Abstract
Two novel, highly fluorinated macrocyclic chelators with highly branched and spherically symmetric fluorocarbon moieties have been designed and efficiently synthesized. This is achieved by conjugating a spherically symmetric fluorocarbon moiety to the macrocyclic chelator DOTA, with or without a flexible oligo-oxyethylene linker between these two parts. As a result of the spherical symmetry, all 27 fluorine atoms in each fluorinated chelator give a sharp singlet 19F NMR signal. The hydrophilicity and the 19F relaxation behavior of fluorinated chelators can be modulated by the insertion of a flexible linker between the fluorocarbon moiety and the macrocyclic linker. These chelators serve as prototypes for 1H-19F dual-nuclei magnetic resonance imaging agents.
Keywords: fluorinated macrocyclic chelator, fluorinated amphile, 19F NMR, spherical symmetry, DOTA
We are interested in using fluorocarbon liquid nanoparticles, formulated as microemulsions, as multifunctional drug delivery vehicles for 19F MR image-guided targeted drug therapy, particularly for radionuclide therapy.1 In our first paper of this series, the synthesis of prototypical fluorinated oils (F-oils) and fluorinated amphiles (Famphiles) was described.2 F-oils and F-amphiles (serving as emulsifiers) will constitute the inner core and the outer shell of the fluorocarbon nanoparticles, respectively. To facilitate 19F MR imaging, the fluorocarbon moiety, containing three perfluoro-tert-butyl groups centered around a pentaerythritol hub, is designed to be highly branched and spherically symmetric so that all fluorine atoms will give a sharp singlet 19F signal. To enable the nanoparticles with 1H-19F dual nuclei MR imaging capacity, some highly fluorinated chelators are needed. Detailed discussions of the design principles of the nanoparticle (e.g., rationale for 1H-19F dual nuclei MR imaging) and that of F-oils and F-amphiles (e.g., the rationale for size and shape matching and branching) can be found in references 1 and 2, respectively.
In this paper, the syntheses of two highly fluorinated macrocyclic chelators are described. For targeted radionuclide therapy, a macrocylic chelator serves two functions: carrying metallic radionuclides (e.g., 90Y3+) for radiotherapy and carrying Gd3+ for contrast-enhanced 1H MR imaging (See Figure 1 of reference 1). The macro-cyclic chelator is conjugated to a fluorocarbon moiety so that it can be incorporated into the outer shell of a fluorocarbon nanoparticle. Note that a fluorinated chelator is an F-amphile as the fluorocarbon moiety is hydrophobic while the chelator is hydrophilic.
Figure 1.
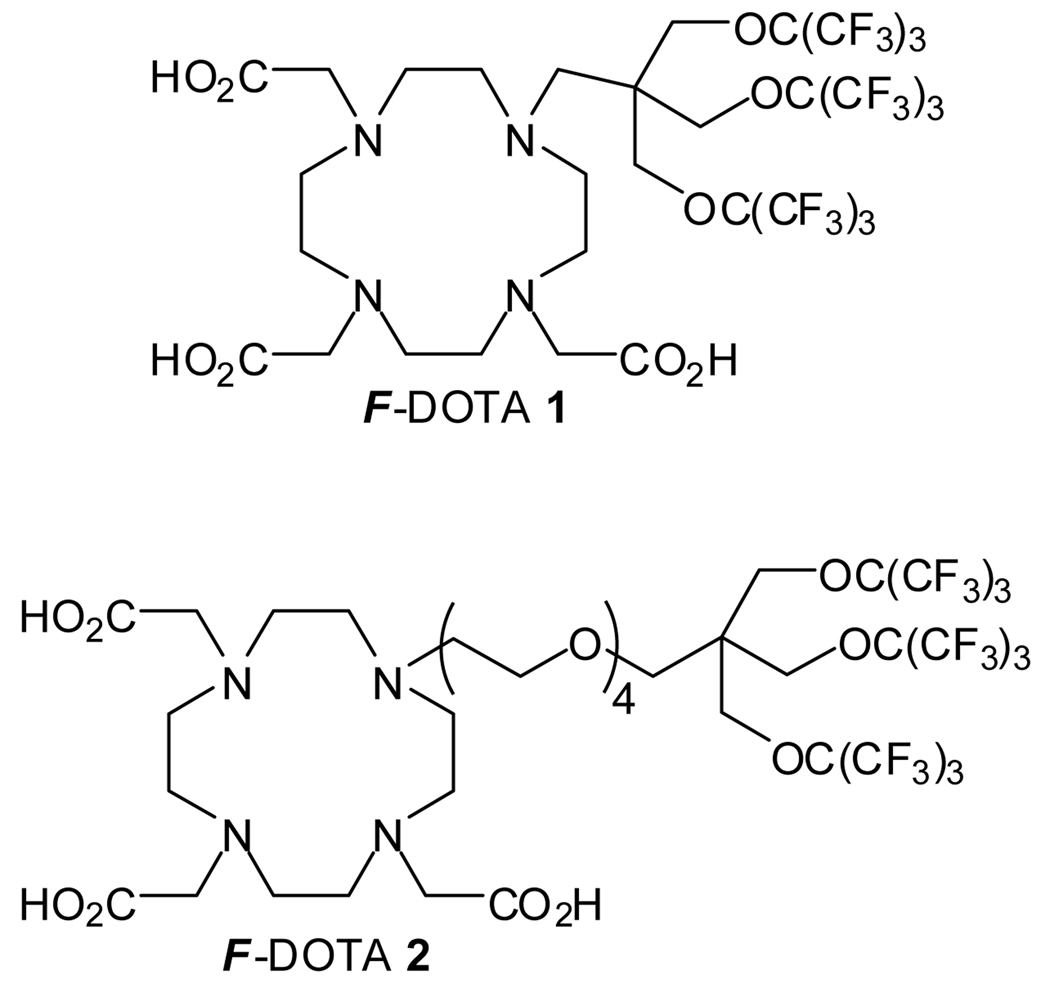
Structures of target molecules F-DOTA 1 and F-DOTA 2. The fluorocarbon moiety of both 1 and 2 has spherical symmetry.
