Abstract
Dopamine receptor activity in the rodent medial preoptic area (mPOA) is crucial for the display of maternal behaviors, as well as numerous other physiological and behavioral functions. However, the origin of dopaminergic input to the mPOA has not been identified through neuroanatomical tracing. To accomplish this, the retrograde tracer Fluorogold was iontophoretically applied to the mPOA of postpartum laboratory rats, and dual-label immunocytochemistry for Fluorogold and tyrosine hydroxylase later performed to identify dopaminergic cells of the forebrain and midbrain projecting to the mPOA. Results indicate that the number of dopaminergic cells projecting to the mPOA is moderate (~90 cells to one hemisphere), and that these cells have an unexpectedly wide distribution. Even so, more than half of the dual-labeled cells were found in what has been considered extensions of the A10 dopamine group (particularly the ventrocaudal posterior hypothalamus and adjacent medial supramammillary nucleus), or in the A10 cells of the ventral tegmental area. The rostral hypothalamus and surrounding region also contained numerous dual-labeled cells, with the greatest number found within the mPOA itself (including in the AVPV and PVpo). Notably, dual-labeled cells were rare in the zona incerta (A13), a site previously suggested to provide dopaminergic input to the mPOA. This study is the first to use anatomical tracing to detail the dopaminergic projections to the mPOA in the laboratory rat, and indicates that much of this projection originates more caudally than previously suggested.
Keywords: catecholamine, dopamine, female, maternal behavior, postpartum
The medial preoptic area (mPOA) of the basal forebrain is necessary for a wide range of physiological and behavioral functions in mammals, including thermoregulation (Bicego et al., 2007; Kumar et al., 2007), arousal (Kumar et al., 2007; Szymusiak et al., 2007), sodium and fluid balance (Bourque et al., 1994), gonadotropin release (Funibashi et al., 2002; Mahesh and Brann, 2005), feeding (Patterson et al., 2006; Leibowitz et al., 2007), and sexual responses (Xiao et al., 2005; Hull and Dominguez, 2006; Balthazart and Ball, 2007). The mPOA has also long been implicated in the control of maternal motivation and behavior in mammals (Numan, 1974; Numan and Insel, 2003; Lonstein and Morrell, 2006). With its high concentration of ovarian steroid receptors (Pfaff and Keiner, 1973; Parsons et al., 1982), receipt of multisensory inputs (Kevetter and Winans, 1981; Rizvi et al., 1992; Chadha and Hubscher, 2008), and connections with regions mediating motor activity and reward (Fahrbach et al., 1986; Sinnamon, 1992), the mPOA is well-equipped to organize parental responses toward offspring. Indeed, postpartum rats with lesions of the mPOA display impaired retrieval and licking of pups, and poor nest construction (Numan and Insel, 2003). Conversely, hormonal or electrical stimulation of the mPOA activates maternal behavior in normally non-maternal virgin female rats (Numan et al, 1977; Fahrbach and Pfaff, 1986; Bridges et al., 1990; Morgan et al., 1999).
Many neurochemicals released in the mPOA are critical for the display of maternal behaviors (Numan and Insel, 2003). Recent work has highlighted dopamine (DA) neurotransmission, and we have shown that infusing the D1 receptor antagonist, SCH-23390, into the mPOA of lactating rats impairs retrieval and licking of pups. This effect is specific to the D1 receptor, as neither behavior is affected by antagonizing D2 receptors in the mPOA (Miller and Lonstein, 2005). D2 receptor antagonism facilitates nursing, though, indicating that maternal behaviors are under differential control by different dopamine receptors in the mPOA. D1 receptor modulation of maternal retrieval and licking is further supported by work from Stolzenberg and colleagues (2007) demonstrating that infusing a D1 receptor agonist into the mPOA facilitates the onset of maternal responding in pregnancy-terminated rats.
D1 and D2 receptor expressions do not change between early and late pregnancy (Bakowska and Morrell, 1997). Instead, DA content of the mPOA fluctuates across pregnancy and lactation, with an increase during the first postpartum week compared to the low levels found during late pregnancy or on the day of parturition (Lonstein et al., 2003). DA levels are also higher in maternally-acting adult virgin female rats compared to non-maternal females (Olazabal et al., 2004), indicating that high DAergic tone in the mPOA is associated with high maternal responsiveness.
Although DA in the mPOA is essential for maternal behaviors, the source of DAergic input to the female rat mPOA is unclear. The mPOA receives afferents of varying strength from DA-rich sites including the periventricular hypothalamus, zona incerta, and ventral tegmental area (VTA) (Chiba and Murata, 1985; Simerly and Swanson, 1986; Wagner et al., 1995). Previous research lesioning specific DA cell groups, or transecting fiber tracts leading to the basal forebrain, suggested that the mPOA and most of the hypothalamus receive almost all their DAergic input from within the forebrain (Jonsson et al., 1972; Weiner et al., 1972; Björklund et al., 1975; Kizer et al., 1976; Palkovits et al., 1977; Horvath et al., 1993; Day et al., 1980). However, there have never been anatomical tracing studies that confirm this for the mPOA. In the present study, we identified the origins of DAergic input to the mPOA by iontophoretically applying the retrograde tracer Fluorogold (FG) to the mPOA of postpartum rats, and then using double-label immunocytochemistry (ICC) to reveal cells containing immunoreactivity for both FG and tyrosine hydroxylase (TH - the rate-limiting enzyme for catecholamine synthesis). Locating the neurons responsible for DA’s presence in the mPOA is a necessary step toward understanding the mPOA’s role in maternal behaviors, and its other physiological and behavioral functions.
Experimental Procedures
Subjects
Subjects were adult female Long-Evans rats born and raised in our laboratory, descended from rats purchased from Harlan Laboratory (Indianapolis, IN), and mated with sexually experienced Long-Evans male rats from our colony. After mating, females were housed with other mated females, 2–3 per cage, in clear polypropylene cages (48 × 28 × 16) with wood shavings for bedding, food and water available ad libitum, and a 12:12 h light/dark cycle. Approximately 5 days before parturition, females were individually housed. Litters were culled to contain 8 pups (4 females and 4 males) within 48 hours after parturition. The experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), as well as the Institutional Animal Care and Use Committee at Michigan State University.
Stereotaxic Surgery and Iontophoresis
Within 48 hours after parturition, litters were removed and placed in an incubator set at nest temperature (~34°C), and dams were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg; Butler Co., Dublin OH) followed by an intramuscular injection of xylazine (4 mg/kg; Butler Co, Columbus, OH). After placement in a Kopf stereotaxic instrument, the subject’s scalp was cleaned with ethanol and anesthetized with a subcutaneous injection of 0.1 ml of 3% lidocaine. A 1.5 cm incision was made in the scalp, and a hole was drilled above the mPOA (A/P −0.2 mm, M/L +0.5 mm from bregma) on the left side of the brain. Glass micropipettes with tip diameters of approximately 30–50 µm were backfilled with a 4% solution of the retrograde tracer Fluorogold dissolved in saline (FG; Flurochrome, Denver, CO). An FG-filled pipette was lowered slowly into the brain to a depth of −8.8–9.0 mm measured from the top of the skull. Approximately 30 seconds after the pipette was lowered, alternating current (5 µA, 7 seconds on/7 seconds off) was run for 10 minutes with the use of an iontophoretic current generator (Stoeling, Wood Dale, IL) through a stainless-steel wire placed in the pipet. The current was then turned off, the pipet left in the brain for another 5 minutes, and then slowly retracted from the brain. The craniotomy was packed with sterile gelatin foam (Gelfoam, Upjohn, Kalamazoo, MI), the scalp closed with surgical staples, and the subject given a subcutaneous injection of 0.015 mg/kg of the analgesic, buprenorphine. Subjects recovered on a heating pad in their home cage for approximately one hour, after which pups were returned and the cage brought back to the colony room. The following morning, subjects received another injection of buprenorphine.
