Abstract
Dasatinib, a dual Src/Abl tyrosine kinase inhibitor, has significant antileukemic effects against various imatinib mesylate-resistant BCR/ABL mutants. Despite well-documented inhibitory effects of dasatinib on BCR/ABL kinase, the exact downstream cellular events leading to generation of its potent antileukemic effects remain to be defined. We provide evidence that p38 Map kinase (MAPK) pathway is activated leading to increased upregulation of MLK3, MKK3/6, MSK1 and Mapkapk2, upon treatment of BCR/ABL expressing cells with dasatinib, including cells expressing various imatinib-resistant mutants, except for T315I. Our data demonstrate that such dasatinib-dependent activation of p38 MAPK and its effectors plays a critical role in generation of antileukemic responses, since pharmacological inhibition of p38 or siRNA-mediated knockdown of its expression reverse dasatinib-mediated apoptosis, cell cycle arrest and anti-proliferative effects. p38MAPK inhibition also reversed dasatinib-induced suppression of CML patient-derived leukemic CFU-GM progenitor growth in vitro, as well as BCR/ABL expressing KT-1 cell derived leukemic progenitor growth . Altogether, our findings suggest a critical role for p38 MAPK pathway in generation of antileukemic effects of dasatinib, and raise the possibility that development of novel means to enhance p38 MAPK activation in BCR/ABL expressing cells may be an approach to promote antileukemic responses and, possibly, reverse T315I mutation-mediated resistance.
Keywords: p38 Map kinase, CML, dasatinib
Introduction
The recent approval of dasatinib for the treatment of chronic myelogenous leukemia (CML) has had a major impact in the treatment of imatinib-resistant disease 1. It is well established that the major pathogenic event in CML is the expression of the BCR/ABL oncogene, a hybrid gene created by the Philadelphia chromosome translocation, resulting in the abnormal fusion protein p210 BCR/ABL 2. The abnormal BCR/ABL tyrosine kinase is constitutively activated and promotes leukemogenesis by inducing the phosphorylation of multiple downstream protein targets that mediate growth promoting and antiapoptotic signals 2. Multiple pathways are engaged by the activated BCR/ABL kinase, including MYC, Ras/MAPK, c-Raf, MAPK/ERK, SAPK/JNK, STAT, NF-κB, PI3’K (phosphatidylinositol 3-kinase), c-Jun, c-cbl pathways and CrkL pathways, as well as Jak-STAT and Src pathways 2–5. A major mechanism for the inhibition of apoptosis appears to result from BCR/ABL mediated activation of the PI3’K and Ras pathways, with induction through Akt of MYC and Bcl-2 6. Of note, the PI3’K pathway has been implicated in Abl tyrosine kinase-mediated leukemogenesis 7 and its function has been previously shown to be essential for Abl oncogene mediated transformation of B-lineage cells 8. BCR/ABL also causes genetic instability as a result of transcriptional defects 9, while there is accumulating evidence that the suppression of apoptosis constitutes an important mechanism by which BCR/ABL drives the expansion of myeloid cells 10.
Although, the advent of the abl tyrosine kinase inhibitor, imatinib, has revolutionized the field of CML leading to long term remissions 11,12, approximately 30% of CML patients will develop intolerance or resistance to imatinib 13 either due to point mutations or gene amplification 14–16. More recently, there is emerging evidence that other mechanisms, such as activation of Src-kinases, also contribute to resistance in some cases 17,18.
Dasatinib is an oral dual BCR/ABL and Src family tyrosine kinases inhibitor approved for the treatment of patients with CML who develop resistance to imatinib treatment, as well as for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL) 19. The ability of dasatinib to overcome resistance to imatinib may relate to differences in binding affinity for the BCR/ABL tyrosine kinase, and dasatinib has been shown to overcome the resistance to imatinib of CML cells with several BCR/ABL kinase domain point mutations 20. Dasatinib has been previously shown to be about 2 orders of magnitude more potent than imatinib in wild-type BCR/ABL expressing cells and to be active against 18 of 19 BCR/ABL mutations associated with imatinib resistance 20, with the only exception being the T315I mutation 21. However, the molecular mechanisms and cellular events that ultimately lead to dasatinib-dependent induction of growth arrest and apoptosis of CML cells are not fully understood. Considerable attention has been recently focused on the role played by different kinase cascades in regulating apoptosis and exerting anti-proliferative effect of tyrosine kinases downstream of Abl kinase inhibition. Recently, Nguyen et al, demonstrated the importance of inhibition of the MEK kinase pathway in sensitizing cells to the effects of dasatinib, and demonstrated that MEK inhibitors enhance dasatinib responses, and that such effects were associated with regulation of different signals, including inactivation of Erk1/2 and STAT5; and downregulation of Bcl-x(L) and MCL1 22. However, such combinations failed to reverse resistance to T315I 22.
In previous work, we demonstrated that the p38 pathway is activated during treatment of BCR/ABL expressing cells with imatinib mesylate 23, in contrast to the PI3’K/mTOR pathway that is suppressed 24. As the p38 MAPK pathway primarily mediates pro-apoptotic and/or growth inhibitory signals, our previous observations strongly suggested that its engagement during treatment of cells with imatinib mesylate may participate in the generation of antileukemic responses. In the present study, we sought to precisely determine whether the p38 MAPK pathway plays a role in mediating the antileukemic effects of dasatinib. Our data demonstrate that treatment of various BCR/ABL expressing leukemia cell lines with dasatinib leads to activation of p38 and we identify several upstream and downstream effectors of the pathway in BCR/ABL expressing cells. In addition, our studies establish that pharmacological or molecular targeting of the p38 MAPK pathway results in reversal of the inhibitory effects of dasatinib on CML cell lines and primary leukemic progenitors, underscoring the functional relevance of the pathway in the induction of antileukemic effects.
Materials and Methods
Cells
The CML derived BCR/ABL expressing K562 25, KT1 26 and KBM7 27 cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Murine peripheral blood B-cell BA/F3 cell line transfected with wild type (p210) BCR/ABL, empty vector (pSRα), or with different mutations such E255K, M351T, T315I, Y253F, H396P were kindly provided by Dr. Brian Druker (Howard Hughes Medical Institute, Oregon Health Sciences University, Portland, OR) 28.
Reagents
Dasatinib was a gift from Bristol Meyers Squibb, Inc or was purchased from American Custom Chemicals Corporation (San Diego, CA). Antibodies against the phosphorylated forms of p38 MAPK (Thr180/Tyr182), pMsk1 (Ser 376), pMKK3/6 (Ser189/207), pMLK3(Thr277/Ser281), pMapkapK-2(Thr334), pErk (p44/42 MAP Kinase (Thr202/Tyr204)) were purchased from Cell Signaling Technology (Beverly, MA). Total MLK3, MKK3, MSK1, were obtained from Cell Signaling Technology (Beverly, MA). Total MapkapK2 and p38α antibody were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA.). The p38 MAP kinase inhibitors SB203580, was purchased from Calbiochem Inc. (San Diego, CA).
Cell lysis and Immunoblotting
Cells were lysed in a lysis buffer containing 1% Triton X-100, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenyl- methylsulfonyl fluoride (PMSF). For immunoblot analysis, samples were prepared by mixing an aliquot of cell lysates containing 50µg of protein with an equal volume of 2 X Laemmli’s sample buffer and heating at 100°C. Samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred to Immobilon PVDF membrane (Millipore, Bedford, MA, USA). The membranes were blocked with 5% milk in TBST (Tris buffered saline-0.1% Tween 20) for 1 hour at room temp and then probed with primary and secondary antibodies as per manufacturer’s instructions. Blots were washed with TBST and antigen-antibody complex were visualized using ECL reagents (Amersham, Pittsburgh, PA) and SuperSignal West Dura Extended Duration Substrate, (Pierce Technologies, Rockford, IL) according to manufacturer’s instructions. Where reprobing of blots was performed, membranes were stripped with Restore stripping buffer, (Pierce technologies, Rockford, IL) for 30 min at room temperature before being washed and blocked as above. ImageJ software was used for quantification of phosphorylation.
