD at 2 years was 23% (95% CI 12–34%).
Transplant related mortality and relapse
The cumulative incidence of TRM at 100 d, 1 and 2 years was 6·3%, 6·3% and 9·9% (95% CI 2·3–17·5%) respectively. TRM occurred in only one related donor recipient, from chronic GVHD, just over 2 years post-transplant; the remainder of the TRM (seven patients, 11%) occurred in recipients of UCB with four early deaths (≤day 50) from infection and three late deaths from chronic GVHD (Table II). TRM occurred in six patients with high-risk disease (HR CR2, CR3, CR4) and two patients with intermediate risk disease (CR1, IR CR2).
The main cause of treatment failure was relapse, which occurred in 17 patients (27%), with a cumulative incidence at 2 years of 25·5% (95% CI 14–37). Most relapses occurred within the first year of transplant (67%), with no relapses occurring after 2·3 years. Although the cumulative incidence of relapse was higher in early relapse CR2 and CR3 patients compared to CR1 and intermediate risk CR2 patients (32% vs. 19%), this difference was not statistically significant (P = 0·15).
Event free and overall survival
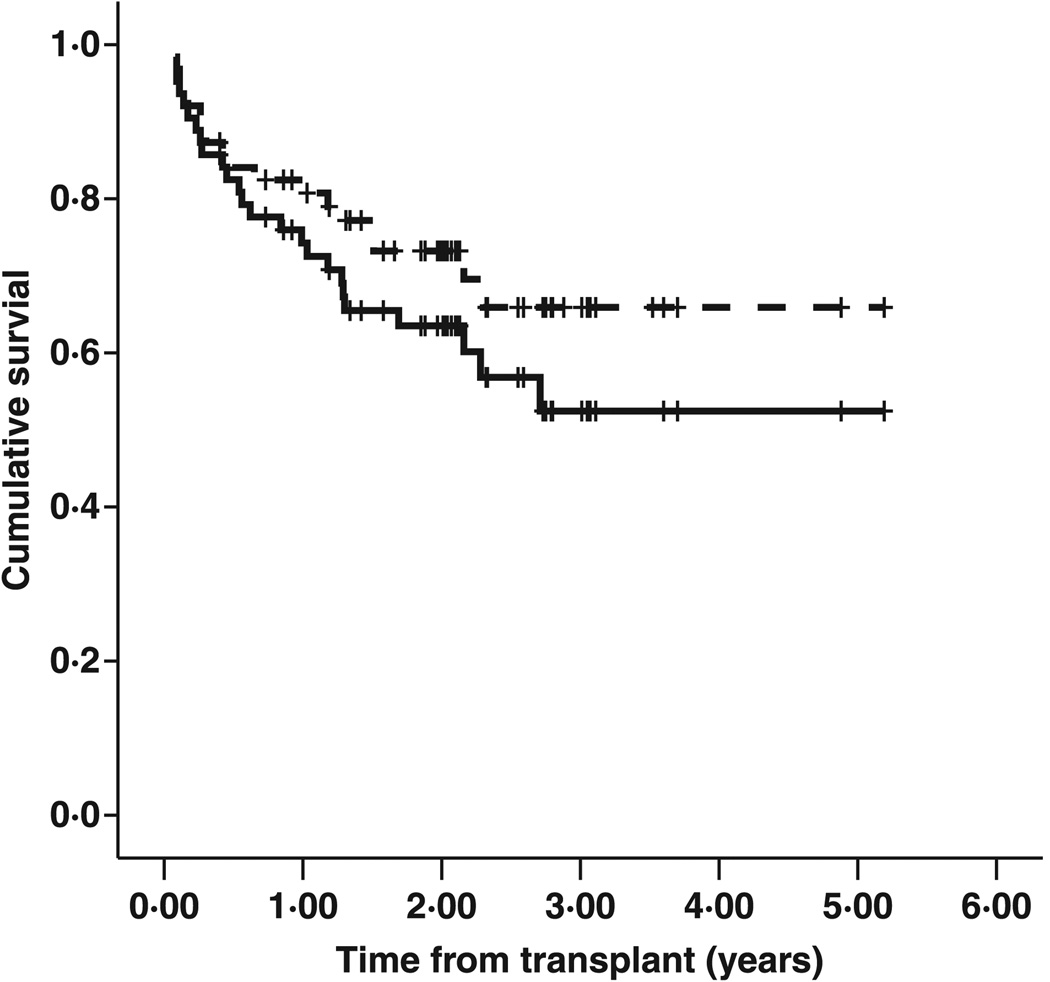
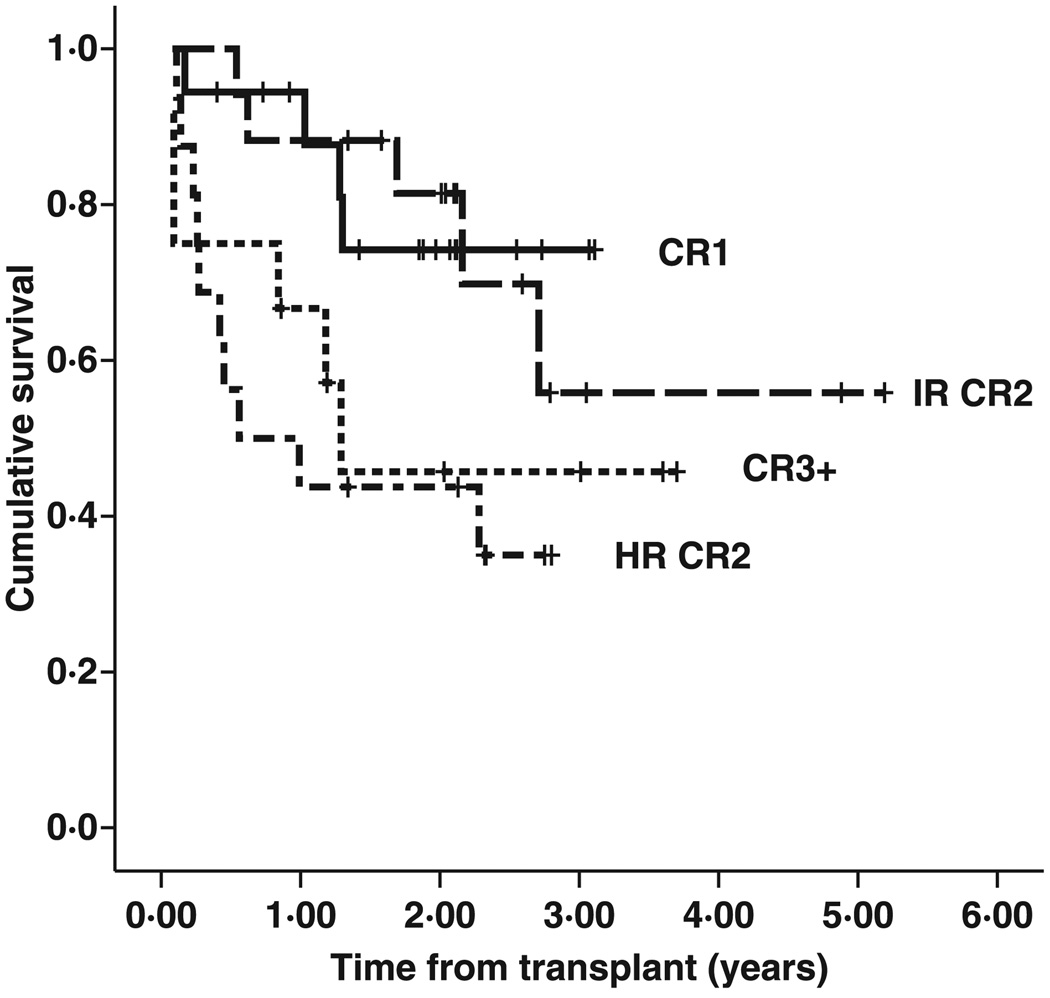
Two-year EFS and overall survival (OS) were 64% (standard error [SE] 6·3) and 73% (SE 5·8) respectively (Fig 1). Univariate analysis showed significant differences in 2-year EFS and OS according to risk category (Fig 2). Kaplan–Meier survival curves of CR1 patients and BFM intermediate risk CR2 patients visually overlapped and were statistically indistinguishable [2-year EFS 74% (SE 11) for CR1 vs. 81% (SE10) for IR CR2; P = 0·966]. Outcomes of the HR CR2 and CR3+ groups were also similar [2-year EFS 44% (SE 12) for HR CR2 vs. 46% (SE 16) for CR3+; P = 0·641]. Both of the better risk groups had superior outcomes compared either individually to one of the poor risk groups or when the better risk groups combined were compared with the poor risk groups. A combined analysis of the risk groups showed superior 2-year EFS (78% vs. 45%, P = 0·006) and 2-year OS (94% vs. 48%, P = 0·001) in the CR1/IR CR2 cohort compared to the HR CR2/CR3+ cohort.
