Abstract
Background
Prenatal alcohol exposure is a leading preventable cause of birth defects and developmental disabilities in the United States.
Design
A randomized controlled trial (2002–2005; data analyzed 2005–2006) of a brief motivational intervention to reduce the risk of an alcohol-exposed pregnancy (AEP) in preconceptional women by focusing on both risk drinking and ineffective contraception use.
Setting/Participants
A total of 830 nonpregnant women, aged 18–44 years, and currently at risk for an AEP were recruited in six diverse settings in Florida, Texas, and Virginia. Combined settings had higher proportions of women at risk for AEP (12.5% overall) than in the general population (2%).
Interventions
Participants were randomized to receive information plus a brief motivational intervention (n=416) or to receive information only (n=414). The brief motivational intervention consisted of four counseling sessions and one contraception consultation and services visit.
Main Outcome Measures
Women consuming more than five drinks on any day or more than eight drinks per week on average, were considered risk drinkers; women who had intercourse without effective contraception were considered at risk of pregnancy. Reversing either or both risk conditions resulted in reduced risk of an AEP.
Results
Across the follow-up period, the odds ratios (ORs) of being at reduced risk for AEP were twofold greater in the intervention group: 3 months, 2.31 (95% confidence interval [CI]=1.69–3.20); 6 months, 2.15 (CI=1.52–3.06); 9 months, 2.11 (CI=1.47–3.03). Between-groups differences by time phase were 18.0%, 17.0%, and 14. 8%, respectively.
Conclusions
A brief motivational intervention can reduce the risk of an AEP.
Introduction
Alcohol is a known teratogen and a leading preventable cause of birth defects and developmental disabilities in the United States.1,2 Each year 500,000 pregnant women report alcohol use in the past month, and 80,000 report binge drinking.3 Fetal exposure to alcohol results in a spectrum of adverse effects known as Fetal Alcohol Spectrum Disorders,4 with the brain and central nervous system being particularly susceptible to alcohol throughout gestation.5 Fetal Alcohol Syndrome, a severe lifelong condition, has an estimated overall prevalence of up to 2 of every 1000 live births in the United States6 and an estimated lifetime cost of $2 million per case.7
Most women reduce alcohol consumption after learning that they are pregnant,8 but many do not recognize that they are pregnant during the early critical weeks of gestation and continue hazardous drinking.9 Studies from the Centers for Disease Control and Prevention (CDC) show that approximately one in two women of childbearing age (18–44 years) report alcohol use in the past month, and one in eight report binge drinking.10 Furthermore, in the United States, almost half of pregnancies are unplanned,11 of which about half occur in women who are using contraception ineffectively or intermittently.12 Enhancing effective contraception use in women of childbearing age who are risky drinkers (consuming eight or more drinks per week, or five or more drinks on one occasion, or binge drinking) could avert many alcohol-exposed pregnancies (AEP).
Randomized controlled trials (RCTs) of women of childbearing age who are risky drinkers have shown brief interventions to be a promising strategy for reducing AEP.13,14 The Project CHOICES Feasibility Study, a single-arm trial, evaluated a motivational intervention for women determined to be at risk for an alcohol-exposed pregnancy (AEP).15 The intervention was based on theory and research in brief interventions,16 motivational interviewing,17 and the Transtheoretical Model.18 At 6 months postenrollment, 68.5% of the women had reduced their risk for AEP by reducing drinking, using effective contraception methods, or both. This paper presents the major findings from an RCT that followed the feasibility study.
Methods
Participants
Project CHOICES was a multisite collaborative study involving the CDC, Nova Southeastern University in Ft. Lauderdale FL, the University of Texas Health Science Center in Houston TX, and Virginia Commonwealth University in Richmond VA. The study was conducted in six community-based settings. Recruitment was from July 1, 2002 to January 30, 2004, and the follow-up period ended August 15, 2005. An earlier epidemiologic study estimated the risk of AEP in these combined settings to be 12.5%, compared with 2% of fertile women of childbearing age in the United States overall.19 The settings included jails, drug and alcohol treatment centers, suburban primary care practices, a hospital-based gynecology clinic, a Medicaid health maintenance organization, and a media-recruited sample. Recruitment strategies included the use of flyers (posted and mailed) and newspaper and radio announcements. In the jails and treatment centers, presentations were made to groups of women aged 18–44 years who were interested in the study.
