Abstract
Background
To clarify the role of inflammation in the pathogenesis of small vessel disease of the brain, we investigated the association between common variation in the CRP and IL6 genes, plasma CRP and IL6 levels, and presence of MRI-defined white matter lesions (WML) and brain infarcts (BI) in elderly participants of the Cardiovascular Health Study.
Methods and Results
Tag single nucleotide polymorphisms (SNPs) in the CRP and IL6 genes were selected from the SeattleSNPs database. In cross-sectional analyses, logistic regression models adjusting for known CVD risk factors were constructed to assess the associations of plasma CRP and IL6 levels and common CRP and IL6 gene haplotypes with presence of WML or BI in Blacks (N=532) and Whites (N=2,905). Plasma IL6 and CRP levels were associated with presence of WML and BI in both races. In Whites, common haplotypes of the IL6 gene were significantly associated with WML and BI. The common haplotype tagged by the −174G/C promoter polymorphism was associated with an increased risk of WML (OR=1.14; 95% CI: (1.02; 1.28)). The common haplotype tagged by the −572G/C promoter polymorphism was associated with an increased risk of BI (OR=1.57; 95% CI: (1.15; 2.14)). Significant associations were lacking for WML or BI with IL6 gene variation in Blacks, or with CRP gene variation in either race.
Conclusions
This study provides evidence of a genetic basis underlying the relationship between plasma biomarkers of inflammation and small vessel disease of the brain. Further studies to elucidate the specific role of IL6 in disease pathogenesis are warranted.
INTRODUCTION
Vascular disease of the brain is the third leading cause of death and the leading cause of severe long-term disability in the United States.1 The burden of brain vascular disease is far greater than that of the clinically-recognizable acute neurological event, stroke.2 Brain imaging techniques, such as magnetic resonance imaging (MRI), have revealed that white matter lesions (WML) and brain infarcts (BI) are remarkably common in the elderly.3–6 While the majority of these MRI-detectable abnormalities do not produce acute clinical symptoms, they cannot be considered benign as they are often associated with an increased risk for cognitive deficits,7, 8 motor function impairment,9, 10 and future stroke.11–13 The pathogenesis of these abnormalities remains poorly understood but may reflect vascular damage to the deep penetrating vessels of the brain resulting from arteriolosclerosis and leading to localized ischemic areas of necrosis and cavitation (i.e., BI) or diffuse rarefaction in the white matter (i.e., WML).14
Evidence is mounting suggesting that chronic inflammation contributes to the development and consequences of cardiovascular disease.15 Numerous prospective studies have shown associations of serum levels of biomarkers of inflammation such as C-reactive protein (CRP), interleukin 6 (IL6), and fibrinogen with myocardial infarction, stroke, cardiovascular death, and peripheral arterial disease.16–19 Little is known, however, about the relationship between inflammation and small vessel disease of the brain.20 Previous studies have shown evidence of inflammatory activation and endothelial dysfunction in individuals with lacunar infarction and WML.21, 22 More recently, higher CRP levels have been associated with presence and progression of WML in a population-based sample of non-demented elderly Europeans.23 A significant association between higher CRP and IL6 levels and presence of silent BI was also reported in Japanese individuals.24 While these studies provide support for a link between inflammatory markers and small vessel disease pathogenesis, it is yet unclear whether small vessel disease of the brain is directly and causally influenced by increased levels of inflammatory markers, or itself induces increased synthesis of inflammatory molecules, or both.
To further investigate the relationship between molecular markers of inflammation and small vessel disease of the brain, we conducted an association study of the sequence variation in 2 inflammation genes, CRP and IL6, with presence of WML and BI among elderly participants in the Cardiovascular Health Study (CHS).
MATERIAL AND METHODS
Subjects
Details of the study design and characteristics of the CHS cohort participants have been previously published.25, 26 Briefly, between June 1989 and May 1990, 5,201 adults 65 years or older were recruited from a random sample of people on Medicare eligibility lists in 4 US communities: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh (Allegheny County), PA. They were non-institutionalized, able to give informed consent, and able to respond to questions without the help of a surrogate respondent. To enhance minority representation in the cohort, 687 African-American participants were recruited to the study in 1992. Between enrollment and 1998–99, participants were seen in the clinic annually, and contacted by phone at 6-month intervals to collect information about hospitalizations and potential cardiovascular events. At clinic examinations, measures of cardiovascular risk were assessed. Between 1992 and 1994, 3,660 (62%) participants (3,073 Whites, 571 Blacks, and 16 belonging to other ethnic groups) underwent a MRI of the brain. Those who underwent MRI were younger and healthier than those who did not.27
Data Collection
Participants underwent an extensive baseline evaluation, including standard questionnaires, clinical examination, cognitive function assessment, and clinical laboratory testing. Parts of the baseline evaluation have been repeated annually. Variables considered in these analyses as potential risk factors or confounders were those from examination closest in time and before the MRI scan. IL6 was measured in serum by an ultra-sensitive ELISA method (R&D Systems, Minneapolis, MN) at the baseline examination and CRP was measured at the Year 5 examination by ELISA assay developed at the CHS central blood laboratory.28
Detailed descriptions of the MRI techniques and methods of analyses have been published.29 Briefly, scanning protocols included sagittal T1-weighted localizer images and axial T1-weighted, spin density-, and T2-weighted images. All axial images had 5-mm thickness without interslice gaps. Blinded to any information about participants, neuroradiologists at the reading center identified brain infarcts 30 and estimated white matter grade using a 10-point system from 0 to 9 (most abnormal), using a library of templates.27 In these analyses, presence of WML was defined as a white matter grade greater or equal to 2 and absence of WML was defined as a white matter grade of 0 or 1 (No or barely detectable WML, respectively). A brain infarct was defined as presence on MRI of an area of abnormal signal intensity 3 mm in size or greater in a vascular distribution and without mass effect.
