Abstract
OBJECTIVES
To investigate the effect of CSF abnormalities on rate of decline in everyday function in normal aging, MCI, and mild AD.
DESIGN
T-tau, p-tau181, and Aβ42 were immunoassayed in CSF obtained from participants in the AD Neuroimaging Initiative. Random effects regressions were used to examine the relationship between CSF abnormalities, cognitive impairment (assessed with the ADAS-Cog), and functional decline (assessed with Pfeffer’s FAQ); and to determine whether the impact of CSF abnormality on functional decline is mediated by cognitive impairment.
SETTING
Fifty-eight sites in the US and Canada.
PARTICIPANTS
One hundred fourteen cognitively-intact adults, 195 MCI patients, and 100 mild AD patients.
MAIN OUTCOME MEASURE
Decline in Pfeffer’s FAQ.
RESULTS
All CSF analytes were associated with functional decline in MCI and all but t-tau/Aβ42 were associated with functional decline in controls. No CSF analyte was associated with functional decline in AD. Among controls, p-tau181 was the most sensitive to functional decline whereas in MCI it was Aβ42. CSF biomarkers were uniformly more sensitive to functional decline than the ADAS-Cog among controls and variably so in MCI, whereas the ADAS-Cog was unequivocally more sensitive than CSF biomarkers in AD. The impact of CSF abnormalities on functional decline in MCI was partially mediated by their impact on cognitive status. Across all diagnostic groups, persons with both tau and Aβ42 abnormalities exhibited the steepest rate of functional decline.
CONCLUSIONS
CSF abnormalities are associated with functional decline, and thus with future development of AD in controls and MCI patients. However, they do not predict further functional degradation in AD. Persons with comorbid tau and Aβ42 abnormalities are at greatest risk of functional loss.
Keywords: CSF, FAQ, ADAS-Cog, activities of daily living, functional decline, MCI, AD
INTRODUCTION
Cerebrospinal fluid (CSF) concentrations of total tau (t-tau), phosphorylated tau (particularly at epitope 181, p-tau181), and β-amyloid 1-42 (Aβ42) have emerged as core Alzheimer’s disease (AD) biomarkers due to their intrinsic linkage to the pathognomonic features of AD (i.e., neurofibrillary tangles and amyloid plaques).1–4 In contrast with the demonstrations of associations between CSF abnormalities and some indices of disease severity and progression such as cognitive decline,5 plaque density,6 and cerebral alterations,7, 8 the relationship between CSF abnormalities and decline in everyday function has received limited attention.5, 9, 10 This is a significant knowledge gap for several reasons.
First, functional restriction is a hallmark of AD and other dementias.11, 12 Indeed, widely-used dementia staging instruments (e.g., the CDR) lean heavily on reports of an individual’s daily functioning in ascertaining dementia severity. Thus, decline in everyday function likely signals disease onset or progression among cognitively-normal older adults and those with mild cognitive impairment (MCI) respectively. Secondly, everyday function is an important outcome in AD clinical trials.13 Therefore, it is useful to understand how it is related to biomarkers of AD. Third, unraveling associations between CSF abnormalities and functional decline, especially in preclinical AD, might be valuable information for patients and their care providers, as they often wish to know what the future holds.
In this paper, we investigate: (i) whether CSF abnormalities are associated with decline in everyday function, (ii) whether such associations, if existent, are comparable or differential across CSF analytes, (iii) whether CSF analytes are more sensitive to functional decline than cognitive measures, (iv) whether the impact of CSF abnormalities on functional decline is mediated by their impact on cognition, (v) whether the combination of abnormally high t-tau or p-tau181 and abnormally low Aβ42 concentrations confers increased risk of functional decline, and finally (vi) whether the foregoing effects are similarly present throughout the continuum from healthy cognitive aging to AD.
METHODS
Participants
The analyses presented here were based on data from the AD Neuroimaging Initiative (ADNI; http://www.loni.ucla.edu/ADNI/). The ADNI was launched in 2003 by the National Institute on Aging and other entities (see Acknowledgments) as a 5-year public-private partnership. Enrolment target was 800 participants—200 normal controls, 400 patients with amnestic MCI, and 200 patients with mild AD—at 58 sites in the United States and Canada.
Diagnosis of amnestic MCI required memory complaints, objective memory difficulties (impaired delayed recall of Story A from the Logical Memory test14), essentially normal functional activities, CDR global score of 0.5, and MMSE score ≥ 24. Patients with AD met NINCDS/ADRDA criteria12 for probable AD, had MMSE scores between 20 and 26 (inclusive), and CDR global scores of 0.5 or 1.0. Participants were evaluated at six-month intervals for 2 (mild AD) or 3 (controls and MCI) years. Further details about ADNI, including participant selection procedures and complete study protocol, have been presented elsewhere,1, 15, 16 and may be found online at http://www.nia.nih.gov/Alzheimers/ResearchInformation/ClinicalTrials/ADNI.htm.
The present analyses included all participants—114 controls, 195 MCI patients, and 100 mild AD patients—who had valid test results for all CSF biomarkers (i.e., t-tau, Aβ42, p-tau181, t-tau/Aβ42, and p-tau181/Aβ42) when data download occurred in November 2008. Table 1 details the participants’ baseline characteristics. Informed consent was obtained from study participants and their families, and the study was approved by the local institutional review board at participating sites.
Table 1.