Inclusion criteria were: (1) 18–44 years old; (2) no condition causing infertility (tubal ligation, hysterectomy, menopause, or other reason); (3) not pregnant or planning to become pregnant in the next 9 months; (4) had vaginal intercourse during the previous 3 months (or 3 months before going to jail or residential treatment) with a fertile man (not surgically sterile) without using effective contraception (defined in Outcome Measures); (5) engaged in risky drinking (defined in Outcome Measures); and (6) available for the follow-up period. Participants provided written informed consent, and study protocols were approved by the Institutional Review Boards at CDC and at each participating university. A Certificate of Confidentiality was obtained from CDC.
Intervention
The goal of the intervention was to encourage women to change either or both of the target behaviors of risky drinking and ineffective contraception use. The intervention focused on increasing the participants’ commitment to change through the use of motivational interviewing20 and content aimed at increasing motivation. Motivational interviewing is a directive but client-centered counseling style intended to minimize resistance to change. Counselors used motivational interviewing to express empathy, manage resistance without confrontation, and support the participants’ self-efficacy. Procedures to increase motivation were delivered in four motivational interviewing counseling sessions and one contraception counseling visit with a healthcare provider (Table 1). Although both behaviors leading to risk for AEP were targeted, counselors could emphasize the target behavior favored by the participant.
Table 1.
Components of counseling sessions
| Session One |
|
| Session Two |
|
| Session Three |
|
| Session Four |
|
| Contraceptive counseling visit |
|
The contraception consultation visit included participants’ medical history and a discussion of her contraception options. For some women, a physical examination, pregnancy test, and free contraception were provided. Typically, the contraception visit occurred between the second and third counseling sessions, giving the motivational interviewing counselors the opportunity to debrief the visit with the participant. The intervention was delivered by 21 trained counselors (master’s level and above) supervised by the Project CHOICES Efficacy Study Research Team, and six contraceptive care providers (physicians and family planning nurses). Reimbursement for participants’ time was offered for intervention sessions.
Study Design
The study was a two-group parallel RCT. It was hypothesized that women at risk for an AEP who received the brief motivational intervention would be considerably more likely to reduce their risk of an AEP than at-risk women who received information only. Participants were randomized into two groups, information only (IO; the control group) and information plus counseling (IPC; the intervention group). Random allocation was controlled by a data coordinating center. A randomization program was developed using Microsoft Visual Basic 6.0 Professional Edition to generate 200 unique identifiers (IDs) separately for each site and to randomly assign each ID to either the intervention or control group with an equal number (n=100) in each group. Each unique study ID number was then printed on an opaque envelope. A card inside the envelope indicated the group status to which the study participant receiving that ID number was to be assigned. Envelopes were sealed, boxed in numeric order, and mailed to the sites with instructions to the staff to draw the envelopes in numeric order. The study sites were blinded to the ID number’s group status until the envelopes were opened. As the field-collection period reached its final stages, unused IDs were rerandomized in blocks of ten for the Florida and Virginia sites to ensure an equal number of intervention and control participants. Fifty additional IDs were necessary for the Texas jail site.
Newly recruited participants opened the envelopes after the baseline interview. It was not possible to blind the study participants or those administering the intervention to group assignment. Women assigned to the control group received brochures on alcohol use and women’s health in general, and a referral guide to local resources. The intervention was delivered over 14 weeks, with approximately 2 to 3 weeks between sessions. The counseling sessions and the contraception consultation visit were each 45 to 60 minutes. Participants were contacted at 3, 6, and 9 months for follow-up assessments. Staff blinded to the group assignment conducted follow-up interviews. All participants were reimbursed for time and travel except jail inmates who were not allowed to receive reimbursements.
Outcome Measures
Primary outcomes were risky drinking, ineffective contraception, and risk for AEP. Risky drinking was defined as consuming five or more standard drinks in a day (binge drinking), shown to be particularly deleterious to fetal development and subsequent outcomes,21-23 or, on average, eight or more drinks in a week (frequent drinking), which corresponds with the lower threshold for which measurable effects on growth, neurodevelopment, and cognition have been reported.24 A standard drink contained 0.6 ounces of ethanol.1 Ineffective contraception was defined as any occurrence of vaginal intercourse when contraception was either not used or was used ineffectively. Ineffective use was defined as the participant’s reported deviations from the published guidelines for use of a method. At baseline, all woman reported risky drinking and ineffective contraception use. At follow-up, women were categorized as “at reduced risk of AEP” if they reported no risky drinking, effective contraception, or both.