Polymorphisms selection and genotyping
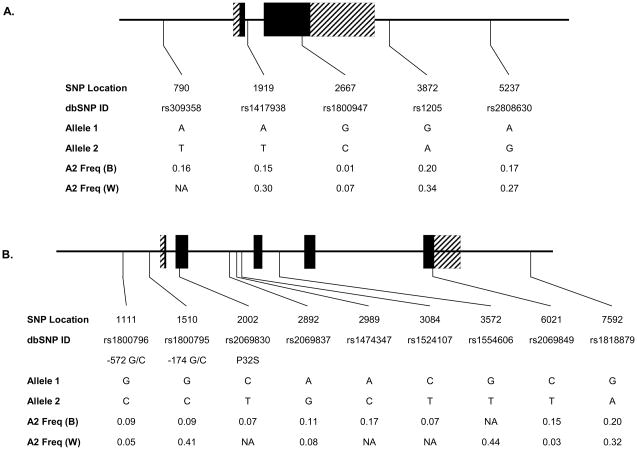
Single Nucleotide Polymorphisms (SNPs) were chosen based on pairwise linkage disequilibrium (LD) relationships 31 among common SNPs identified in African-Americans and European-Americans as part of the Seattle SNPs Variation Discovery resource.32 A total of 5 SNPs in the CRP gene, and 9 SNPs in the IL6 gene were genotyped in 3,437 CHS MRI participants (532 Blacks, 2,905 Whites) who had a DNA sample and had given informed consent for use of their genetic material. The gene location and identity of these polymorphisms are shown in Figure 1. Genotyping was performed using TaqMan Assays by Design (Applied Biosystems) under conditions recommended by the manufacturer. Probe and primer sequences for each assay are available upon request.
Figure 1.
Gene location and allele frequency of the assayed polymorphisms in the CRP (A.) and IL6 (B.) genes by race. Exons are indicated by boxes and are numbered. Coding sequence is shown in black. Polymorphism position is given according to the SeattleSNPs numbering. The corresponding dbSNP ID and common polymorphism designation are also shown. A2 Freq: Frequency of allele 2; B: Blacks; W: Whites.
Statistical Analysis
For each polymorphism, agreement of genotype frequencies with Hardy Weinberg equilibrium expectations was tested using a χ2 goodness-of-fit test. All analyses were carried out stratified on race to minimize potential confounding due to population stratification. Non-normally distributed variables were log-transformed.
In each racial group, logistic regression models were used to evaluate the associations between MRI-findings and individual tagSNPs. For each SNP, genotypes were coded as number of copies (0, 1, or 2) of the variant allele. Significance of genotype effects were assessed by the Armitage’s test, which is equivalent to the score test in the logistic regression model.33 Models were adjusted for age, gender, current smoking, hypertension status, body mass index, diastolic blood pressure, education, diabetes status and HDL-cholesterol, and further for plasma levels of IL6 or CRP, as appropriate. Correction for multiple testing was performed using the direct simulation approach, a fast approximation to permutation.34 Global tests of significance for association of the combined set of polymorphisms in each gene with MRI traits were evaluated by Fisher’s product method.35
Significance of haplotype effects on presence of MRI findings was evaluated using a regression method for unphased haplotypes based on score statistics and implemented in the Haplo. Stats software.36 Score statistics are constructed to test the null hypotheses of no haplotype effects on the probability of having MRI-defined brain vascular disease, adjusting for covariates. Statistical significance was evaluated by permutation tests.36 Only haplotypes with a frequency greater or equal to 3% were considered in the analyses to reduce risk of false positive findings related to low frequency haplotypes.37 To estimate the magnitude of effects of haplotypes on traits, each individual was assigned the most probable pair of haplotypes, as estimated by EM algorithm implemented in the score method above, and haplotype effect was estimated using logistic regression models contrasting odds of having MRI findings between individuals carrying 0, 1, or 2 copies of the given haplotype.