Fig 1.
Event-free and overall survival. Two-year EFS and OS for the cohort were 64% [standard error (SE) 6·3] and 73% (SE 5·8).
Fig 2.
Event-free survival by risk group. Two-year EFS for the risk groups were 74% [standard error (SE) 11] for CR1, 81% (SE 10) for IR CR2, 44% (SE 12) for HR CR2 and 46% (SE 16) for CR3+.
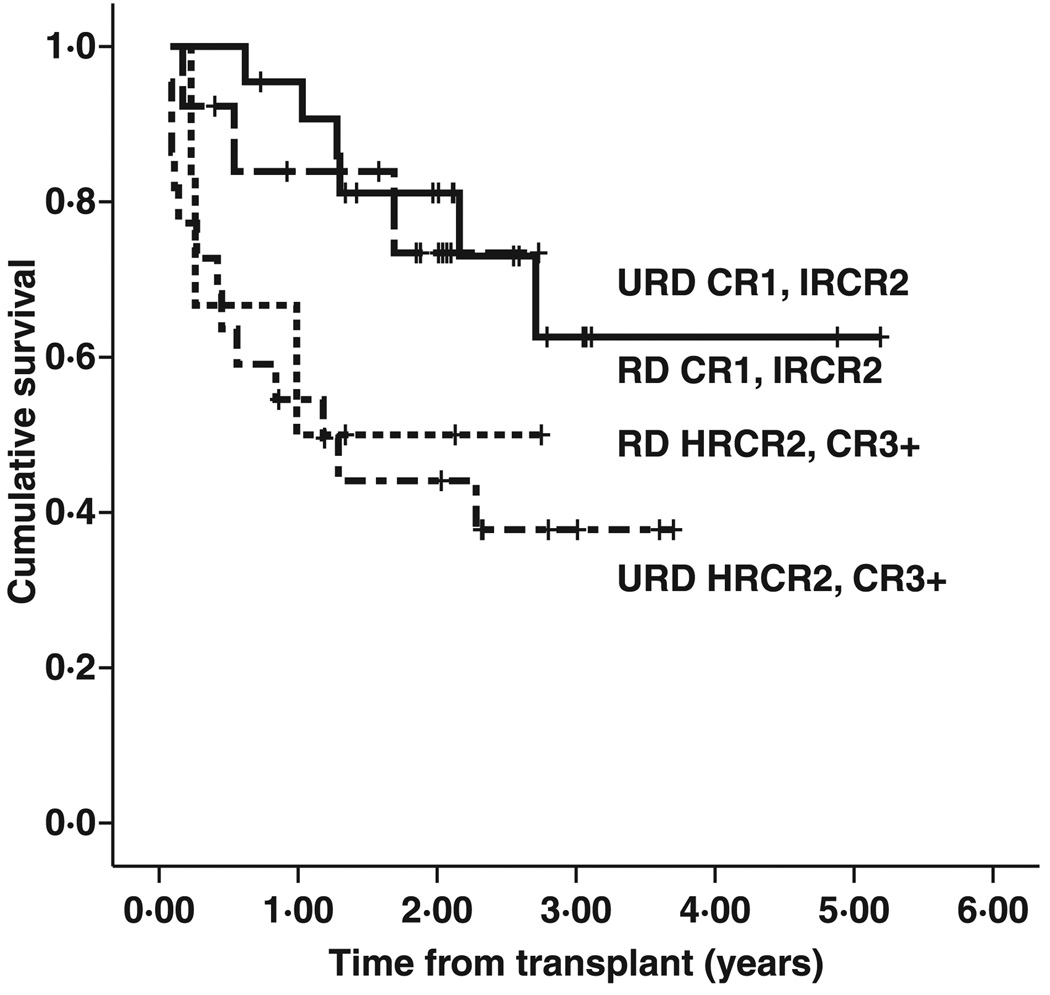
We performed univariate analyses on other donor and recipient characteristics, including donor source (UCB versus URD versus RD), donor/recipient CMV status, presence of acute and chronic GVHD, donor/recipient ABO mismatches, and T-cell versus B-cell disease in the recipient. While URD and UCB outcomes initially appeared to be inferior to related donor outcomes, when adjusted for transplant risk group, related and unrelated donor outcomes were indistinguishable (Fig 3). Acute GVHD or chronic GVHD of any degree had no effect on survival outcomes in this cohort. CMV status in the donor and the presence of ABO mismatches also had no statistically measurable effect. Although outcomes in patients with T-cell disease were not statistically different from those with B-cell disease in this cohort, the number of T-cell patients was low, and valid comparisons would require a larger cohort of T-cell patients.
