Abstract
Interictal spikes (IIS) are paroxysmal discharges commonly observed in patients with epilepsy which represent an abnormally-synchronized population of hyperexcitable neurons firing as an aggregate. Due to conflicting studies on the clinical significance of IIS, research focusing on IIS has been sparse. However, recent attention on IIS has increased for patients undergoing surgery for intractable epilepsy as a means to identify epileptic foci for surgical resection. There is growing evidence that IIS are not asymptomatic as has been commonly accepted. Other than epilepsy, IIS have been associated with a wide range of behavioral and psychiatric disorders, including attention deficit disorder, anxiety disorders and psychoses. For these reasons, a well-characterized animal model of interictal spiking which accurately mimics the human phenomenon would be a valuable tool to gain insights both into the pathophysiology of epilepsy as well as a broad variety of human neuropsychiatric diseases. Here, we review the literature on the clinical significance of IIS in humans and on animal models where IIS has been observed. We then demonstrate the utility of using tetanus toxin to generate a reproducible pattern of progressive IIS for future studies into their clinical significance.
Keywords: Animal Model; Behavioral Disorder; Seizure; Spikes, Interictal; Tetanus Toxin
INTRODUCTION
IIS in epilepsy
A major goal in the management of patients with intractable epilepsy is to reduce or eliminate clinical seizures which often lead to major disability and sometimes death. While an electrographic seizure is a rhythmic burst of epileptic discharges that can start focally and spread to large regions of the brain, interictal spikes (IIS) generally occur singly or in small groups, remain localized to specific brain regions, and are more frequent than seizures. One widespread opinion is that IIS may prevent rather than provoke seizures. This opinion is largely based on scalp encephalogram (EEG) studies that examined IIS frequency immediately before and after seizures. The finding that IIS are not increased before the seizure onset has led some to speculate that IIS are not required for seizure generation.1–4 While some even interpret these findings to mean that IIS may actually be protective for seizures, these studies were limited by the use of scalp EEG which can only detect IIS that involve very large regions of cortex that often exceed 10cm.2 5
Further experimental evidence in vitro also argues against a pathologic role of IIS in epilepsy. In hippocampal slice preparations, the presence of sustained interictal spiking reduced the probability of generating an ictal discharge.6–11 In this same system, ictal events could still be generated while interictal activity was abolished with blockers of synaptic transmission.12,13 A potential problem with all these in vitro preparations is that it is unclear how well the IIS generated from these acute preparations compare to IIS generated over months to years in living animals. While acute slice models require some sort of irritant to produce discharges, an epileptic brain generates discharges spontaneously with no outside intervention, suggesting a fundamental difference in intrinsic excitability.
More recently, a number of studies support a pathologic role for IIS in maintaining epileptic foci. IIS, in fact, develop long before actual seizures and thus may represent an important driving force in epileptogenesis.14 IIS are routinely detected on surface EEG and their presence is used to identify epileptic brain regions. Determining their location, patterns of propagation, latency, and frequency can be helpful clinically to identify ictal-onset zones and thereby improve post-surgical out comes.15–21 In vitro, interictal spiking and seizure activity can arise from the same neuronal networks22 and, in hippocampal slice cultures, these neuronal networks become fully active when there is sufficient neurotransmitter release to initiate action potentials in a critical number of neurons.23 It has been hypothesized that positive feedback from synapses of neurons lead to the propagation of excitation throughout the neural network,24 that, in turn, becomes an important driving force for the generation of seizures. Finally, our laboratory has recently found that genes which are differentially induced at human epileptic foci were induced in proportion to the amount of IIS, rather than the frequency of seizures, suggesting that interictal activity may be an important driving force for activity-dependent gene expression and plasticity at epileptic foci.25,26
IIS in psychiatric disorders
EEG abnormalities have been noted in a wide range of psychiatric disorders for decades. In many of these studies, however, all EEG abnormalities were lumped into a single “abnormal EEG” description and were not categorized specifically as IIS or focal slowing. Several studies describe a high incidence of EEG abnormalities in anxiety disorders, panic disorders, and obsessive-compulsive disorder.27,28 However, one large community study by Bridgers29 found that epileptiform discharges, specifically, were correlated with symptoms of anorexia nervosa, depression, mania, personality disorders, suicidality without depression, nonpsychotic explosive behavior, and the effects of psychotropic medications. Individuals displaying antisocial and aggressive tendencies have also been found to display a wide range of EEG abnormalities, including epileptiform discharges.30–34 Most remarkably, IIS have often been associated with attention deficit hyperactivity disorder (ADHD), with a prevalence ranging from 4.9% to 60% while estimates in normal school-aged children are around 2–3%.35–41 Given the wide array of psychiatric symptoms and the comorbidity of psychiatric diseases with epilepsy, long-term human recordings and focused behavioral studies in animals will be needed to establish any causality between these abnormal brain activities and behaviors.