RESULTS
Selected characteristics of the participants are shown in Table 1 by race. In the group of elderly individuals, prevalence of WML (65%) and that of BI (28%) at the initial examination were similar in black and white participants.
Table 1.
Mean (standard deviation) or proportion for selected characteristics of participants present at the initial examination
| Whites (N=3,073) | Blacks (N=571) | P | |
|---|---|---|---|
| Age (years) | 75.4 (5.0) | 73.9 (5.7) | <0.0001 |
| Men (%) | 42.5 | 37.3 | 0.02 |
| Body mass index (kg/m2) | 26.3 (4.4) | 28.2 (4.9) | <0.0001 |
| Current smokers (%) | 8.5 | 15.0 | <0.0001 |
| Education (years) | 14.3 (4.6) | 12.9 (5.1) | <0.0001 |
| Hypertension (%) | 55.0 | 75.1 | <0.0001 |
| Diabetes (%) | 12.5 | 23.4 | <0.0001 |
| LDL cholesterol (mg/dL) | 127.2 (33.3) | 129.2 (36.4) | 0.19 |
| HDL cholesterol (mg/dL) | 53.0 (14.6) | 57.1 (14.6) | <0.0001 |
| Systolic blood pressure (mm Hg) | 133.9 (20.4) | 140.4 (21.4) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 69.8 (10.5) | 75.3 (10.9) | <0.0001 |
| WML prevalence (%) | 65.6 | 63.9 | 0.44 |
| Brain infarcts prevalence (%) | 27.7 | 28.3 | 0.78 |
| Interleukin 6 (pg/mL) | 2.0 (1.7) | 2.3 (2.1) | <0.0001* |
| C-Reactive Protein (mg/L) | 4.7 (8.4) | 6.3 (8.5) | <0.0001* |
P value for comparison of geometric means
There was a significant and graded association between plasma IL6 levels and presence of WML in both races. Individuals with higher plasma IL6 levels had a significantly greater risk of having WML (odds ratio (OR) per standard deviation of Log(IL6) (95% confidence interval): 1.28 (1.11; 1.47) for Whites; 1.55 (1.12; 2.15) for Blacks) (Table 2). Adjustment for covariates did not significantly attenuate this relationship (Table 2). Similar results were obtained for plasma CRP (OR: 1.13 (1.06; 1.22), Whites; 1.09 (0.93; 1.28), Blacks), although statistical significance was reached only in Whites. Consistently, there was a graded association between plasma IL6 levels and presence of BI (OR: 1.41 (1.21; 1.63), Whites; 1.32 (0.94; 1.85), Blacks) (Table 2), but statistical significance was reached only in Whites. Adjustment for covariates did not modify this association (Table 2). Plasma CRP levels were also associated, although marginally, with presence of BI in the two racial groups (OR: 1.09 (1.01; 1.19), Whites; 1.21 (1.00; 1.47), Blacks).
Table 2.
Association of plasma IL6 and CRP levels with presence of white matter lesions and brain infarcts at the 1st MRI examination
| Odds Ratio (95% Confidence Interval) for association with presence of WML | Odds Ratio (95% Confidence Interval) for association with presence of BI | |||||||
|---|---|---|---|---|---|---|---|---|
| Whites (N=3,073) | Blacks (N=571) | Whites (N=3,073) | Blacks (N=571) | |||||
| IL6 | CRP | IL6 | CRP | IL6 | CRP | IL6 | CRP | |
| Model 1 | ||||||||
| 1st Quartile | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 2nd Quartile | 1.24 (0.99; 1.55) | 1.28 (1.03; 1.59) | 1.87 (1.09; 3.19) | 0.82 (0.49; 1.38) | 1.38 (1.07; 1.78) | 0.99 (0.78; 1.25) | 1.05 (0.57; 1.92) | 1.05 (0.58; 1.89) |
| 3rd Quartile | 1.32 (1.05; 1.65) | 1.31 (1.05; 1.62) | 1.26 (0.75; 2.13) | 0.89 (0.53; 1.51) | 1.43 (1.10; 1.85) | 1.16 (0.92; 1.47) | 1.17 (0.63; 2.17) | 1.07 (0.60; 1.93) |