We choose the macrocyclic chelator DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra-acetate) and its derivative for our application because DOTA forms very stable complexes with metallic ions,3 which is essential for radionuclide therapy. DOTA and its derivative are already in clinical use as MR imaging contrast agents (Dotarem® and ProHance®). DOTA is also the chelator used in the radiotherapeutic drug candidate (OctreoTher®), which is under clinical trial.4 Hence, DOTA has a proven record in radionuclide therapy and MR imaging.
In its simplest form, DOTA is conjugated directly to the fluorocarbon moiety, forming the chelator F-DOTA 1, as shown in Figure 1. However, it might be necessary to insert a flexible hydrophilic linker between the macro-cyclic chelator and the fluorocarbon moiety to increase amphipathicity and to alleviate potential steric hindrance to chelation caused by the bulky fluorocarbon moiety. An obvious choice for the flexible linker is oligo-oxyethylene as oxyethylene is hydrophilic and biocompatible. One such chelator with a tetra-oxyethylene linker (F-DOTA 2) is presented in Figure 1. The fluorocarbon moieties of both molecules have spherical symmetry.
The purpose of this synthesis paper is to establish the feasibility of conjugating a bulky fluorocarbon moiety to the macrocyclic DOTA and the feasibility of inserting an oligo-oxyethylene linker between the fluorocarbon moiety and DOTA. Detailed investigation of metal ion chelation and MR imaging capacity will be conducted in the future.
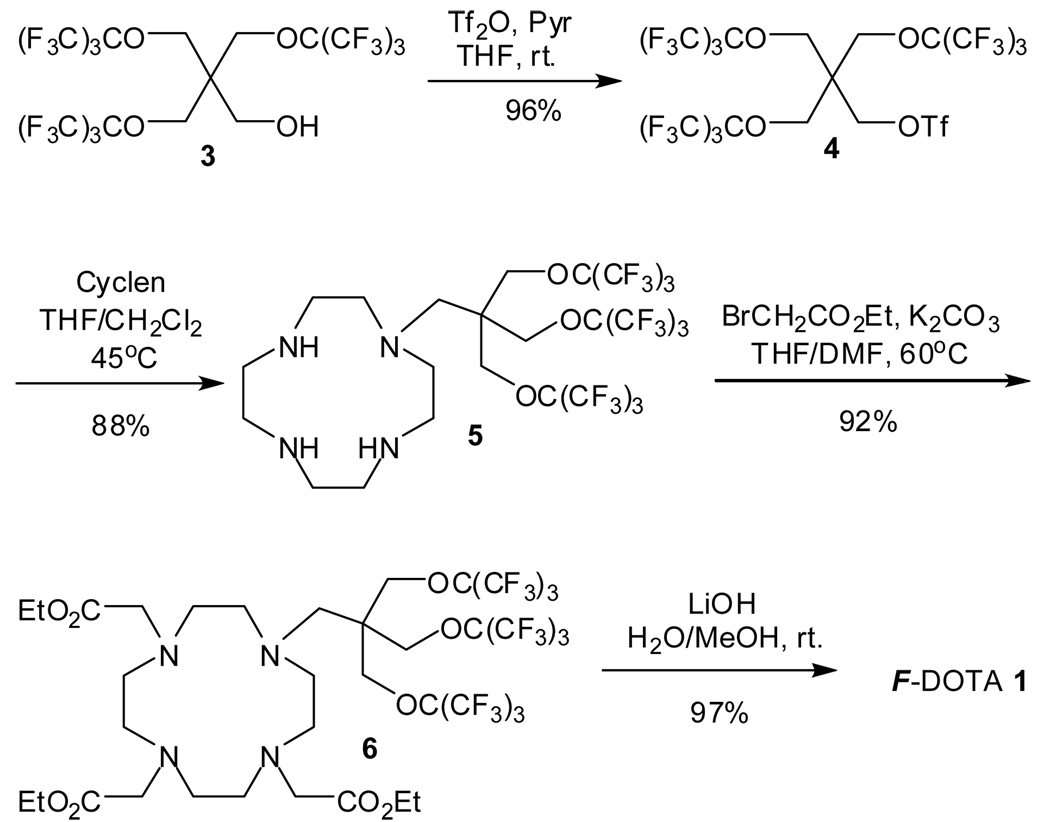
The synthesis of F-DOTA 1 is depicted in Scheme 1. By employing the method described in our previous paper,2 the highly fluorinated alcohol 3 was prepared on a 50-gram scale that will be used as the common starting material for both F-DOTA 1 and F-DOTA 2 synthesis. Treatment of alcohol 3 with