Characteristics of study participants at baseline
| Variable | Controls, n = 114 | MCI, n = 195 | AD, n = 100 |
|---|---|---|---|
| Age, mean (SD) | 75.54 (5.19) | 74.46 (7.50) | 74.85 (7.89) |
| Female, % | 49.10 | 33.30 | 42.0 |
| Caucasian, % | 91.20 | 95.40 | 99.0 |
| Education, mean (SD) | 15.74 (2.86) | 15.82 (3.00) | 15.11 (3.30) |
| On anti-dementia medication, % | 0 | 54.90 | 92.0 |
| APOE ε4 +, % | 23.7 | 53.8 | 69.0 |
| GDS, mean (SD) | .86 (1.10) | 1.67 (1.36) | 1.67 (1.36) |
| CDR-global, % | |||
| 0.0 | 100.0 | 0.0 | 0.0 |
| 0.5 | 0.0 | 100.0 | 57.0 |
| 1.0 | 0.0 | 0.0 | 43.0 |
| MMSE, mean (SD) | 29.09 (1.03) | 26.91 (1.79) | 23.54 (1.91) |
| ADAS-Cog, mean (SD) | 6.41 (2.90) | 11.65 (4.50) | 18.15 (6.18) |
| FAQ, mean (SD) | .16 (.66) | 3.81 (4.45) | 12.71 (6.71) |
MCI = mild cognitive impairment; AD = Alzheimer disease; APOE ε4 + = possession of one or more copies of apolipoprotein E ε4 allele; GDS = Geriatric Depression Scale; CDR = Clinical Dementia Rating scale; MMSE = Mini-Mental State Examination; ADAS-Cog = the Alzheimer Disease Assessment Scale—Cognitive subscale; FAQ = Pfeffer Functional Activities Questionnaire.
CSF collection and analysis
Full details of the collection and analysis of CSF samples in ADNI have been provided elsewhere.1 Briefly, lumbar puncture was performed in the morning following an overnight fast. T-tau, Aβ42, and p-tau181 were assayed from 0.5 ml aliquots using the multiplex xMAP Luminex platform (Luminex Corp., Austin, TX) with Innogenetics immunoassay kit-based reagents (INNO-BIA AlzBio3; Ghent, Belgium; for research use-only reagents).
Functional assessment
Everyday function was assessed with the Pfeffer Functional Activities Questionnaire (FAQ).17 The FAQ is an informant-report inventory that inquires into an older adult’s ability to manage finances, complete forms, shop, perform games of skill or hobbies, prepare hot beverages, prepare a balanced meal, follow current events, attend to television programs, books or magazines, remember appointments, and travel out of the neighborhood. Ratings range from normal (0) to dependent (3), for a total of 30 points. Higher scores indicate worse functional status. The FAQ has good reliability (item-total correlations ≥ .80) and validity (correlations ≥ .70 with measures of mental status, daily function, and clinical diagnosis).17 Within this ADNI sample, the FAQ demonstrated excellent reliability (Cronbach’s alpha=.93). And, at baseline, with the exception of control participants who, not surprisingly, mostly had zeros on the FAQ, FAQ scores in this cohort were largely devoid of floor and ceiling effects. For instance, no MCI or AD patient had a score of 30.
Cognitive assessment
Global cognition was assessed with the Alzheimer Disease Assessment Scale—Cognitive subscale (ADAS-Cog).18 The ADAS-Cog is the most widely used cognitive measure in AD clinical trials. It is brief, structured, and assesses verbal learning and memory, language, orientation, ideational praxis, and constructional praxis. Scores range from 0 to 70, with higher scores reflecting poorer cognitive function.
Data analyses
Group differences on the CSF measures were tested using single-degree of freedom contrast tests, corrected for inequality of variance. To examine the association between CSF abnormality, cognitive impairment, and functional decline within each diagnostic group, we fitted a series of random coefficient regressions19, 20 that modeled change in FAQ scores as a function of baseline values on CSF biomarkers and the ADAS-Cog. Abnormality on CSF biomarkers was defined using previously-established ADNI thresholds (t-tau=93 pg/ml, Aβ42=192 pg/ml, p-tau181=23 pg/ml, t-tau/Aβ42=.39 pg/ml, and p-tau181/Aβ42=.10 pg/ml).1 For the ADAS-Cog, we modeled the effect of performance that is 1 SD above (i.e., worse than) group-specific means.21 The biomarker*time terms were the primary effects of interest because they would reveal the impact of CSF abnormality or cognitive impairment on the rate of change in FAQ.
To quantify and compare the variation in functional decline accounted for by each CSF biomarker or ADAS-Cog, we calculated the proportional reduction—a pseudo R2 statistic—in FAQ’s rate of change residual variation that was attained when each biomarker and its interaction with time was introduced into a model that only contained age, baseline FAQ, and their interactions with time.20 Higher R2 values indicated that the variable being modeled accounted for a larger proportion of the unexplained variation in—and, thus, is more sensitive to—rate of change in FAQ.
To examine whether the effect of CSF biomarkers on functional decline is mediated by their impact on cognition, we tested a series of random coefficient regressions that added terms for ADAS-Cog and ADAS-Cog*time to each CSF biomarker model. Full mediation was assumed when a previously significant biomarker*time interaction became nonsignificant. Partial mediation was indicated when the biomarker*time effect was attenuated but remained significant. The percentage of the CSF biomarker functional decline relationship mediated by cognition was computed as: [(original estimate – ADAS-Cog-adjusted estimate) ÷ original estimate]. Because “mediation” requires that both substantive and mediator variables be associated with the outcome, these analyses were only performed within diagnostic groups in which CSF biomarkers and ADAS-Cog were both significantly related to functional decline.
