Abstract
We used behavioral measures and functional magnetic resonance imaging (fMRI) to study the effects of parametrically varied task-irrelevant pitch changes in attended sounds on loudness-discrimination performance and brain activity in cortical surface maps. Ten subjects discriminated tone loudness in sequences that also included infrequent task-irrelevant pitch changes. Consistent with results of previous studies, the task-irrelevant pitch changes impaired performance in the loudness discrimination task. Auditory stimulation, attention-enhanced processing of sounds and motor responding during the loudness discrimination task activated supratemporal (auditory cortex) and inferior parietal areas bilaterally and left-hemisphere (contralateral to the hand used for responding) motor areas. Large pitch changes were associated with right hemisphere supratemporal activations as well as widespread bilateral activations in the frontal lobe and along the intraparietal sulcus. Loudness discrimination and distracting pitch changes activated common areas in the right supratemporal gyrus, left medial frontal cortex, left precentral gyrus, and left inferior parietal cortex.
Keywords: auditory processing, attention, change detection, distraction, fMRI
While effective cognitive performance requires focusing of attention on relevant information and ignoring irrelevant sensory inputs, one must retain the ability to respond to potentially important novel events in the environment. Previous studies have used the so-called auditory distraction paradigm [3, 7, 26, 32] to examine the effect of task-irrelevant sound changes on behavior and event-related potentials (ERPs). In this paradigm, subjects discriminate sounds on one dimension (e.g., sound duration) while task-irrelevant changes occur in some other dimension (e.g., pitch) of the same sounds. Typically, deviant auditory stimuli produce a brief degradation of performance (reaction times increase and hit rates decrease) that is accompanied by two characteristic responses in event-related brain potentials (ERPs), the mismatch negativity (MMN) and the subsequent P3a response, occurring within 100–400 ms from the change onset. The MMN is associated with auditory change detection, while the P3a is associated with involuntary shifting of attention to task-irrelevant features of the sounds [8]. Several cortical areas including the supratemporal and inferior frontal cortices and the temporo-parietal junction (TPJ) have been implicated in these functions by ERP source analyses [1, 23, 27, 30] and functional magnetic resonance imaging (fMRI) [19, 21, 24]. However, previous studies have not focused directly on mapping the brain activation underlying the behavioral distraction effect. Here, we examine in detail the cortical circuits involved in auditory change detection and distraction of focused auditory attention using cortical surface mapping techniques that provide increased spatial precision [10].
Twelve right-handed healthy subjects (20–27 years old; 6 males) participated in the experiment. The data from one subject was rejected from the analysis due to a high error rate (over 35 %) in the behavioral task and the data from another subject due to excessive head movements during fMRI scanning. Informed written consent was obtained from each subject prior to the experiment. The study protocol was approved by the ethical committee of the Hospital District of Helsinki and Uusimaa, Finland.
The subjects performed a forced-choice discrimination task. Harmonic tones (fundamental frequency and first four harmonics with equal intensity; duration 100 ms; rise and fall time 5 ms) were presented at constant 1.1 s onset-to-onset intervals. Soft and loud tones (70 and 75 dB SPL at the eardrum, respectively) were presented equiprobably. Subjects indicated the loudness of each tone by pressing a button with their right index or middle finger for soft and loud tones, respectively. The harmonic tones also varied in frequency content. Most tones (standards; P = 0.8) had a fundamental frequency of 236 Hz. Fundamental frequency of the infrequent tones (deviants) was lowered by 10, 20, 30, 40 or 50 Hz (P = 0.04 for each), and the harmonics in the same relation. The tones were delivered diotically through earplugs (EAR, USA) via pneumatic headphones (Avotec, USA) and were presented in random order except that each pitch change was preceded by at least two tones with a standard pitch. MRI scanner noise (approximately 102 dB SPL, A-weighted measurement inside the head coil; relatively low energy at 100 – 500 Hz) was attenuated to approximately 70 dB SPL at the eardrum by acoustic shielding material wrapped around the headcoil, headphones and earplugs. Because of differences in the spectra and subjective sound location (diotic sounds were perceived as if their source was inside the head) of the tones and scanner noise, both soft and loud tones were clearly perceived. Subjects maintained constant fixation on a fixation mark (projected to a mirror fixed to the head coil). The tone sequence and task performance was interrupted by 5 short (27.5 s) periods. During these resting periods, the subjects were only required to maintain fixation. The experiment was performed with Presentation software (Neurobehavioral Systems, Albany, CA).