| 4th Quartile | 1.48 (1.18; 1.86) | 1.59 (1.27; 1.98) ** | 2.16 (1.26; 3.73) * | 1.28 (0.74; 2.19) | 1.81 (1.40; 2.33)** | 1.18 (0.93; 1.49) | 1.37 (0.74; 2.51) | 1.45 (0.81; 2.58) |
| P trend | 0.007 | 0.0006 | 0.02 | 0.37 | 0.0001 | 0.29 | 0.74 | 0.56 |
| Per SD (log) | 1.28 (1.11; 1.47) | 1.13 (1.06; 1.22) | 1.55 (1.12; 2.15) | 1.09 (0.93; 1.28) | 1.41 (1.21; 1.63) | 1.11 (1.03; 1.20) | 1.32 (0.94; 1.85) | 1.16 (0.98; 1.38) |
| Model 2 | ||||||||
| 1st Quartile | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 2nd Quartile | 1.22 (0.97; 1.53) | 1.21 (0.97; 1.51) | 1.83 (1.05; 3.18) | 0.80 (0.47; 1.37) | 1.35 (1.04; 1.75) | 0.95 (0.74; 1.21) | 1.09 (0.58; 2.04) | 1.04 (0.56; 1.93) |
| 3rd Quartile | 1.27 (1.01; 1.60) | 1.20 (0.97; 1.50) | 1.25 (0.71; 2.17) | 0.86 (0.50; 1.48) | 1.40 (1.07; 1.83) | 1.10 (0.86; 1.41) | 1.12 (0.58; 2.18) | 1.11 (0.60; 2.04) |
| 4th Quartile | 1.43 (1.12; 1.82) * | 1.48 (1.17; 1.84) ** | 2.20 (1.23; 3.90) * | 1.16 (0.67; 2.04) | 1.75 (1.34; 2.28)** | 1.11 (0.86; 1.42) | 1.37 (0.71; 2.65) | 1.62 (0.85; 3.11) |
| P trend | 0.03 | 0.01 | 0.03 | 0.55 | 0.0008 | 0.54 | 0.80 | 0.43 |
| Per SD (log) | 1.24 (1.07; 1.44) | 1.11 (1.03; 1.19) | 1.55 (1.10; 2.19) | 1.06 (0.90; 1.25) | 1.37 (1.17; 1.60) | 1.09 (1.01; 1.19) | 1.34 (0.93; 1.93) | 1.21 (1.00; 1.47) |
| Model 3 | ||||||||
| 1st Quartile | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 2nd Quartile | 1.09 (0.84; 1.41) | 1.15 (0.89; 1.50) | 1.95 (1.02; 3.71) | 0.84 (0.44; 1.61) | 1.33 (0.99; 1.80) | 0.96 (0.72; 1.29) | 1.28 (0.62; 2.67) | 0.81 (0.39; 1.66) |
| 3rd Quartile | 1.28 (0.97; 1.67) | 1.13 (0.86; 1.47) | 1.22 (0.63; 2.34) | 0.83 (0.43; 1.58) | 1.38 (1.01; 1.87) | 1.07 (0.80; 1.44) | 1.19 (0.56; 2.56) | 1.03 (0.51; 2.08) |
| 4th Quartile | 1.42 (1.08; 1.87)* | 1.33 (1.01; 1.75)* | 2.13 (1.08; 4.20)* | 0.90 (0.44; 1.86) | 1.87 (1.38; 2.54)** | 1.14 (0.84; 1.54) | 1.68 (0.81; 3.52) | 1.47 (0.70; 3.08) |
| P trend | 0.06 | 0.26 | 0.06 | 0.94 | 0.0009 | 0.69 | 0.55 | 0.47 |
| Per SD (log) | 1.23 (1.05; 1.46) | 1.09 (0.99; 1.21) | 1.54 (1.03; 2.30) | 1.18 (0.94; 1.48) | 1.46 (1.22; 1.74) | 1.09 (0.99; 1.21) | 1.42 (0.96; 2.11) | 1.18 (0.94; 1.48) |
P<0.05;
P<0.01 for comparison with 1st quartile;
P trend over quartiles; SD: standard deviation; log: logarithm-transformed biomarker plasma levels; Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, current smoking, education, diastolic blood pressure, body mass index, and hypertension (MRI-defined WML); adjusted for age, sex, current smoking, systolic blood pressure, diastolic blood pressure, HDL cholesterol, body mass index, diabetes, and hypertension (MRI-defined BI). Model 3: adjusted for variables in Model 2 and excluding individuals with prevalent stroke, transient ischemic attack, coronary heart disease, and heart failure (N=2,283, Whites; N=426, Blacks). Cutoff for Quartiles of plasma IL6 in Whites and Blacks, respectively: 1st: 0.35–1.08; 0.36–1.18 pg/mL; 2nd: 1.09–1.55; 1.19–1.70 pg/mL; 3rd: 1.56–2.31; 1.71–2.58 pg/mL; 4th: 2.32–44.17; 2.59–23.37 pg/mL. Cutoff for Quartiles of plasma CRP in Whites and Blacks, respectively: 1st: 0.13–1.13; 0.16–1.47 mg/L; 2nd: 1.14–2.43; 1.49–3.45 mg/L; 3rd: 2.44–5.34; 3.46–7.86 mg/L; 4th: 5.35–143.0; 7.89–79.40 mg/L.