Finally, we examined whether individuals with a combination of abnormal tau and Aβ42 experience a faster rate of functional decline relative to those with no or one CSF abnormality, by fitting a series of random coefficient regressions in which the rate of functional decline among persons with “normal tau, normal Aβ42,” was contrasted with the rate of decline in the “abnormal tau, normal Aβ42,” “normal tau, abnormal Aβ42,” and “abnormal tau, abnormal Aβ42” groups.
As a precondition for examining the effects of CSF abnormalities and ADAS-Cog on functional decline, we first examined the temporal course and rate of functional decline within each group by fitting group-specific random effects regressions that modeled change in FAQ scores as a function of time.20 To determine temporal course of functional decline, we compared the relative fit of linear (time) and curvilinear (time*time) polynomials for time using the Bayesian Information Criterion (BIC).22 On the BIC, lower values indicate better fit. The polynomial specification for time (i.e., linear or quadratic) that emerged as optimal was employed in all subsequent analyses.
All random coefficient regressions outlined above included random intercept and random slope terms to account/test for potential inter-individual variability in baseline scores and rate of change, respectively.20 In addition, they all included age, baseline FAQ, and their interactions with time as covariates; and, to further adjust for variations in baseline FAQ, analyses were begun at the six-month assessment. Data analyses were performed using SPSS 16.
RESULTS
Group differences in baseline CSF analytes
As reported in prior studies,1, 2, 5 CSF levels of t-tau, p-tau181, t-tau/AB42, and p-tau181/AB42 were significantly higher, whereas Aβ42 was significantly lower, in MCI and AD compared to controls, and in AD compared to MCI (see Table 2).
Table 2.
Cerebrospinal fluid biomarker concentrations and ratios at baseline
| Biomarker | Controls, n = 114 | MCI, n = 195 | AD, n = 100 |
|---|---|---|---|
| t-tau, mean (SD) | 69.65 (30.32) | 103.54 (60.93)a | 121.57 (57.56)a,b |
| % abnormal | 18.4 | 44.6 | 65.0 |
| Aβ42, mean (SD) | 205.63 (55.07) | 163.31 (54.93)a | 143.51 (41.01)a,b |
| % abnormal | 37.7 | 74.4 | 91.0 |
| p-tau181, mean (SD) | 24.84 (14.59) | 35.68 (18.10)a | 41.73 (19.96)a,b |
| % abnormal | 36.0 | 70.3 | 87.0 |
| t-tau/Aβ42, mean (SD) | .39 (.27) | .75 (.62)a | .92 (.48)a,b |
| % abnormal | 34.2 | 69.7 | 88.0 |
| p-tau/Aβ42, mean (SD) | .14 (.13) | .26 (.18)a | .32 (.19)a,b |
| % abnormal | 47.4 | 77.9 | 94.0 |
Cerebrospinal fluid biomarker concentrations were measured in pg/ml.
% abnormal refers to the percentage of cases, within each diagnostic group, whose cerebrospinal fluid biomarker values were worse than the cut offs (t-tau = 93 pg/ml, Aβ42 = 192 pg/ml, p-tau181 = 23 pg/ml, t-tau/Aβ42 = .39 pg/ml, and p-tau181/Aβ42 = .10 pg/ml) established in a prior ADNI study.1
MCI = mild cognitive impairment; AD = Alzheimer disease, t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau181= tau phosphorylated at threonine 181
significantly different from controls
significantly different from MCI
Temporal pattern of change in FAQ across dementia spectrum
Within each diagnostic group, the model that examined change in FAQ as a function of linear time had a lower BIC compared to the model that specified a quadratic function for time. For example, within the MCI group, BIC was 3618.45 for the linear model whereas it was 3628.87 for the quadratic model. This was taken as evidence that, within each group, change in the FAQ was better characterized as proceeding linearly. Accordingly, all subsequently analyses were performed using a linear function for time.