More direct associations of IIS with specific cognitive behaviors have been noted. A recent double-blind study that reduced interictal spiking using add-on seizure therapies showed significant behavioral improvements in patients with epilepsy, raising the possibility that interictal spiking is deleterious to normal brain functioning.42 Some studies suggest an association between high frequency IIS and cognitive and behavioral decline in children with autism.43,44 Inui et al45 found that spike variants were significantly higher among patients with mood-incongruent psychotic mood disorder (33%), schizoaffective disorder (33%), and schizophreniform disorder (30%) as compared to patients with nonpsychotic mood disorder (3.2%) and schizophrenia (0%).45 IIS have even been associated with self-injurious behavior among tuberous sclerosis patients.46 With a potential role in such a wide range of disorders, it would be advantageous to develop a better understanding of IIS through animal models.
Animal models
Given the inherent property of excitability in the brain, it is not surprising that it is a relatively simple task to generate epileptic discharges and seizures in animals using a wide array of irritative, electrical, and cytotoxic methods.47 Animal models have been used to investigate epilepsy for decades, and most of them generate IIS in addition to seizures. Rarely, however, are IIS the focus of study, and their contribution to both pathology and epileptogenesis is difficult to disentangle from that of seizures. An ideal model for the study of clinically-important IIS in animals would be to mimic the human phenomenon as closely as possible. This would require IIS in the absence of frank seizures that are similar to those observed in humans, are chronic, spontaneous, and are not associated with significant neuronal toxicity or death.
Both acute and chronic IIS have been observed in animal models. In hippocampal slice culture, gamma-aminobutyric-acid-A (GABAA) blockade, low extracellular magnesium concentration, low extracellular calcium, kainic acid, and 4-aminopyridine have all been used to generate interictal-like activities.47 The advantage of these methods is that interictal activity without ictal discharges can be generated in order to study the electrophysiological properties of IIS independently from seizures. Disadvantages are that the relationship of in vitro discharges to those generated in intact animals is unclear and nothing can be said about “higher order” phenomena such as behavior with in vitro models. Acute in vivo models are better suited to address these problems. In intact animals, focal IIS can be seen immediately after penicillin injection or electrical stimulation. Systemic administration of GABA-blocking substances, kainic acid, or pilocarpine produces IIS, but these can also be injected focally. After penicillin injection into the cortex, focal IIS as well as seizures occur and can last for 6–8 hours. Kainic acid and pilocarpine given systemically produce acute status epilepticus that can last for several hours, after which IIS are observed. Pentylenetetrazol is generally used as a convulsant agent, but in the right dose can produce spikes with no detected seizures and produces minimal tissue damage.48 One question that remains with all of these models of acute epileptiform activity is whether they are truly identical to the spontaneous IIS generated chronically in patients with epilepsy.
Chronic models of epilepsy are generally produced by an initial lesion, followed by a latent period, and the subsequent development of spontaneous epileptiform activities.47 Most models focus on the hippocampus, and commonly used methods of modeling human hippocampal epilepsy are electrical kindling, or systemic administration of pilocarpine or kainic acid. Kindling involves repeated electrical stimulations which cause seizures. If done enough times, kindling can lead to the production of spontaneous IIS and seizures. Status epilepticus induced by either pilocarpine or kainic acid cause significant neuronal damage in the hippocampus. After a latent period, large IIS mixed with spontaneous seizures occur chronically in a subset of animals. For chronic neocortical epilepsy, the most common techniques are application of either toxic metals or tetanus toxin. When metals, such as cobalt, are directly applied to the cortex, spontaneous interictal and ictal activities are observed and can persist for one or more weeks, depending on the method of application. Limitations of using metals are that the epileptiform activity requires continued presence of the irritant and extensive tissue destruction is seen at the site. This is likely different from lesions that produce epileptic foci in humans, such as brain injury, which are usually a single initial insult.