In Vitro Kinase Assays
Protein (200ug) from whole cell lysates was incubated for 12 hr at 4°C with 5µl of antibodies against p38. Extracts were then incubated with 50µl of protein A sepharose bead suspension (Pharmacia Biotech Inc., Piscataway, NJ) for another 2 hr. The immune complexes were washed 3 times with lysis buffer and once with kinase reaction buffer (30mM Tris, pH8, 20 mM Mg CL2; 2mM MnCl2; 25 mM β-glycerol phosphate; 0.1 mM sodium vanadate). Beads were resuspended with 30µl of kinase reaction buffer (7ug of MEF2C substrate (kind gift from Dr Melanie Cobb, UT Southwestern Medical Center, Dallas), 3uCi of (g-32) ATP, 10uM ATP) and incubated at 30°C for 30 min in a shaking incubator. Reaction was stopped by addition of 10µl of 5X Laemmli SDS sample buffer. Samples were subjected to SDS −10% PAGE and products were visualized by autoradiography. The in vitro kinase activity of p38 was also measured using p38 MAP Kinase Assay Kit (non radioactive), Cell Signaling Technology (Beverly, MA), according to the manufacturer’s instructions. Cell extracts were incubated overnight with immobilized phospho-p38 MAPK (Thr180/Tyr182) mAb. Kinase reaction was performed in the presence of 100 µM of cold ATP and 2 µg of ATF-2 fusion protein. Phosphorylation of ATF2 was measured by Western blot using Phospho-ATF2 (Thr71) Antibody.
Hematopoietic Progenitor Cell Assays
The effect of dasatinib on the growth of hematopoietic progenitors from patients with CML was determined in methylcellulose assays as described previously 23. Briefly, peripheral blood (PB) or bone marrow (BM) aspirate specimens were obtained under local anesthesia from patients with CML, after obtaining Institutional Review Board (IRB)-approved informed consent. PB or BM mononuclear cells (MNC) were separated by Ficoll-Hypaque sedimentation, and cells were cultured in a methylcellulose mixture containing hematopoietic growth factors 23, in the presence or absence of dasatinib (10nM) and/or SB203580 (10µM) as indicated. Granulocyte/macrophage colony-forming units (CFU-GM) from CML patient samples were scored on day 14 of culture.
Proliferation assays
Cell proliferation was determined by the sodium 3’-[19phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitroobenzene sulfonic acid hydrate(XTT) colorimetric assay using the Cell Proliferation Kit II (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer’s instructions. In brief, cells were cultured in a 96 -well plate at a density of 5×104 cells per each well in a final volume of 100 µl with indicated concentrations of dasatinib for 48 hrs in presence or absence of indicated inhibitors. Then, 50 µl of XTT labeling agent was added and cells were re-incubated for 4 hrs before the optical density at 490 nm was measured with a microplate reader.
Apoptosis assay
In brief, cells were seeded in six-well plates and exposed to dasatinib in the presence or absence of the indicated inhibitors for 48 hrs. Subsequently, 1 × 106 cells were resuspended in 100 µl of binding buffer containing annexin V-FITC and propidium iodide (PI), and incubated for 20 min at RT. After 400 µl of binding buffer was added, and the percentage of apoptotic cells were analyzed using Becton Dickinson FACS scan flow cytometer (Mountain View, CA) 29.
Cell cycle analysis
The distribution of cells at different stages in the cell cycle was estimated using propiodium iodide (PI) staining and flow cytometric DNA analysis as described previously 30. Briefly, 5× 105 cells were incubated overnight in 6-well dishes in medium containing 10% FBS+ 4mM Glutamine, then for different times with various concentrations of dasatinib with or without SB203580 (10µm) pretreatment for 60 min, in BA/F3 cells. Cells were harvested, washed twice with cold phosphate buffered saline (PBS, pH 7.4) and fixed with 70% ethanol/ 30% PBS at 4 °C. The fixed cells were incubated with 0.5 ml PBS containing 0.1 mg/ml RNase for 30 min at 37 °C, then stained with 50µg/ml PI (Sigma, St. Louis, MO, USA) for 30 min in the dark at room temperature, and finally analyzed on a FACS cytometer (Caliburä, Becton Dickinson, USA). A minimum of 1 × 104 cells/sample was evaluated, and the percentage of cells in each cell cycle phase was calculated using the CELLQUEST software packages (Becton Dickinson).
p38 MAPK siRNA transfection
Non-specific control and mouse MAPK14 (p38) SMARTpool siRNA duplexes (proprietory target sequences) were purchased from Dharmacon Research, Inc. (Lafayette, CO). The transfections of BAF3/p210 cell line were done with the Amaxa nucleofector device using the Nucleofector kit V (Amaxa, Koeln, Germany) in 6-well plates. Transfection protocols were performed following the manufacturer's instructions. Briefly, the cells were resuspended in 100 µL nucleofector V solution. This cell suspension at a density of 2 × 106 cell/ml was mixed with 2 µg of pmax green fluorescent protein (GFP) vector for a positive control or with 100nM of p38 or non-specific control siRNA. This nucleofection sample was then transferred to a cuvette and nucleofected with an Amaxa nucleofector apparatus. The cells were transfected using the X-01 program parameter and were immediately transferred into wells containing 37°C pre-warmed culture media in 6-well plates. Untreated cells and cells transfected with non-specific siRNA were used as a negative control. The cells were collected forty-eight hours post-transfection.
Statistical Analysis
Student’s paired t-test was used to analyze statistically significant differences in number of CFU-GM colonies in the dasatinib treated wells in presence or absence of the p38 MAPK inhibitor as well as to identify differences in degree of phosphorylation. Anova: Two-Factor With Replication test was used to analyze statistically significant difference between the proliferation index between p38 inhibitor (SB203580 or p38siRNA) and the different concentrations of dasatinib.
Results
Dasatinib induces phosphorylation/activation of the p38MAPK pathway in BCR/ABL expressing cells
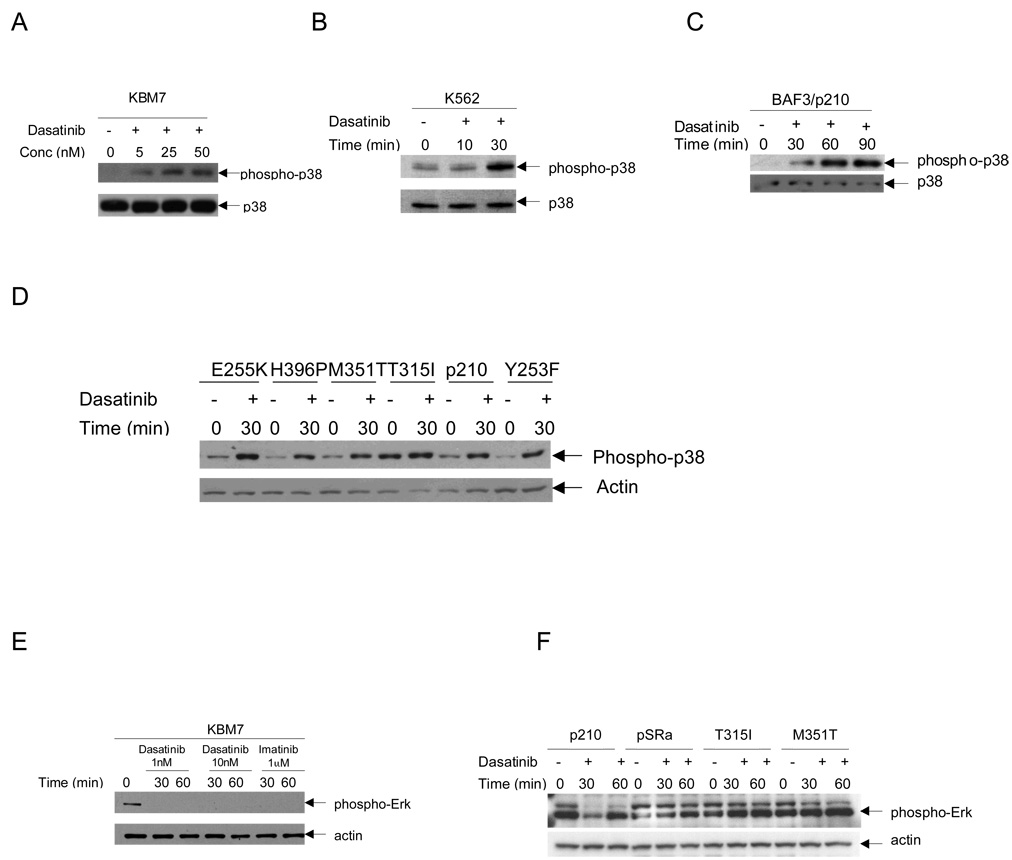
To determine the effects of dasatinib on the phosphorylation/activation of p38MAPK, we initially performed studies using the BCR/ABL expressing imatinib-sensitive, K562 25 and imatinib-insensitive, KBM7 cell lines 27. Actively growing, KBM7 or K562 cells were treated with escalating concentrations of dasatinib and, after cell lysis, equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho p38MAPK antibody. Increased levels of p38 phosphorylation at Thr180/Tyr182 were clearly seen after treatment of the cells with as low concentrations of dasatinib as 5nm in KBM7 cells (Fig 1A) and as early as after 30 minutes of dasatinib treatment in K562 cells (Fig 1B). A dose- and time- dependent increase in p38 phosphorylation in response to dasatinib was also seen in BAF3 transfectants stably expressing wild type (p210) BCR/ABL (Fig 1C) as well as those expressing BCR/ABL mutations, except for T315I (Fig 1D). Some baseline elevation in the levels of p38MAPK phosphorylation was observed in T315I cells (Fig. 1D). In parallel studies, the effects of dasatinib on Erk phosphorylation/activation were analyzed, and consistent with previous reports 22, we found that dasatinib treatment leads to de-phosphorylation of Erk1/2 kinases in KBM7 cells (Fig. 1E). However, such effect was restricted to Erk1 dephosphorylation in BAF3/p210 and BAF3/M351T cells (Fig. 1F). On the other hand no effects were noticeable in BAF3 cells carrying the dasatinib resistant T315I mutation(Fig 1F).