Rate of change in FAQ across dementia spectrum
FAQ scores increased (i.e., worsened) at a mean biannual rate (±SE; p value) of .04 (±.04; p=.276) among controls, 1.23 (±.16, p<.001) among MCI patients, and 1.77 (±.19; p<.001) among AD patients. Although the mean rate of deterioration in FAQ among controls was nonsignificant, inspection of the random slope term revealed that there was significant inter-individual variability around this mean value (estimate= .10, SE=.02, p<.001). Taken together, these findings suggest that the FAQ duly captures longitudinal decline in everyday function across the dementia spectrum, albeit potentially less so among controls. Furthermore, the observed inter-individual variability in slope trajectory, which was seen within each group, provided the basis for examining the impact of predictors (i.e., CSF measures and the ADAS-Cog) on rate of change in the FAQ.20
CSF biomarkers, ADAS-Cog, and rate of change in FAQ across dementia spectrum
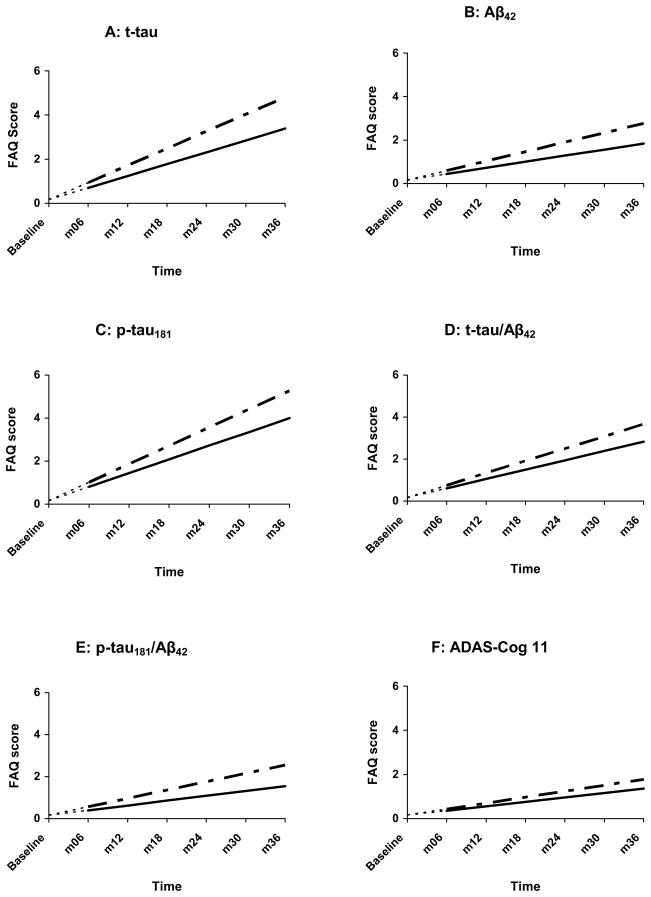
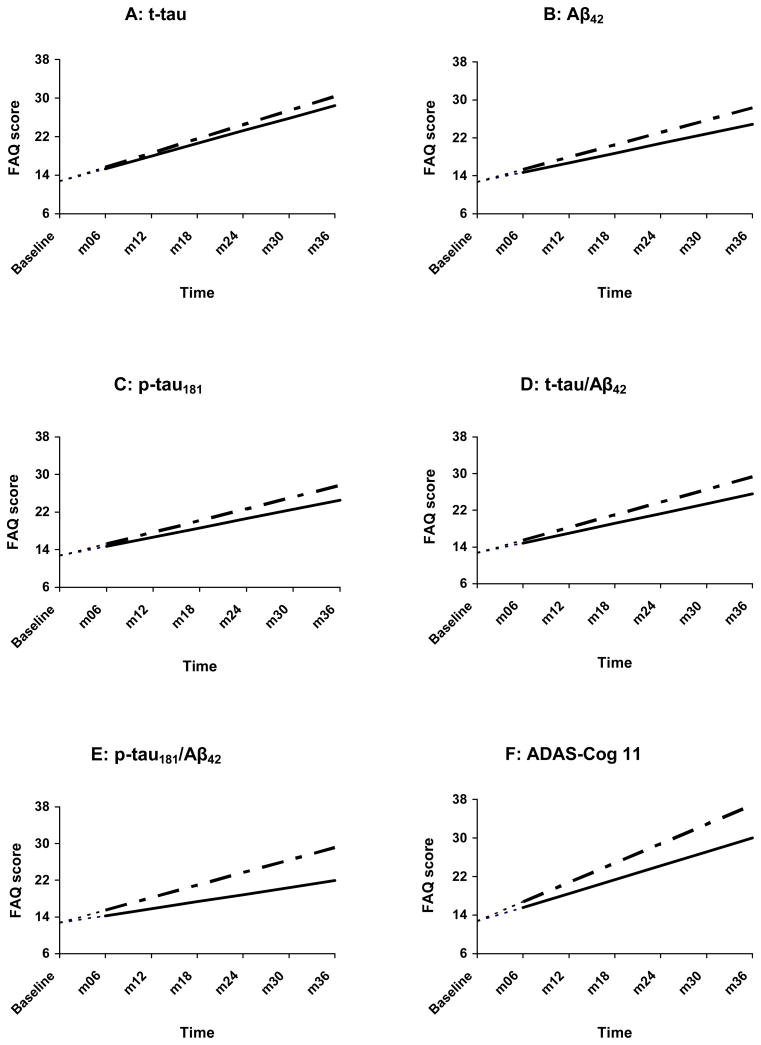
Among controls, only t-tau, Aβ42, p-tau181, and p-tau/Aβ42 abnormalities were associated with faster rate of functional decline. In MCI, all CSF measures and the ADAS-Cog were significantly associated with rate of functional decline. Finally, within the AD group, no CSF measure predicted rate of decline on the FAQ. In contrast, the ADAS-Cog significantly predicted FAQ decline (see Table 3; Fig. 1–3). Of note, the random slope term in the foregoing analyses was significant (p <.001), indicating substantial between-person deviations from the mean/prototypical rate of change. The plots (Fig. 1–3) present the prototypical change trajectories, for illustrative purposes (e.g., Fig. 1A displays trajectories for the prototypical control with normal t-tau versus the prototypical control with abnormal t-tau).20
Table 3.