Chronic models of epilepsy using tetanus toxin
Tetanus toxin injected directly into either the neocortex or hippocampus produces a variety of transient excitatory patterns dependent on the injection site. It is a nice model of chronic epilepsy because it does not produce significant neuronal toxicity as many of the other focal models do and does not produce the tissue destruction observed after prolonged status epilepticus (Figure 1). Tetanus toxin produces a transient inhibition of inhibitory neurons that is known to lead to the gradual generation of spontaneous epileptic discharges that over time lead to the development of epileptic seizures.49 An initial injection of tetanus toxin is followed by a latent period, after which IIS and spontaneous seizures can persist almost indefinitely (>7 months). Most intriguingly, Jeffreys and colleagues50 noted that, unlike injections into hippocampus or motor cortex that produce seizures early on, injection of small amounts of tetanus toxin into the somatosensory cortex results predominantly in IIS with only infrequent seizures.50 This model seemed to offer the ideal characteristics to study IIS in animals since it is a chronic, spontaneous in vivo model, without seizures early on and minimal neuronal cell loss.
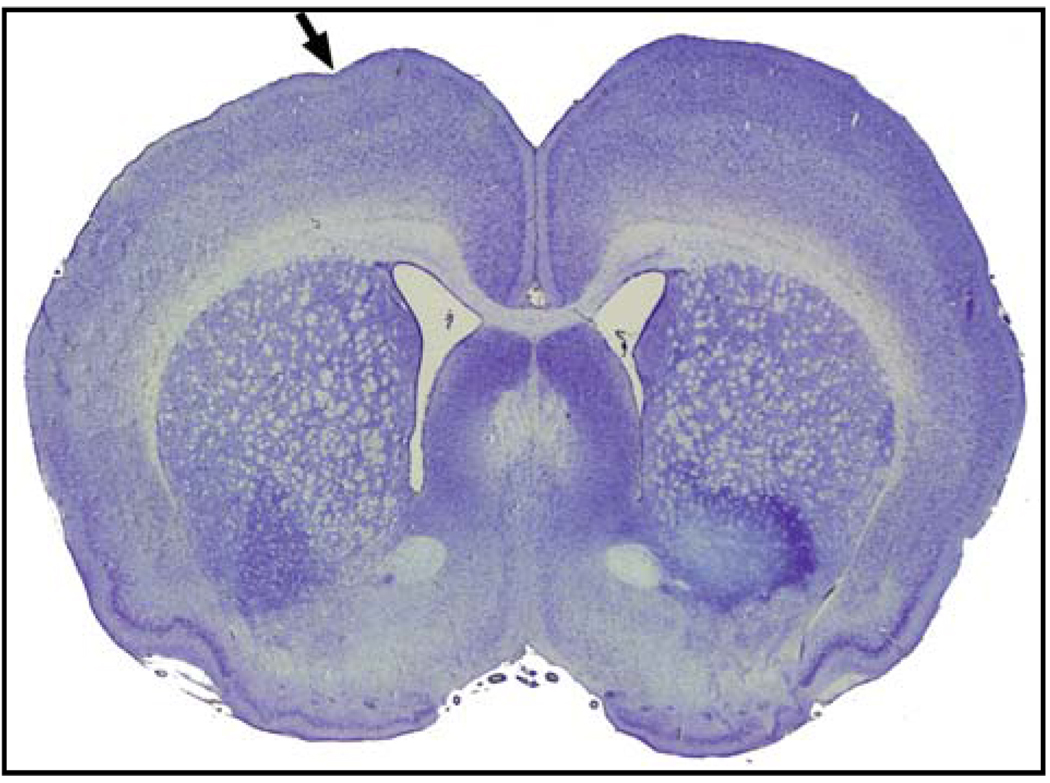
Figure 1. Tetanus toxin injection causes minimal to no histopathologic damage.
A 20µm coronal section within 400µm of the injection site of a spiking rat’s brain two weeks after tetanus toxin injection stained with cresyl violet demonstrates no major pathologic changes (arrow signifies injection site). The absence of frank trauma to the brain eliminates many potential confounding variables in the experiment.