trifluoromethanesulfonic anhydride in the presence of pyridine gave triflate 4 with excellent yield, which was isolated through phase separation after the addition of 10% water (v/v) to the reaction mixture. It is noteworthy that tetrahydrofuran, instead of the commonly used dichloromethane for such esterification, turned out to be the ideal solvent for this reaction because alcohol 3 has very limited solubility in dichloromethane. It is necessary to use excess of trifluoromethanesulfonic anhydride and pyridine to achieve high conversion of the well buried highly fluorinated alcohol 3 to triflate 4. Triflate 4 was then conjugated to the cyclen ring after reacting with 2 equivalents of cyclen in a mixture of tetrahydrofuran and dichloromethane (1/1, v/v) at 45 °C overnight. The product 5 was extracted by FC-72 (perfluorohexanes) from the dried reaction residue (dissolved in dichloromethane) with an 88% yield. Again, the choice of solvent is crucial for the conjugation. Instead of reacting with dissolved cyclen, triflate 4 decomposed slowly, when dichloromethane or chloroform alone was used as the solvent, because it is not stable at ambient temperature and has low solubility in dichloromethane or chloroform. The reaction of compound 5 with ethyl bromoacetate in the presence of potassium carbonate in a mixture of tetrahydrofuran/dimethylformamide (1/1, v/v) at 60 °C provided ester 6 with an excellent yield after flash chromatography purification on neutral aluminum oxide. Finally, hydrolysis of the three ethyl esters in 6 with lithium hydroxide in water and methanol afforded the target molecular FDOTA 1 with a 97% yield.
Scheme 1.
Synthesis of F-DOTA 1.
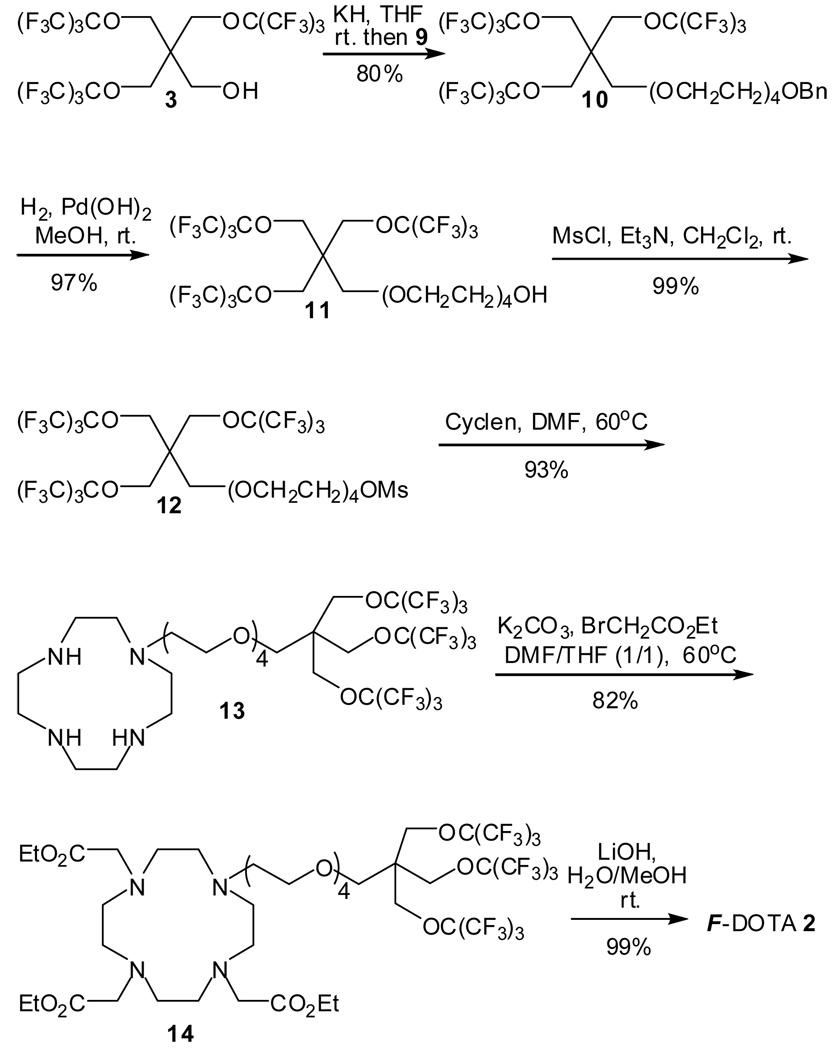
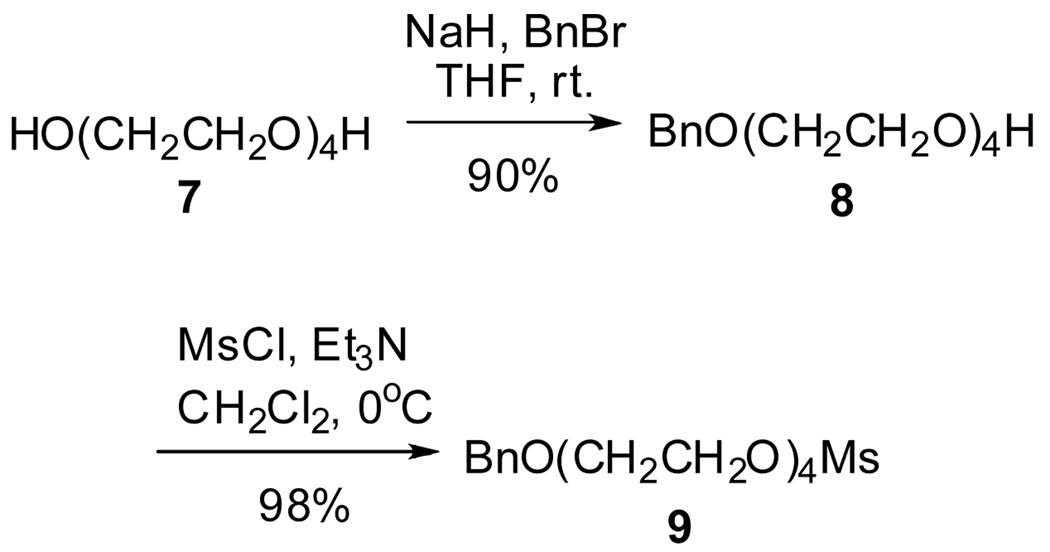