Trajectories of functional change across AD spectrum as a function of CSF biomarkers and ADAS-Cog scores
| Time | Biomarker | Biomarker*time | Δ R2 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Est. (SE) | p | Est. (SE) | p | Est. (SE) | p | |||
| Controls | t-tau | .54 (.53) | .313 | −.15 (.15) | .330 | .24 (.10) | .015 | 6.38 |
| Aβ42 | .28 (.52) | .593 | −.02 (.12) | .881 | .16 (.08) | .046 | 5.32 | |
| p-tau181 | .64 (.53) | .233 | −.26 (.13) | .042 | .21 (.08) | .009 | 9.57 | |
| t-tau/Aβ42 | .44 (.53) | .409 | .02 (.13) | .865 | .14 (.08) | .084 | 4.26 | |
| p-tau/Aβ42 | .23 (.52) | .656 | −.12 (.12) | .321 | .17 (.07) | .023 | 7.45 | |
| ADAS-Cog | .23 (.53) | .658 | −.03 (.06) | .663 | .04 (.04) | .326 | 1.06 | |
| MCI | t-tau | 1.29 (1.60) | .423 | −1.47 (.61) | .017 | .79 (.32) | .015 | 5.21 |
| Aβ42 | 1.21 (1.56) | .442 | −.87 (.71) | .220 | 1.24 (.37) | .001 | 11.84 | |
| p-tau181 | 1.10 (1.60) | .496 | −.62 (.66) | .347 | .99 (.34) | .004 | 5.76 | |
| t-tau/Aβ42 | 1.17 (1.57) | .454 | −.92 (.67) | .169 | 1.21 (.34) | .001 | 11.39 | |
| p-tau/Aβ42 | 1.05 (1.59) | .509 | −.49 (.74) | .513 | 1.22 (.38) | .002 | 7.71 | |
| ADAS-Cog | 1.93 (1.58) | .224 | .34 (.31) | .272 | .43 (.16) | .009 | 7.40 | |
| AD | t-tau | 2.62 (1.76) | .141 | −.76 (1.19) | .525 | .31 (.38) | .422 | 1.60 |
| Aβ42 | 2.02 (2.09) | .338 | 1.84 (2.06) | .375 | .58 (.70) | .413 | −9.63 | |
| p-tau181 | 1.97 (2.01) | .330 | .38 (1.72) | .827 | .52 (.56) | .358 | 3.08 | |
| t-tau/Aβ42 | 2.14 (1.92) | .269 | 1.07 (1.79) | .552 | .62 (.64) | .336 | −.54 | |
| p-tau/Aβ42 | 1.53 (2.16) | .479 | −.77 (2.61) | .769 | 1.20 (1.07) | .263 | −3.74 | |
| ADAS-Cog | 3.45 (1.60) | .035 | −.56 (.65) | .393 | .57 (.22) | .010 | 33.69 | |
Top panel = controls, middle panel= MCI, bottom panel= AD
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p- tau181= tau phosphorylated at threonine 181, ADAS-Cog = the Alzheimer Disease Assessment Scale—Cognitive subscale.
Models adjusted for age, baseline FAQ, and their interactions with time. In addition, analyses were begun at month 6 to further correct for potential group differences in FAQ at baseline. “Time” is the estimated semiannual rate of change in FAQ for those who have normal biomarker levels, or whose ADAS-Cog scores are at the mean for their group.
“Biomarker*time” is the estimated differential in semiannual rate of change in FAQ for those who have abnormal biomarker levels, or whose ADAS-Cog scores are one standard deviation above (i.e., worse than) their group’s mean.
Δ R2= proportional reduction in FAQ’s rate of change residual variation attained when each biomarker and its interaction with time was introduced into a “base” model that only contained age, baseline FAQ, and their interactions with time. These R2 statistics were computed thus: [(“base model” residual variation “substantive model” residual variation) ÷ “base model” residual variation].
Figure 1.
Change in FAQ as a function of CSF biomarkers and ADAS-Cog among controls
-----------= normal values — — — - = abnormal values
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau = tau phosphorylated at threonine-181, ADAS-Cog = the Alzheimer Disease Assessment Scale—Cognitive subscale.
Analyses were begun at month 6, with baseline FAQ treated as a covariate. The net effect of this adjustment is that, at baseline, all controls were assigned an FAQ score of 0.16—the within-group FAQ mean at that assessment point. This is represented by the leading dashes in each line.
Figure 3.
Change in FAQ as a function of CSF biomarkers and ADAS-Cog among AD patients
---------= normal values — — — - = abnormal values
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau = tau phosphorylated at threonine-181, ADAS-Cog = the Alzheimer Disease Assessment Scale—Cognitive subscale.
Analyses were begun at month 6, with baseline FAQ treated as a covariate. The net effect of this adjustment is that, at baseline, all AD patients were assigned an FAQ score of 12.71—the within-group FAQ mean at that assessment point. This is represented by the leading dashes in each line.
Variance in functional decline explained by CSF biomarkers and ADAS-Cog
Within controls, p-tau181 emerged the most sensitive to decline in FAQ (R2 =9.57), and ADAS-Cog the least. In MCI, Aβ42 accounted for the most variance in FAQ (R2 =11.84), though t-tau/Aβ42 was virtually as sensitive (R2 =11.39). Among AD patients, ADAS-Cog accounted for 34% of the variance whereas no CSF measure accounted for more than 3% (see Table 3).
Cognition as a mediator of CSF biomarkers’ effect on rate of decline
The mediation analyses were performed only in the MCI group, because they were the only group in which both CSF biomarkers and ADAS-Cog significantly predicted rate of functional decline. Adjustment for ADAS-Cog did not obliterate the relationship between any CSF biomarker and rate of change in FAQ. However, the relationships were attenuated—17% for p-tau181, 13% for t-tau/Aβ42 and p-tau/Aβ42, 12% for Aβ42, and 7% for t-tau—consistent with partial mediation.