Perfusion and tissue collection
One week after surgery, rats were overdosed with sodium pentobarbital and perfused through the heart with 200 ml saline followed by 200 ml 4% paraformaldehyde in sodium phosphate buffer (NaPB, pH 7.6). Brains were submerged in 4% paraformaldehyde overnight, then in 20% sucrose for at least three days before being cut into 35 µm sections with a freezing microtome. During sectioning in the coronal plane, a hole was punched with an 18-gauge, blunt-tipped syringe needle through the right hemisphere of the cortex or dorsal striatum to maintain section orientation during mounting and differentiate between the FG-infused and non-infused side of the brain. Brain sections were stored at −20°C in a sucrose-based cryoprotectant until immunocytochemical processing.
Dual-label immunocytochemistry
A one-in-three series of sections through the forebrain and midbrain was rinsed three times with Trisma-buffered saline (TBS), incubated in 0.1% sodium borohydride for 15 minutes, rinsed three times with TBS, incubated in 1% hydrogen peroxide in 0.3% Triton-X for 10 minutes, rinsed again with TBS, incubated in 20% normal goat serum in 0.3% Triton-X for at least 30 minutes, and then incubated in a solution of 2% normal goat serum in 0.3% Triton-X containing a rabbit polyclonal primary antiserum for FG (AB153, 1:3000; Chemicon, Temecula, CA) overnight at room temperature. The next day, sections were rinsed three times with TBS, incubated in a goat anti-rabbit biotinylated secondary antiserum (dilution 1:500; Vector Labs, Burlingame, CA) for 60 minutes, rinsed with TBS, incubated in avidin-biotin complex for 60 minutes, rinsed with TBS and then visualized using a solution of hydrogen peroxide and 3–3’-diaminobenzadine, which revealed the FG as a dark brown punctuate label throughout the cells.
The tissue was then rinsed four times with TBS, incubated in 20% normal goat serum in 0.3% Triton-X for at least 30 minutes, then incubated in a solution of 2% normal goat serum in 0.3% Triton-X that contained a mouse polyclonal primary antiserum for tyrosine hydroxylase (TH) (AB5986, 1:2000; Chemicon International, Temecula, CA) overnight at room temperature. The next day, the tissue was rinsed three times in TBS, then incubated in a rabbit anti-mouse biotinylated secondary antiserum (dilution 1:500; Vector Labs, Burlingame, CA) for 60 minutes, rinsed in TBS, incubated in avidin-biotin complex for 60 minutes, rinsed in TBS, then visualized using Vector SG (Vector Laboratories, Burlingame, CA), which provided a diffuse light blue label thoughout the cytoplasm. Tissue was then rinsed four times in TBS, mounted onto glass microscope slides, dehydrated and coverslipped. Cross-reactivity was not expected, given that the primary antisera were obtained from different species, and visual inspection of the tissue revealed that numerous single-labeled cells of both types were clearly visible in many brain areas. We also performed immunocytochemical controls that included omitting one or both of the primary and secondary antisera, which eliminated the corresponding specific labeling.
Microscopic Analysis
Eight dams were selected for detailed analysis because of their accurately placed and relatively restricted FG deposits in the mPOA (see Results below for details). An additional 51 females were not analyzed because they had considerable FG deposited outside the mPOA, including along the pipet track, in the ventral septum, anterior commissure, optic tract, and/or extensively into the anterior hypothalamus. All TH-immunoreactive cells in both hemispheres of the forebrain and midbrain were examined with a Nikon E400 light microscope at 200× magnification to identify potential dual-labeled cells, and each candidate cell re-examined under 400× magnification to confirm the presence or absence of double-labeling. A cell was considered double-labeled only if its soma and any visible processes contained unmistakably overlapping FG- (brown) and TH- (blue) immunoreactive products. Any ambiguously labeled cells were not considered dual-labeled. Dual-labeled cells were mapped onto representative plates from Swanson’s (1998) atlas of the rat brain. Pilot work demonstrated that confocal analysis of fluorescing secondary antisera was unfeasible because the small number and very wide distribution of dual-labeled cells, including many outside the dense accumulations of DAergic cells in the A8–15 groups, required slow and thorough visual scanning of all TH-immunoreactive cells in the forebrain and midbrain. This resulted in tremendous loss of fluorescence before brains could be comprehensively examined, so using permanent immunoreactive labels was the only method possible to identify all TH-immunoreactive cells projecting to the mPOA.
To estimate the total number of dual-labeled cells in each brain, the number of these cells for each subject found in the one-in-three series of sections was first multiplied by three, and this number then subjected to an Abercrombie correction factor of 0.7 (Abercrombie, 1946). This factor was calculated based on an average somal length of 15 µm, which is conservative given the ~10–15 µm average length of TH-immunoreactive neurons in the brain areas we found to contain dual-labeled cells (Björklund and Nobin, 1973; Chan-Palay et al., 1984; Hayakawa and Zyo, 1994; Defazio et al., 2000; Huot et al., 2007). A number of sites (LPOA, AVP, SCH, PVHm, REa, SBPV, REl, MEin, ml, SPFm, PF, fr, PMd, IF, rust, RN, IPNr, mlf, III, RR, and LDT) contained 1–3 dual-labeled cells in not more than one of the eight subjects. These were thought to be very minor projections, if not spurious, so were not further considered.
Results
Location of iontophoretically applied Fluorogold
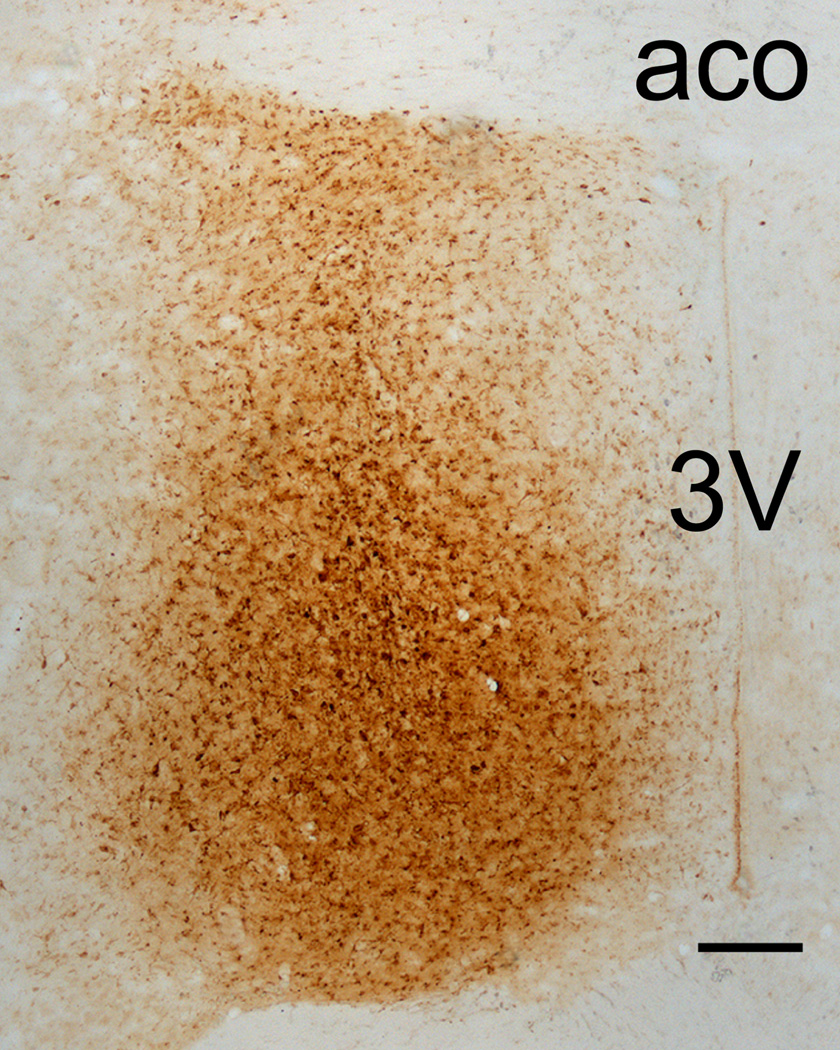
The eight subjects analyzed in detail each had a dense core of FG focused on the medial portion of the mPOA (Figures 1 and 2), an area that contains many TH-immunoreactive fibers (Simerly et al., 1986; Figure 3). In all subjects, these deposits included portions of the MPN and AVP. In most subjects (exception #53), the deposits also involved the AVPV, ADP, and/or PSCH. In some subjects (#35, 52, 37, 57, 53), FG extended into medial portions of the LPOA, NDB and/or BSTv. Lastly, the core FG deposit in one subject (#53) extended more caudally than others to include the rostral AHN (Figure 1). These core deposits were surrounded by a “halo” of lighter FG immunoreactivity, but previous work suggests this diffusion zone does not contain sufficient tracer to be retrogradely transported (Leak and Moore, 1997; Linke, 1999; Linke et al. 2004).