Before starting the synthesis of the target molecule F-DOTA 2, the tetra-oxyethylene linker 9 between the fluorocarbon moiety and the DOTA moiety was synthesized with good yield on a 50-gram scale from commercially available tetraethylene glycol 7 by selectively protecting one of the hydroxyl groups with benzyl bromide5 and transforming the other hydroxyl group into mesylate (Scheme 2). It is an improvement to our former synthesis2a by changing the protective group for the hydroxyl group in tetraethylene glycol 7 from the tert-butyldimethylsilyl (TBDMS) group to the benzyl (Bn) group because the mesylate 9 can be prepared on a larger scale and in a much simpler and cheaper way. This also reduces side reaction (when coupling 9 with alcohol 3 in Scheme 3) and simplifies the purification process (when deprotecting 10 in Scheme 3) for subsequent steps.
Figure 2.
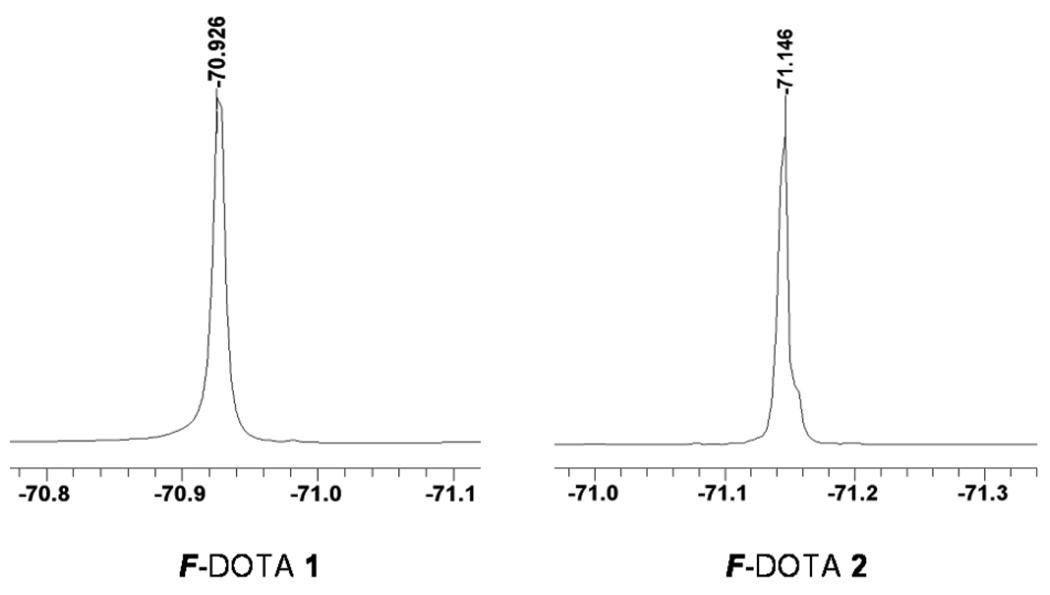
19F NMR spectra of F-DOTA 1 and F-DOTA 2 (0.025 M in CD3OD, 376 MHz).
Scheme 3.
Synthesis of F-DOTA 2.
With 9 in hand, the synthesis of F-DOTA 2 proceeded (Scheme 3). The highly fluorinated alcohol 3 was first attached to the hydrophilic tetra-oxyethylene linker 9 to afford compound 10 with good yield. Then, removal of the benzyl group in compound 10 by palladium hydroxide catalyzed hyodrogenolysis gave alcohol 11 with an excellent yield which was then treated with methanesulfonyl chloride and triethyl amine to give the mesylate 12 with a quantitative yield. Attaching cyclen to the fluorocarbon moiety was achieved by treating compound 12 with 2 equivalents of cyclen. However, the resulting cyclen derivative 13 can hardly be isolated from the reaction mixture by flash chromatography. Fortunately, solid-phase extraction of the reaction mixture on fluorinated silica gel6 provided amine 13 with a 93% yield. Compound 13 was then reacted with ethyl bromoacetate to afford ester 14 with a good yield. Finally, treatment of compound 14 with lithium hydroxide gave the target molecule F-DOTA 2 with a 99% yield.