Combination of tau and Aβ42 abnormalities and rate of functional decline
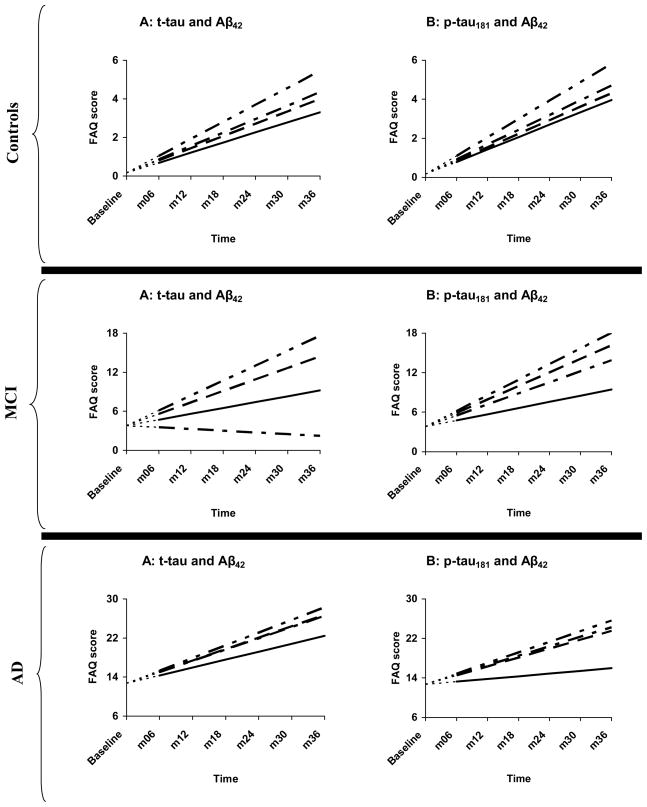
Within each diagnostic group, the “abnormal t-tau, abnormal Aβ42” subgroup experienced the steepest rate of functional decline. However, within the AD group, their rate of decline was statistically indistinguishable from that of the other three subgroups. Interestingly, among MCI patients, those who had “normal t-tau, abnormal Aβ42” declined faster than those who had “normal t-tau, normal Aβ42” whereas those who had “abnormal t-tau, normal Aβ42” did not. These findings were essentially replicated in the p-tau181–Aβ42 analyses (see Table 4; Fig. 4).
Table 4.
Rate of change in FAQ for groups defined by combination of tau and Aβ42 abnormalities
| Effect | Estimate | Standard Error | p | |
|---|---|---|---|---|
| Controls | Time | .52 | .52 | .317 |
| Abnormal t-tau, Normal Aβ42*time | .17 | .13 | .196 | |
| Normal t-tau, Abnormal Aβ42*time | .11 | .09 | .191 | |
| Abnormal t-tau, Abnormal Aβ42*time | .36 | .12 | .005 | |
| Time | .63 | .53 | .231 | |
| Abnormal p-tau181, Normal Aβ42*time | .12 | .10 | .240 | |
| Normal p-tau181, Abnormal Aβ42*time | .06 | .10 | .572 | |
| Abnormal p-tau181, Abnormal Aβ42*time | .31 | .10 | .002 | |
| MCI | Time | .90 | 1.56 | .566 |
| Abnormal t-tau, Normal Aβ42*time | −1.16 | 1.30 | .373 | |
| Normal t-tau, Abnormal Aβ42*time | .87 | .43 | .042 | |
| Abnormal t-tau, Abnormal Aβ42*time | 1.40 | .40 | .001 | |
| Time | .94 | 1.58 | .554 | |
| Abnormal p-tau181, Normal Aβ42*time | .74 | .77 | .337 | |
| Normal p-tau181, Abnormal Aβ42*time | 1.12 | .60 | .063 | |
| Abnormal p-tau181, Abnormal Aβ42*time | 1.44 | .40 | .001 | |
| AD | Time | 1.62 | 2.14 | .453 |
| Abnormal t-tau, Normal Aβ42*time | .68 | 1.30 | .602 | |
| Normal t-tau, Abnormal Aβ42*time | .71 | .92 | .441 | |
| Abnormal t-tau, Abnormal Aβ42*time | .97 | .91 | .285 | |
| Time | .54 | 2.42 | .823 | |
| Abnormal p-tau181, Normal Aβ42*time | 1.38 | 1.42 | .332 | |
| Normal p-tau181, Abnormal Aβ42*time | 1.26 | 1.28 | .325 | |
| Abnormal p-tau181, Abnormal Aβ42*time | 1.61 | 1.21 | .186 | |
Top two panels are control models; middle two panels are MCI models; bottom two panels are AD models.
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau181= tau phosphorylated at threonine-181.
Models adjusted for age, baseline FAQ, and their interactions with time. In addition, analyses were begun at month 6 to further correct for potential group differences in FAQ at baseline. “Time” is the estimated semiannual rate of change in FAQ for those who have normal tau (t-tau or p-tau, depending on the model being tested) and normal Aβ42. The other terms indicate the estimated difference in semiannual rate of change in FAQ between the “normal tau, normal Aβ42” group and each of the other three groups.
Figure 4.