MATERIALS AND METHODS
To create a focus of IIS, rats were anesthetized with pentobarbital and placed in a stereotactic frame. Three holes were drilled in the skull over each hemisphere at +4mm, −1mm and −6mm relative to the bregma in the cranial-caudal axis and 3.5mm lateral (Figure 2). A seventh hole was drilled at the midline over the nasal sinus. Tetanus toxin (Sigma) was prepared with sterile water to yield a 100ng/ µL solution in 0.01M sodium phosphate. This solution was injected with a Hamilton syringe into the left somatosensory cortex (−1mm relative to bregma, 3.5mm lateral to midline) at a depth of 1.5mm over the course of 4 minutes. The needle was left in place for 10 more minutes to allow diffusion and binding of the toxin. Screws (Small Parts Inc., TX2-4-1C) with the points filed off were placed in each drilled hole and wired to a 9-pin female connector (Winchester Electronics 89-1109S-10D) to act as electrodes, with the nasal sinus screw used as a reference. The apparatus was covered with dental cement (A-M Systems) and the skin tightened around it with a skin staple. One hour of EEG per day was recorded at the same time of day for each rat on a 32-channel, 200Hz Stellate HARMONIE 6.0 digital EEG recording system (Stellate Systems Inc., Quebec, Canada). EEG recordings were reviewed manually by one blinded reviewer to mark the time and location of each interictal spike.
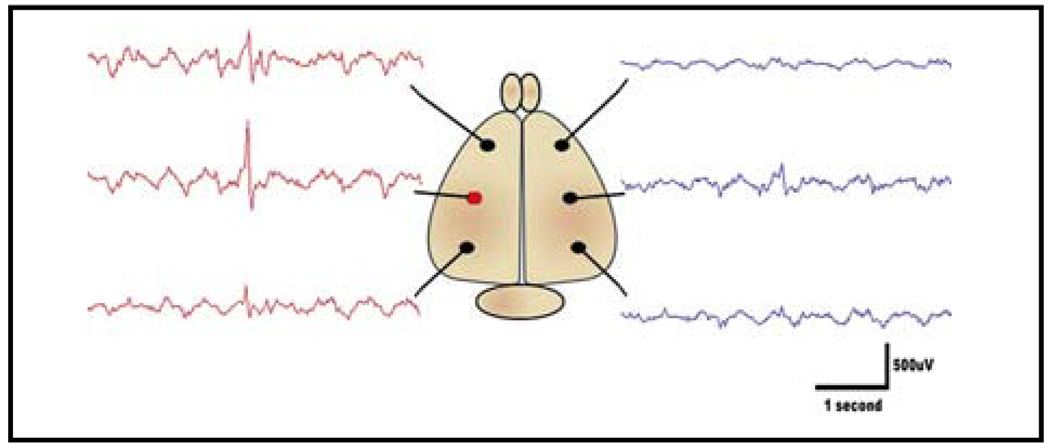
Figure 2. Electrode placement and spike localization.
A schematic of the placement of six recording electrodes with sample EEG traces shows how interictal spikes can be localized. The field is most prominent over the injection site, but can also be detected to a lesser degree in other nearby leads (red circle denotes electrode overlying the injection site).
RESULTS AND DISCUSSION
Based on the initial observation by Jeffreys’ group,50 we have developed and characterized a model of chronic, focal interictal spiking in rats with a long latent period before spontaneous seizures. The goal for this model was to create an epileptic focus for long-term recordings of IIS that closely parallels observations made from human long-term subdural recordings. With this method, we observed a latent period of 4–7 days where no abnormal activity is detected, followed by a steady increase in the frequency of interictal spiking for several weeks. The rate of increase in spiking is dependent upon the dose of tetanus toxin used (Figure 3). Once IIS have built up over time, seizures can be observed originating at or near the injection site approximately 6 weeks after tetanus toxin injection in highly spiking animals (Figure 4).