With F-DOTA 1 and F-DOTA 2 in hand, the 19F NMR spectrum of each chelator was then acquired. As designed, both F-DOTA 1 and F-DOTA 2 gave only one sharp singlet 19F NMR signal with a peak width of around 0.02 ppm (Figure 2). These highly fluorinated chelators are ideal for 19F MR imaging applications because a sharp singlet 19F NMR signal can not only eliminate chemical shift artifacts but also get rid of the signal intensity modulation caused by J-coupling from adjacent 19F or 1H atoms.7
The flexible oligo-oxyethylene linker is the variable portion of our chelator design. Hence, its impact on the physicochemical properties of fluorinated chelators is assessed. The impact of the tetra-oxyethylene linker on the hydrophilicity of the chelator was evaluated by comparing the 1-octanol/water partition coefficients (Poct) of F-DOTA 1 and F-DOTA 2. Poct is a standard physicochemical parameter in assessing the hydrophilicity of pharmaceuticals.8 Poct of F-DOTA 1 and F-DOTA 2 was determined by 19F NMR.9 Poct of F-DOTA 1 and F-DOTA 2 are 7.63 and 1.40 × 10−2, respectively. Hence, the tetra-oxyethylene linker significantly increased the hydrophilicity of the fluorinated chelator.
The NMR signal relaxation times, T1 and T2 are important parameters for MR imaging because MRI contrasts are T1- or T2- weighted. The impact of the tetra-oxyethylene linker on 19F relaxation behavior was evaluated by NMR spectroscopy (376 MHz). F-DOTA 1 and F-DOTA 2 in methanol-d4 at a concentration of 0.025 M gave the following T1 and T2 values: F-DOTA 1, T1 = 708 ms, T2 = 424 ms; F-DOTA 2, T1 = 1067 ms, T2 = 824 ms (As a reference point, the trifluoromethyl group of perfluorooctyl bromide, a fluorocarbon compound previously used in 19F MRI, has a T1 of 2881 ms and a T2 of 2184 ms in methanol-d4 under the same condition10). This demonstrates clearly that, in addition to modulating hydrophilicity, the oligo-oxyethylene linker can also modulate T1 and T2 of fluorinated chelators.
In short, both the hydrophilicity and the 19F relaxation behavior of fluorinated chelators can be modulated by the addition of the flexible linker. Hence, one way to improve the physicochemical properties of future generation of fluorinated chelators for MR imaging applications is to engineer the flexible linker to achieve desired aqueous solubility and 19F relaxation profile.
Experimental Section
1H NMR spectra were recorded with trimethylsilane as internal reference and 19F NMR spectra were recorded with hexafluorobenzene as internal reference on a Varian 400 spectrometer. Measurements of pH in mobile phases for high performance liquid chromatography (HPLC) were taken after pH calibration with aqueous standard solutions. Molecular mass was obtained using a Voyager-DE MALDI-TOF mass spectrometer.
Triflate 4
To a stirred solution of alcohol 3 (7.9 g, 10.0 mmol) and pyridine (8.2 mL, 7.9 g, 100.0 mmol) in tetra-hydrofuran (250 mL) was added dropwise a solution of trifluoromethanesulfonic anhydride (8.2 mL, 14.1 g, 50.0 mmol) in tetrahydrofuran (20 mL) at 0 °C. After stirring at this temperature for 1 h, the reaction was quenched by slow addition of water (27 mL). The mixture was then transferred to a separatory funnel and the lower phase was collected. Washing the oil with dichloromethane gave the pure triflate 4 as a clear oil (8.8 g, 96% yield) (This compound is unstable at rt. and needs to be used immediately after preparation). 1H NMR (400 MHz, Acetone-d6) δ 4.82 (s, 2H), 4.39 (s, 6H); 19F NMR (376 MHz, Acetone-d6) δ 71.21 (s, 27F), 75.93 (s, 3F).
Amine 5
A suspension of triflate 4 (8.5 g, 9.3 mmol) and cyclen (3.3 g, 18.9 mmol) in a mixture of tetrahydrofuran and dichloromethane (50 mL/50 mL) was stirred at rt. for 2 h. Then the reaction temperature was slowly raised to 45 °C and the mixture was then stirred at this temperature overnight. After concentrating the reaction mixture to dryness under vacuum, the residue was dissolved in dichloromethane (50 mL) and extracted with FC-72 (50 mL, three times). Evaporation of the combined FC-72 phase gave the product 5 as a clear oil (7.7 g, 88% yield). 1H NMR (400 MHz, CDCl3) δ 4.19 (s, 6H), 2.72–2.75 (m, 6H), 2.58–2.60 (m, 4H), 2.51–2.54 (m, 8H); 19F NMR (376 MHz, CDCl3) δ −73.31 (s); 13C NMR (100.7 MHz, CDCl3) δ 120.1 (q, J = 293.3 Hz), 79.1–80.0 (m), 68.4, 54.4, 53.4, 46.9, 46.0, 45.7, 45.5; MS (CI) m/z 945 ((M+H)+, 100); HRMS (CI) calcd for C25H28F27N4O3 945.1730, found 945.1717.