Analyses were also carried out excluding individuals with prevalent stroke, coronary heart disease, heart failure, and transient ischemic attacks (N=988). Associations of the 2 inflammatory biomarkers with WML and BI were essentially unchanged (Table 2).
We also determined whether sequence variation in the 2 genes may be associated with presence of WML and BI. Within each racial group, all polymorphisms were in Hardy Weinberg equilibrium, except for SNP 2892 (rs2069837) in Whites. This polymorphism was excluded from further analysis in this group.
IL6 gene polymorphisms were associated with both WML and BI in Whites but not in Blacks. In Whites, a common IL6 haplotype (frequency=40%) was associated with a significantly greater risk of WML (OR: 1.14 (1.02; 1.28)) (Table 3). This haplotype was tagged by the promoter SNP 1510 (−174 G/C) and SNP 3572 in intron 3, both of which were in significant linkage disequilibrium (r2=0.86). Consistently, these 2 SNPs showed similar associations with WML, although only that of SNP 1510 with WML reached statistical significance after correction for multiple tests (Table 3). Magnitude of associations of IL6 haplotypes and genotypes with WML was not modified after adjusting for plasma IL6 or CRP levels or both, although statistical significance was attenuated (not shown).
Table 3.
Association of IL6 haplotypes and genotypes with presence of MRI-defined WML in Whites.
| SNP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1111 | 1510 | 3572 | 6021 | 7592 | |||||
| Haplotype Index | Haplo. Freq. | OR* (95% CI) | P† | Global P† | |||||
| 1 | 40.5 | G | C | T | C | A | 1.14 (1.02; 1.28) | 0.03 | 0.07 |
| 2 | 3.1 | C | G | G | C | C | 0.98 (0.72; 1.33) | 0.91 | |
| 3 | 23.2 | G | G | G | C | A | 0.93 (0.81; 1.07) | 0.32 | |
| 4 | 27.7 | G | G | G | C | C | 0.98 (0.86; 1.12) | 0.68 | |
| OR ‡ (95% CI) | 0.89 (0.69; 1.15) | 1.13 (1.01; 1.27) | 1.10 (0.99; 1.23) | 0.84 (0.60; 1.18) | 0.97 (0.86; 1.10) | ||||
| P¶ | 0.13 | 0.03 | 0.08 | 0.46 | 0.58 | ||||
| Global P | 0.03 | ||||||||
Odds ratio per copy of haplotype adjusted for age, sex, current smoking, education, diastolic blood pressure, body mass index, and hypertension.
Permutation-based P value from score test. P values for individual haplotypes test the null hypothesis that the odds of having WML of a particular haplotype are the same as that of all other haplotypes combined. The global P value tests the null hypothesis that the odds of having WML are the same for all haplotypes.
Odds ratio per copy of variant allele adjusted for age, sex, current smoking, education, diastolic blood pressure, body mass index, and hypertension.
adjusted for multiple tests.
Haplo. Freq.: Haplotype frequency. SNP: Single nucleotide polymorphism
Another IL6 haplotype (frequency=3%) was significantly associated with greater risk of BI (OR: 1.57 (1.15; 2.14)) (Table 4) and this association persisted after adjusting for plasma IL6 or CRP levels, or both. This haplotype was tagged by SNP 1111 (−572 G/C), located in the gene promoter. While this SNP showed a trend toward a significant association with BI in single-SNP analysis (OR: 1.23 (0.94; 1.62)), statistical significance was not reached after correction for multiple testing (P=0.13) (Table 4). Moreover, the association of the IL6 haplotype with BI was not modified after adjusting for the effects of SNP 1111 (OR: 2.6 (1.3; 5.3), P=0.005).
Table 4.
Association of IL6 haplotypes and genotypes with presence of MRI-defined BI in Whites.
| SNP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1111 | 1510 | 3572 | 6021 | 7592 | |||||
| Haplotype Index | Haplo. Freq. | OR* (95% CI) | P† | Global P† | |||||
| 1 | 40.5 | G | C | T | C | A | 1.02 (0.90; 1.16) | 0.78 | 0.01 |
| 2 | 3.1 | C | G | G | C | C | 1.57 (1.15; 2.14) | 0.001 | |
| 3 | 23.2 | G | G | G | C | A | 0.98 (0.85; 1.13) | 0.76 | |
| 4 | 27.7 | G | G | G | C | C | 0.97 (0.84; 1.12) | 0.70 | |
| OR ‡ (95% CI) | 1.23 (0.94; 1.62) | 1.02 (0.90; 1.15) | 1.01 (0.89; 1.14) | 0.90 (0.61; 1.32) | 0.98 (0.85; 1.13) | ||||
| P ¶ | 0.13 | 0.48 | 0.51 | 0.53 | 0.87 | ||||
| Global P | 0.61 | ||||||||
Odds ratio per copy of haplotype adjusted for age, sex, current smoking, systolic blood pressure, diastolic blood pressure, HDL cholesterol, body mass index, diabetes, and hypertension.