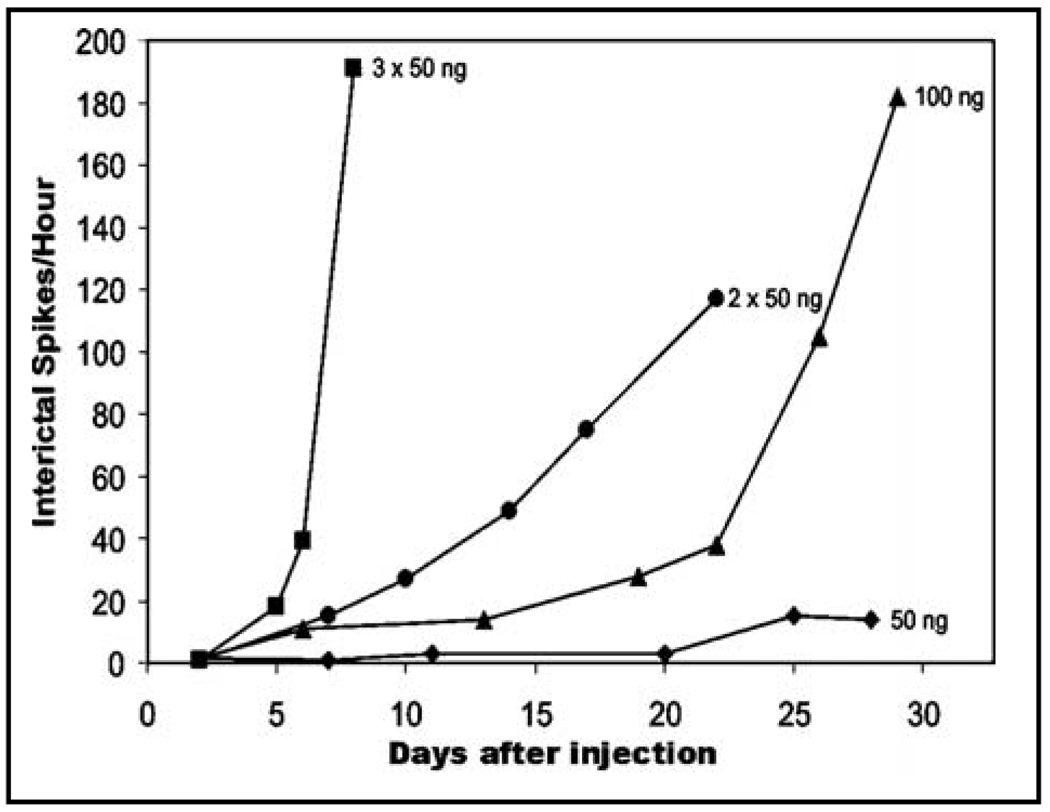
Figure 3. The development of interictal spiking is dose-dependent.
The rate of increase in interictal spiking frequency increases with the dose of tetanus toxin injected. An injection of 50ng results in very little spiking even 4 weeks after injection, while three injections of 50ng (150ng total) within 1mm2 of cortex quickly results in a rapid increase in spike frequency. Dose and the number of nearby injection sites can thus be modulated to vary the rate of IIS generation in the absence of seizures.
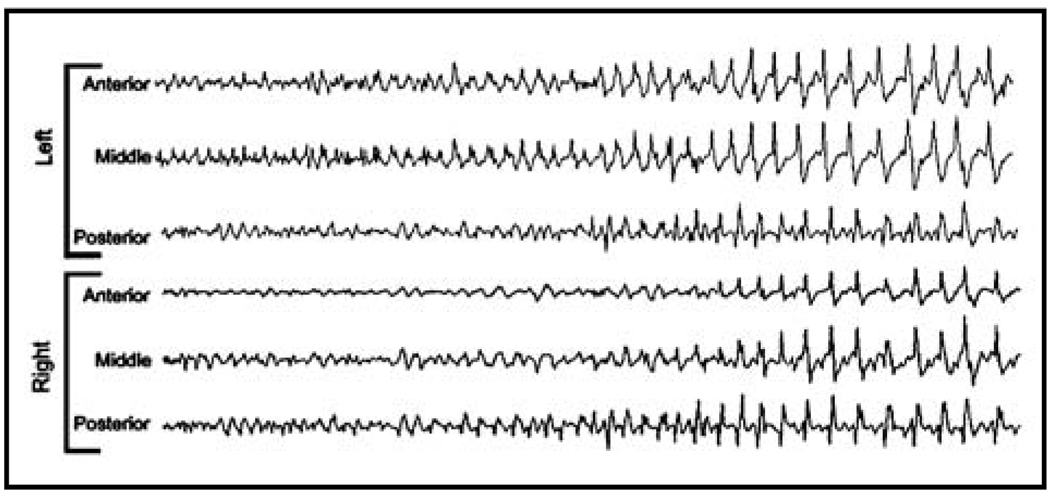
Figure 4. Spiking eventually leads to secondarily-generalized seizures.