Change in FAQ as a function of concurrent tau and Aβ42 abnormalities
--------------- = normal tau, normal Aβ42 — — — — — = normal tau, abnormal Aβ42
— — — — — = abnormal tau, normal Aβ42 — — — — — = abnormal tau, abnormal Aβ42
Top two panels = controls, middle two panels = MCI, bottom two panels = AD
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau = tau phosphorylated at threonine-181. Analyses were begun at month 6, with baseline FAQ treated as a covariate. The net effect of this adjustment is that, at baseline, all AD patients were assigned an FAQ score of 12.71—the within-group FAQ mean at that assessment point. This is represented by the leading dashes in each line.
COMMENT
With reference to the core questions this study investigated, our key findings were: (i) all CSF analytes were associated with functional decline in MCI and all but t-tau/Aβ42 were associated with functional decline in controls, whereas no CSF analyte was associated with functional decline in AD, (ii) among controls, p-tau181 was the most sensitive to functional decline whereas in MCI it was Aβ42, (iii) CSF biomarkers were more sensitive than ADAS-Cog among controls and variably so in MCI, whereas the ADAS-Cog was unequivocally more sensitive than CSF biomarkers in AD, (iv) the impact of CSF biomarkers on functional decline in MCI is partially mediated by their impact on cognitive status, and (v) across all diagnostic groups, persons with a combination of tau and Aβ42 abnormalities exhibited the fastest rate of functional decline.
Progressive diminution in, and eventual loss of, the ability to perform daily activities is a hallmark feature of AD.11 Consequently, decline in everyday function is a veritable measure of disease progression in AD.13 The findings from this study therefore suggest that p-tau181 is the strongest predictor of possible disease progression among controls whereas Aβ42 is most potent in MCI. This conclusion is consistent with histopathological studies that suggest a temporal sequence in the manifestation of AD-related brain lesions wherein intraneuronal alterations precede the deposition of amyloid plaques.23–25 Even so, we acknowledge that the temporal ordering of AD lesions and their presumed downstream effects on CSF analytes remain controversial issues deserving continued investigation.6, 7, 26, 27 For instance, it may be that t-tau and p-tau181 were stronger correlates of FAQ decline (compared to Aβ42) among controls because Aβ42 levels were already reduced in the earliest phase of AD.7, 28, 29 Nonetheless, because levels of p-tau181 reflect hyperphosphorylation of tau (a putatively AD-specific process),3, 30, 31 our control findings suggest that, among cognitively-intact elders, functional decline and eventual progression to AD may be most probable for those individuals who already demonstrate pathognomonic features of AD.
Within the MCI and control groups, we found that ratio of tau proteins to Aβ42 were strongly correlated with functional decline. Prior reports have suggested that biomarker ratios may be more promising AD biomarkers compared to absolute biomarker levels.5, 32–35 However, a potential drawback to their application is that, by virtue of being ratios, they mask a likely nontrivial distinction between individuals who have “normal tau, abnormal Aβ42” and those who have “abnormal tau, normal Aβ42.” For instance, in the present study we found that MCI patients with normal tau–abnormal Aβ42 experienced a more rapid functional decline compared to those with normal–tau normal Aβ42 whereas those with abnormal tau–normal Aβ42 did not. This observation buttresses the earlier-noted finding that, among MCI patients, Aβ42 abnormalities were better prognostic of functional degradation and disease progression than tau alterations.36–38
We were surprised to find that no CSF biomarker was predictive of functional decline among AD patients. The reason for this is not immediately clear, though might be due to reduced variability in the CSF biomarkers. This would be consistent with prior studies that have shown that upon becoming abnormal, CSF biomarkers subsequently tend to remain stable for several years even as dementia progresses.7, 9, 39–41 In addition, other studies have also failed to find associations between CSF biomarkers and indices of disease risk and burden in AD.42
CSF analytes hold great promise as biomarkers of AD30 and, therefore, have potentially pivotal clinical utility.43–45 However, their routine implementation in clinical practice is hampered by several factors including lumbar puncture’s relative invasiveness and potential for iatrogenesis, though the latter may not be as inexorable as originally believed.45–47 Thus, clinical measures and peripheral-fluid biomarkers are increasingly explored as viable alternatives.31, 32, 48 Accordingly, in this study, we examined the comparative sensitivity of CSF biomarkers and the ADAS-Cog, a brief measure of global cognition, to the rate of functional decline within each diagnostic group. Overall, our findings suggest that a cognitive screen that is brief, noninvasive, and easy to administer competes favorably with CSF biomarkers with regard to sensitivity to functional decline, and hence disease progression, especially among AD patients.49
Interestingly, our mediation analyses revealed that the greatest reduction in the variance accounted for by CSF biomarkers occurred for p-tau181. There is evidence that p-tau181 reflects neurofibrillary tangle formation,3, 31 and that the density of tangles correlates better with cognitive decline and dementia than plaque load.50, 51 Accordingly, it stands to reason that adjusting for cognition most attenuated the original relationship between p-tau181 and rate of functional decline. Finally, consistent with reports from prior investigations,5, 33, 35 we found that, within each diagnostic group, individuals who had pathological concentrations of tau and Aβ42 experienced the steepest functional decline. This was most pronounced in the MCI group where those with “abnormal tau, abnormal Aβ42” declined at about 2.5 times the rate at which the “normal tau, normal Aβ42” group declined (e.g., t-tau, Aβ42 = [.90+1.40]/.90). As concurrent disturbances in tau and Aβ42 is considered diagnostic for AD, the accelerated decline in everyday function manifested by control and MCI patients with these defining CSF alterations might represent a harbinger of their eventual progression to AD.52
Potential limitations of this study include the use of relatively gross measures of everyday function (FAQ) and cognition (ADAS-Cog), and the low ethnic diversity of the sample. In addition, the participants studied were enrolled in a clinical study, not an epidemiological study. It is unclear how these factors may have influenced our findings. Despite these limitations, this study is unique in being the first to examine several interrelated questions concerning the relationship between CSF biomarkers and rate of functional decline across the AD spectrum.