Permutation-based P value from score test.
Odds ratio per copy of variant allele adjusted for age, sex, current smoking, systolic blood pressure, diastolic blood pressure, HDL cholesterol, body mass index, diabetes, and hypertension.
adjusted for multiple tests.
Haplo. Freq.: Haplotype frequency. SNP: Single nucleotide polymorphism
Despite their significant association with plasma CRP levels in this cohort 38, we did not find any evidence that CRP haplotypes and/or genotypes were associated with WML or BI in either Blacks or Whites (not shown).
DISCUSSION
In the CHS cohort of elderly individuals, we showed a significant association of circulating IL6 and CRP with WML and BI. In addition, common variation in the IL6 gene was significantly associated with increased risk of WML and BI in Whites but not Blacks. A common IL6 haplotype and the −174 G/C (rs1800795) promoter polymorphism that tags it were significantly associated with a modest increase in risk of WML. Another common IL6 haplotype, tagged by the −572 G/C (rs1800796) promoter polymorphism, was significantly associated with a modest increase in risk of BI. No significant associations of CRP sequence variation with MRI traits were observed in either race.
Our findings of significant associations between circulating IL6 and CRP with MRI-defined brain small vessel disease are consistent with previous reports. In the population-based Rotterdam Scan Study of 1,033 individuals of similar age as the CHS participants, higher plasma CRP levels were significantly associated with presence and progression of WML, and marginally associated with greater prevalence and incidence of lacunar infarcts.23 Higher plasma levels of CRP and IL6 were associated with increased risk for silent BI in two independent cohorts of elderly Japanese.24, 39
Sequence variation in the IL6 and CRP genes has previously been shown to influence circulating levels of the two cytokines in several studies, including CHS.38, 40 In particular, the −174G/C polymorphism has been associated with increased plasma IL6 and CRP 40–42, raising the possibility that the observed association between IL6 gene variation and WML may be mediated via effects of this gene on levels of circulating inflammatory proteins. IL6 sequence variation and IL6 plasma levels remained significantly and independently associated with presence of WML in statistical models estimating their simultaneous effects, suggesting that the mechanism of the association between −174 G/C and WML may not be solely through the polymorphism influence on IL6 levels. Indeed, the −174 C/G SNP or its related haplotype accounted for only about 1% of the inter-individual variation in IL6 levels in Whites (not shown). This polymorphism may have additional functional properties relevant to cardiovascular disease pathogenesis. Indeed, a recent proteomic screen of the human serum from 151 healthy men identified apolipoprotein C1 and HSP60 as circulating proteins whose serum levels were significantly associated with IL6 −174 genotypes.43 These data raise the possibility that sequence variation in the IL6 gene may have pleiotropic effects on multiple, yet-unrecognized protein components or pathways, which may be of importance to pathogenesis of brain small vessel disease.
Several additional arguments must also be considered to explain the independent associations of IL6 polymorphism and plasma levels in our analyses. The single CRP and IL6 plasma level measurements in this study may not accurately represent the cumulative inflammatory burden experienced over the period of disease development and, thus, may not comprehensively reflect the true relationship between genotype, intermediate biomarker levels, and disease. Moreover, plasma IL6 and CRP measurement variability, including that due to intra-individual variation and to assay experimental variability 28, 44, 45 may have also occasioned incomplete adjustment for potential mediation among gene, gene products, and phenotypes.
The strong epidemiological associations that exist among risk factors, WML and BI suggest that they may share a common pathophysiology.46 Our data showing an association of variation in the IL6 gene with both WML and BI are consistent with such a hypothesis, although the underlying specific genetic variants that may influence pathophysiology may or may not be shared between the two diseases. A previous case-control study in a small sample of Spanish individuals found a significant association of the −174G/C polymorphism with increased risk of lacunar stroke.47 In a subset of the CHS cohort, this polymorphism was significantly associated with MRI-defined BI.48 In our study, the haplotype associated with increased risk of BI was tagged by the −572 G/C promoter polymorphism. Nonetheless, effects of this polymorphism, alone, are unlikely to fully account for the observed association, suggesting that a yet-unrecognized variant or variants on this haplotype background may influence risk of BI in this population.
There was no association of sequence variation in the IL6 gene with WML or BI in Blacks, despite evidence of a relationship between plasma IL6 and the two MRI traits. Possible explanations include a reduced statistical power in the smaller sample of Blacks, and/or differences in allele frequencies between the 2 racial groups, or both. For example, the power to detect a 1.2 fold increased risk of WML associated with the −174G/C polymorphism in our sample of Blacks was only 15%. Analyses in larger cohorts are warranted to further investigate the role of the IL6 gene in small-vessel disease in African-Americans.