Fig. 1. Dasatinib induces phosphorylation of p38 and dephosphorylation of Erk1/2 in BCR/ABL expressing cell lines.
A, KBM7 cells were incubated for indicated dasatinib concentrations for 30 minutes, as indicated. Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the phosphorylated/activated forms of p38MAPK on Threonine 180 and Tyrosine 182. The blot was then stripped and reprobed with an antibody against p38 MAPK to control for protein loading. B, Similar experiment to one shown in panel A except K562 cells were treated with dasatinib at 150 nm for indicated times. C, Similar experiment to one shown in panel A except BAF3/p210 cells were treated dasatinib (10nM) for indicated time. D, BAF3 cells expressing wild type BCR/ABL p210 and those carrying mutations E255K, H396P, M351T, T315I, and Y253F were treated with dasatinib (10nM) for 30 min, as indicated. Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the phosphorylated/activated forms of p38MAPK on Threonine 180 and Tyrosine 182. The blot was then stripped and reprobed with an antibody against actin to control for protein loading. E, KBM7 cells were incubated with the indicated doses of dasatinib and imatinib for 30 and 60 minutes respectively. Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the phosphorylated/activated forms of Erk 1/2. The blot was then stripped and reprobed with an antibody against actin to control for protein loading. F, Similar experiment to one shown in panel E except BAF3/p210, BAF3/M351T and BAF3/T315I cells were incubated with dasatinib (10nM)s for the indicated time intervals respectively. The empty vector pSRa was used as a control. The blots are representative of at least 3 independent experiments.
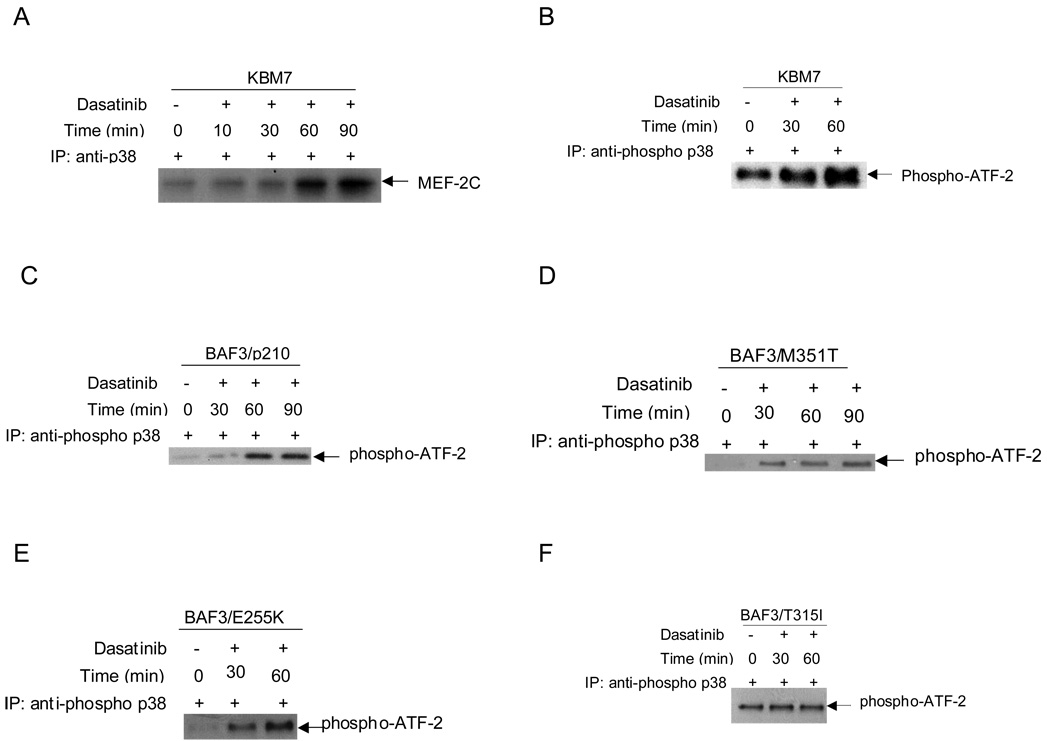
To directly examine whether dasatinib-dependent phosphorylation of p38 results in induction of its kinase activity, in vitro kinase assays were performed in anti-p38 immunoprecipitates from dasatinib-treated KBM7 cells. As shown in Fig. 2A, dasatinib treatment resulted in time-dependent induction of p38 MAPK activity, evidenced by the phosphorylation of MEF2C used as an exogenous substrate in the kinase assay (Fig 2A). Furthermore, using ATF2 as the exogenous substrate, a strong activation of p38 MAPK kinase activity was also evident in anti-phosphop38 immunoprecipitates from dasatinib-treated KBM7 cells (Fig 2B). Similar results were obtained when the BAF3/p210 (Fig 2C) or BAF3/M351T (Fig 2D) or BAF3/E255K (Fig 2E) cells were studied. No effect of dasatinib treatment on the p38 MAPK kinase activity was seen in T315I cells (Fig 2F).
Fig. 2. Dasatinib-dependent induction of p38 MAPK kinase activity.
A, KBM7 cells were treated with dasatinib (150 nM) for the indicated times. The cells were lysed and the total cells were immunoprecipitated (IP) with an antibody against p38. Immunoprecipitated proteins were subjected to in vitro kinase assays, using MEF2C as an exogenous substrate. The proteins were resolved by SDS-PAGE and transferred to Immobilon membrane. The upregulation of MEF2C was detected by autoradiography. B, KBM7 cells were treated with dasatinib (10 nM) for the indicated times. The cells were lysed and immobilized phospho-p38 MAPK on Threonine 180 and Tyrosine 182 mAb was used to immunoprecipitate p38 MAP kinase. Then an in vitro kinase assay was performed using ATF2 as a substrate. ATF2 phosphorylation was detected by Western blotting using Phospho-ATF2 (Thr71) Antibody. Similar experiment to one shown in panel B except in C, BAF3/p210 cells D, BAF3/M351T. E, BAF3/E255K F, BAF3/T315I cells were treated with dasatinib (10nM) for the indicated times. The signals for the kinase activity for each cell line was quantified by densitometry and was compared for statistical analysis using student paired t-test, dasatinib 30 min vs untreated, p=0.08; dasatinib 60 min vs untreated, p=0.05.