Figure 2.
Change in FAQ as a function of CSF biomarkers and ADAS-Cog among MCI patients
--------= normal values — — — - = abnormal values
t-tau = total tau, Aβ42 = β-amyloid 1-42 peptide, p-tau = tau phosphorylated at threonine-181, ADAS-Cog = the Alzheimer Disease Assessment Scale—Cognitive subscale.
Analyses were begun at month 6, with baseline FAQ treated as a covariate. The net effect of this adjustment is that, at baseline, all MCI patients were assigned an FAQ score of 3.81—the within-group FAQ mean at that assessment point. This is represented by the leading dashes in each line.
Acknowledgments
Data collection and sharing for this project was funded by the ADNI (Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the AD Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Footnotes
Ozioma C. Okonkwo had full access to all of the data reported in this manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunderland T, Linker G, Mirza N, et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 3.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurology. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 4.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 5.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 6.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 7.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–83. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrie EC, Cross DJ, Galasko D, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–7. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snider BJ, Fagan AM, Roe C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–45. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: Predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. text revision ed. [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Black R, Greenberg B, Ryan JM, et al. Scales as outcome measures for Alzheimer’s disease. Alzheimers Dement. 2009;5:324–39. doi: 10.1016/j.jalz.2009.05.667. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler D. Wechsler Memory Scale - Revised edition. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 15.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 19.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SASR for Mixed Models. 2. Cary, North Carolina: SAS Institute Inc; 2007. [Google Scholar]
- 20.Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 21.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: A challenge to current thinking. Br J Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz G. Estimating the dimensions of a model. Annals of Statistics. 1978;6:461–4. [Google Scholar]
- 23.Schonheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. doi: 10.1016/j.neurobiolaging.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Del Tredici K. Alzheimer’s disease: Intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol Aging. 2004;25:713–8. doi: 10.1016/j.neurobiolaging.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–71. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 26.Price JL, Morris JC. So what if tangles precede plaques? Neurobiol Aging. 2004;25:721–3. doi: 10.1016/j.neurobiolaging.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Korczyn AD. The amyloid cascade hypothesis. Alzheimers Dement. 2008;4:176–8. doi: 10.1016/j.jalz.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–31. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 29.Jack CRJ, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hampel H, Mitchell A, Blennow K, et al. Core biological marker candidates of Alzheimer’s disease - perspectives for diagnosis, prediction of outcome and reflection of biological activity. J Neural Transm. 2004;111:247–72. doi: 10.1007/s00702-003-0065-z. [DOI] [PubMed] [Google Scholar]
- 31.Frank RA, Galasko D, Hampel H, et al. Biological markers for therapeutic trials in Alzheimer’s disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging. 2003;24:521–36. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 32.Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurology. 2008;7:704–14. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 34.Kapaki E, Paraskevas GP, Zalonis I, Zournas C. CSF tau protein and beta-amyloid (1-42) in Alzheimer’s disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. Eur J Neurol. 2003;10:119–28. doi: 10.1046/j.1468-1331.2003.00562.x. [DOI] [PubMed] [Google Scholar]
- 35.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurology. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 36.Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9:705–10. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 37.Schoonenboom SN, Visser PJ, Mulder C, et al. Biomarker profiles and their relation to clinical variables in mild cognitive impairment. Neurocase. 2005;11:8–13. doi: 10.1080/13554790490896785. [DOI] [PubMed] [Google Scholar]
- 38.Ivanoiu A, Sindic CJ. Cerebrospinal fluid TAU protein and amyloid beta42 in mild cognitive impairment: Prediction of progression to Alzheimer’s disease and correlation with the neuropsychological examination. Neurocase. 2005;11:32–9. doi: 10.1080/13554790490896901. [DOI] [PubMed] [Google Scholar]
- 39.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 40.Sunderland T, Wolozin B, Galasko D, et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry. 1999;46:750–5. doi: 10.1016/s0006-3223(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 41.Zetterberg H, Pedersen M, Lind K, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–60. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- 42.Engelborghs S, Sleegers K, Cras P, et al. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer’s disease. Brain. 2007;130:2320–6. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 43.Hampel H, Buerger K, Zinkowski R, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 44.Riemenschneider M, Wagenpfeil S, Vanderstichele H, et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–7. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 45.Andreasen N, Minthon L, Davidsson P, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–9. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 46.Peskind E, Nordberg A, Darreh-Shori T, Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer’s disease. Current Alzheimer Research. 2009;6:290–2. doi: 10.2174/156720509788486509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peskind ER, Riekse R, Quinn JF, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005;19:220–5. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 48.Carrillo MC, Blackwell A, Hampel H, et al. Early risk assessment for Alzheimer’s disease. Alzheimers Dement. 2009;5:182–96. doi: 10.1016/j.jalz.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–9. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 50.Trojanowski JQ, Shin RW, Schmidt ML, Lee VM. Relationship between plaques, tangles, and dystrophic processes in Alzheimer’s disease. Neurobiol Aging. 1995;16:335–40. doi: 10.1016/0197-4580(94)00176-2. [DOI] [PubMed] [Google Scholar]
- 51.Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 52.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]