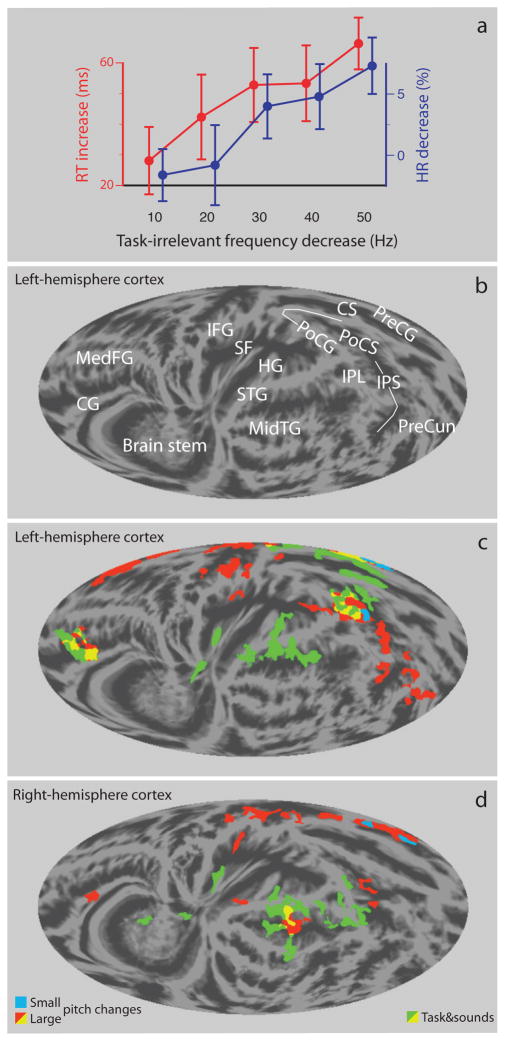
The loudness discrimination task was intentionally difficult (77% of responses were correct, s.e.m. 2%) and therefore required constant focused attention. Consistent with behavioral distraction effects reported in previous studies [8, 26, 31], task performance was impaired by the task-irrelevant pitch changes (Fig. 1a): reaction time (RT) increased (within-subject difference in performance between infrequent pitch change and frequent sound; repeated-measures ANOVA, main effect of Pitch-Change Level: F(4,36) = 9.0, P < 0.001; linear trend: F(1,9) = 43.6, P < 0.001) and the hit rate (HR) decreased (main effect: F(4,36) = 5.1, P < 0.01; linear trend: F(1,9) = 27.0, P < 0.001) with increasing pitch deviance. Note that even the smallest pitch change (10 Hz) had a distinct effect on RT (mean RT increase 28.1 ms ± 11 ms s.e.m), while HR decreased only for the large (30–50 Hz) pitch changes.
Fig 1.
Cortical networks of focused and distracted behavior. (a) Distraction of performance (within-subject difference in performance between infrequent pitch change and frequent sound) in the loudness-discrimination task. Mean reaction time (RT, red line) increased and hit rate (HR, blue line) decreased with the increasing magnitude of task-irrelevant pitch change (error bars show s.e.m.). (b) Anatomical landmarks for the left-hemisphere cortical surface (for abbreviations, see text). (c, d) Left- and right-hemisphere cortical activations (N=10; threshold Z > 2.3, corrected cluster threshold P < 0.01) overlaid onto grand-average cortical anatomy. Green = Activations associated with focused discrimination-task performance. Red = Activations to large (≥ 30 Hz) task-irrelevant pitch changes. Light blue = Activations to small pitch changes (≤ 20 Hz). Yellow = Areas activated by both the discrimination task and large distracting pitch changes.