Figure 1.
Schematic representations of the dense core of FG deposits in the mPOA (indicated with gray shading) of each of the subjects examined in detail. Subject numbers referenced in text are at top left of panels. Subjects arranged across from the top left to bottom right according to the rostrocaudal placement of FG deposit. Abbreviations as in list above.
Figure 2.
Photomicrograph of the mPOA of Subject #57 after immunocytochemical processing for FG and TH. aco = anterior commissure. 3V = third ventricle. Bar = 200 µm.
Figure 3.
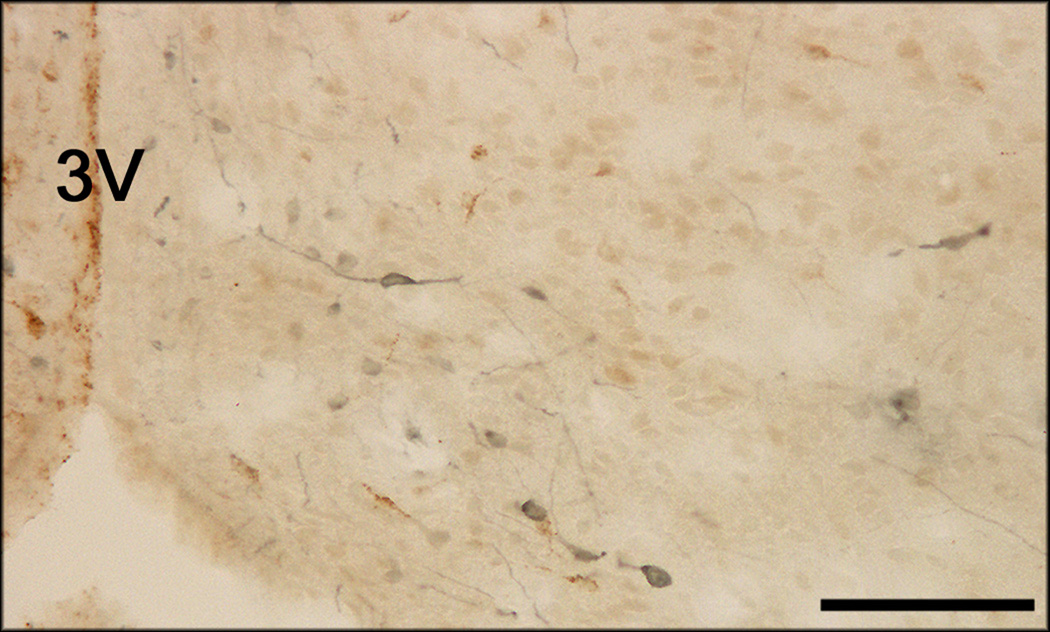
Photomicrograph of TH-immunoreactive fibers in the mPOA of a lactating rat, contralateral to a deposit of FG. 3V = third ventricle. Bar = 200 µm.
Distribution of cells containing Fluorogold and TH immunoreactivity
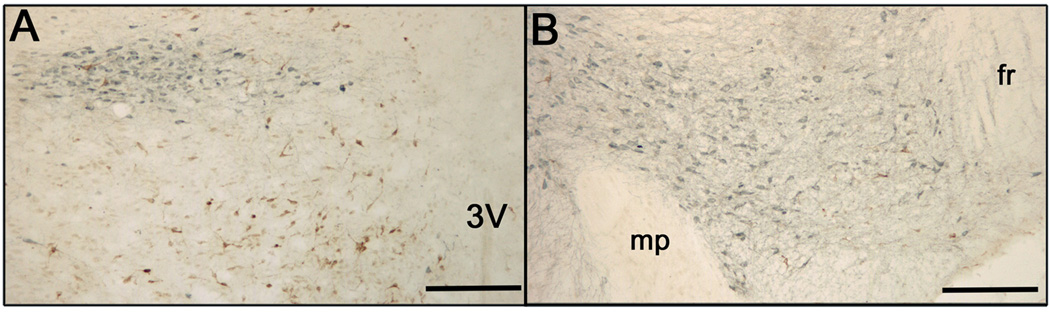
The distribution of cells containing FG immunoreactivity after retrograde tracer application into the mPOA was similar to numerous published reports (e.g., Chiba and Murata, 1985; Simerly and Swanson, 1986), with many FG-labeled cells in major projections to the mPOA, such as the MeApd (Figure 4). Similarly, the distribution of cells containing only TH immunoreactivity was similar to previous descriptions (Dahlstrom and Fuxe, 1964; Björklund et al., 1975; Chan-Palay et al., 1984; van den Pol et al., 1984), with many TH-immunoreactive cells found in the hypothalamus and surrounding region (e.g., zona incerta - Figure 5a), as well as in the brainstem (e.g., VTA - Figure 5b).
Figure 4.
Photomicrograph of FG-immunoreactive cells in the MeApd of a lactating rat after FG was iontophoretically applied to the mPOA one week before sacrifice. opt = optic tract. Bar = 200 µm.
Figure 5.
TH-immunoreactive (blue labeled) and FG-immunoreactive (brown labeled) cells in the A) zona incerta and B) VTA of a lactating rat after FG was iontophoretically applied to the mPOA one week before sacrifice. 3V = third ventricle, mp = mammillary peduncle, fr = fasciculus retroflexus. Bars = 200 µm.
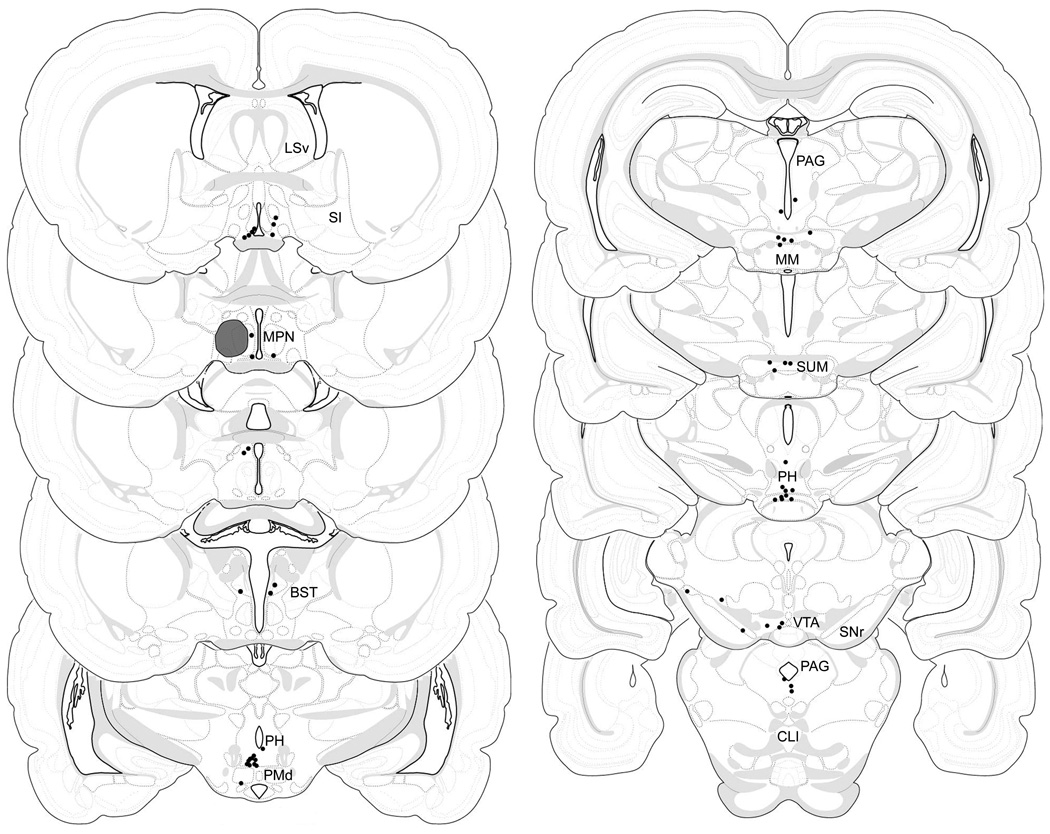
Semi-quantitative analysis revealed that the average number of cells found to contain both TH and FG immunoreactivity in the one-in-three series of brain sections analyzed for each subject was 42 ± 8. Given this, we used an Abercrombie correction (Abercrombie, 1946) to estimate the average number of dual-labeled cells in the brain that project to one mPOA hemisphere to be 89 ±16. These dual-labeled cells had a very widespread distribution throughout the forebrain and midbrain (Figure 6; Table 1). Even so, more than one-third of the dual-labeled cells were found in the ventrocaudal PH (~26% of total) and immediately adjacent SUM (~13% of total), with most of these cells ipsilateral to the FG deposit sites (Figure 7). The VTA (~8% of total), SN (~7% of total), and some subdivisions of the PAG (PAGm, PAGrm, PAGvl; collectively ~6% of total) also contained numerous dual-labeled cells. The rostral hypothalamic region with the greatest number of cells containing both FG and TH immunoreactivity was the ipsilateral mPOA outside the FG deposits (including the AVPV, MPN, and PVpo), where ~10% of all dual-labeled cells were found. It is notable, however, that many TH-immunoreactive cells in this area immediately adjacent to the FG deposit sites were not retrogradely labeled (Figure 8). Lastly, a small number of dual-labeled cells were found in at least two subjects within the PSCH, BSTd, AHN, ADP, PVHp, LHA, RCH, PV, ZI, TU, VMHdm, DMH, cpd, smd, RL, CLI, and DR (Table 1; Figure 9).