Fig 3.
Event-free survival by risk group/stem cell source. Recipients of related donor (RD) transplants were more often CR1 or intermediate risk CR2 (21 RD vs. 13 URD), while recipients of unrelated donor (URD) transplantation were more often high risk CR2 or CR3 (20 URD vs. 5 RD). When stratified by risk, survival after related or unrelated donor transplantation was similar {2-year EFS 81% [standard error (SE) 8·5] vs. 73% (SE 13·4), RD versus URD CR1 or intermediate risk (dashed line URD, solid line RD); 2-year EFS 50% (SE20) vs. 44% (SE 11), RD versus URD high risk CR2 or CR3 (dotted line RD, dot-dash line URD)}.
Discussion
The principle areas of concern of the feasibility portion of this study was whether we could deliver sirolimus orally to young children in the midst of an intensive myeloablative transplant, achieve appropriate levels, and avoid significant toxicities and TRM. Delivery of the medication was not an issue. The pill form was preferred and tolerated well even in young children. Those too young to swallow pills easily took the liquid form either orally or via a nasal gastric tube, appropriate levels were achieved, and no patients went off study because they were unable to take the medication. Overall TRM was very low (8/63, 13%), in spite of the fact that the majority of transplants were performed with unrelated donor sources.
We closely followed specific toxicities associated with sirol-imus use in adult studies. Investigators have shown increased risk TAM and VOD associated with the use of sirolimus after allogeneic HCT, and in the case of VOD, the risk has been reported to be higher when methotrexate was given as part of the GVHD prophylactic regimen (Cutler et al, 2005, 2008). TAM was seen in three patients in our cohort. In one patient who started sirolimus on day −3, it was part of a larger picture of sepsis and MSOF. In the other two patients, the resultant renal damage was reversible by decreasing levels of sirolimus and tacrolimus or holding one or both of these medications until symptoms resolved, as has been described by Cutler et al (2005). Our TAM rate of 5% was less than half of the published experience with this combination in adults, and similar to rates expected after TBI-based allogeneic transplantation using tacrolimus for GVHD prophylaxis (4–13%) (Miano et al, 2008).
Veno-occlusive disease has been reported in 10–20% of allogeneic transplants performed in paediatrics (Reiss et al, 2002; Cesaro et al, 2005; Miano et al, 2008). The overall rate in our population (11%) is lower than what would be expected with a TBI-based preparative regimen in children. Our approach differs from the Dana Farber regimen, in that sirolimus starts on the day of transplant, after completion of the preparative regimen, and a loading dose is not administered. Our experience of significant toxicity in two patients who had overlap of tacrolimus/sirolimus with cyclophosphamide is reminiscent of the toxicity and GVHD described with overlap of cyclophosphamide with this GVHD prophylaxis regimen by the Seattle group (Furlong et al, 2008). It is possible that avoiding overlap of sirolimus with cyclophosphamide (the Dana Farber group overlapped with TBI) lessens VOD risk. That said, given how rarely this event occurs, a much larger experience in children would be needed to discern whether the risk of VOD is increased with our approach. This question should be answered by an ongoing Children’s Oncology Group phase III trial randomising the addition of sirolimus to a tacrolimus/methotrexate GVHD prophylactic regimen in children with ALL.
More than 80% of paediatric patients with ALL are cured with chemotherapy approaches (Pui & Evans, 1998; Gaynon et al, 2000; Schrappe et al, 2000), leaving only a small fraction of patients identified as poor risk either by incomplete response to therapy, cytogenetic associations, relapse or, more recently, gene expression profiles, who may benefit from allogeneic transplantation (Arico et al, 2000; Borgmann et al, 2003; Nachman et al, 2007; Borowitz et al, 2008; Yang et al, 2009). Use of TBI-based regimens may decrease relapse rates in this high-risk group (Eapen et al, 2006; Marks et al, 2006), but approaches aimed at enhancing GVHD have generally led to more toxicity without improved survival. Because the limits of intensity of TBI-based preparative regimens have been reached, the introduction of anti-ALL agents after achieving minimal tumour burden post-transplant may be a fruitful approach (Pulsipher et al, 2008). Ideal approaches to relapse prevention in the post-HCT would need to (i) avoid worsening GVHD or introducing other toxicities and (ii) treat leukemic blasts, either directly or by modulating the allogeneic immune system to increase tumour kill.