It could be argued that these behavioral distraction effects were not due to distraction but to intrinsic differences in loudness-discrimination performance depending on pitch. This possibility was tested in a separate behavioral control condition (N = 13, 8 subjects participated in the main experiment). In this control condition (without fMRI scanning), the effect of pitch per se on the loudness discrimination task was measured while the possible contributions of auditory change detection and involuntary attention switching on task performance were minimized. Harmonic sounds used in the fMRI experiment were presented in constant-pitch blocks of 6–10 sounds (fundamental frequency 236, 226, 216, 206, 196 or 186 Hz; 160 sounds for each). The blocks were presented in random order, except that the pitch difference between two successive blocks was 30 Hz or less. The two first sounds of each block were excluded from the analysis. The results of the control condition (Fig. 2) showed that RT in the loudness-discrimination task was not affected by pitch in the absence of change (repeated-measures ANOVA, main effect of Pitch: F(5,60) = 1.45; linear trend: F(1,12) = 0.16). However, HR depended on pitch (main effect: F(5,60) = 4.52, P < 0.01; linear trend: F(1,12) = 6.89, P < 0.05). Although this result is mainly due to low HR for the 186 Hz tones (Fig. 2), such dependency of HR on pitch could partially explain the HR effects in the main experiment (Fig. 1a). However, the results of the control condition verified that the pitch of the tones per se did not affect loudness-discrimination performance in a way that would be enough to fully explain the behavioral distraction effects obtained in the main experiment. Thus, the deterioration of loudness-discrimination performance in the main experiment was mainly caused by the unpredictability and rarity of the task-irrelevant pitch changes.
Fig 2.
Performance in the loudness-discrimination task with constant-pitch blocks (i.e., in the absence of change). The results of this behavioral control experiment show that the pitch of the tones per se does not explain the deterioration of loudness-discrimination performance seen in the main experiment. Red line = Reaction time (RT). Blue line = Hit rate (HR). Error bars show s.e.m.
As the subjects performed the discrimination task (main experiment), continuous whole-head fMRI data (3.0 T GE Signa retrofitted with an Advanced NMR operating console and a quadrature birdcage coil, gradient-echo planar imaging, TE 32 ms, TR 2800 ms, flip angle 90 degrees) were acquired. The imaged area consisted of 28 contiguous 3.4 mm thick axial oblique slices. The in-plane resolution was 3.4 mm × 3.4 mm (voxel matrix 64 × 64, FOV 22 cm × 22 cm). For each subject, a total of 644 functional whole-brain volumes were acquired. Anatomical images were obtained in a separate session (Siemens Sonata 1.5 T, voxel matrix 176 × 512 × 512, resolution 1 mm × 0.5 mm × 0.5 mm, resliced to 1 mm × 1 mm × 1 mm resolution). All individual functional images were co-registered and resliced into the 1 mm × 1 mm × 1 mm anatomical space using SPM99 (http://www.fil.ion.ucl.ac.uk/spm).
fMRI activations were analyzed on the cortical surface of each subject using the following steps: First, cortical surfaces were extracted from high-resolution anatomical images, transformed to spherical standard space, and anatomically normalized on the basis of the cortical gyral and sulcal patterns using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) [6, 9]. SurfRelax (http://www.cns.nyu.edu/~jonas/software.html) [18] was used to preprocess (segmentation and removal of subcortical structures including the cerebellum) some of the images. Next, the three dimensional (3D) spherical cortical surfaces were rotated and projected to a two dimensional (2D) space separately for each hemisphere using equal area Mollweide projections (Fig. 1b) permitting a view of the entire cortical surface (see http://www.ebire.org/hcnlab/cortical-mapping for further details). This procedure produced 3D-to-2D anatomical transformation matrices for each subject that were then applied separately for each functional image. Finally, the functional maps on the 2D surface were analyzed using the tools developed by the Analysis Group at the Oxford Centre for Functional MRI of the Brain (FMRIB) and implemented as software tools within FMRIB’s software library (FSL, release 3.3, http://www.fmrib.ox.ac.uk/fsl) [34]. The first five images were excluded from analysis to eliminate magnetization effects. The data were spatially smoothed with a Gaussian kernel of 3 mm full-width half-maximum and high-pass filtered (cutoff 40 s). First-level statistical analysis was carried out using general linear modeling (FMRIB’s Improved Linear Model). The time of occurrence of each task-irrelevant pitch change and the time of 27.5-s silent periods were entered separately to the model. Thus, the other periods during which the subject was performing non-distracted loudness discrimination with standard-pitch tones (i.e., constant-pitch trials) were used as baseline. However, the first 12 s of each performance block after the 27.5-s breaks were excluded (i.e., these periods were modeled separately) due to the possible enhanced activation levels caused by the beginning of a tone sequence [20]. The hemodynamic responses to the 27.5-s breaks (and to the 12-s block-onset periods) were modeled with a gamma-function (mean lag 6 s, sd 3 s) and its temporal derivative. The responses to task-irrelevant pitch changes were modeled with a double-gamma-function and its temporal derivative. The model and data underwent similar temporal filtering. Finally, several contrasts were specified to create Z statistic images comparing task-irrelevant pitch changes and 27.5-s breaks against the baseline (non-distracted discrimination task). In the group analyses (FMRIB’s Local Analysis of Mixed Effects), Z statistic images were thresholded with Z >2.3 and a (corrected) cluster significance threshold of P < 0.01.