Figure 6.
Diagrammatic representation of the distribution of dual-labeled cells throughout the brain of Subject #57. Each dot represents one cell retrogradely labeled after Fluorogold application into the mPOA, and also immunoreactive for TH. Shaded area on second panel indicates the FG deposit. Figures modified from Swanson (1998).
Table 1.
Relative number of cells dual labeled for TH and FG in a one-in-three series of sections through the brains of eight postpartum female rats after mPOA application of FG. − = no dual-labeled cells, + = 1–3 cells, ++ = 4–9 cells, +++ = 10–15 cells, ++++ = 16–30 cells. Sites arranged top to bottom at left in approximate rostrocaudal order. Subject numbers arranged left to right across the top according to rostrocaudal location of the core of their FG deposit.
| FG 28 | FG 35 | FG 51 | FG 52 | FG 37 | FG 55 | FG 57 | FG 53 | |
|---|---|---|---|---|---|---|---|---|
| Brain Site | ||||||||
| mPOA | − | + | + | + | + | ++ | ++ | ++ |
| ADP | − | − | − | − | − | + | − | + |
| PSCH | − | + | + | − | − | − | + | + |
| BSTd | + | − | − | + | + | + | − | − |
| PV | + | − | − | + | + | + | + | + |
| RCH | − | + | − | + | − | − | − | − |
| AHN | − | + | − | + | ++ | + | − | − |
| TU | + | − | − | + | − | − | − | − |
| PVHp | − | − | + | + | − | + | + | + |
| LHA | + | − | − | − | + | + | − | + |
| ZI | + | − | − | − | − | − | − | + |
| cpd | + | − | + | − | − | − | − | − |
| VMHdm | + | − | − | − | + | − | − | + |
| DMH | + | − | − | − | + | + | − | + |
| PH | + | ++ | ++ | ++ | +++ | ++++ | +++ | +++ |
| SUM | + | + | + | − | ++ | +++ | +++ | ++ |
| smd | − | − | − | − | − | + | + | − |
| MM | − | − | − | − | − | + | + | ++ |
| RL | − | + | + | − | + | + | − | − |
| VTA | ++ | ++ | ++ | − | ++ | + | + | + |
| SN | ++ | + | ++ | − | + | − | + | − |
| CLI | − | − | ++ | − | − | − | − | + |
| PAGm | − | − | − | − | + | ++ | + | + |
| PAGrm | − | − | − | − | − | − | + | + |
| PAGvl | − | − | + | − | + | + | + | + |
| DR | − | − | + | − | + | − | − | + |
Figure 7.
Photomicrographic representation of dual-labeled cells in the PHv of Subject #55. Top panel – Location of PHv, with gray-shaded box indicating area shown in photomicrograph immediately below. Figure modified from Swanson (1998). Lower panel - 200× magnification of area indicated in top panel. Brown-labeled cells contain FG immunoreactivity, while blue-labeled cells contain TH immunoreactivity. Bar = 50 µm. Boxes indicate representative cells shown further below at 600× magnification containing (A) FG immunoreactivity, (B) TH immunoreactivity, (C) both FG and TH immunoreactivity. Bars in 600× magnification photos = 10 µm.
Figure 8.
Photomicrograph of TH-immunoreactive cells immediately adjacent to the FG deposit site of Subject #53. Arrows point to light blue-shaded cells whose cytoplasm contains TH-immunoreactivity, but not FG immunoreactivity, which is indicated in many nearby cells by dense brown cytoplasmic staining. 3V = third ventricle. Bar = 100 µm.
Figure 9.
Schematic representation of a horizontal section through the rat brain, indicating sites providing TH-immunoreactive projections to the postpartum rat mPOA. Gray-shaded area indicates an FG deposit in the mPOA. Bold-faced text highlights the sites containing the greatest number of TH-immunoreactive cells projecting to the mPOA. Figure modified from Paxinos and Watson (2007). Abbreviations as in list above.
Discussion
Our semi-quantitative analysis revealed that fewer than 90 TH-immunoreactive cells project to one hemisphere of the mPOA of the postpartum female rat, which is completely consistent with the relatively low DA content of the mPOA, even when it is compared to the levels found in nearby hypothalamic regions (Kizer et al., 1976; Palkovitz et al., 1977; Lookingland and Moore, 1984; Simerly, Gorski and Swanson, 1986; Simerly and Swanson 1987). These dual-labeled cells were widely distributed across the brain (see Figure 9), including in relatively caudal regions containing the extended and intrinsic A10 group, as well as within the preoptic area and hypothalamus.
Extended and intrinsic A10 group
More than one-third of the cells immunoreactive for both TH and FG were found at the juncture of the forebrain and midbrain, in a collection of midline catecholaminergic neurons sometimes suggested to be extensions of the A10 group based on their proximity to and somewhat similar anatomical projections as cells of the intrinsic A10 group (Dahlstrom and Fuxe, 1964; Swanson, 1982; Chan-Palay et al., 1984; Hokfelt et al., 1984; Descarries et al., 1986; Stratford and Wirtshafter, 1990; Hasue and Shammah-Lagnado, 2002; although Shepard et al., 1988; Abrahamson and Moore, 2001). These sites include the PHv, medial SUM, PAGvl, DR, and the rostral and central linear nuclei of the raphe. Of these sites, we found that the PHv and immediately adjacent medial SUM contained the most dual-labeled cells. Each has been shown to project to the mPOA (Simerly and Swanson, 1986; Vertes, 1992; Vertes et al., 1995), and it is herein indicated that some of these projections are catecholaminergic. Neurons in these sites, and all others sites we describe herein as TH-immunoreactive, are presumed to be DAergic. This is supported by the fact that catecholaminergic cells of the forebrain and midbrain of laboratory rats are immunoreactive for DA, but rarely or never contain the enzymes necessary for epinephrine or norepinephrine synthesis (see Smeets and Gonzalez, 2000).