The primary study outcomes were assessed using the timeline followback (TLFB) method.25 The TLFB has been extensively evaluated with clinical and nonclinical populations,26 and has been shown to be a generally reliable and valid method.27 A modification of the TLFB approach for assessing vaginal intercourse and contraception behavior was tested and refined in the Project CHOICES Feasibility Study, and those procedures were used in this study. To enhance reliability of the TLFB data, trained interviewers administered the instrument using memory aids or anchors (i.e., birthdays, special events, holidays) and standardized measures of alcohol consumption (standard drink) and effective contraception (published guidelines). The TLFB data provided a continuous record of daily drinking, vaginal intercourse, and contraceptive practices from 90 days prior to enrollment to 9 months postenrollment. At the baseline, 3-, 6-, and 9-month interviews, participants provided TLFB reports for the previous 90 days. These data were subdivided into 30-day segments over each 90-day period for calculating outcomes as follows: if a woman consumed five or more drinks on any day, or on average eight or more drinks per week during the 30-day period, she was categorized as a risky drinker; if she had any occurrence of vaginal intercourse without effective contraception use, she was categorized as an ineffective contraceptive user for that 30-day period. Women engaging in risky drinking, and/or using ineffective contraception for any of the 30-day segments within a given 90-day period, were considered at risk for that 90-day period.
Baseline Measures
Participants were assessed in person at baseline, and at 3 and 9 months postenrollment, with an abbreviated assessment of main outcomes at 6 months by telephone. Besides the primary outcomes, other measures captured demographic information and psychosocial parameters including the Alcohol Use Disorder Identification Test (AUDIT),28 the Brief Symptom Inventory of psychologic distress,29 and the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) checklist for alcohol disorders.30 Participants’ readiness to change risky drinking and ineffective contraception was measured independently for each behavior using Readiness Rulers.31 The Decisional Balance for Alcohol questionnaire32 and the Decisional Balance for Contraception questionnaire33 assessed the pros and cons of alcohol use and contraception use, and Processes of Change were assessed for alcohol34 and contraception using a questionnaire modeled after the alcohol version.15 Confidence in resisting alcohol use was assessed using the Brief Situational Confidence Questionnaire (BSCQ),35 and temptation to drink by the BSCQ-T, which parallels the BSCQ. Confidence in using contraception was measured using the Self-Efficacy for Contraception Scale,33 and temptation to not use contraception was measured with the Temptation for Contraception Scale developed in an earlier study.15 All psychosocial parameters measured were considered potential confounders, as they have been previously associated with risky drinking or AEP.14,15,20,36,37
Sample Size
Two studies that employed brief interventions with both men and women38,39 showed 19% to 34% reductions in problem drinking. Based on this, it was estimated that 20% of the control group at each site would reduce their risk. In the feasibility study, the proportion of women who achieved a reduction in risk for AEP at 6 months postintervention ranged from 57% to 79% across the six settings. Based on this, it was estimated that 50% of intervention women at each site would reduce risk. Assuming a 30% greater effect in the intervention group (50% vs. 20%), power estimates using the Power and Precision software40 indicated that a minimum of 60 intervention cases and 60 control cases were required at each of the six settings, to produce an estimated power of 0.82, with a Type I error α=0.01.
Statistical Analyses
Demographic and behavioral characteristics were compared across the intervention and control groups at baseline on all (n=830) randomized participants using Fisher’s exact tests on nominal variables such as race, gender, marital status, education, and income. Group means on the remaining variables such as age, AUDIT score, number of drinks, and number of binge episodes were compared using a Satterthwaite t test. Intent-to-treat analysis41 of outcome measures was performed on all randomized participants in the intervention group (n=416) and in the control group (n=414). To determine the efficacy of the motivational intervention in reducing behaviors that lead to AEP, the odds were estimated that women in the intervention group were at reduced risk relative to women in the control group. Logistic regression models were used that expressed the log odds that a participant is at risk for an AEP as a function of the intervention or control group phase (3, 6, and 9 months), and demographic and behavioral confounders measured at baseline. Outcome measures for a given participant are repeated measures on the same subject over time at 3, 6, and 9 months; therefore, a generalized estimating equations analysis approach was used that incorporates the intrasubject correlation resulting from the repeated measurements assuming a symmetric variance covariance error matrix that accounted for the longitudinal behavioral changes in individual women over the three time periods in the computation of the odds ratio (OR) estimates.42 Odds ratios (ORs) and regression coefficients were computed using SAS43 for each outcome measure: AEP, drinking, and contraception use.