In order to reveal activations associated with the focused discrimination-task performance (including activations associated with presentation of sounds and active listening), the non-distracted (constant-pitch) trials were contrasted with short no-task periods without sounds where subjects were only required to maintain fixation. Activations were observed in the auditory areas of the supratemporal gyrus (STG) and Heschl’s gyri (HG) bilaterally, due to auditory stimulation and attention-enhanced processing of sounds [13, 22, 25] (Fig. 1c and d, green and yellow, yellow areas were also activated by large distracting pitch changes, see caption). In the left hemisphere, focused discrimination activated sensorimotor areas around the hand area in the central sulcus (CS) consistent with the use of the right hand for responding [5] (Fig. 1c). In addition, left-hemisphere activations were seen in the inferior parietal cortex encompassing parts of the post-central gyrus and sulcus (PoCG/PoCS) and inferior parietal lobule (IPL), and the medial frontal cortex including the medial frontal gyrus (MedFG) and cingulate gyrus (CG). In the right-hemisphere temporal areas, task-related activation extended from STG to the middle temporal gyrus (MidTG) but excluded activations of parietal areas around PoCG/PoCS and frontal areas (Fig. 1d). In addition, activations were detected bilaterally in the insula (in the Sylvian fissure, SF).
To reveal the cortical network involved in deviant-tone processing, the brain activity following task-irrelevant pitch changes was contrasted with brain activity associated with the constant-pitch trials. As seen in Figure 1(c, d), the large pitch changes (≥30 Hz) activated widespread temporal, frontal and parietal cortical areas (red/yellow areas). In contrast to the bilateral activations of STG associated with focused discrimination performance (green/yellow areas), activations in the vicinity of auditory cortex were strongly right–hemisphere dominant following large and distracting pitch changes (red/yellow areas). Further bilateral activations associated with large pitch changes were observed in the inferior frontal gyrus (IFG), medial frontal cortex and intraparietal sulcus (IPS). Interestingly, although the right hand was used in the discrimination task, the right-hemisphere (ipsilateral) motor areas in the precentral gyrus (PreCG) were activated by the large pitch changes. In the left hemisphere, these pitch changes also activated regions of the inferior and superior parietal cortex including the IPS and precuneus (PreCun). Activations to small pitch changes were detected only in the left inferior parietal cortex and in the left and right PreCG. Note that these areas were also activated by large pitch changes. This suggests that the cortical network involved in deviant tone processing was also activated, but to lesser degree, by the small pitch changes.
The results suggest that the distraction of focused performance by unexpected sound changes were caused by several functional systems. First, large pitch changes activated supratemporal cortex of the right hemisphere. This activity might be associated with a right-hemisphere dominant auditory change-detection mechanism initiating involuntary attention to a task relevant auditory feature [15, 19, 21, 24] while the bilateral activations in the IFG and/or insula might be related to the actual switching of attention [11, 23, 24]. Alternatively, as pitch uncertainty is known to decrease detectability of a tone [12, 14] due to relatively narrow listening bands, these activations might be related to tuning of attention to a new pitch. Processes involved in possible inhibition of responses to irrelevant information might also contribute to the IFG/insular activity [2, 24]. The parietal areas in and around IPS, the surpramarginal gyrus (part of IPL in Fig. 1) and the TPJ, here activated by the distracting sounds, have been found to be involved in target detection, processing of sound changes and novel sounds in the auditory environment, and voluntary control of attention [4, 16, 17, 19, 29, 33]. The present left-hemisphere dominance of the parietal activations suggests that these activations were related to the control of motor attention [28] after the distracting events. Consistently, the task-irrelevant pitch changes also enhanced activity in the medial motor areas in the left MedFG and in the CG bilaterally [5]. Thus, task-irrelevant, distracting pitch changes appear to trigger activation in several brain areas involved in auditory change detection, involuntary attention, control of the motor task, and voluntary reconfiguration of focused performance.
The cortical network of loudness-discrimination performance and the network activated following unexpected pitch changes overlapped in a few cortical areas (yellow in Fig. 1c, d) including the right STG, the left medial frontal cortex, the left PreCG, and the left inferior parietal cortex. These might be areas where focused task performance and the processing of task-irrelevant, pitch changes shared common resources and came into conflict contributing to deterioration in performance speed and accuracy.