It is notable that, even though DA release in the mPOA is essential for maternal and other reproductive behaviors (Dominguez and Hull, 2005; Miller and Lonstein, 2005; Stolzenberg et al., 2007), the number of DAergic cells projecting from the PHv and SUM to the mPOA was actually quite small. Indeed, for most subjects, the one-in-three series of tissue analyzed had fewer than 15 dual-labeled cells in these two sites. Most of the projection from the PHv and SUM to the mPOA, therefore, is probably not DAergic. In support, the number of cells projecting from either the PHv or SUM to just the sexually dimorphic nucleus (SDN) within the mPOA appears to be much greater than the number of DAergic cells we found projecting from these sites (Simerly and Swanson, 1986; Vertes, 1988). The phenotype of non-TH cells projecting from the PHv to the mPOA is unknown, but many cells projecting from the medial SUM to the mPOA are glutamatergic (Kocsis et al., 2003). DAergic cells of the SUM instead preferentially project to the lateral septum and other mammillary nuclei (Swanson, 1982; Gonzalo-Ruiz et al., 1992).
The functions of the PHv and SUM are consistent with a potential role in postpartum physiology and behavior. These include PHv ability to modulate blood pressure, heart rate, and core body temperature in rats (e.g., Spencer et al., 1990), and the SUM to regulate memory, anxiety, and behavioral inhibition (Pan and McNaughton, 1997, 2002; Day et al., 2004; Shahidi et al., 2004; Aranda et al., 2006; Smith and Lonstein, 2008). Swanson (2000) suggests that the medial mammillary region is necessary for motivated behaviors, such as maternal behavior. Stimulation of the PHv and SUM is rewarding (Ikemoto et al., 1997; 2004; Ikemoto, 2005), and through projections to the mPOA might contribute to maternal motivation. Indeed, mutant mice with disrupted mammillary body development do not display maternal behaviors (Wehr et al., 1997). However, lesions of the entire mammillary region that likely involved the PHv do not abolish postpartum maternal behaviors in rats (Galindo-Estaun, 1983), but instead somewhat hasten females’ retrieval of pups (Galindo-Estaun, 1983). Therefore, DAergic projections from the PHv and SUM to the mPOA may not be essential for maternal behaviors, but instead more involved in determining the rate that some subcomponents of the behavioral repertoire are displayed. If the same DAergic projections from the PHv and SUM to the mPOA exist in male rats, they could be relevant for males’ sexual motivation and behavior (Dominguez and Hull, 2005; Hull and Dominguez, 2006). In fact, very similar to maternal behaviors, lesions of the entire mammillary region and surrounding tissue do not eliminate copulation in male rats, but rather, disinhibit it (Lisk, 1966, 1969). Lastly, female rhesus monkeys with posterior hypothalamic lesions extending into the mammillary bodies show advanced puberty (Terasawa et al., 1984; Windsor-Engnell et al., 2007), the onset of which involves DAergic influence in the mPOA (Wuttke et al., 1980; Docke et al., 1987). Thus, DAergic projections from the PHv and mammillary region to the mPOA may be an important contributor to the balance of inhibitory and excitatory inputs influencing a range of reproductive processes.
Dual-labeled cells were also found within the VTA, which contains most of the A10 catecholamine group. A small to moderate projection from the VTA to the mPOA has previously been demonstrated, but the chemical phenotype has been unknown (Fallon and Moore, 1978; Simon et al., 1979; Kita and Oomura, 1982; Simerly and Swanson, 1986; Vertes, 1988). 6-hydroxydopamine lesions of A10 cells in the VTA disrupt maternal retrieval of pups (Hansen et al., 1991), as does severing the connections between the mPOA and VTA (Numan and Smith, 1984). Disruption of DAergic projections from the VTA to the mPOA could, in part, be responsible for these effects on maternal behaviors.
We were surprised to find some dual-labeled cells in the A9 group of the substantia nigra because, although a small projection from the mPOA to the SN may exist (Chiba and Murata, 1985), we are unaware of any report of the reverse projection in rodents. The number of dual-labeled cells in the SN was relatively small (average of ~3 cells per subject), but this was a reliable finding involving 5 of our 8 subjects. This very small projection could have been overlooked in previous studies of mPOA afferents or nigral efferents, or perhaps only exists in postpartum females, as nigral projections to the mPOA have been examined only in male or non-lactating female rats (Beckstead et al., 1979; Swanson, 1982; Deutch et al., 1986; Simerly and Swanson, 1986; Vertes, 1988). Severing the lateral connections of the mPOA of postpartum female rats with a knife covered with HRP does not retrogradely label cells in the SN, though (Numan et al., 1990). Nonetheless, a small DAergic projection from the SN to the mPOA does exist in Japanese quail (Coturnix japonica; Balthazart and Absil, 1997), which lends support to our findings, and suggests that further examination of this projection in rats is warranted.
Preoptic area
The mPOA outside the Fluorogold deposits contained numerous dual-labeled cells. Some of these cells were in the rostral A14 periventricular dopamine group, although dual-labeled cells were also found in more ventral and lateral portions of the mPOA. These results are consistent with previous work indicating a mostly ipsilateral projection from the A14 cells to medial regions of the POA (Day et al., 1980; Simerly and Swanson 1986, 1988; Horvath et al., 1993; Gu and Simerly, 1997). Because many TH-immunoreactive cells in or near the A14 group were not retrogradely labeled, they must provide DAergic input to yet unknown extra-mPOA sites. It is interesting that one of our subjects (#28) had no dual-labeled cells in the A14 or elsewhere within the mPOA. This subject had the most rostral FG deposit of all of our subjects, and this may be particularly relevant, because few or no A14 cells project to the rostral POA in sheep, either (Tillet et al., 1993; Scott et al., 2003).
The A14 group of the female rodent AVPV contains 2–5-fold more TH-immunoreactive cells than that of males (Simerly et al., 1985a,b; Zup et al., 2003; Lansing and Lonstein, 2006), and these cells are sensitive to gonadal hormones circulating during adulthood (Simerly, 1989; Lansing and Lonstein, 2006). We were particularly interested in DAergic regulation of maternal behaviors so, therefore, studied DAergic projections in postpartum female rats. Given the hormonal sensitivity of A14 and other DA cell groups in the brain, it could be useful to determine if our findings generalize to male rats or female rats in other reproductive/endocrine states.
Midbrain versus hypothalamic sources of DA to the mPOA
It was unexpected that approximately half of the dual-labeled cells in this study were found very caudally within, or completely outside, the hypothalamus. Even recently, we and others have stated that the primary source of DA to the mPOA is from the A13 and A14 cells of the hypothalamus (e.g., Paredes and Agmo, 2004; Dominguez and Hull, 2005; Miller and Lonstein, 2005; Guarraci et al., 2008). This is despite the fact that the A13 and surrounding cells of the medial ZI provide a paucity of projections to most of the mPOA (Simerly and Swanson, 1986; Horvath et al., 1993; Wagner et al., 1995; Sita et al., 2007). Indeed, only two of our eight subjects (#28 and #53) had any dual-labeled cells in the A13 group, and the number of these cells was very small (1–3 cells each).
Attributing the source of DAergic input to the mPOA as the A13 and A14 cells is probably based on early work emphasizing self-containment of DA systems within the hypothalamus. For example, after using electrolytic lesions or knifecuts to destroy A9 and A10 projections, Björklund et al., (1975) found that catecholamine fibers throughout the preoptic area and hypothalamus remained intact via visual inspection of catecholamine fluorescence. However, they state that some intrinsic A10 cells did escape lesioning, and many extended A10 cells were probably rostral to their lesions or knifecuts. Additionally, catecholamine fluorescence in the brain is not linearly related to changes in catecholamine tissue content, and a very large decrease in tissue content may be necessary before changes in fluorescence are readily detectable (Kopin et al., 1974; Landis et al., 1975). Kizer and colleagues (1976) further examined the source of basal forebrain DA by electrolytically lesioning the A8, A9, and A10 groups in male rats; these lesions were explicitly stated to extend to the supramammillary decussation, and involved both the PHv and medial SUM. They found a 20% drop in DA content in the mPOA, although this change was not statistically significant. Similarly, pharmacological inhibition of A9 and A10 cells that increases DA stores in cell terminals produces a 30% increase in DA content in the MPN (Lookingland and Moore, 1984). Thus, previous data do indicate A9 and A10 cells as a source of some DAergic input to the mPOA.