Before proceeding, the effects of the diverse study settings on AEP risk were evaluated. Setting was included in the basic longitudinal model as a random effect to determine if the settings were appreciably different. Results showed no statistically significant effect, and therefore, data were combined across the settings to calculate the results of the longitudinal analysis. Given that odds ratios can be considerably affected by confounding demographic and behavioral variables, potential confounders identified a priori along with others identified in the univariate analyses of the current study data were assessed using logistic regression. A backward elimination procedure was used that considered the significance of the potential confounder along with the standard error of the OR associated with the intervention effect to determine a parsimonious model. Based on this procedure, six of the measures—number of male intercourse partners, scores on the AUDIT, Readiness for Change for Contraception, Processes of Change for Alcohol, Decisional Balance for Alcohol, and Temptation for Alcohol—were significant at the p=0.01 level, and were retained in a final model as confounders.44
Because 29% of the participants who initially enrolled in the study at baseline were lost to follow-up at 9 months, additional analyses were conducted to assess potential bias in the overall results. A comparison was conducted of the baseline demographics for participants completing the study with those who were lost to follow-up at 9 months. Another analysis examined the 3-month outcomes of participants who were lost to follow-up after the 3-month interview. To test the sensitivity of the estimated ORs to a possible bias in the assumption that those lost to follow-up had similar outcome distributions as those participants who completed the study, a second intent-to-treat analysis was conducted wherein all the women lost to follow-up in both treatment groups were considered treatment failures and assigned an outcome status of “at risk for AEP.”41
Results
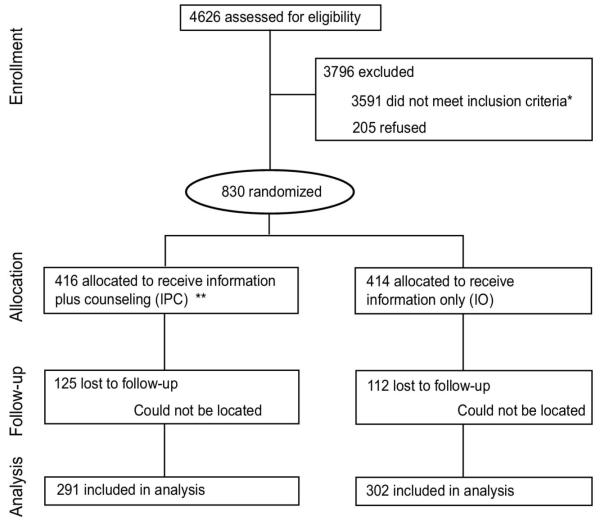
Figure 1 summarizes the study flow and shows that 4626 women were screened at the six sites. Three fourths (3591) of those screened were ineligible, including 3019 who did not meet the ineffective contraception criteria and 1396 who did not meet drinking criteria (not mutually exclusive). In addition, 205 eligible women (19.8%) refused to participate after being informed about the study. Their distribution across sites was proportionally similar. There were 830 eligible, consenting women who participated in the study. Seven women in the intervention group and 15 women in the control group had pregnancies unrecognized at baseline that were reported later in the study. Because these women were not using effective contraception and reported alcohol use during this period, their AEP outcome was “at risk for AEP” in the intent-to-treat analysis.
Figure 1.
Study flow. *The following inclusion criteria categories are not mutually exclusive: 1398 did not meet drinking criteria, 3019 did not meet pregnancy-risk criteria, 826 had other exclusion reasons (moving out of town, unable to understand English, out of age range). **IPC, intervention group; IO, control group.
No significant differences were found in the sociodemographic and clinical characteristics of the intervention and control groups at baseline (Table 2). Overall, study participants had a mean age of 30 years, were predominately African American (48%), had never been married (51%), and had annual incomes of <$20,000 (55%). Fifty-six percent met the criteria for alcohol dependence on a DSM-IV checklist, and illicit drug use (>90%) and tobacco smoking (>70%) were highly prevalent in the population. Approximately 30% consumed on average eight drinks per occasion and 36 drinks per week; about one third reported no contraception use, with the remainder reporting using contraception inconsistently or ineffectively. More than 98% of the women in the intervention group received at least one session, and 63% received all four sessions. On average, they attended 3.2 counseling sessions, and approximately 70% attended a contraception consultation visit. Overall, 71% of participants completed the 9-month follow-up interview. The longitudinal analysis included 665 participants who completed the 3-month follow-up interview, 604 who completed the 6-month follow-up interview, and 593 who completed the 9-month follow-up interview, with approximately equal numbers in treatment and control groups at each of the phases.
Table 2.