Activation of upstream and downstream p38 effectors by dasatinib
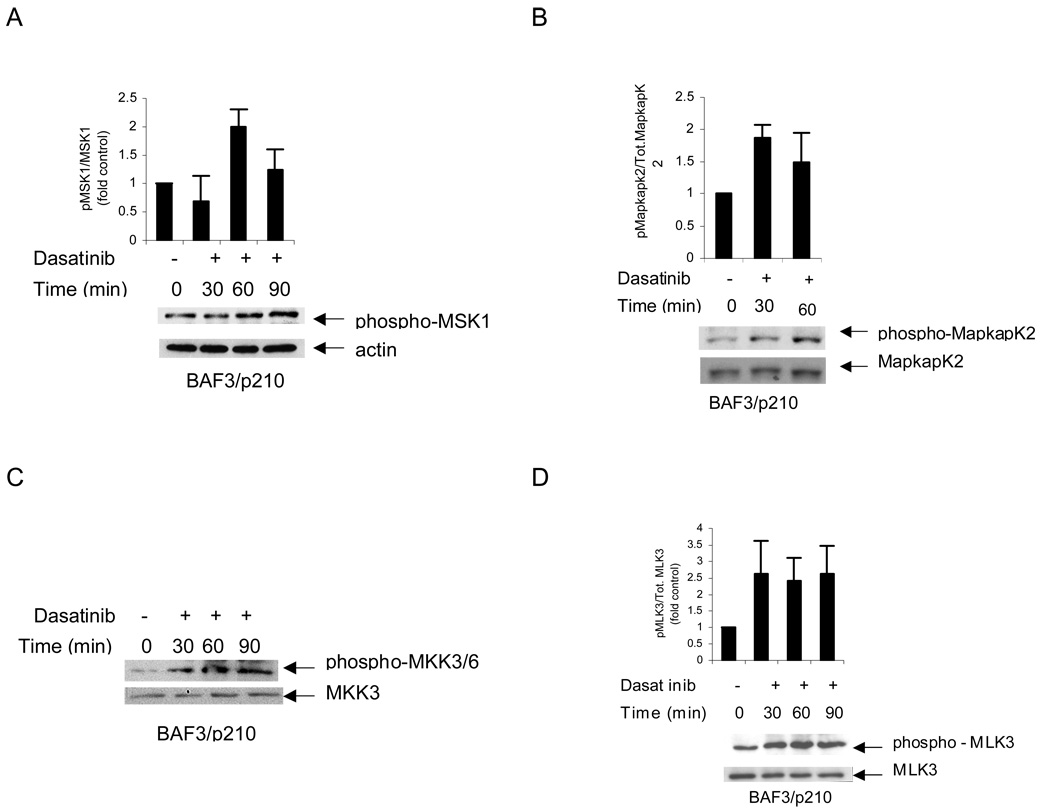
In subsequent studies, we sought to determine the effects of dasatinib on known upstream regulators of p38, as well as downstream effector kinases. Mitogen and stress kinase (MSK1) is a key downstream effector of the p38 MAPK pathway in several systems that play important roles in the regulation of p38-regulated immediate gene expression 31. This kinase is activated by undergoing phosphorylation on at least three sites by the upstream MAPK 32, resulting in its activation. It is also well established that depending on the type of stimulus and cellular context, both Erk1/2 and p38 can phosphorylate MSK1 32,33 on the same site. It should be noted that MSK1 activity is required for the mitogen- or stress-induced phosphorylation of the transcription factors CREB and ATF1 downstream of both the Erk1/2 and p38 MAPK signaling cascades in a variety of cell types 34–36. When BCR/ABL expressing cells were treated with dasatinib, there was induction of phosphorylation of MSK1 serine 376 (Fig 3A). Similarly, treatment of cells with dasatinib resulted in phosphorylation/activation of another downstream effector that plays important roles in the generation of p38-mediated responses, MAPK-activated protein (Mapkap) kinase 2 (Fig. 3B). Treatment of BAF3/M351T cells with dasatinib at various time points led to increased phosphorylation of MapkapK2 on threonine 334 (Fig 3B), a finding consistent with activation of the p38 MAPK. Altogether, these studies established that dasatinib activates p38 and two key downstream effector elements of the pathway that are known to play important roles in the generation of p38-mediated responses.
Fig 3. Dasatinib leads to phosphorylaton of MSK1, MLK3, MapkapK2, MKK3/6.
A BAF3/p210 cells were treated with dasatinib (10nM) for the indicated times. Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the phosphorylated/activated forms of MSK1 on serine 376. The blot was then stripped and reprobed with an antibody against actin to control for protein loading. The signals for phospho-MSK1 and actin from three independent experiments (including the one shown in A) were quantitated by densitometry, and the intensity of phosphorylation of MSK1 relative to actin expression was calculated. Data are expressed as the fold increase of the mean of ratios of phospho-MSK1 to actin levels in dasatinib treated vs. untreated samples ±S.E. for each experimental condition. B. Similar experiment to one shown in panel A except BAF3/p210 cells were treated with dasatinib (10nM) for the indicated times and analyzed for phosphorylated/activated form of MapkapK2 on Threonine 334. The blot was then stripped and reprobed with an antibody against total MapkapK2 to control for protein loading. The signals for phospho-MapkapK2 and actin from three independent experiments (including the one shown in B) were quantitated by densitometry using ImageJ software, and the intensity of phosphorylation of MapkapK2 relative to total MapkapK2 expression was calculated. Data are expressed as the fold increase of the mean of ratios of phosphorylated to total MapkapK2 levels in dasatinib treated vs. untreated samples ±S.E. for each experimental condition. C. Similar experiment to one shown in panel A except BAF3/p210 cells were treated with dasatinib (10nM) for the indicated times and analyzed for phosphorylated/ activated form of MKK3/MKK6 at Serine 189 and Serine 207. The blot was then stripped and reprobed with an antibody against total MKK3 to control for protein loading. D. Similar experiment to one shown in panel A except BAF3/p210 cells were treated with dasatinib (10nM) for the indicated times and analyzed for phosphorylated/activated form of MLK3 on Threonine 277 and Serine 281. The blot was then stripped and reprobed with an antibody against total MLK3 to control for protein loading. The signals for phospho-MLK3 and actin from four independent experiments (including the one shown in D) were quantitated by densitometry, and the intensity of phosphorylation of MLK3 relative to total MLK3 expression was calculated. Data are expressed as the fold increase of the mean of ratios of phosphorylated to total MLK3 levels in dasatinib treated vs. untreated samples ±S.E. for each experimental condition
In subsequent studies, we evaluated the effects of dasatinib on the activation of upstream regulatory effector kinases for p38. In initial studies, we sought to determine whether the effects occur through two different upstream kinases, mitogen-activated protein kinase kinase 3 (MKK3) and MKK6 that directly phosphorylate and activate p38. Treatment of the BAF3/p210 cells with dasatinib led to phosphorylation of MKK3/6 at serines 189/207 (Fig 3C), strongly suggesting that these kinases may act as MAPKKs upstream of p38. In addition, dasatinib-treatment led to activation of mixed lineage kinase 3 (MLK3) (Fig. 3D), a widely expressed mammalian MAPK kinase kinase (MAPKKK) that has also been previously shown to activate JNK or p38 MAPK via its activation 37. We showed that treatment of the BAF3/p210 cells with dasatinib lead to phosphorylation of MLK3 at threonine 277/serine 281 (Fig 3D). These findings raised the possibility that dasatinib treatment of the CML cells results in sequential stimulation of MLK3→MKK3/6→p38, leading to downstream phopshorylation and engagement of the kinases Mapkapk2 and MSK1.