Conclusions and Implications
Most DA-producing neurons in the rat brain are located in catecholamine cell groups A8-A15 (Dahlstrom and Fuxe, 1964), but many DAergic cells are scattered outside these collections (Hökfelt et al., 1984; van den Pol et al., 1984). We found that no single DA group contained the majority of DA cells projecting to the mPOA, and that more than half of these cells were outside the A8–15 groups. Cells of the extended A10 group, and those of the A10 group itself, collectively contributed the largest component of the DAergic projection to the mPOA. This source is substantially more caudal than often suggested. Still, some of the DAergic projection to the mPOA did originate from cells in or near the hypothalamus. The literature on laboratory rats suggests that this widespread distribution might be unexpected, but very similar to our results, Balthazart and Absil (1997) found that ~100 TH-immunoreactive cells could be retrogradely labeled from one hemisphere of the nucleus preopticus medialis (POM – analogue of mammalian mPOA) of male Japanese quail, but that only ~60% of these cells were in or near the hypothalamus. The remainder was found in the A8, A9 and A10 groups, leading them to conclude that the contribution of the midbrain to the DAergic projection to the POM was “not negligible” (Balthazart and Absil, 1997). Similarities between our results and those of Balthazart and Absil (1997) indicate some conservation of the DAergic projections to the mPOA across vertebrate classes. Additionally, that a relatively small number of neurons can drive the many DA-dependent functions of the rat mPOA or quail POM is consistent with how some other neural systems produce their effects. For example, fewer than ten cells producing gonadotropin-releasing hormone (GnRH) can drive gonadal function in male mice (Silverman et al., 1985), and even a handful of motor cortex neurons can control arm and wrist movements in monkeys (Moritz et al., 2008).
DA release in the mPOA is essential for maternal behaviors (Lonstein et al., 2003; Olazabal et al., 2004; Lonstein and Miller, 2005; Stoltzenberg et al., 2007), as well as for male sexual behaviors (Hull et al., 1986; Hull and Dominguez, 2006), and may be a neurochemical message broadly necessary for mammalian sociosexual behaviors (Stern, 1990; Newman, 1999; Lonstein, 2002). Because the mPOA contains fibers and receptors for many other neurotransmitters (e.g., Petetti et al., 1982; Halpain et al., 1984; Nock et al., 1985; Simerly et al., 1986; Simerly and Swanson, 1987) and neuropeptides (e.g., Hammer, 1984; Dubois-Dauphin et al., 1996; Simerly et al., 1986; Simerly and Swanson, 1987; Yoshimura et al., 1993), convergence between DA and other chemical signals in the mPOA is surely functionally significant (as in Liu and Wang, 2003). Destroying the cells providing DAergic input to the mPOA would help determine this, but the widespread distribution of TH cells retrogradely labeled from the postpartum rat mPOA seems to render any manipulations of its DA at the source quite difficult. Based on our results, destruction of the extended and intrinsic A10 groups might only eliminate about one-third of the DA content in the mPOA. Targeting DAergic fibers in the mPOA is equally problematic, as it contains little DA transporter (Meister and Elde, 1993; Lorang et al., 1994), and 6-hydroxydopamine infusions in the mPOA produce variable and incomplete lesions (Bazzett et al., 1992; Sood et al., 1997; Dhawan et al., 1998). Thus, pharmacological manipulations of DA receptor activity within the mPOA seem to be the best strategy for determining how DA produces its behavioral and physiological effects by acting within this site.
Acknowledgements
The authors would like to thank Dr. Geert J. De Vries for assistance with establishing the methodology used for this project, and Dr. Sharleen Sakai for useful comments on drafts of this manuscript. This work was supported by NIH grant HD40894 to J.S. Lonstein.
Abbreviations
- III
oculomotor nucleus
- aco
anterior commissure
- ADP
anterodorsal preoptic nucleus
- AHN
anterior hypothalamic nucleus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral preoptic area
- BSTd
bed nucleus of the stria terminalis, dorsomedial
- BSTv
bed nucleus of the stria terminalis, ventromedial
- CLI
central linear nucleus raphe
- cpd
cerebral peduncle
- DMH
dorsomedial hypothalamic nucleus
- DR
dorsal raphe
- fr
fasciculus reroflexus
- fx
fornix
- IF
interfascicular nucleus raphe
- IPNr
interpeduncular nucleus, rostral part
- LHA
lateral hypothalamic area
- LDT
laterodorsal tegmental nucleus
- LPOA
lateral preoptic area
- LSv
lateral septum, ventrolateral
- MeApd
medial amygdala, posterodorsal
- MEin
median eminence interal lamina
- mfb
medial forebrain bundle
- ml
medial lemniscus
- mlf
medial longitudinal fascicle
- mPOA
medial preoptic area
- MPN
medial preoptic nucleus
- NDB
nucleus of the diagonal band
- och
optic chiasm
- opt
optic tract
- PAGm
periaqueductal gray, medial
- PAGrm
periaqueductal gray, rostromedial
- PAGvl
periaqueductal gray, ventrolateral
- PF
parafascicular nucleus
- PH
posterior hypothalamus
- PHv
posterior hypothalamic nucleus, ventrocaudal
- PMd
dorsal premammillary nucleus
- PV
periventricular hypothalamic nucleus
- PVHm
paraventricular hypothalamic nucleus, magnocellular
- PVHp
paraventricular hypothamic nucleus, parvicellular
- Pvpo
preoptic periventricular nucleus
- PSCH
suprachiasmatic preoptic nucleus
- RCH
retrochiasmatic nucleus
- REa
nucleus reuniens, anterior
- REl
nucleus reuniens, lateral
- RL
rostral linear nucleus raphe
- RN
red nucleus
- RR
retrorubral field
- rust
rubrospinal tract
- SBPV
subparaventricular zone of the hypothalamus
- SCH
suprachiastmatic nucleus
- SI
substantia innominata
- smd
supramammillary decussation
- SPFm
subparafascicular nucleus of the thalamus, magnocellular
- SUM
supramammillary nucleus
- SN
substantia nigra
- TU
tuberal nucleus
- VMHdm
ventromedial hypothalamus, dorsomedial
- VTA
ventral tegmental area
- ZI
zona incerta
- 3V
third ventricle
References
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- Aranda L, Santín LJ, Begega A, Aquirre JA, Arias JL. Supramammillary and adjacent nuclei lesions impair spatial working memory and induce anxiolitic-like behavior. Behavioural Brain Res. 2006;167:156–164. doi: 10.1016/j.bbr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bakowska JC, Morrell JI. Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J Comp Neurol. 1997;386:161–177. doi: 10.1002/(sici)1096-9861(19970922)386:2<161::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382(3):401–428. [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28(4):161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett T, Lumley L, Bitran D, Markowski V, Warner R, Hull E. Male rat copulation following 6-OHDA lesions of the medial preoptic area: resistance to repeated administration and rapid behavioral recovery. Brain Res. 1992;580(1–2):164–170. doi: 10.1016/0006-8993(92)90940-b. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175(2):191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Bicego KC, Barros RC, Branco LG. Physiology of temperature regulation: comparative aspects. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(3):616–639. doi: 10.1016/j.cbpa.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O, Nobin A. Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Res. 1975;89:29–42. doi: 10.1016/0006-8993(75)90131-6. [DOI] [PubMed] [Google Scholar]
- Björklund A, Nobin A. Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrenaline cell groups in the rat diencephalon. Brain Res. 1973;51:193–205. doi: 10.1016/0006-8993(73)90372-7. [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Evidence for a major spinal cord projection from the diencephalic A11 dopmaine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res. 1979;177(1):170–175. doi: 10.1016/0006-8993(79)90927-2. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol. 1994;15(3):231–274. doi: 10.1006/frne.1994.1010. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha HK, Hubscher CH. Convergence of nociceptive information in the forebrain of female rats: reproductive organ response variations with stage of estrus. Exp Neurol. 2008;210(2):375–387. doi: 10.1016/j.expneurol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Záborszky L, Köhler C, Goldstein M, Palay SL. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of rats. J Comp Neurol. 1984;227(4):467–496. doi: 10.1002/cne.902270403. [DOI] [PubMed] [Google Scholar]
- Chiba T, Murata Y. Afferent and efferent connections of the medial preoptic area in the rat: a WGA-HRP study. Brain Res Bull. 1985;14(3):261–272. doi: 10.1016/0361-9230(85)90091-7. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;64:1–55. [PubMed] [Google Scholar]