There was no association of common variation in the CRP gene with presence of WML or BI in either racial group. These results are in agreement with recent findings from the Rotterdam Scan study and the MEMO study.49 Significant associations of CRP haplotypes with cardiovascular events, including stroke, have been recently reported in the CHS cohort.38 We cannot exclude the possibility that common CRP gene variation may have an influence on large-vessel disease pathogenesis or on the transition from covert disease to clinical disease.38
Despite the clear strengths of our study, including a well-characterized sample of older adults and a comprehensive tagSNP strategy in genes encoding two major markers of inflammation, some limitations must be acknowledged. First, although in vitro experiments showed that sequence variants in the IL6 promoter, including −174G/C, are functional 50, 51, it is important to recognize that association studies cannot distinguish the causal SNP (or combination of SNPs), among those in linkage disequilibrium (LD). Analysis of pairwise LD among polymorphic markers located in the IL6 gene region of chromosome 7 and genotyped as part of the HapMap project, shows that the −174G/C polymorphism is in significant LD with multiple SNPs within the IL6 gene but not beyond (not shown). Second, the cross-sectional nature of our analyses opens the possibility that the results obtained here were confounded by an unrecognized association of IL6 polymorphisms with associated prevalent cardiovascular conditions. However, although statistical significance of the associations between polymorphisms and traits was attenuated, the magnitude of these associations remained unchanged after exclusion of individuals with prevalent clinical disease (not shown). Finally, we have not examined possible interactions between the 2 inflammation genes. Hence, genetic variation, whose individual contribution is minimal – and perhaps undetectable – but when considered in the context of the other gene is non-negligible, may have been missed.
In summary, the present findings add to the growing body of evidence of a link between inflammation and small vessel disease of the brain and provide support for a genetic basis underlying this relationship. The molecular mechanisms by which the IL6 gene influences risk for WML and BI remain to be established. Small vessel atherosclerosis has been causally implicated in a proportion of BI.52 Atherosclerosis lesion formation in different vascular beds may share common etiologic mechanisms. Increased levels of IL6 have been recently associated with intra-cranial large vessel atherosclerosis 53, raising the possibility that IL6’s role in atherogenesis may be similar in small and large vessels. IL6 is the principal regulator of acute-phase proteins and, therefore, plays a major role in the activation of the coagulation-fibrinolysis system.54 WML have been associated with hypercoagulable conditions.55 Elevated circulating endothelial-derived adhesion molecules have been reported in patients with WML or lacunar infarcts.21, 22, 56 Leukocyte-mediated injury to the small vessels and associated upregulation of endothelial adhesion molecules can lead to blood brain barrier disruption, which has been implicated in the pathogenesis of white matter lesions.57, 58 Whether IL6 plays a role in these pathogenetic mechanisms remains to be explored.
Acknowledgments
ACKNOWLEDGEMENTS & FUNDING
The authors thank the staff and participants of the Cardiovascular Health Study for their important contributions. The authors acknowledge Jill Perrotte, Kate M. Durda, and J. Peter Durda in the Department of Pathology, University of Vermont College of Medicine, Burlington, for assistance with genotyping. The research reported in this article was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01-HC-15103, N01-HC-55222, and U01-HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org. Funds for genotyping were from grant R01-HL-071862 to A.P.R. M.F. is supported by grant R01-HL-084099 from the National Heart, Lung, and Blood Institute.
References
- 1.AHA. Heart Disease and Stroke Statistics - Update. 2005. [Google Scholar]
- 2.Longstreth WT., Jr Brain vascular disease overt and covert. Stroke. 2005;36:2062–2063. doi: 10.1161/01.str.0000179040.36574.99. [DOI] [PubMed] [Google Scholar]
- 3.Bryan RN, Wells SW, Miller TJ, Elster AD, Jungreis CA, Poirier VC, Lind BK, Manolio TA. Infarct-like lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly--data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 4.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Shahar E, Nieto J, Mosley T, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16:149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 5.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Pantoni L, Leys D, Fazekas F, Longstreth WT, Jr, Inzitari D, Wallin A, Filippi M, Scheltens P, Erkinjuntti T, Hachinski V. Role of white matter lesions in cognitive impairment of vascular origin. Alzheimer Dis Assoc Disord. 1999;13 (Suppl 3):S49–54. [PubMed] [Google Scholar]
- 8.Mosley TH, Jr, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, Folsom AR, Cooper LS, Burke GL, Liao D, Szklo M. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 9.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2005;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 10.Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Whalley LJ. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, Price T. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 12.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 14.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 15.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 18.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, Polak JF, Sutton-Tyrrell K, Herrington DM, Price TR, Cushman M. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 20.Di Napoli M, Papa F. C-reactive protein and cerebral small-vessel disease: an opportunity to reassess small-vessel disease physiopathology? Circulation. 2005;112:781–785. doi: 10.1161/CIRCULATIONAHA.104.516237. [DOI] [PubMed] [Google Scholar]
- 21.Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M. Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large- and small-vessel disease. Stroke. 1999;30:1647–1650. doi: 10.1161/01.str.30.8.1647. [DOI] [PubMed] [Google Scholar]
- 22.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–432. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 24.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke. 2005;36:768–772. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 26.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 28.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 29.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the Cardiovascular Health Study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 30.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 31.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SeattleSNPs Variation Discovery Resource. http://pga.mbt.washington.edu.
