Abstract
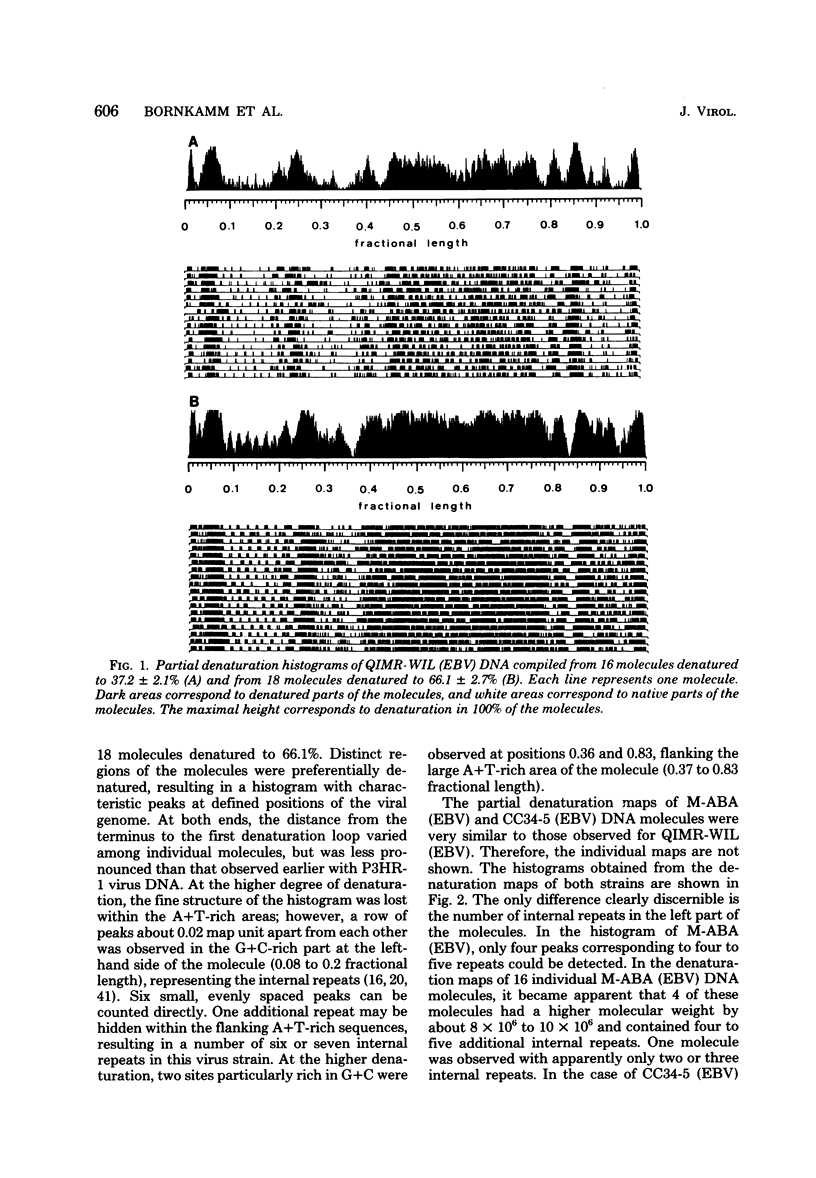
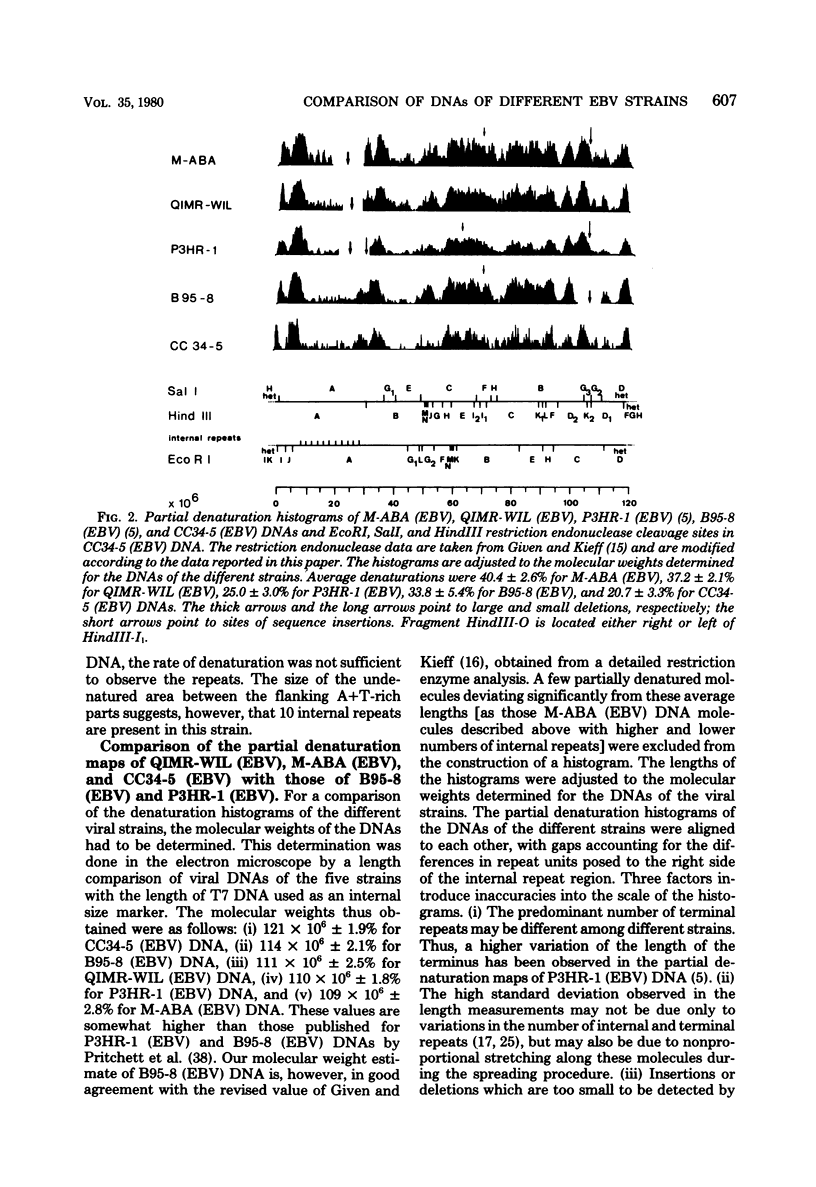
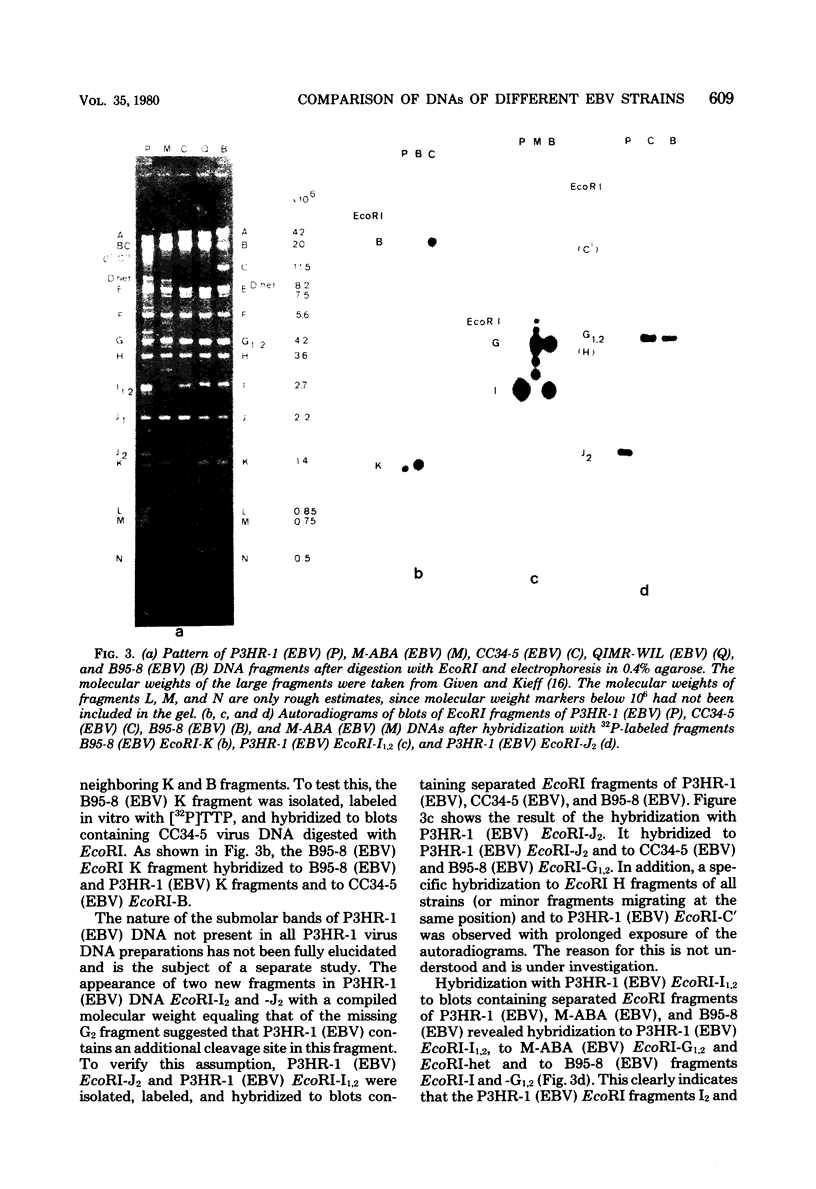
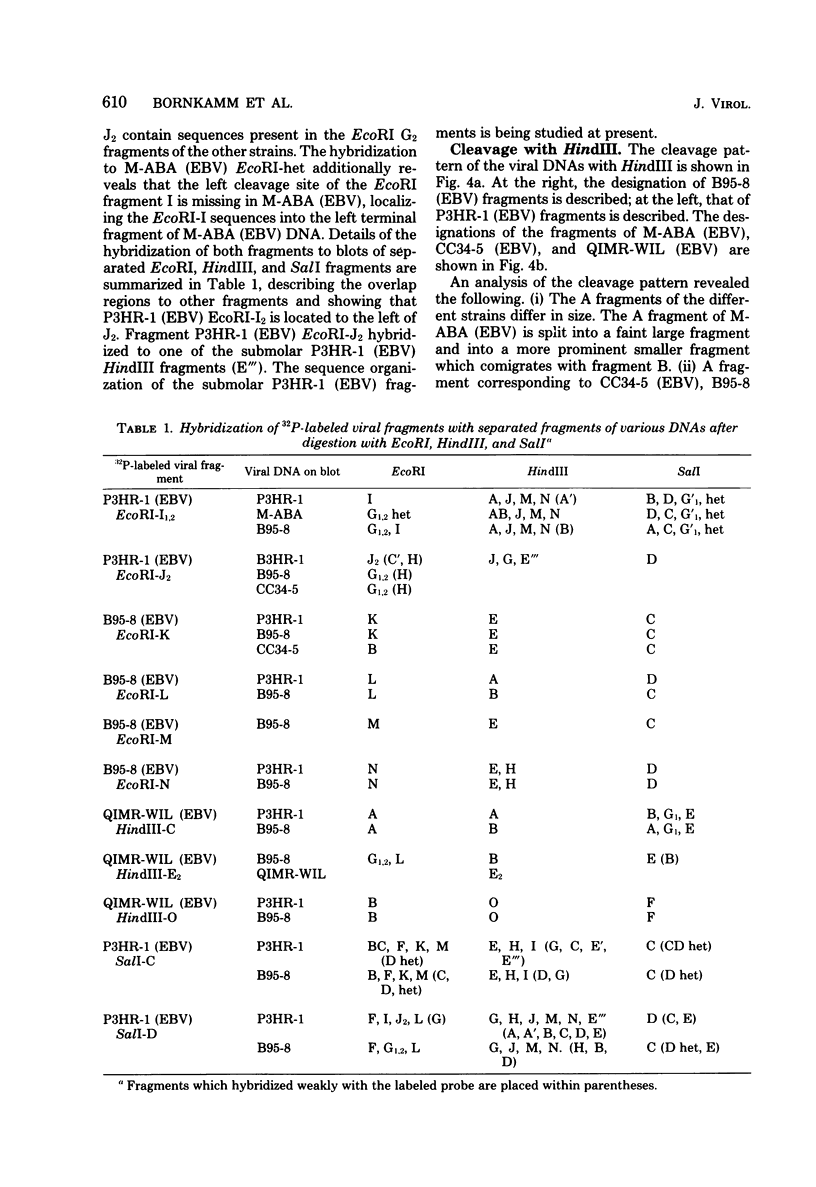
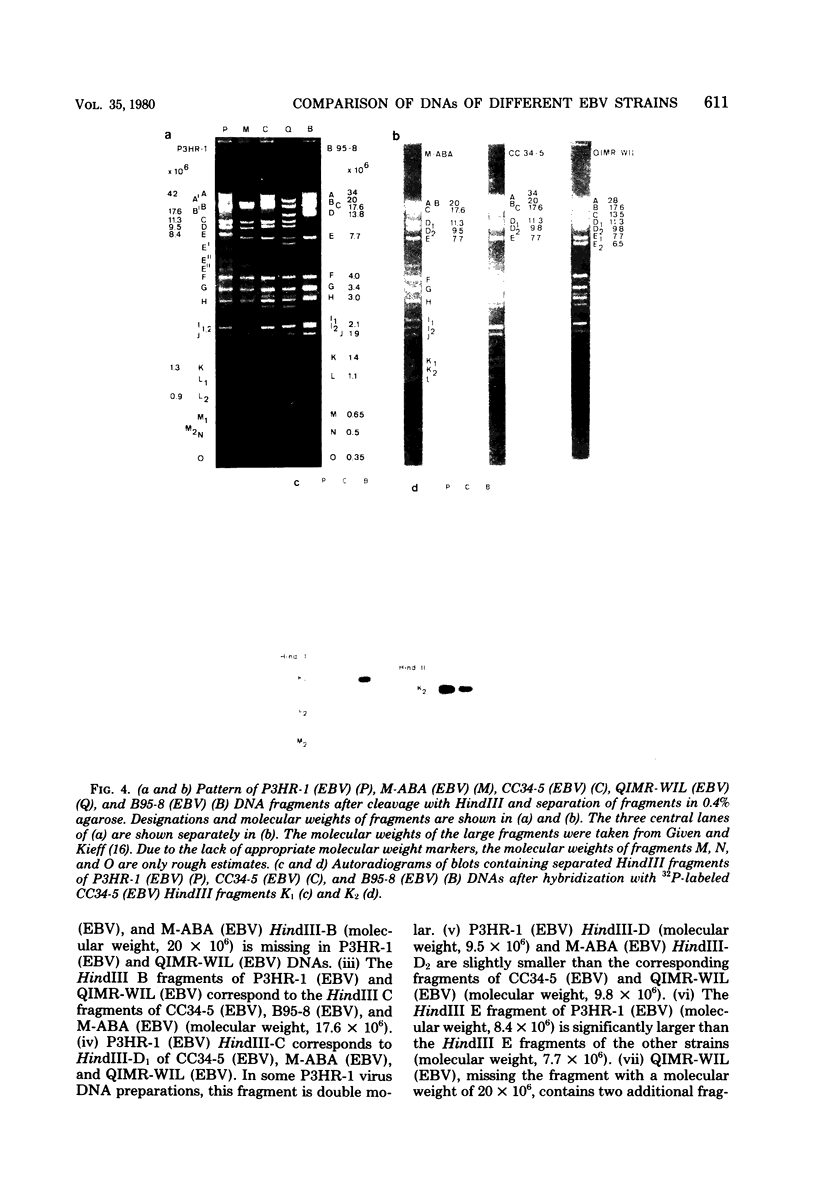
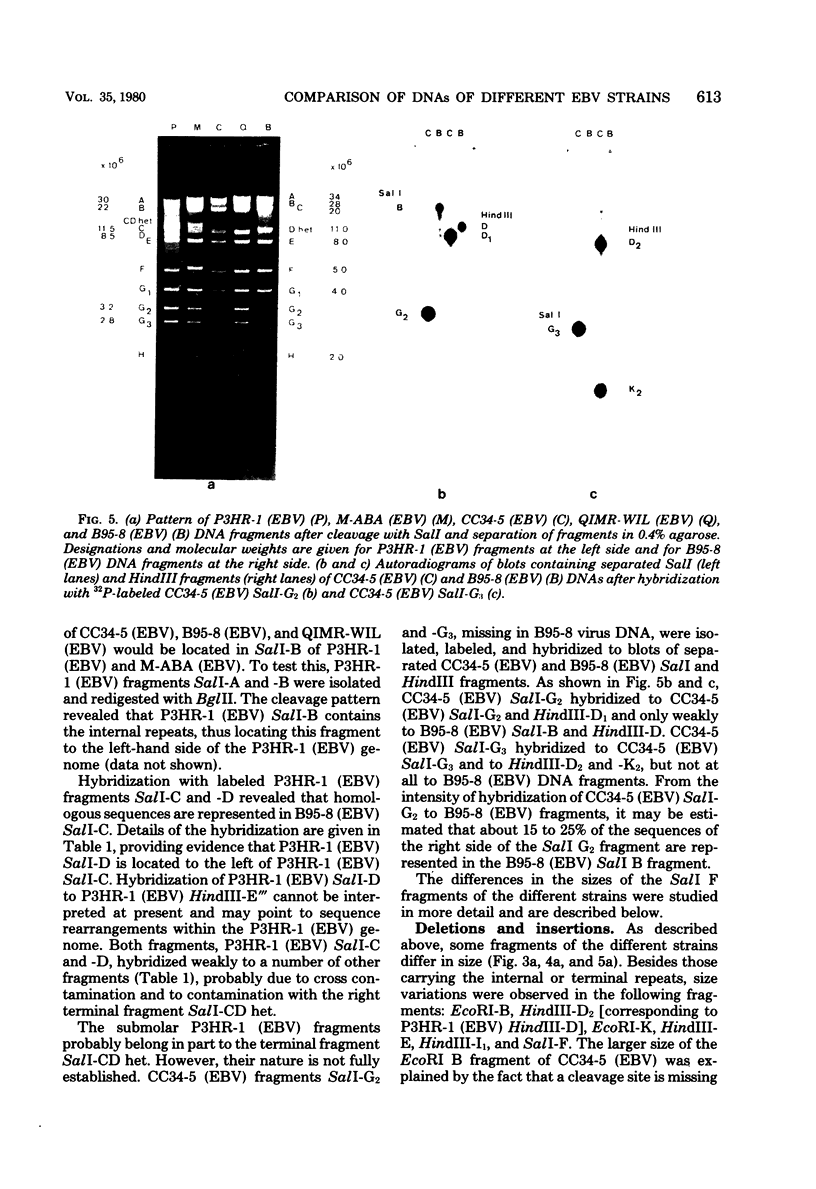
Epstein-Barr virus (EBV) originating from Burkitt's lymphoma (P3HR-1 and CC34-5), nasopharyngeal carcinoma (M-ABA), transfusion mononucleosis (B95-8), and a patient with acute myeloblastic leukemia (QIMR-WIL) was isolated from virus-carrying lymphoid cell lines after induction with the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Viral DNA was analyzed by partial denaturation mapping and by use of the restriction endonucleases EcoRI, HindIII, and SalI and separation of fragments in 0.4% agarose. By using the restriction enzyme data of B95-8 (EBV) and W91 (EBV) obtained by Given and Kieff (D. Given and E. Kieff, J. Virol. 28:524-542, 1978), maps were established for the other virus strains. Comigrating fragments were assumed to be identical or closely related among the different strains. Fragments of different strains migrating differently were isolated, purified, radioactively labeled, and mapped by hybridization against blots of