- Day TA, Blessing W, Willoughby JO. Noradrenergic and dopaminergic projections to the medial preoptic area of the rat. A combined horseradish peroxidase/catecholamine fluorescence study. Brain Res. 1980;193:543–548. doi: 10.1016/0006-8993(80)90185-7. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025(1–2):139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Defazio RA, Pong K, Knusel B, Walsh JP. Neurotrophin-4/5 promotes dendritic outgrowth and calcium currents in cultured mesencephalic dopamine neurons. Neuroscience. 2000;99(2):297–304. doi: 10.1016/s0306-4522(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, Beaudet A. Dopaminergic projection from nucleus raphe dorsalis to neostriatum in the rat. J Comp Neurol. 1986;249(4):511–520. doi: 10.1002/cne.902490407. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Roth RH. The ascending projections of the dopaminergic neurons of the substantia nigra, zona reticulata: a combined retrograde tracer-immunohistochemical study. Neurosci Lett. 1986;71:257–263. doi: 10.1016/0304-3940(86)90630-0. [DOI] [PubMed] [Google Scholar]
- Dhawan JK, Kumar VM, Govindaraju V, Raghunathan P. Changes in magnetic resonance imaging and sex behavior after 6-OHDA injection in the medial preoptic area. Brain Res Bull. 1998;45(3):333–339. doi: 10.1016/s0361-9230(97)00404-8. [DOI] [PubMed] [Google Scholar]
- Döcke F, Rohde W, Oelssner W, Schleussner E, Gutenschwager I, Dörner G. Influence of the medial preoptic dopaminergic activity on the efficiency of the negative estrogen feedback in prepubertal and cyclic female rats. Neuroendocrinology. 1987;46(5):445–452. doi: 10.1159/000124859. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86(3):356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Barberis C, de Bilbao F. Vasopressin receptors in the mouse (Mus musculus) brain: sex-related expression in the medial preoptic area and hypothalamus. Brain Res. 1996;743(1–2):32–39. doi: 10.1016/s0006-8993(96)01019-0. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol. 1986;247(3):364–382. doi: 10.1002/cne.902470307. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW. Effects of preoptic region implants of dilute estradiol on the maternal behavior of ovariectomized, nulliparous rats. Horm Behav. 1986;20:354–363. doi: 10.1016/0018-506x(86)90043-7. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Mitsushima D, Nakamura TJ, Uemura T, Hirahara F, Shinohara K, Suyama K, Kimura F. Gonadotropin-releasing hormone (GnRH) surge generator in female rats. Prog Brain Res. 2002;141:165–173. doi: 10.1016/S0079-6123(02)41091-6. [DOI] [PubMed] [Google Scholar]
- Galindo-Estraún I. Effect of mammillary body lesions in adult rats on the survival and development of their young. Rev Esp Fisiol. 1983;39(4):447–454. [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinás RR. A dopaminergic projection to the rat mammillary nuclei demonstrated by retrograde transport of wheat germ agglutinin-horseradish peroxidase and tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1992;321(2):300–311. doi: 10.1002/cne.903210209. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384(1):142–164. [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Hines D, Navaira E, Smith J, Wampler L. Intracranial infusions of amphetamine into the medial preoptic area but not the nucleus accumbens affect paced mating behavior in female rats. Pharmacol Biochem Behav. 2008;89(3):253–262. doi: 10.1016/j.pbb.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Halpain S, Wieczorek CM, Rainbow TC. Localization of L-glutamate receptors in rat brain by quantitative autoradiography. J Neurosci. 1984;4(9):2247–2258. doi: 10.1523/JNEUROSCI.04-09-02247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RP. The sexually dimorphic region of the preoptic area in rats contains denser opiate receptor binding sites in females. Brain Res. 1984;308:172–176. doi: 10.1016/0006-8993(84)90932-6. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105(4):588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454(1):15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Fine structure of the supramammillary nucleus of the rat: analysis of the ultrastructural character of dopaminergic neurons. J Comp Neurol. 1994;346(1):127–136. doi: 10.1002/cne.903460109. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS, Series Handbook of Chemical Neuroanatomy. vol. 2. Amsterdam: Elsevier; 1984. pp. 277–379. [Google Scholar]
- Horvath TL, Naftolin F, Leranth C. Luteinizing hormone-releasing hormone and gamma-aminobutyric acid neurons in the medial preoptic area are synaptic targets of dopamine axons originating in anterior periventricular areas. J Neuroendocrinol. 1993;5:71–79. doi: 10.1111/j.1365-2826.1993.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res. 1986;370(1):73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126(1):66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Huot P, Lévesque M, Parent André. The fate of striatal dopaminergic neurons in parkinson’s disease and huntington’s chorea. Brain. 2007;130:222–232. doi: 10.1093/brain/awl332. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABAA receptors. Neuropsychopharmacology. 2005;30:1088–1095. doi: 10.1038/sj.npp.1300660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111(2):369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24(25):5758–5765. doi: 10.1523/JNEUROSCI.5367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson G, Fuxe K, Hökfelt T. On the catecholamine innervation of the hypothalamus, with special reference to the median eminence. Brain Res. 1972;40(2):271–281. doi: 10.1016/0006-8993(72)90133-3. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the "olfactory amygdala". J Comp Neurol. 1981;197(1):99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kita H, Oomura Y. An HRP study of the afferent connections to rat medial hypothalamic region. Brain Res Bull. 1982;8(1):53–62. doi: 10.1016/0361-9230(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Kizer JS, Palkovits M, Brownstein MJ. The projections of the A8, A9, and A10 dopaminergic cell bodies: evidence for a nigral-hypothalamic-median eminence dopaminergic pathway. Brain Res. 1976;108(2):363–370. doi: 10.1016/0006-8993(76)90192-x. [DOI] [PubMed] [Google Scholar]
- Kocsis K, Kiss J, Csáki A, Halász B. Location of putative glutamatergic neurons projecting to the medial preoptic area of the rat hypothalamus. Brain Res Bull. 2003;61(4):459–468. doi: 10.1016/s0361-9230(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Kopin IJ, Palkovits M, Kobayashi RM, Jacobowitz DM. Quantitative relationship of catecholamine content and histofluorescence in brain of rats. Brain Res. 1974;80(2):229–235. doi: 10.1016/0006-8993(74)90687-8. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochem Int. 2007;50(6):783–790. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Landis SC, Shoemaker WJ, Schlumpf M, Bloom FE. Catecholamines in mutant mouse cerebellum: fluorescence microscopic and chemical studies. Brain Res. 1975;93(2):253–266. doi: 10.1016/0006-8993(75)90349-2. [DOI] [PubMed] [Google Scholar]
- Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus orchrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66(3):197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Identification of retinal ganglion cells projecting to the lateral hypothalamic area of the rat. Brain Res. 1997;770:105–114. doi: 10.1016/s0006-8993(97)00761-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ. Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol. 2007;19(10):753–766. doi: 10.1111/j.1365-2826.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci. 1999;11:187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R, Faber-Zuschratter H, Seidenbecher T, Pape HC. Axonal connections from posterior paralaminar thalamic neurons to basomedial amygdaloid projection neurons to the lateral entorhinal cortex in rats. Brain Res Bull. 2004;63:461–469. doi: 10.1016/j.brainresbull.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lisk RD. Increased sexual behavior in the male rat following lesions in the mammillary region. J Exp Zool. 1966;161(1):129–136. doi: 10.1002/jez.1401610112. [DOI] [PubMed] [Google Scholar]
- Lisk RD. Reproductive potential of the male rat: enhancement of copulatory levels following lesions of the mammillary body in sexually non-active and active animals. J Reprod Fertil. 1969;19(2):353–356. doi: 10.1530/jrf.0.0190353. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Frank A. Beach Award: Parenting--the other reproductive behavior. Horm Behav. 2002;42(3):258–262. doi: 10.1006/hbeh.2002.1818. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Dominguez JM, Putnam SK, De Vries GJ, Hull EM. Intracellular preoptic and striatal monoamines in pregnant and lactating rats: possible role in maternal behavior. Brain Res. 2003;970(1–2):149–158. doi: 10.1016/s0006-8993(03)02315-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI. Neuropharmacology and neuroendocrinology of maternal behavior and motivation. In: Blaustein JD, editor. Behavioral Neurobiology, Handbook of Neurochemistry and Molecular Biology. vol. 18. Kluwer Press; 2006. pp. 195–245. [Google Scholar]