- 33.Sasieni PD. From genotypes to genes: doubling the sample size. Biometrics. 1997;53:1253–1261. [PubMed] [Google Scholar]
- 34.Seaman SR, Muller-Myhsok B. Rapid simulation of P values for product methods and multiple-testing adjustment in association studies. Am J Hum Genet. 2005;76:399–408. doi: 10.1086/428140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher RA. Statistical methods for research workers. London: Oliver and Boyd; 1932. [Google Scholar]
- 36.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comeron JM, Kreitman M, De La Vega FM. On the power to detect SNP/phenotype association in candidate quantitative trait loci genomic regions: a simulation study. Pac Symp Biocomput. 2003:478–489. [PubMed] [Google Scholar]
- 38.Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, Kuller LH, Nickerson DA, Psaty BM, Tracy RP, Reiner AP. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa J, Tamura Y, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K. Low-grade inflammation is a risk factor for clinical stroke events in addition to silent cerebral infarcts in Japanese older hypertensives: the Jichi Medical School ABPM Study, wave 1. Stroke. 2007;38:911–917. doi: 10.1161/01.STR.0000258115.46765.f1. [DOI] [PubMed] [Google Scholar]
- 40.Walston J, Fallin DM, Cushman M, Lange LA, Psaty BM, NSJ, Browner W, Tracy RP, Durda P, Reiner AP. IL-6 gene variation is associated with IL-6 and C-reactive protein levels but not cardiovascular outcomes in the Cardiovascular Health Study. Hum Genet. 2007 doi: 10.1007/s00439-007-0428-x. (in press) [DOI] [PubMed] [Google Scholar]
- 41.Basso F, Lowe GD, Rumley A, McMahon AD, Humphries SE. Interleukin-6 -174G>C polymorphism and risk of coronary heart disease in West of Scotland coronary prevention study (WOSCOPS) Arterioscler Thromb Vasc Biol. 2002;22:599–604. doi: 10.1161/01.atv.0000013283.84306.1a. [DOI] [PubMed] [Google Scholar]
- 42.Vickers MA, Green FR, Terry C, Mayosi BM, Julier C, Lathrop M, Ratcliffe PJ, Watkins HC, Keavney B. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res. 2002;53:1029–1034. doi: 10.1016/s0008-6363(01)00534-x. [DOI] [PubMed] [Google Scholar]
- 43.Hegedus CM, Skibola CF, Bracci P, Holly EA, Smith MT. Screening the human serum proteome for genotype-phenotype associations: an analysis of the IL6 −174G>C polymorphism. Proteomics. 2007;7:548–557. doi: 10.1002/pmic.200600366. [DOI] [PubMed] [Google Scholar]
- 44.Ledue TB, Rifai N. Preanalytic and analytic sources of variations in C-reactive protein measurement: implications for cardiovascular disease risk assessment. Clin Chem. 2003;49:1258–1271. doi: 10.1373/49.8.1258. [DOI] [PubMed] [Google Scholar]
- 45.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 46.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 47.Revilla M, Obach V, Cervera A, Davalos A, Castillo J, Chamorro A. A −174G/C polymorphism of the interleukin-6 gene in patients with lacunar infarction. Neurosci Lett. 2002;324:29–32. doi: 10.1016/s0304-3940(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 48.Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 49.Reitz C, Berger K, de Maat MP, Stoll M, Friedrichs F, Kardys I, Witteman JC, Breteler MM. CRP Gene Haplotypes, Serum CRP, and Cerebral Small-Vessel Disease. The Rotterdam Scan Study and the MEMO Study. Stroke. 2007;38:2356–2359. doi: 10.1161/STROKEAHA.107.482661. [DOI] [PubMed] [Google Scholar]
- 50.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 52.Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relation between interleukin-6 level and subclinical intracranial large-artery atherosclerosis. Atherosclerosis. 2007 Jun 28; doi: 10.1016/j.atherosclerosis.2007.05.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 55.Tomimoto H, Akiguchi I, Ohtani R, Yagi H, Kanda M, Shibasaki H, Yamamoto Y. The coagulation-fibrinolysis system in patients with leukoaraiosis and Binswanger disease. Arch Neurol. 2001;58:1620–1625. doi: 10.1001/archneur.58.10.1620. [DOI] [PubMed] [Google Scholar]
- 56.Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005;36:1410–1414. doi: 10.1161/01.STR.0000169924.60783.d4. [DOI] [PubMed] [Google Scholar]
- 57.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol (Berl) 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- 58.Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]