Evidence from a number of trials points to sirolimus as an agent that may result in a survival benefit after transplantation by decreasing relapse. Studies in recipients of solid organ transplants have shown that switching to sirolimus from other immune suppressive agents can successfully treat post-transplant lymphoproliferative disorders and Kaposi sarcoma (Garcia et al, 2003; Campistol et al, 2004). The agent has been shown to inhibit primary and metastatic tumour growth by antiangiogenesis (Guba et al, 2002). Sirolimus treatment leads to apoptosis and cell death of ALL blasts, and synergy with methotrexate in treating ALL blasts has also been described (Brown et al, 2003; Teachey et al, 2008). The Dana Farber group showed a distinct advantage in survival of lymphoma patients undergoing reduced intensity allogeneic transplantation when sirolimus was used for GVHD prophylaxis compared to other approaches (Armand et al, 2008). This survival difference was not related to changes in GVHD incidence. Whether use of sirolimus-based GVHD prophylaxis results in a survival advantage in children undergoing allogeneic transplantation for ALL requires further study.
In summary, transplantation of children with ALL using sirolimus-based GVHD prophylaxis leads to stable engraftment and low rates of TRM and relapse, resulting in excellent 2-years EFS rates. The approach of this multi-institutional pilot protocol is being tested in a large phase III COG study, which will define whether the addition of sirolimus after transplantation improves survival in children with ALL undergoing allogeneic HSCT.
Acknowledgements
We would like to express appreciation to the research assistants at each institution for their diligence and attentiveness to detail, and the outstanding clinical care delivered by the transplant centre staffs.
Support: NIH CA102646, CA1116660, ACS RSG0507101, and the Weinberg Fund of the Children’s Hospital of Philadelphia (SAG).
Footnotes
Authorship and conflict of interest statement
Contribution: Michael A. Pulsipher had primary responsibility for study design, data analysis, data interpretation, and manuscript writing. Also, Dr Pulsipher had primary responsibility for the entire paper as an accurate and verifiable report. Donna Wall participated in study design, patient accrual, data analysis, interpretation of data, and manuscript writing. Michael Grimley participated in patient accrual, interpretation of data and manuscript writing. Rakesh Goyal participated in study design, patient accrual, interpretation of data and manuscript writing. Ken Boucher had responsibility for study design, data file preparation, statistical analysis and manuscript writing. Patricia Hankins participated in study design, protocol writing, patient accrual, data analysis, interpretation of data and manuscript writing. Stephan A. Grupp participated in study design, patient accrual, data analysis, interpretation of data and manuscript writing. Nancy Bunin had responsibility for study design, patient accrual, data analysis, data interpretation and manuscript writing. Also, Dr Bunin had responsibility for the entire paper as an accurate and verifiable report.
References
- Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, Antin JH. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biology of Blood & Marrow Transplantation. 2008;14:920–926. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, Hochberg EP, Wu CJ, Alyea EP, Soiffer RJ. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G, Kamps W, Pui CH, Masera G. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. New England Journal of Medicine. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, LaCasce AS, Jacobsen ED, Fisher DC, Brown JR, Canellos GP, Freedman AS, Soiffer RJ, Antin JH. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. Journal of Clinical Oncology. 2008;26:5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, Barth A, Borkhardt A, Peters C, Handgretinger R, Sykora KW, Holter W, Kabisch H, Klingebiel T, von Stackelberg A. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. Journal of Clinical Oncology. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, Henze G. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, Linda S, Martin PL, Pullen DJ, Viswanatha D, Willman CL, Winick N, Camitta BM. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin N, Carston M, Wall D, Adams R, Casper J, Kamani N, King R. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: a successful treatment for posttransplantation Kaposi’s sarcoma. Transplantation. 2004;77:760–762. doi: 10.1097/01.tp.0000115344.18025.0b. [DOI] [PubMed] [Google Scholar]
- Cejka D, Preusser M, Fuereder T, Sieghart W, Werzowa J, Strommer S, Wacheck V. mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo. Anticancer Research. 2008;28:3801–3808. [PubMed] [Google Scholar]
- Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, Strugo L, Destro R, Gazzola MV, Varotto S, Errigo G, Carli M, Zanesco L, Messina C. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396–1404. [PubMed] [Google Scholar]
- Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplantation. 2004;34:471–476. doi: 10.1038/sj.bmt.1704604. [DOI] [PubMed] [Google Scholar]
- Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, Ho V, Lee SJ, Soiffer R, Antin JH. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biology of Blood & Marrow Transplantation. 2005;11:551–557. doi: 10.1016/j.bbmt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, Revta C, Ebert R, Warren D, Choi S, Koreth J, Armand P, Alyea E, Carter S, Horowitz M, Antin JH, Soiffer R. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, Billett A, Homans A, Camitta B, Carroll WL, Davies SM. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, Flowers ME, Mielcarek M, Nash RA, Storb R, Martin PJ. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biology of Blood & Marrow Transplantation. 2008;14:531–537. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia VD, Bonamigo Filho JL, Neumann J, Fogliatto L, Geiger AM, Garcia CD, Barros V, Keitel E, Bittar AE, Ferrera des Santos A, Roithmann S. Rituximab in association with rapamycin for post-transplant lymphoproliferative disease treatment. Transplant International. 2003;16:202–206. doi: 10.1007/s00147-002-0487-9. [DOI] [PubMed] [Google Scholar]
- Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, Bleyer WA. Children’s Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14:2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Medicine. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, Ferrara J, Soiffer R, Giralt S. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biology of Blood & Marrow Transplantation. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Klein J, Moeschberger M. Survival Analysis: Statistical Methods for Censored and Truncated Data. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- Marks DI, Forman SJ, Blume KG, Perez WS, Weisdorf DJ, Keating A, Gale RP, Cairo MS, Copelan EA, Horan JT, Lazarus HM, Litzow MR, McCarthy PL, Schultz KR, Smith DD, Trigg ME, Zhang MJ, Horowitz MM. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biology of Blood & Marrow Transplantation. 2006;12:438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Annals of Internal Medicine. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C, Gooley T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- Miano M, Faraci M, Dini G, Bordigoni P. Early complications following haematopoietic SCT in children. Bone Marrow Transplantation. 2008;41(Suppl. 2):S39–S42. doi: 10.1038/bmt.2008.53. [DOI] [PubMed] [Google Scholar]
- Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, Dastugue N, Schrappe M, Pui CH, Basso G, Silverman LB, Janka-Schaub GE. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini M, Wang Z, Schneider L. CIBMTR Summary Slides 2007-Part 1. Center for International Blood and Marrow Transplant Research Newsletter. 2007;13:5–8. [Google Scholar]
- Pui CH, Evans WE. Acute lymphoblastic leukemia. New England Journal of Medicine. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- Pulsipher MA, Bader P, Klingebiel T, Cooper LJ. Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biology of Blood & Marrow Transplantation. 2008;15:62–71. doi: 10.1016/j.bbmt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Reiss U, Cowan M, McMillan A, Horn B. Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. Journal of Pediatric Hematology/Oncology. 2002;24:746–750. doi: 10.1097/00043426-200212000-00013. [DOI] [PubMed] [Google Scholar]
- Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. International Journal of Clinical Oncology. 2008;13:66–70. doi: 10.1007/s10147-007-0733-3. [DOI] [PubMed] [Google Scholar]
- Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, Gadner H, Odenwald E, Riehm H. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995 Berlin–Frankfurt–Munster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, Carroll AJ, Heerema NA, Rubnitz JE, Loh ML, Raetz EA, Winick NJ, Hunger SP, Carroll WL, Gaynon PS, Camitta BM. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber C, Mayerhofer M, Bohm A, Vales A, Gruze A, Aichberger KJ, Esterbauer H, Pfeilstocker M, Sperr WR, Pickl WF, Haas OA, Valent P. Evaluation of antileukaemic effects of rapamycin in patients with imatinib-resistant chronic myeloid leukaemia. European Journal of Clinical Investigation. 2008;38:43–52. doi: 10.1111/j.1365-2362.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, Houghton PJ, Brown VI, Grupp SA. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107:1149–1155. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, Reid GS, Seif AE, Norris R, Chang YJ, Carroll M, Grupp SA. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Cheng C, Yang W, Pei D, Cao X, Fan Y, Pounds SB, Neale G, Trevino LR, French D, Campana D, Downing JR, Evans WE, Pui CH, Devidas M, Bowman WP, Camitta BM, Willman CL, Davies SM, Borowitz MJ, Carroll WL, Hunger SP, Relling MV. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. Journal of the American Medical Association. 2009;301:393–403. doi: 10.1001/jama.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]