Acknowledgments
Supported by the Academy of Finland (#202562, 207180, 201160, 211486), VA Research Service, and NIDCD DC005814.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alho K, Winkler I, Escera C, Huotilainen M, Virtanen J, Jääskeläinen IP, Pekkonen E, Ilmoniemi RJ. Processing of novel sounds and frequency changes in the human auditory cortex: magnetoencephalographic recordings. Psychophysiology. 1998;35:211–24. [PubMed] [Google Scholar]
- 2.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Berti S, Roeber U, Schröger E. Bottom-up influences on working memory: behavioral and electrophysiological distraction varies with distractor strength. Experimental Psychology. 2004;51:249–57. doi: 10.1027/1618-3169.51.4.249. [DOI] [PubMed] [Google Scholar]
- 4.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 5.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–85. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- 6.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 7.Escera C, Alho K, Schröger E, Winkler I. Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiology & Neuro-Otology. 2000;5:151–66. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- 8.Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- 9.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 10.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–40. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg GZ, Larkin WD. Frequency-response characteristic of auditory observers detecting signals of a single frequency in noise: The probe-signal method. The Journal of the Acoustical Society of America. 1968;44:1513–1523. doi: 10.1121/1.1911290. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–53. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 14.Hafter ER, Schlauch RS, Tang J. Attending to auditory filters that were not stimulated directly. Journal of the Acoustical Society of America. 1993;94:743–7. doi: 10.1121/1.408203. [DOI] [PubMed] [Google Scholar]
- 15.Hari R, Hämäläinen M, Ilmoniemi R, Kaukoranta E, Reinikainen K, Salminen J, Alho K, Näätänen R, Sams M. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man. Neuroscience Letters. 1984;50:127–32. doi: 10.1016/0304-3940(84)90474-9. [DOI] [PubMed] [Google Scholar]
- 16.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 17.Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Research. 1989;502:109–16. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- 18.Larsson J. PhD Thesis. Karolinska Institutet; Stockholm: 2001. Imaging vision: functional mapping of intermediate visual processes in man, Vol. [Google Scholar]
- 19.Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex. 2005;15:545–51. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- 20.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 21.Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schröger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 2002;15:167–74. doi: 10.1006/nimg.2001.0970. [DOI] [PubMed] [Google Scholar]
- 22.Petkov CI, Kang X, Alho K, Bertrand O, Yund EW, Woods DL. Attentional modulation of human auditory cortex. Nature Neuroscience. 2004;7:658–63. doi: 10.1038/nn1256. [DOI] [PubMed] [Google Scholar]
- 23.Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–9. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- 24.Rinne T, Degerman A, Alho K. Superior temporal and inferior frontal cortices are activated by infrequent sound duration decrements: an fMRI study. Neuroimage. 2005;26:66–72. doi: 10.1016/j.neuroimage.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Rinne T, Pekkola J, Degerman A, Autti T, Jaaskelainen IP, Sams M, Alho K. Modulation of auditory cortex activation by sound presentation rate and attention. Human Brain Mapping. 2005;26:94–9. doi: 10.1002/hbm.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinne T, Särkkä A, Degerman A, Schröger E, Alho K. Two separate mechanisms underlie auditory change detection and involuntary control of attention. Brain Research. 2006;1077:135–43. doi: 10.1016/j.brainres.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Rosburg T. Left hemispheric dipole locations of the neuromagnetic mismatch negativity to frequency, intensity and duration deviants. Cognitive Brain Research. 2003;16:83–90. doi: 10.1016/s0926-6410(02)00222-7. [DOI] [PubMed] [Google Scholar]
- 28.Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. Journal of Cognitive Neuroscience. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- 29.Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. Journal of Neuroscience. 2001;21:5262–71. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherg M, Vajsar J, Picton T. A source analysis of the human auditory evoked potentials. Journal of Cognitive Neuroscience. 1989;1:336–355. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- 31.Schröger E. A neural mechanism for involuntary attention shifts to changes in auditory stimulation. Journal of Cognitive Neuroscience. 1996;8:527–539. doi: 10.1162/jocn.1996.8.6.527. [DOI] [PubMed] [Google Scholar]
- 32.Schröger E, Wolff C. Behavioral and electrophysiological effects of task-irrelevant sound change: a new distraction paradigm. Cognitive Brain Research. 1998;7:71–87. doi: 10.1016/s0926-6410(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 33.Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. Journal of Neuroscience. 2006;26:435–9. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]