Electrographic and behavioral seizures can be observed approximately 6 weeks after tetanus toxin injection. Seizures begin at the injection site, but rapidly spread to include the entire brain. This confirms the model as one of true epileptogenesis, which closely mimics the time course of human, acquired, neocortical epilepsy.
This model, therefore, provides an excellent parallel to acquired, focal epilepsy in humans where an initial lesion is followed by a latent period before spontaneous seizures occur. During this time, a focus of IIS develops and progresses, which allows a large window in which to study the effects of neocortical spiking in the absence of seizures. Additionally, the focal nature of this model permits the use of the opposite hemisphere as an internal control for tissue and molecular studies, and the potential to lateralize any behavioral changes. Unlike in the common kainic acid and pilocarpine models that produce severe brain injuries from hours-long status epilepticus, there is no mortality from this procedure and minimal tissue damage. Confounding effects from brain trauma, the disruption of the blood-brain barrier, and inflammation are thereby minimized. While all treated animals developed progressive interictal spiking, there is substantial variation in the frequency of IIS by 3 weeks out making it necessary to quantify IIS frequency when relating IIS to behavior or seizure development. Finally, because this model creates a chronic, self-sustaining focus of spontaneous spiking in a living animal, it may be a far more accurate model of the human condition than in vitro slices or acute in vivo models.
ACKNOWLEDGMENTS
This work was funded by NIH/NINDS R01NS045207 and R01NS058802 (JAL) and a predoctoral fellowship from the Epilepsy Foundation of America (DTB). We thank Drs. Thomas Babb for help in setting up this model and Nash Boutros for helpful comments and collaborations.
Footnotes
DISCLOSURE AND CONFLICT OF INTEREST
D.T. Barkmeier and J.A. Loeb have no conflicts of interest in relation to this article.
REFERENCES
- 1.Lieb JP, Woods CS, Siccardi A, Crandall PH, Walter TD, Leake B. Quantitative analysis of depth spiking in relation to seizure foci in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1978;44:641–663. doi: 10.1016/0013-4694(78)90130-x. [DOI] [PubMed] [Google Scholar]
- 2.Lange HH, Lieb JP, Engel JJ, Crandall PH. Temperospatial patterns of preictal spike activity in human temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1983;56:543–555. doi: 10.1016/0013-4694(83)90022-6. [DOI] [PubMed] [Google Scholar]
- 3.Kats A, Marks DA, McCarthy G, Spencer SS. Does interictal spiking change prior to seizures? Electroencephalogr Clin Neurophysiol. 1991;79:153–156. doi: 10.1016/0013-4694(91)90054-8. [DOI] [PubMed] [Google Scholar]
- 4.Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18:573–576. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- 5.Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilkus R, Dodrill CB, Troupin AS. Carbamazepine and the electroencephalogram of epileptics: a double blind study in comparison to phenytoin. Epilepsia. 1978;19:283–291. doi: 10.1111/j.1528-1157.1978.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 7.Engel JJ, Rausch R, Lieb JP, Kulh DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patients considered for surgical therapy of epilepsy. Ann Neurol. 1981;9:215–224. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- 8.Engel JJ, Ackermann R. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdala kindling. Brain Res. 1982;241:75–86. doi: 10.1016/0006-8993(80)90296-6. [DOI] [PubMed] [Google Scholar]
- 9.Swartzwelder HS, Lewis DV, Anderson WW, Wilson WA. Seizure-like events in brain slices: suppression by interictal activity. Brain Res. 1987;410:362–366. doi: 10.1016/0006-8993(87)90339-8. [DOI] [PubMed] [Google Scholar]
- 10.Jeffreys JGR. Chronic epileptic foci in vitro in hippocampal slices from rats with the tetanus toxin epileptic syndrome. J Neurophysiol. 1989;62:458–468. doi: 10.1152/jn.1989.62.2.458. [DOI] [PubMed] [Google Scholar]