Tri-ethyl ester 6
Powdered potassium carbonate (8.3 g, 60.2 mmol) and ethyl bromoacetate (4.2 mL, 6.3 g, 37.5 mmol) were added to a stirred solution of amine 5 (7.1 g, 7.5 mmol) in a mixture of tetrahydrofuran and dimethyl-formamide (35 mL/ 35 mL) at rt. and the resulting suspension was stirred at 60 °C overnight. After filtration, the solvent was removed under vacuum and the residue was purified by flash chromatography on neutral aluminum oxide (CH2Cl2/Methanol = 10/1) to give the product 6 as a clear oil (6.3 g, 92% yield). 1H NMR (400 MHz, CDCl3) δ 4.03–4.10 (m, 12H), 3.34 (s, 2H), 3.25 (s, 4H), 2.62–2.76 (m, 18H), 1.16–1.21 (m, 9H); 19F NMR (376 MHz, CDCl3) δ −73.42 (s); 13C NMR (100.7 MHz, CDCl3) δ 171.7, 171.4, 120.3 (q, J = 293.3 Hz), 79.1–80.0 (m), 68.3, 60.2, 56.0, 54.9, 54.8, 54.0, 52.6, 52.2, 51.9, 46.7, 29.8, 14.3, 14.1; MS (MALDI-TOF) m/z 1203 ((M+H)+, 100); HRMS (MALDI-TOF) calcd for C37H46F27N4O9 1203.2834, found 1203.2883.
F-DOTA 1
(2,2',2''-(10-(3-(1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-yloxy)-2,2-bis((1,1,1,3,3,3 hexa fluoro-2-(trifluoromethyl)propan-2-yloxy)methyl) prpyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl) triacetic acid). To a solution of lithium hydroxide (1.4 g, 60.0 mmol) in water (10 mL) was added a solution of ester 6 (6.0 g, 5.0 mmol) in methanol (200 mL) at rt. The resulted mixture was stirred at rt. overnight. Then 2N HCl was added to the cloudy suspension to adjust the reaction mixture to pH 1. The solid was collected through centrifugation and then washed with water and diethyl ether. Removal of solvent under vacuum gave the product 1 as a white solid (5.4 g, 97% yield). 1H NMR (400 MHz, CD3OD) δ 4.20 (s, 6H), 3.59 (s, 2H), 3.41 (s, 3H), 3.22 (br, 4H), 3.06 (br, 4H), 2.98 (s, 8H), 2.80 (br, 3H); 19F NMR (376 MHz, CD3OD) δ −70.93 (s); 13C NMR (100.7 MHz, CD3OD) δ 180.1, 177.4, 121.6 (q, J = 292.5 Hz), 81.0 (m), 69.9, 58.5, 56.8, 52.2, 51.9, 48.9, 45.9, 45.7, 22.2; MS (MALDI-TOF) m/z 1119 ((M+H)+, 100); HRMS (CI) calcd for C31H36F27N4O9 1121.2051, found 1121.2792.
Alcohol 8
To a stirred solution of tetraethylene glycol 7 (97.0 g, 500.0 mmol) in tetrahydrofuran (450 mL) at 0 °C was added sodium hydride (60% in paraffin, 20.8 g, 520.0 mmol) slowly and the resulting mixture was stirred at rt. for 30 min. Then benzyl bromide (51.2 g, 300.0 mmol) was added and the resulting mixture was stirred at rt. overnight. After quenching the reaction with water (200 mL), the mixture was extracted with ethyl acetate (100 mL, 4 times). The combined organic phase was dried over anhydrous magnesium sulfate. After concentration under vacuum, the residue was purified by flash chromatography on silica gel (n-hexane/ethyl acetate = 1/1) to give alcohol 8 as a clear oil (76.5 g, 90%). 1H NMR (400 MHz, CDCl3) δ 7.28–7.29 (m, 5H), 4.51 (s, 2H), 3.52–3.66 (m, 16 H).
Mesylate 9
To a stirred solution of alcohol 8 (69.3 g, 243.7 mmol) and triethylamine (49.2 g, 68.4 mL, 487.0 mmol) in dichloromethane (700 mL) at 0 °C was added methanesulfonyl chloride (41.9 g, 28.3 mL, 365.6 mmol). The resulting mixture was stirred at rt. overnight and quenched with water (400 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate. The combined organic phase was washed with 2N HCl (100 mL), brine (100 mL) and dried over anhydrous magnesium sulfate. Concentrating the solution under vacuum gave the mesylate 9 as a clear oil (86.6 g, 98%). 1H NMR (400 MHz, CDCl3) δ 7.25–7.32 (m, 5H), 4.54 (s, 2H), 4.32–4.34 (m, 2H), 3.71–3.73 (m, 2H), 3.59–3.66 (m, 12H), 3.03 (s, 3H).