Pharmacological or molecular inhibition of p38 reverses dasatinib-induced apoptosis, cell cycle arrest and growth inhibitory effects in BCR/ABL expressing cells
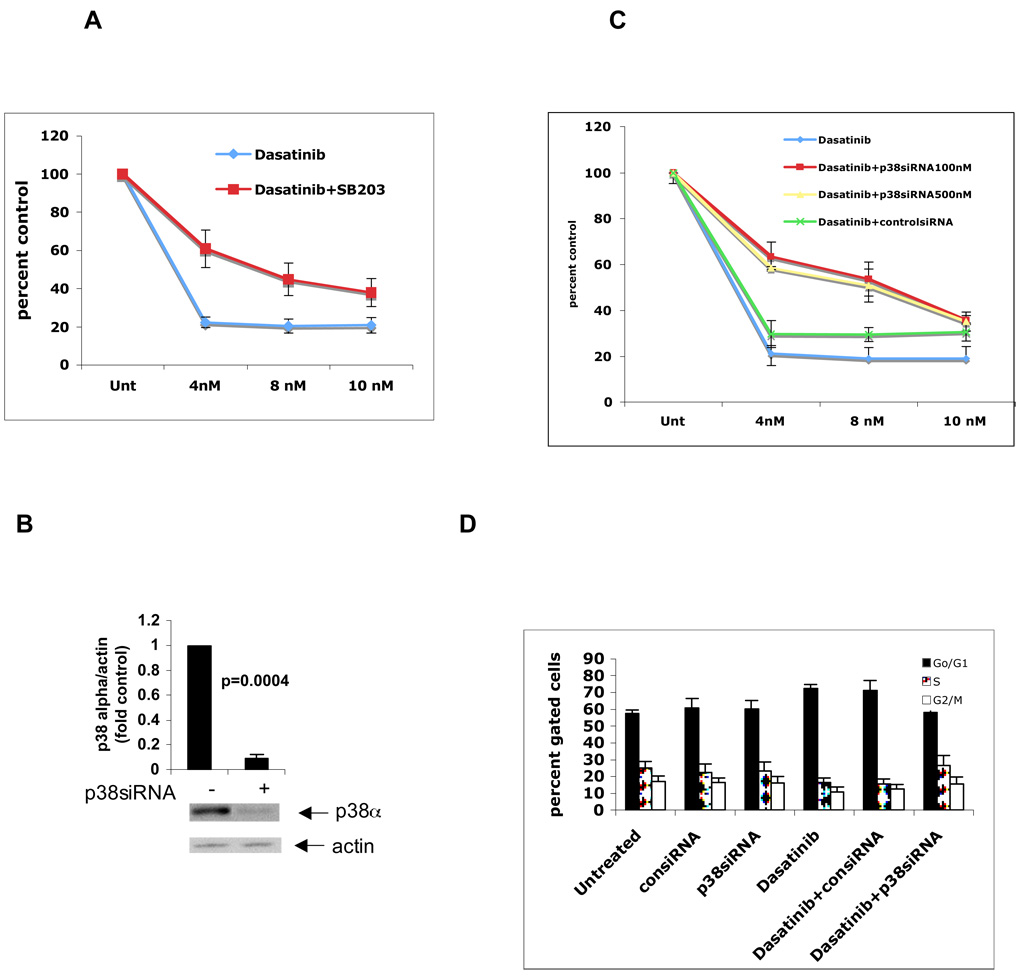
In subsequent studies, we sought to define the role of dasatinib-inducible activation of p38 MAPK in BCR/ABL-expressing cells. BAF3 cells expressing BCR/ABL, or different imatinib-resistant mutants, were pre-incubated in the presence or absence of the p38 MAPK inhibitor SB203580 prior to treatment with dasatinib and cell proliferative responses were assessed using an XTT assay. As expected, dasatinib led to strong inhibition of cell proliferation of BAF3/p210 cell line (Fig 4A). Concomitant treatment of cells with the pharmacological inhibitor of p38 MAPK, SB203580, led to partial reversal of the antiproliferative effects of dasatinib, strongly suggesting that the p38 MAPK pathway plays an important role in the generation of growth inhibitory responses. This partial reversal was statistically significant when including all the different concentration levels of dasatinib (ANOVA: two-factor with replication, p=0.019). To definitively establish the relevance of p38 in the generation of such effects, we used MAPK14 (p38) SMARTpool to knockdown p38 expression and assess the generation of dasatinib responses in cells in which p38 is knocked down. As shown in Fig. 4B, transfection of BAF3/p210 cells with p38α MAPK siRNA (100nm) led to inhibition of p38α Consistent with the findings using SB203580, siRNA-mediated knockdown of p38 also led to partial reversal of the anti-proliferative effects of dasatinib, further establishing a key role for p38 in the process (Fig 4C).
Fig 4. Inhibition of p38 Map Kinase partially reverses the anti-proliferative effect and cell cycle arrest induced by dasatinib in BAF3/p210 cells.
A. BAF3/p210 cells were incubated with different doses of dasatinib in the presence or absence of SBS203580 (10 µM) for 48 hrs as indicated. Cell proliferation was assessed using XTT assays. Figure represents mean of 4 different experiments. Percent control was calculated by dividing observed value by untreated value × 100. (ANOVA: Two-Factor With Replication, p=0.019). B. BAF3/p210 cells were transfected with p38siRNA at 100nm concentrations as per Amaxa protocol. Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the p38α isoform. The blot was then stripped and reprobed with an antibody against actin to control for protein loading. The signals for p38∝ and actin from three independent experiments (including the one shown in B) were quantitated by densitometry, and the intensity of expression of p38∝ relative to actin expression was calculated. Data are expressed as the fold increase of the mean of ratios of p38∝ to actin levels in p38siRNA transfected vs. untreated samples ±S.E. for each experimental condition. (untreated vs p38siRNA, paired t-test, p=0.0004). C. BAF3/p210 cells were incubated with different doses of dasatinib in the presence or absence of control or p38 siRNA transfection for 48 hrs as indicated. Cell proliferation was assessed using XTT assays. Figure represents mean of 3 different experiments. (ANOVA: Two-Factor With Replication, dasatinib vs. dasatinib + p38siRNA100nM, p=0.09; dasatinib vs. dasatinib +p38siRNA500nM, p=0.01). D. BAF3/p210 cells were incubated with dasatinib (1nM) and/or transfected with control or p38 siRNA (100nM) for 48 hrs as indicated. Cell cycle analysis was done using PI staining. Figure represents mean of 4 different experiments. (2-tailed paired t-test, dasatinib vs. dasatinib+p38siRNA, p=0.01).
Subsequently, cell cycle analysis was performed to better understand the mechanism of CML cell growth arrest by dasatinib. After 48 hrs of continued incubation, dasatinib significantly increased the G0/G1 fraction of the BAF3/p210 cells and decreased proportion of S phase cells compared to the untreated cells (2-tailed paired t-test, untreated vs. dasatinib, p=0.02) (Fig 4D). Using control or p38siRNA did not affect the baseline pattern of the cell cycle. However, this degree of G0/G1 cell cycle arrest induced by dasatinib treatment was reverted to the control values upon inhibition of p38MAPK expression using p38α siRNA (2-tailed paired t-test, dasatinib vs. dasatinib+p38siRNA, p=0.01). Similarly, the number of cells pushed into G2/M +S phase upon treatment with dasatinib went back to baseline levels upon silencing of p38 (2-tailed, p=0.001) (Fig 4D).