- 11.Barbarosie M, Avoli M. CA3-Driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen MS, Yaari Y. The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann Neurol. 1988;24:591–598. doi: 10.1002/ana.410240502. [DOI] [PubMed] [Google Scholar]
- 13.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 14.Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- 15.Asano E, Muzik O, Shah A, Juhasz C, Chugani DC, Sood S, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44:425–434. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- 16.Niedermeyer E, Lopes da Silva FH, editors. ElectroencephaLography: Basic Principles, Clinical Applications, and Related Fields. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 17.Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Hufnagel A, Elger CE, Pels H, Zentner J, Wolf HK, Schramm J, et al. Prognostic significance of ictal and interictal epileptiform activity in temporal lobe epilepsy. Epilepsia. 1994;35:1146–1153. doi: 10.1111/j.1528-1157.1994.tb01781.x. [DOI] [PubMed] [Google Scholar]
- 19.Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40:880–890. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 20.Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, et al. Origin and propagation of interictal discharges in the acute electrocorticogram: implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120(Pt 12):2259–2282. doi: 10.1093/brain/120.12.2259. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9:407–411. doi: 10.1053/seiz.2000.0423. [DOI] [PubMed] [Google Scholar]
- 22.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23:7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamberlin NL, Traub RD, Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990;64:1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- 24.O’Donovan MJ, Chub N. Population behavior and self-organization in the genesis of spontaneous rhythmic activity by developing spinal networks. Semin Cell Dev Biol. 1997;8:21–28. doi: 10.1006/scdb.1996.0117. [DOI] [PubMed] [Google Scholar]
- 25.Rakhade SN, Shah AK, Agarwal R, Yao B, Asano E, Loeb JA. Activity-dependent gene expression correlates with interictal spiking in human neocortical epilepsy. Epilepsia. 2007;48:86–95. doi: 10.1111/j.1528-1167.2007.01294.x. [DOI] [PubMed] [Google Scholar]
- 26.Rakhade SN, Yao B, Ahmed S, Asano E, Beaumont TL, Shah AK, et al. A common pattern of persistent gene activation in human neocortical epileptic foci. Ann Neurol. 2005;58:736–747. doi: 10.1002/ana.20633. [DOI] [PubMed] [Google Scholar]
- 27.Weilburg JB, Schachter S, Worth J, Pollack MH, Sachs GS, Ives JR, et al. EEG abnormalities in patients with atypical panic attacks. J Clin Psychiatry. 1995;56:358–362. [PubMed] [Google Scholar]
- 28.Hughes JR. A review of the usefulness of the standard EEG in psychiatry. Clin Electroencephalogr. 1996;27:35–39. doi: 10.1177/155005949602700106. [DOI] [PubMed] [Google Scholar]
- 29.Bridgers SL. Epileptiform abnormalities discovered on electroencephalographic screening of psychiatric inpatients. Arch Neurol. 1987;44:312–316. doi: 10.1001/archneur.1987.00520150056022. [DOI] [PubMed] [Google Scholar]
- 30.Hill D, Watterson D. Electroencephalographic studies of psychopathic personalities. J Neurol Psychiatry. 1942;5:47–65. doi: 10.1136/jnnp.5.1-2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribas JC, Baptistete E, Fonseca CA, Tiba I, Coutinho Filho HS. Behaviour disorders with predominance of aggressiveness, irritability, impulsiveness, and instability: clinical electroencephalographic study of 100 cases. Arquivos de Neuro-Psiquiatria. 1974;32:187–194. doi: 10.1590/s0004-282x1974000300002. [DOI] [PubMed] [Google Scholar]
- 32.Pillmann F, Rohde A, Ullrich S, Draba S, Sannemüller U, Marneros A. Violence, criminal behaviour, and the EEG: significance of left hemispheric focal abnormalities. J Neuropsych Clin Neurosci. 1999;11:454–457. doi: 10.1176/jnp.11.4.454. [DOI] [PubMed] [Google Scholar]