Compound 10
A solution of alcohol 3 (5.9 g, 7.5 mmol) in tetrahydrofuran (50 mL) was stirred at 0 °C and potassium hydride (25% in paraffin, 1.4 g, 8.5 mmol) was added slowly to the solution. After the addition, the mixture was stirred for an additional 10 min at 0 °C and mesylate 9 (4.2 g, 7.5 mmol) was then added in one portion. The resulting mixture was stirred at rt. overnight and quenched with water (100 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate. The combined organic phase was washed with 2N HCl (100 mL) and brine (100 mL) and dried over anhydrous magnesium sulfate. Concentration under vacuum and flash chromatography on silica gel (n-hexane/ethyl acetate = 10/1) gave compound 10 as a clear oil (6.4 g, 80%). 1H NMR (400 MHz, CDCl3) δ 7.26–7.33 (m, 5H), 4.56 (s, 2H), 4.07 (s, 6H), 3.54–3.69 (m, 16H), 3.45 (s, 2H); 19F NMR (376 MHz, CDCl3) δ −73.28 (s); 13C NMR (100.7 MHz, CDCl3) δ 138.3, 128.3, 127.7, 127.5, 120.1 (q, J = 292.6 Hz), 78.9–7 79.8 (m), 73.2, 70.7, 70.65, 70.6, 70.56, 70.3, 69.4, 66.3, 65.5, 46.2; MS (MALDI-TOF) m/z 1079 ((M+Na)+, 100); HRMS (MALDI-TOF) calcd for C32H31F27NaO8 1079.1458, found 1079.1517.
Alcohol 11
A suspension of compound 10 (6.0 g, 5.7 mmol) and palladium hydroxide (10%, 1.2 g) in methanol (80 mL) was stirred under an atmosphere of hydrogen for 2 h. After filtration, the mixture was concentrated under vacuum and purified by flash chromatography on silica gel (n-hexane/ethyl acetate = 8/1) to give alcohol 11 as a clear oil (5.3 g, 97%). 1H NMR (400 MHz, CDCl3) δ 3.99 (s, 6H), 3.53–3.60 (m, 16H), 3.39 (s, 2H).
Mesylate 12
To a stirred solution of alcohol 11 (5.1 g, 5.3 mmol) and triethylamine (3.2 g, 31.8 mmol) in dichloromethane (80 mL) at 0 °C was added methanesulfonyl chloride (1.9 g, 15.9 mmol). The resulting mixture was stirred at rt. for 1h and quenched with water (50 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate. The combined organic phase was dried over anhydrous magnesium sulfate. After concentration under vacuum, the residue was purified by flash chromatography on silica gel (n-hexane/ethyl acetate = 10/1) to give mesylate 12 as a clear oil (5.5 g, 99%). 1H NMR (400 MHz, CDCl3) δ 4.34–437 (m, 2H), 4.04 (s, 6H), 3.73–3.76 (m, 2H), 3.53–3.67 (m, 12H), 3.43 (s, 2H), 3.04 (s, 3H).
Amine 13
A suspension of mesylate 12 (5.3 g, 5.1 mmol) and cyclen (1.8 g, 10.2 mmol) in a mixture of dimethylformamide (50 mL) was stirred at 60 °C overnight. After concentrating the reaction mixture to dryness under vaccum, the residue was purified by solid-phase extraction on FluoroFlash® silica gel (After loading the sample to fluorous silica gel, it was firstly washed with methanol/water (4/1 v/v, 200 mL) and then washed with methanol (400 mL). The product was yielded by concentrating the methanol eluant) to give the product 13 as a clear oil (5.2 g, 93% yield). 1H NMR (400 MHz, CD3OD) δ 4.14 (s, 6H), 3.57–3.69 (m, 14H), 3.49 (s, 2H), 2.95–3.04 (m, 7H), 2.85–2.89 (m, 5H), 2.76–2.82 (m, 2H), 2.69 (s, 4H); 19F NMR (376 MHz, CD3OD) δ −71.14 (s); 13C NMR (100.7 MHz, CD3OD) δ 121.6 (q, J = 292.5 Hz), 80.5–81.4 (m), 78.3, 72.0, 71.9, 71.73, 71.7, 71.67, 71.6, 71.56, 71.5, 71.4, 71.41, 71.4, 71.2, 69.6, 67.5, 67.2, 50.9, 46.4, 44.4; MS (MALDI-TOF) m/z 1121 ((M+H)+, 100); HRMS (MALDI-TOF) calcd for C33H44F27N7O4 1021.2779, found 1021.2790.
Tri-ethyl ester 14
To a stirred solution of amine 13 (5.1 g, 4.6 mmol) in tetrahydrofuran (25 mL) and dimethyl-formamide (25 mL) was added powdered anhydrous potassium carbonate (6.3 g, 46.0 mmol) and ethyl bromoacete (2.6 mL, 3.8 g, 23.0 mmol). The resulting mixture was stirred overnight at 60 °C. After filtration, the solvent was removed under vacuum and the residue was purified by flash chromatography on neutral aluminum oxide (CH2Cl2/Methanol = 10/1) to give the product 14 as a clear oil (5.2 g, 82% yield). 1H NMR (400 MHz, CD3OD) δ 4.16–4.29 (m, 8H), 4.14 (s, 6H), 3.56–3.67 (m, 16H), 3.48 (s, 4H), 2.70–3.45 (m, 4H) 2.41–2.76 (m, 14H), 1.27–1.30 (m, 9H); 19F NMR (376 MHz, CD3OD) δ −71.12 (s); 13C NMR (100.7 MHz, CD3OD) δ 175.3, 175.1, 121.6 (q, J = 292.6 Hz), 80.7–81.5 (m), 71.9, 71.5, 71.4, 71.3, 71.2, 70.8, 68.5, 67.4, 67.1, 62.3, 62.26, 56.4, 56.1, 53.6, 51.9, 51.1, 48.4, 47.4, 14.5, 14.3; MS (MALDI-TOF) m/z 1379 ((M+H)+, 100); HRMS (MALDI-TOF) calcd for C45H62F27N4O13 1379.3882, found 1379.3902.