separated viral fragments. The results were as follows. (i) All strains studied were closely related. (ii) The number of internal repeats was variable among and within viral strains. (iii) B95-8 (EBV) was the only strain with a large deletion of about 12,000 base pairs at the right-hand side of the molecule. At the same site, small deletions of about 400 to 500 base pairs were observed in P3HR-1 (EBV) and M-ABA (EBV) DNA. (iv) P3HR-1 (EBV), the only nontransforming EBV strain, had a deletion of about 3,000 to 4,000 base pairs in the long unique region adjacent to the internal repeats carrying a HindIII site. (v) Small inserted sequences of 150 to 400 base pairs were observed in M-ABA (EBV) and B95-8 (EBV) at identical sites in the middle of the long unique region. (vi) Near this site, an insertion of about 1,000 base pairs was found in P3HR-1 (EBV) DNA. (vii) The cleavage patterns of P3HR-1 virus DNA and the results of blot hybridizations with P3HR-1 virus fragments are not conclusive and point to the possibility that in addition to the normal cleavage pattern some viral sequences may be arranged differently. Even though it is possible that small differences in the genome organization may have significant biological effects, the great similarity among different EBV strains does not favor the hypothesis that disease-specific subtypes exist.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Bünemann H., Müller W. Base specific fractionation of double stranded DNA: affinity chromatography on a novel type of adsorbant. Nucleic Acids Res. 1978 Mar;5(3):1059–1074. doi: 10.1093/nar/5.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. H., Epstein M. A., Bornkamm G. W., Achong B. G., Finerty S., Thompson J. L. Biological and biochemical observations on isolates of EB virus from the malignant epithelial cells of two nasopharyngeal carcinomas. Int J Cancer. 1979 Sep 15;24(3):294–302. doi: 10.1002/ijc.2910240305. [DOI] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Diehl V., Henle G., Henle W., Kohn G. Demonstration of a herpes group virus in cultures of peripheral leukocytes from patients with infectious mononucleosis. J Virol. 1968 Jul;2(7):663–669. doi: 10.1128/jvi.2.7.663-669.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., BARR Y. M. CHARACTERISTICS AND MODE OF GROWTH OF TISSUE CULTURE STRAIN (EB1) OF HUMAN LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Natl Cancer Inst. 1965 Feb;34:231–240. doi: 10.1093/jnci/34.2.231. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HENLE G., ACHONG B. G., BARR Y. M. MORPHOLOGICAL AND BIOLOGICAL STUDIES ON A VIRUS IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Exp Med. 1965 May 1;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresen K. O., Cho M. S., Gissmann L., zur Hausen H. NC37-R1 Epstein-Barr virus (EBV): a possible recombinant between intracellular NC37 viral DNA and superinfecting P3HR-1 EBV. Intervirology. 1980;12(6):303–310. doi: 10.1159/000149089. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. VI. Mapping of the internal tandem reiteration. J Virol. 1979 Aug;31(2):315–324. doi: 10.1128/jvi.31.2.315-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Yee D., Griem K., Kieff E. DNA of Epstein-Barr virus. V. Direct repeats of the ends of Epstein-Barr virus DNA. J Virol. 1979 Jun;30(3):852–862. doi: 10.1128/jvi.30.3.852-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Wolf H., Bornkamm G. W. Expression of Epstein-Barr virus genes in different cell types after microinjection of viral DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):433–436. doi: 10.1073/pnas.77.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Nogee L., Hayward G. S. Organization of repeated regions within the Epstein-Barr virus DNA molecule. J Virol. 1980 Jan;33(1):507–521. doi: 10.1128/jvi.33.1.507-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudewentz J., Bornkamm G. W., Zur Hausen H. Effect of the diterpene ester TPA on Epstein-Barr virus antigen- and DNA synthesis in producer and nonproducer cell lines. Virology. 1980 Jan 15;100(1):175–178. doi: 10.1016/0042-6822(80)90563-2. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Sugden B. The structure of the termini of the DNA of Epstein-Barr virus. Cell. 1979 Jul;17(3):661–671. doi: 10.1016/0092-8674(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H., Bünemann H., Müller W. The isolation of DNA from agarose gels by electrophoretic elution onto malachite green-polyacrylamide columns. Gene. 1978 Nov;4(3):227–239. doi: 10.1016/0378-1119(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Yajima Y., Nonoyama M. Mechanism of infection by Epstein-Barr virus. II. Comparison of viral DNA from HR-1 and superinfected Raji cells by restriction enzymes. Virology. 1977 Aug;81(1):17–24. doi: 10.1016/0042-6822(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Magrath I. T., Chattopadhyay S. K., Biggar R. J., Gerber P. A new tumour-derived transforming strain of Epstein-Barr virus. Nature. 1978 Apr 13;272(5654):629–631. doi: 10.1038/272629a0. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Achong B. G., Epstein M. A., Biddulph J. Burkitt lymphoma in New Guinea: establishment of a line of lymphoblasts in vitro and description of their fine structure. J Natl Cancer Inst. 1967 Nov;39(5):933–945. [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rymo L., Forsblom S. Cleavage of Epstein-Barr virus DNA by restriction endonucleases EcoRI, HindIII and BamI. Nucleic Acids Res. 1978 Apr;5(4):1387–1402. doi: 10.1093/nar/5.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E., Seebeck T., Li J. L., Pagano J. S. Epstein-Barr virus DNA synthesized in superinfected Raji cells. Virology. 1977 Apr;77(2):762–771. doi: 10.1016/0042-6822(77)90497-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Electron microscopy of DNA: determination of absolute molecular weights and linear density. Mol Gen Genet. 1977 Sep 9;154(3):299–303. doi: 10.1007/BF00571286. [DOI] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]