- 33.Monroe RR. Anticonvulsants in the treatment of aggression. J Nerv Ment Dis. 1975;160:119–126. doi: 10.1097/00005053-197502000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Wong MT, Lumsden J, Fenton GW, Fenwick PB. Electroencephalography, computed tomography and violence ratings of male patients in a maximum-security mental hospital. Acta Psychiatr Scand. 1994;90:97–101. doi: 10.1111/j.1600-0447.1994.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 35.Richer LP, Shevell MI, Rosenblatt BR. Epileptiform abnormalities in children with attention-deficit-hyperactivity disorder. Pediatr Neurol. 2002;26:125–129. doi: 10.1016/s0887-8994(01)00370-8. [DOI] [PubMed] [Google Scholar]
- 36.Abdeldayem HH, Salim OAA. Cognitive function and skills performance of children with attention deficit disorder. Epilepsia. 2005;49:179. [Google Scholar]
- 37.Holtmann M, Becker K, Kentner-Figura B, Schmidt MH. Increased frequency of rolandic spikes in ADHD Children. Epilepsia. 2003;44:1241–1244. doi: 10.1046/j.1528-1157.2003.13403.x. [DOI] [PubMed] [Google Scholar]
- 38.Becker K, Sinzing JK, Holtmann M. Attention deficits and subclinical epileptiform discharges: are EEG diagnostics in ADHD optional or essential. Dev Med Child Neurol. 2004;46:501–502. [PubMed] [Google Scholar]
- 39.Boutros N, Fraenkel L, Feingold A. A four-step approach for developing diagnostic tests in psychiatry: EEG in ADHD as a test case. J Neuropsychiatr Clin Neurosci. 2005;17:455–464. doi: 10.1176/jnp.17.4.455. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca LC, Tedrus GM, de Moraes C, de Vicente Machado A, de Almeida MP, de Oliveira DO. Epileptiform abnormalities and quantitative EEG in children with attention-deficit/hyperactivity disorder. Arq Neuropsiquiatr. 2008;66:462–467. doi: 10.1590/s0004-282x2008000400004. [DOI] [PubMed] [Google Scholar]
- 41.Silvestri R, Gagliano A, Calarese T, Aricò I, Cedro C, Condurso R, et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75:130–137. doi: 10.1016/j.eplepsyres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Pressler RM, Robinson RO, Wilson GA, Binnie CD. Treatment of interictal epileptiform discharges can improve behavior in children with behavioral problems and epilepsy. J Pediatr. 2005;146:112–117. doi: 10.1016/j.jpeds.2004.08.084. [DOI] [PubMed] [Google Scholar]
- 43.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–730. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 44.Tharp BR. Epileptic encephalopathies, their relationship to developmental disorders: do spikes cause autism? Ment Retard Dev Disabil Res Rev. 2004;10:132–134. doi: 10.1002/mrdd.20025. [DOI] [PubMed] [Google Scholar]
- 45.Inui K, Motomura E, Okushima R, Kaige H, Inoue K, Nomura J. Electroencephalographic findings in patients with DSM-IV mood disorder, schizophrenia, and other psychotic disorders. Biol Psychiatry. 1998;43:69–75. doi: 10.1016/s0006-3223(97)00224-2. [DOI] [PubMed] [Google Scholar]
- 46.Staley BA, Montenegro MA, Major P, Muzykewicz DA, Halpern EF, Kopp CM, et al. Self-injurious behavior and tuberous sclerosis complex: frequency and possible associations in a population of 257 patients. Epilepsy Behav. 2008;13:650–653. doi: 10.1016/j.yebeh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizure and Epilepsy. London: Academic Press; 2006. [Google Scholar]
- 48.Rodin E, Rodin M, Lavine L. Electroclinical and ultrastructural changes associated with subconvulsant doses of pentylenetetrazol. Exp Neurol. 1979;64:386–400. doi: 10.1016/0014-4886(79)90278-4. [DOI] [PubMed] [Google Scholar]
- 49.Nilsen KE, Walker MC, Cock HR. Characterization of the tetanus toxin model of refractory focal neocortical epilepsy in the rat. Epilepsia. 2005;46:179–187. doi: 10.1111/j.0013-9580.2005.26004.x. [DOI] [PubMed] [Google Scholar]
- 50.Brener K, Amitai Y, Jefferys JG, Gutnick MJ. Chronic epileptic foci in neocortex: in vivo and in vitro effects of tetanus toxin. Eur J Neurosci. 1991;3:47–54. doi: 10.1111/j.1460-9568.1991.tb00810.x. [DOI] [PubMed] [Google Scholar]