- Lookingland KJ, Moore KE. Dopamine receptor-mediated regulation of incertohypothalamic dopaminergic neurons in the male rat. Brain Res. 1984;304(2):329–338. doi: 10.1016/0006-8993(84)90337-8. [DOI] [PubMed] [Google Scholar]
- Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci. 1994;14(8):4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Regulatory role of excitatory amino acids in reproduction. Endocrine. 2005;28(3):271–280. doi: 10.1385/ENDO:28:3:271. [DOI] [PubMed] [Google Scholar]
- Meister B, Elde R. Dopamine transporter mRNA in neurons of the rat hypothalamus. Neuroendocrinology. 1993;58(4):388–395. doi: 10.1159/000126568. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119(4):1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long effects of electrical simulation of the medial preoptic area and medial amygdale on maternal behavior in female rats. Behav Brain Res. 1999;99(1):61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008 doi: 10.1038/nature07418. Online Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nock B, Johnson AE, Feder HH, McEwen BS. Tritium-sensitive film autoradiography of guinea pig brain alpha 2-noradrenergic receptors. Brain Res. 1985;336(1):148–152. doi: 10.1016/0006-8993(85)90426-3. [DOI] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol. 1974;87(4):746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel T. The Neurobiology of Parental Behavior. New York: Spring-Verlag; 2003. [Google Scholar]
- Numan M, McSparren J, Numan MJ. Dorsolateral connections of the medial preoptic area and maternal behavior in rats. Behav Neurosci. 1990;104(6):964–979. doi: 10.1037//0735-7044.104.6.964. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98(4):712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Abercrombie E, Rosenblatt JS, Morrell JI. The content of dopamine, serotonin, and their metabolites in the neural circuit that mediates maternal behavior in juvenile and adult rats. Brain Res Bull. 2004;63:259–268. doi: 10.1016/j.brainresbull.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Fekete M, Makara GB, Herman JP. Total and partial hypothalamic deafferentations for topographical identification of catecholaminergic innervations of certain preoptic and hypothalamic nuclei. Brain Res. 1977;127:127–136. doi: 10.1016/0006-8993(77)90384-5. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res. 1997;764(1–2):101–108. doi: 10.1016/s0006-8993(97)00431-9. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci. 2002;16(9):1797–1809. doi: 10.1046/j.1460-9568.2002.02267.x. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73(3):179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2(10):1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, Ghatei MA, Bloom SR. Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol. 2006;18(10):742–747. doi: 10.1111/j.1365-2826.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2007. [Google Scholar]
- Petitti N, Karkanias GB, Etgen AM. Estradiol selectively regulates alpha 1B-noradrenergic receptors in the hypothalamus and preoptic area. J Neurosci. 1982;12(10):3869–3876. doi: 10.1523/JNEUROSCI.12-10-03869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315(1):1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Scott CJ, Clarke JJ, Tilbrook AJ. Neuronal inputs from the hypothalamus and brain stem to the medial preoptic area of the ram: neurochemical correlates and comparison to the ewe. Biol Reprod. 2003;68(4):1119–1133. doi: 10.1095/biolreprod.102.010595. [DOI] [PubMed] [Google Scholar]
- Shahidi S, Motamedi F, Naghdi N. Effect of reversible inactivation of the supramammillary nucleus on spatial learning and memory in rats. Brain Res. 2004;1026(2):267–274. doi: 10.1016/j.brainres.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Mihailoff GA, German DC. Anatomical and electrophysiological characterization of presumed dopamine-containing neurons within the supramammillary region of the rat. Brain Res Bull. 1988;20(3):307–314. doi: 10.1016/0361-9230(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Zimmerman EA, Gibson MJ, Perlow MJ, Charlton HM, Kokoris GJ, Krieger DT. Implantation of normal fetal preoptic area into hypogonadal mutant mice: temporal relationships of the growth of gonadotropin-releasing hormone neurons and the development of the pituitary/testicular axis. Neuroscience. 1985;16(1):69–84. doi: 10.1016/0306-4522(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6(4):297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Gorski RA, Swanson LW. Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J Comp Neurol. 1986;246(3):343–363. doi: 10.1002/cne.902460305. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985;330(1):55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Calas A. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Res. 1979;178(1):17–40. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Microstimulation mapping of the basal forebrain in the anesthetized rat: the "preoptic locomotor region". Neuroscience. 1992;50(1):197–207. doi: 10.1016/0306-4522(92)90392-f. [DOI] [PubMed] [Google Scholar]
- Sita LV, Elias CF, Bittencourt JC. Connectivity pattern suggests that incerto-hypothalamic area belongs to the medial hypothalamic system. Neuroscience. 2007;148(4):949–969. doi: 10.1016/j.neuroscience.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, González A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Rev. 2000;33(2–3):308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res. 2008;190(2):193–200. doi: 10.1016/j.bbr.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Dhawan JK, Ramesh V, John J, Gopinath G, Kumar VM. Role of medial preoptic area beta adrenoceptors in the regulation of sleep-wakefulness. Pharmacol Biochem Behav. 1997;57(1–2):1–5. doi: 10.1016/s0091-3057(96)00384-x. [DOI] [PubMed] [Google Scholar]
- Spencer SE, Sawyer WB, Loewy AD. L-glutamate mapping of cardioreactive areas in the rat posterior hypothalamus. Brain Res. 1990;511(1):149–157. doi: 10.1016/0006-8993(90)90234-3. [DOI] [PubMed] [Google Scholar]
- Stern JM. Multisensory regulation of maternal behavior and masculine sexual behavior: a revised view. Neurosci Biobehav Rev. 1990;14(2):183–200. doi: 10.1016/s0149-7634(05)80219-2. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine d1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121(5):907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 1990;511(1):173–176. doi: 10.1016/0006-8993(90)90239-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the rat brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Gvilia I, McGinty D. Hypothalamic control of sleep. Sleep Med. 2007;8(4):291–301. doi: 10.1016/j.sleep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Noonan JJ, Nass TE, Loose MD. Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. Endocrinology. 1984;115(6):2241–2250. doi: 10.1210/endo-115-6-2241. [DOI] [PubMed] [Google Scholar]
- Tillet Y, Batailler M, Thibault J. Neuronal projections to the medial preoptic area of the sheep, with special reference to monoaminergic afferents: immunohistochemical and retrograde tract tracing studies. J Comp Neurol. 1993;330(2):195–220. doi: 10.1002/cne.903300205. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, Herbst RS, Powell JF. Tyrosine hydroxylase-immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience. 1984;13(4):1117–1156. doi: 10.1016/0306-4522(84)90292-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brainstem afferents to the basal forebrain in the rat. Neuroscience. 1988;24(3):907–935. doi: 10.1016/0306-4522(88)90077-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326(4):595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: Pha-l analysis in the rat. J Comp Neurol. 1995;359(1):90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing a13 dopaminergic neurons: a PHA-L anterograde tract-tracing study in the rat. Brain Res. 1995;677:229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- Wehr R, Mansouri A, De Maeyer T, Gruss P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development. 1997;124(22):4447–4456. doi: 10.1242/dev.124.22.4447. [DOI] [PubMed] [Google Scholar]
- Weiner RI, Shryne JE, Gorski RA, Sawyer CH. Changes in the catecholamine content of the rat hypothalamus following deafferentation. Endocrinology. 1972;90(4):867–873. doi: 10.1210/endo-90-4-867. [DOI] [PubMed] [Google Scholar]
- Windsor-Engnell BM, Kasuya E, Mizuno M, Keen KL, Terasawa E. An increase in in vivo release of LHRH and precocious puberty by posterior hypothalamic lesions in female rhesus monkeys (Macaca mulatta) Am J Physiol Endocrinol Metab. 2007;292(4):E1000–E1009. doi: 10.1152/ajpendo.00493.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke W, Honma K, Lamberts R, Höhn KG. The role of monoamines in female puberty. Fed Proc. 1980;39(7):2378–2383. [PubMed] [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology. 2005;81(1):56–62. doi: 10.1159/000084893. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23(6):2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]