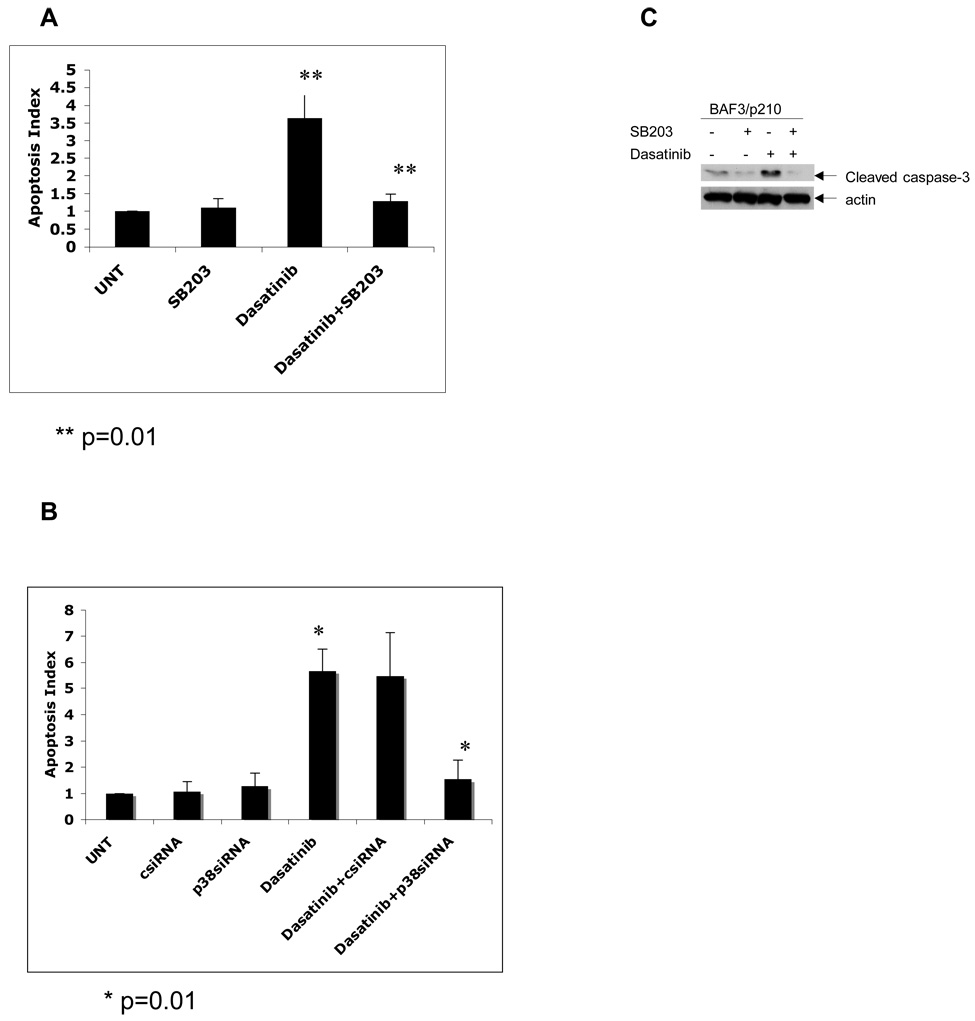
In other studies we examined the effects of pharmacological or molecular inhibition of p38 in the generation of dasatinib-induced apoptosis by annexinV/PI staining. As expected, dasatinib induced apoptosis in the treated cells. As observed in the cell proliferation assays, we found that SB203580 significantly reverses dasatinib-induced apoptosis (2-tailed, p=0.01) (Fig. 5A). Furthermore, inhibition of p38MAPK expression using p38α siRNA led to reversal of dasatinib mediated apoptosis (2-tailed, p=0.01), whereas no such effect was seen when control siRNA was used (Fig 5B). To further evaluate effect of the p38 MAPK pathway on dasatinib-dependent apoptosis, we studied the induction of cleaved caspase 3 in response to treatment of CML cells with dasatinib. Continuous treatment of BAF3/p210 cells with dasatinib for 48 hrs led to clear upregulation of cleaved caspase 3 at aspartate 175 which was reversed by pharmacological inhibition of the p38MAPK pathway by SB203580 (Fig 5C).
Figure 5. Inhibition of p38 Map Kinase reverses the pro-apoptotic effect of dasatinib in BAF3/p210 cells.
A. Logarithmically growing BAF3/p210 cells were treated with dasatinib (1nM) in the presence or absence of SB203580 (10µM) for 48 hrs. Cells were analyzed by flow cytometry for the degree of apoptosis by using AnnexinV/PI staining. Apoptosis index was calculated by dividing observed value by untreated value. (Dasatinib vs. dasatinib +SB203; 2-tailed p=0.01, n=6) B. Similar experiment to one shown in panel A except BAF3/P210 cells were incubated with dasatinib (1nM) in the presence or absence of p38siRNA (100nM) or controlsiRNA for 48 hrs as indicated. (Dasatinib vs. dasatinib +p38SiRNA; 2-tailed p=0.01, n=3) C. BAF3/p210 cells were incubated with dasatinib (1nM), for 48 hrs in presence or absence of SB203580 (10 µM). Equal amounts of total cell lysates (50µg/lane) were analyzed by SDS-PAGE and immunoblotted with antibody against the Cleaved Caspase-3 on Aspartate 175. The blot was then stripped and reprobed with an antibody against actin to control for protein loading.
P38 activity is essential for the generation of the suppressive effects of p38 on leukemic progenitors
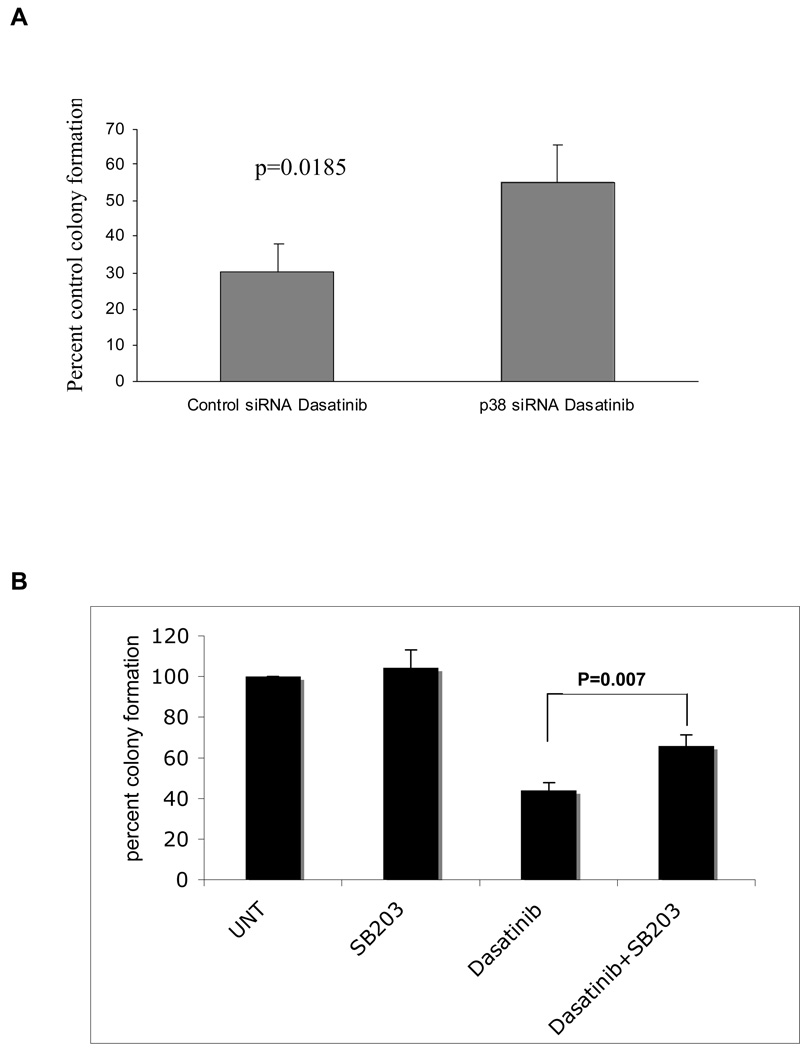
We subsequently determined the role of the p38 MAPK in the generation of the antileukemic effects of dasatinib on primitive leukemic progenitors. In initial experiments, we used p38-specific siRNA to knockdown p38 in BCR/ABL expressing KT1 cells and examine the effects of such inhibition on the growth of leukemic progenitor colonies derived from such cells in clonogenic assays in methylcellulose. As expected, dasatinib treatment suppressed the growth of KT1-derived leukemic progenitor colonies (Fig. 6A). However, such suppressive effects were reversed in cells in which p38 was knocked down (Fig. 6A), indicating that p38 plays an important role in the induction of the effects of dasatinib on primitive leukemic cells. To evaluate whether a similar role is played by p38 in primary leukemic progenitors from CML patients, the effects of dasatinib on leukemic CFU-GM colony formation from bone marrow (BM)– or peripheral-blood (PB)–derived hematopoietic mononuclear cells from CML patients, in the presence or absence of SB203580, were assessed. As expected, treatment with dasatinib strongly suppressed leukemic CFU-GM colony formation in all different patient samples examined (Table 1, Fig 6B). Addition of SB203580 to the cultures led to partial reversal of the inhibitory effects of dasatinib on the leukemic CFU-GM growth in all patient samples, indicating that p38 MAPK plays a critical and essential role in the induction of the antiproliferative effects of dasatinib on leukemic hematopoietic progenitors. (see individual patient results in Table 1 and compilation of the data in Figure 6B). Paired t test analysis to compare the effects of dasatinib alone with those of dasatinib combined with SB203580 demonstrated that this effect was statistically significant (2-tailed, p= 0.007).