F-DOTA 2
(2,2',2''-(10-(18,18,18-trifluoro-14,14-bis((1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-yloxy)methyl)-17,17-bis(trifluoromethyl)-3,6,9,12,16 pentaoxaoctadecyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid) Lithium hydroxide (0.9 g, 37.0 mmol) was added to a solution of compound 14 (5.1 g, 3.7 mmol) in methanol (100 mL) and water (10 mL). The resulting mixture was stirred at rt. for 8 h. Then 1N HCl was added to adjust the solution to pH 1. After removing the solvent under vacuum, the residue was purified by flash column chromatography on neutral aluminum oxide (CH2Cl2/Methanol = 10/1) to give compound 2 as a white solid (4.7 g, 99%). 1H NMR (400 MHz, Acetone-d6) δ 4.20 (s, 6H), 3.53–3.59 (m, 16H), 2.2–3.2 (m, 24H); 19F NMR (376 MHz, CD3OD) δ −71.15 (s); 13C NMR (100.7 MHz, CD3OD) δ 180.9, 179.6, 175.3, 175.1, 121.5 (q, J = 292.6 Hz), 80.5–81.4 (m), 71.9, 71.6, 71.5, 71.4, 71.3, 69.9, 68.8, 67.5, 67.1, 60.6, 59.2, 53.9, 52.4, 51.9, 47.4; MS (MALDI-TOF) m/z 1295 ((M+H)+, 100); HRMS (MALDI-TOF) calcd for C39H50F27N4O13 1295.2943, found 1295.2953.
Supplementary Material
Scheme 2.
Preparation of mesylate 9.
Acknowledgment
This research was supported by the National Institutes of Health under grants EB002880 and EB004416. Here, we also wish to thank G. Mahika Weerasekare for her kind help with NMR experiments.
References
- 1.Yu YB. J. Drug Targeting. 2006;14:663–669. doi: 10.1080/10611860600957887. [DOI] [PubMed] [Google Scholar]
- 2.(a) Jiang Z-X, Yu YB. Tetrahedron. 2007;63:3982–3988. doi: 10.1016/j.tet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang Z-X, Yu YB. J. Org. Chem. 2007;72:1464–1467. doi: 10.1021/jo0616308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi A, Calabi L, Corana F, Fontana S, Losi P, Maiocchi A, Paleari L, Valtancoli B. Coord. Chem. Rev. 2000;204:309–393. [Google Scholar]
- 4.(a) Otte A, Jermann E, Behe M, Goetze M, Buche HC, Roser HC, Heppeler A, Mueller-Brand J, Maecke HR. Eur. J. Nucl. Med. 1997;24:792–795. doi: 10.1007/BF00879669. [DOI] [PubMed] [Google Scholar]; (b) Smith MC, Liu J, Chen T, Schran H, Yeh C-M, Jamar F, Valkema R, Bakker W, Kvols L, Krenning E, Pauwels S. Digestion. 2000;62 suppl 1:69–72. doi: 10.1159/000051858. [DOI] [PubMed] [Google Scholar]
- 5.Grobelny Z, Stolarzewicz A, Czaja M, Demuth W, Maercker A. J. Org. Chem. 1999;64:8990–8994. [Google Scholar]
- 6.Gladysz JA, Curran DP. Handbook of Fluorous Chemistry. Wiley-VCH: Weinheim; 2003. and reference therein. [Google Scholar]
- 7.(a) Kimura A, Narazaki M, Kanazawa Y, Fujiwara H. Magn. Reson. Imag. 2004;22:855–860. doi: 10.1016/j.mri.2004.01.060. [DOI] [PubMed] [Google Scholar]; (b) Singh M, Waluch V. Adv. Drug Del. Rev. 2000;41:7–20. doi: 10.1016/s0169-409x(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski CA, Lmobardo F, Dominy BW, Feeney PJ. Adv. Drug Del. Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 9.Since F-DOTA 1 and F-DOTA 2 each contains three carboxylic groups, the protonation status of which is pH-dependent, the 1-octanol/water partition measurements were conducted with physiological saline buffer (PBS, 50 mM phosphate, 100 mM NaCl, 1 mM EDTA, pH 7.0) as the aqueous phase. Specifically, F-DOTA 1 or F-DOTA 2 (5 mg) was dissolved in mixture of PBS buffer (0.5 mL) and 1-octanol (0.5 mL). The sample was put into an Eppendorff® microcentrifuge tube (1.5 mL), which was then taped to a type 16700 mixer from Thermolyne to be shaken vigorously for 20 min. After phase separation, the aqueous phase and 1-Octanol phases were taken out for 19F NMR, respectively, with a sealed capillary of 1% hexanfluorobenzene in methanol-d4 as the internal standard. Poct was calculated based on the ratio of 19F NMR signal areas in the 1-octanol and water.
- 10.T1 and T2 of of the trifluoromethyl group of perfluorooctyl bromide were measured under the same condition as for F-DOTA 1 and F-DOTA 2 by 19F NMR (376 MHz) in methanol-d4 at a concentration of 0.025 M.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.