Figure 6.
Figure 6: A. Knockdown of p38 reverses dasatinib-mediated suppression of leukemic progenitor. BCR/ABL expressing, KT-1 cells were transfected with control siRNA or p38 siRNA and subsequently incubated in methylcellulose, in the presence or absence of dasatinib (1 nM), and leukemic progenitor colony formation was assessed. Data are expressed as percent control colony formation of untreated samples for each condition and represent means ± S.E. of 3 independent experiments (p=0.0185).
Figure 6: B. Dasatinib inhibits the growth of leukemic myeloid progenitors from CML patients in a p38 MAP kinase-dependent manner. Peripheral blood or bone marrow mononuclear cells from newly diagnosed CML patients were plated in methylcellulose culture assay system with 10nM of dasatinib in the presence or absence of SB203580 as indicated. CML patient derived CFU-GM progenitor colonies were scored on day 14 of culture.
The data are expressed as percent control of CFU-GM colony numbers for untreated cells. Each patient experiment was repeated 1–5 times. A mean of all experiments is presented in the figure. (paired t-test, dasatinib vs. dasatinib +SB203, p=0.007)
Table 1. Dasatinib inhibits the growth of leukemic myeloid progenitors from CML patients in a p38 MAP kinase-dependent manner.
Peripheral blood or bone marrow mononuclear cells from newly diagnosed CML patients were plated in methylcellulose culture assaystem with 10nM of dasatinib in the presence or absence of SB203580 as indicated. CML patient derived CFU-GM progenitor colonies were scored on day 14 of culture. The data are expressed as percent control of CFU-GM colony numbers for untreated cells. Each patient experiment was repeated as follows: (patient 1, n=2; patient 2, n=4; patient 3, n=4, patient 4, n=2; patient 5, n=1). A mean ±S.E. of all experiments is presented in the table.
| Patient no. | SB203580 | Dasatinib | Dasatinib+SB203580 |
|---|---|---|---|
| 1 | 135+/−54 | 35+/−5 | 73+/−7 |
| 2 | 112+/−21 | 50+/−10 | 81+/−13 |
| 3 | 88+/−5 | 37+/−12 | 50+/−14 |
| 4 | 97+/−23 | 41+/−6 | 59+/−10 |
| 5 | 90 | 55 | 65 |
Discussion
Our data provide the first evidence that dasatinib activates the p38 MAPK in CML cells and establishes that such activation is essential for the antileukemic effects of this dual kinase inhibitor. p38MAPK is the mammalian orthologue of the yeast HOG kinase and participates in multiple signaling cascades that regulate functional cellular responses generated in response to cytokines and/or stress-signals 31 38 39. There has been previous evidence that the p38 MAPK pathway is suppressed when embryonic stem (ES) cells are transfected with BCR/ABL and that pharmacological inhibition of p38 leads to expansion of ES-derived hematopoietic progenitors by blocking apoptosis 40. Interestingly, p38 inhibition is sufficient to expand ES-derived hematopoietic progenitors to a substantial degree, approximately 80% to what is seen in response to BCR/ABL transformation 40, suggesting a critical role for BCR/ABL-mediated p38 suppression in its transforming ability. Although, it has been shown that BCR/ABL transfection led to increased phosphorylation of p38MAPK in 293T cells, its functional significance remains unclear 41. Previous work has shown that treatment of BCR/ABL expressing cells with various agents that suppress their growth, such as chlorogenic acid 42, IFNα 43or imatinib mesylate 23, also result in activation of the p38 MAPK pathway. Importantly, in all these cases, pharmacological inhibition of p38 was shown to reverse generation of pro-apoptotic or growth inhibitory effects in response to these agents, suggesting a key role for p38 in the induction of antileukemic responses in BCR/ABL transformed cells.
It should be also noted that other studies have implicated p38 in the multilineage differentiation of BCR/ABL expressing K562 cells in response to imatinib mesylate 44. Altogether, over the last few years there has been strong emerging evidence implicating p38 as a critical negative regulator of BCR/ABL-mediated leukemogenesis, raising the possibility that finding different ways and means to activate the p38 MAPK in BCR/ABL expressing cells may provide a novel complementary approach for the treatment of CML.
In the present study, we used different systems to determine the role of the p38 MAPK pathway in the induction of the antileukemic effects of dasatinib. Although, as in the case of imatinib mesylate, dasatinib also blocks BCR/ABL kinase activity, this agent has dual inhibitory kinase activity and beyond Abl, it also inhibits Src kinases. Thus, it is possible that targeting of more than one pathway is of importance in the induction of dasatinib-induced antileukemic responses in CML, while the relevant contribution of p38-engagement is not known. Our data demonstrate that treatment of BCR/ABL expressing cells with dasatinib induces strong activation of p38 MAPK. Such induction was also noticeable in cells expressing different BCR/ABL mutants known to be resistant to imatinib mesylate, with the exception of the T315I mutant, which is resistant to suppression by either imatinib mesylate or dasatinib. Some increase in the basal levels of p38 MAPK phosphorylation was observed in T315I cells, but the significance of this finding remains unclear. It remains to be determined whether this mutation confers to cells a different survival mechanism, and this should be addressed in future studies.
In other studies, we also identify several upstream and downstream effectors of p38 MAPK activated by dasatinib, providing insights on the mechanisms by which p38 MAPK activity may be blocked by BCR/ABL. Our studies also identify two key downstream p38 effectors activated in a dasatinib-dependent manner, as the kinases Mapkapk2 45 and MSK1 33, suggesting possible mechanisms by which activation of p38 during BCR/ABL-inhibition may lead to transcription of genes that mediate apoptosis or induction of other pro-apoptotic signals. Interestingly, in in vitro kinase assays we were also able to demonstrate that both MEF2C and ATF2 act as in vitro substrates for the dasatinib-activated form of p38MAPK. A sustained upregulation of ATF2 kinase activity was seen similar to previously reported activity with imatinib in CML 23. ATF2, a member of the ATF/cAMP response element-binding protein family, is phosphorylated by p38/Jun NH2-terminal protein kinase and activates the transcription of apoptosis-related genes in response to stress 46. MEF2C has been shown to be a transcriptional factor in myogenesis 47, although a functional role for it in non-muscle cells (e.g., lymphoid cells) has also been described 48. Our data demonstrate that MEF2C is also an in vitro substrate for dasatinib activated form of p38 MAPK in CML cells. Although the ATF2 and MEF2C substrates may target different phosphorylation sites and may have inherent functional differences, their consistent upregulation by dasatinib in CML cells confirms the activation of the kinase domain of p38. The potential activation of these elements in vivo and their functional relevance in BCR/ABL transformed cells remains to be defined in future studies.
In other studies, aimed to identify upstream effectors of the dasatinib-activated p38MAPK pathway, we were able to demonstrate that treatment of cells with dasatinib results in phosphorylation/activation of MKK3/6 and its upstream effector, MLK3 45. Based on our data, we propose that the initiating kinase of the pathway during dasatinib treatment of BCR/ABL expressing cells is the MLK3 kinase. However, it is likely that the suppressive effects of BCR/ABL occur upstream of this kinase, at the levels of small G proteins that are known to activate the kinase cascade that ultimately lead to p38 MAPK activation 45.
The critical role of p38 MAPK in the generation of antileukemic responses by dasatinib in CML cells was further established in studies demonstrating that its antiproliferative effects were partially reversed by pharmacological inhibition of p38 or by siRNA-mediated knockdown of p38. In fact, our data established that p38 activity is required both for the induction of apoptosis, as well as the generation of the suppressive effects of dasatinib on leukemic progenitors from CML patients. Furthermore, p38 MAPK is essential for dasatinib induced cell cycle G0/G1 arrest. Since such reversal was not 100%, we conclude that p38MAPK is one of the essential pathways required for the inhibitory effect of dasatinib in CML. We propose to explore interaction between p38 MAPK and other known pathways downstream of BCR/ABL. We also propose to study p38MAPK knockdown in CML patient derived CD34+ cells using methylcellulose progenitor assays and evaluate the development of resistant disease. Such findings raise the possibility that development of novel approaches to activate p38 in CML or BCR/ABL expressing ALL cells, may provide a novel therapeutic approach to enhance the effects of dasatinib or other BCR/ABL kinase inhibitors on leukemic cells.
Recently MEK kinase inhibitors 22 have been shown to synergize with dasatinib in the induction of antileukemic responses in BCR/ABL expressing cells. Interestingly, there is extensive evidence in the literature that the MEK/ERK pathway antagonizes p38 activation, and under certain circumstances MEK inhibition may lead to p38 activation in other systems 22. It is therefore possible that the enhancing effects of MEK inhibition in the generation of dasatinib responses may be due in part to activation of the p38 MAPK, but this remains to be determined in future studies. It is also possible, that identification of drugs that may activate p38 independently of BCR/ABL inhibition in CML cells may provide a novel approach to overcome resistance, including T315I-mediated resistance. Although at this point no such drugs are available, high throughput screening methodological approaches may lead to the identification of such compounds in the future, and studies in that direction are warranted.
Acknowledgment
We are indebted to Shashi Parmar for her administrative services.
Financial Support: This work was supported by VISN 17 Grant and a Merit Review grant from the Department of Veterans affairs, NIH grants CA121192, HL082946, HL067256 and HL61897.
Footnotes
Conflict of Interest: Authors declare no conflict of interest with the presented work.
References
- 1.Jabbour E, Cortes J, O'Brien S, Giles F, Kantarjian H. New targeted therapies for chronic myelogenous leukemia: opportunities to overcome imatinib resistance. Semin Hematol. 2007;44:S25–S31. doi: 10.1053/j.seminhematol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Goldman JM, Melo JV. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 3.Skorski T, Kanakaraj P, Nieborowska-Skorska M, et al. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86:726–736. [PubMed] [Google Scholar]
- 4.Cortez D, Reuther G, Pendergast AM. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 5.Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 6.Skorski T, Bellacosa A, Nieborowska-Skorska M, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase:mechanism of activation and downstream effectors. Cancer Res. 2005;65:2047–2053. doi: 10.1158/0008-5472.CAN-04-3888. [DOI] [PubMed] [Google Scholar]
- 8.Kharas MG, Deane JA, Wong S, et al. Phosphoinositide 3-kinase signaling is essential for ABL oncogene-mediated transformation of B-lineage cells. Blood. 2004;103:4268–4275. doi: 10.1182/blood-2003-07-2193. [DOI] [PubMed] [Google Scholar]
- 9.Skorski T. Oncogenic tyrosine kinases and the DNA-damage response. Nat Rev Cancer. 2002;2:351–360. doi: 10.1038/nrc799. [DOI] [PubMed] [Google Scholar]
- 10.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: A matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 12.Palandri F, Iacobucci I, Martinelli G, et al. Long-term outcome of complete cytogenetic responders after imatinib 400 mg in late chronic phase, philadelphia-positive chronic myeloid leukemia: the GIMEMA Working Party on CML. J Clin Oncol. 2008;26:106–111. doi: 10.1200/JCO.2007.13.2373. [DOI] [PubMed] [Google Scholar]
- 13.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 14.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 16.Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99:3472–3475. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- 17.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Rahmani M, Corey SJ, Dent P, Grant S. A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J Biol Chem. 2004;279:34227–34239. doi: 10.1074/jbc.M402290200. [DOI] [PubMed] [Google Scholar]
- 19.Hochhaus A. Management of Bcr-Abl-positive leukemias with dasatinib. Expert Rev Anticancer Ther. 2007;7:1529–1536. doi: 10.1586/14737140.7.11.1529. [DOI] [PubMed] [Google Scholar]
- 20.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 21.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109:4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmar S, Katsoulidis E, Verma A, et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of the effects of imatinib mesylate (STI571) in BCR-ABL-expressing cells. J Biol Chem. 2004;279:25345–25352. doi: 10.1074/jbc.M400590200. [DOI] [PubMed] [Google Scholar]
- 24.Parmar S, Smith J, Sassano A, et al. Differential regulation of the p70 S6 kinase pathway by interferon alpha (IFNalpha) and imatinib mesylate (STI571) in chronic myelogenous leukemia cells. Blood. 2005;106:2436–2443. doi: 10.1182/blood-2004-10-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 26.Yanagisawa K, Yamauchi H, Kaneko M, Kohno H, Hasegawa H, Fujita S. Suppression of cell proliferation and the expression of a bcr-abl fusion gene and apoptotic cell death in a new human chronic myelogenous leukemia cell line, KT-1, by interferon-alpha. Blood. 1998;91:641–648. [PubMed] [Google Scholar]
- 27.Scappini B, Gatto S, Onida F, et al. Changes associated with the development of resistance to imatinib (STI571) in two leukemia cell lines expressing p210 Bcr/Abl protein. Cancer. 2004;100:1459–1471. doi: 10.1002/cncr.20131. [DOI] [PubMed] [Google Scholar]
- 28.Griswold IJ, MacPartlin M, Bumm T, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–6093. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimmanapalli R, Fuino L, Stobaugh C, Richon V, Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 30.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 32.McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS. MSK1 activity is controlled by multiple phosphorylation sites. Biochem J. 2005;387:507–517. doi: 10.1042/BJ20041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arthur JS, Fong AL, Dwyer JM, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JM, Greten FR, Wong A, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Muniyappa H, Das KC. Activation of c-Jun N-terminal kinase (JNK) by widely used specific p38 MAPK inhibitors SB202190 and SB203580: A MLK-3-MKK7-dependent mechanism. Cell Signal. 2008;20:675–683. doi: 10.1016/j.cellsig.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouse J, Cohen P, Trigon S, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 39.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 40.Wong S, McLaughlin J, Cheng D, Witte ON. Cell context-specific effects of the BCR-ABL oncogene monitored in hematopoietic progenitors. Blood. 2003;101:4088–4097. doi: 10.1182/blood-2002-11-3376. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Arevalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, et al. Modulation of the p38 MAPK (mitogen-activated protein kinase) pathway through Bcr/Abl: implications in the cellular response to Ara-C. Biochem J. 2005;387:231–238. doi: 10.1042/BJ20040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandyopadhyay G, Biswas T, Roy KC, et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood. 2004;104:2514–2522. doi: 10.1182/blood-2003-11-4065. [DOI] [PubMed] [Google Scholar]
- 43.Mayer IA, Verma A, Grumbach IM, et al. The p38 MAPK pathway mediates the growth inhibitory effects of interferon-alpha in BCR-ABL-expressing cells. J Biol Chem. 2001;276:28570–28577. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- 44.Kohmura K, Miyakawa Y, Kawai Y, Ikeda Y, Kizaki M. Different roles of p38 MAPK and ERK in STI571-induced multi-lineage differentiation of K562 cells. J Cell Physiol. 2004;198:370–376. doi: 10.1002/jcp.10426. [DOI] [PubMed] [Google Scholar]
- 45.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 46.Makino C, Sano Y, Shinagawa T, Millar JB, Ishii S. Sin1 binds to both ATF-2 and p38 and enhances ATF-2-dependent transcription in an SAPK signaling pathway. Genes Cells. 2006;11:1239–1251. doi: 10.1111/j.1365-2443.2006.01016.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Woodring PJ, Bhakta KS, et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]