Sociodemographic and behavioral characteristics of participants at randomization
| Participant characteristics | Control (IO) n=414 | Treatment (IPC) n=416 | p value |
|---|---|---|---|
| Age (years) | 0.44a | ||
| Mean (SD) | 29.45 (7.66) | 29.8 (7.51) | |
| Median | 28 | 28 | |
| Race | 0.21c | ||
| Black/not Hispanic | 205 (49.5%) | 187 (45%) | |
| Marital status | 0.83c | ||
| Single | 209 (50.5%) | 214 (51.4%) | |
| Education | 0.21* | ||
| Grades 1–11 | 125 (30.2%) | 105 (25.2%) | |
| Grade 12 or GED | 145 (35%) | 166 (39.9%) | |
| College 1+ yrs. | 141 (34.1%) | 144 (34.6%) | |
| Income | 0.43c | ||
| <$20,000 | 221 (53.4%) | 235 (56.5%) | |
| AUDIT score | 0.41a | ||
| Mean (SD) | 17.48 (10.01) | 17.81 (9.69) | |
| Median | 15.5 | 16 | |
| Average number of drinks per week past 90 days | 0.35a | ||
| Mean (SD) | 37.08 (58.96) | 35.59 (55.54) | |
| Median | 16.96 | 18.04 | |
| Number of binge episodes past 3 months | 0.06a | ||
| Mean (SD) | 29.09 (29.96) | 30.06 (28.71) | |
| Median | 19 | 22 | |
| Average number of drinks per drinking day past 90 days | 0.64a | ||
| Mean (SD) | 8.01 (8.62) | 7.96 (8.48) | |
| Median | 4.99 | 5.34 | |
| DSM-IV criteria alcohol problems | 0.86* | ||
| 303.90 alcohol dependence | 234 (56.5%) | 230 (55.3%) | |
| 305.00 alcohol abuse | 31 (7.5%) | 27 (6.5%) | |
| V71.09 no diagnosis | 91 (22%) | 83 (20%) | |
| History of treatment for alcohol-related problems | 280 (67.6%) | 291 (70%) | 0.82a |
| Drug use in past 12 months | 379 (91.5%) | 389 (93.5%) | 0.34* |
| Current smoker | 299 (72.2%) | 316 (76%) | 0.30* |
| Number of sexual partners in past 3 months | 0.36a | ||
| Mean (SD) | 12.42 (54.23) | 7.61 (36.31) | |
| Median | 2 | 2 | |
| Contraception use (past 3 months) | 0.88* | ||
| Used contraception, but ineffectivelyb | 276 (66.7%) | 281 (67.5%) | |
| Used no contraception | 136 (32.9%) | 134 (32.2%) |
p values for differences in means across treatment groups were calculated using the Satterthwaite t-test for log-transformed data.
Includes consistent use of contraception method as prescribed by manufacturer.
p values are based upon the collapsed two-level covariate.
Differences in proportion across treatment groups were tested using the Fisher Exact Test (2-tailed) P-values less than .05 indicated statistically significant difference.
AUDIT, Alcohol Use Disorder Identification Test; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GED, general equivalency diploma; IO, information only; IPC, information plus counseling.
Efficacy
Statistically significant benefits of the Project CHOICES intervention were found for all primary outcomes—AEP, risky drinking, and ineffective contraception use in the unadjusted model (not including confounding variables) and adjusted models (including confounders). Across all three phases of follow-up (3, 6, and 9 months), the unadjusted odds of being at reduced risk for an AEP were approximately twofold greater in the intervention group than in the control group. Odds ratios were 2.08 (95% confidence interval [CI]=1.53–2.84) at 3 months; 1.94 (95% CI=1.40–2.67) at 6 months; and 1.90 (95% CI=1.36–2.66) at 9 months (Table 3). Odds ratios were estimated after controlling for the confounders identified in the backwards elimination procedure, and increased slightly with women in the intervention group, again, significantly more likely to be at reduced risk for an AEP. ORs were 2.32 (95% CI=1.69–3.20) at 3 months, 2.15 (95% CI=1.52–3.06) at 6 months, and 2.11 (95% CI=1.47–3.03) at 9 months (Table 3).
Table 3.
Odds ratios for reduced AEP, effective contraception, and reduced risk drinking for intervention versus control group
| Outcome | Model | Phase | Phase OR (95% CI on OR) |
|---|---|---|---|
| Reduced AEP | Unadjusted | 3 | 2.08 (1.53, 2.84) |
| 6 | 1.94 (1.40, 2.67) | ||
| 9 | 1.90 (1.36, 2.66) | ||
| After adjusting for confounders* | 3 | 2.32 (1.69, 3.20) | |
| 6 | 2.15 (1.52, 3.06) | ||
| 9 | 2.11 (1.47, 3.03) | ||
| Effective contraception | Unadjusted | 3 | 2.12 (1.54, 2.92) |
| 6 | 1.84 (1.33, 2.54) | ||
| 9 | 2.10 (1.52, 2.91) | ||
| After adjusting for confounders* | 3 | 2.40 (1.72, 3.34) | |
| 6 | 2.05 (1.45, 2.89) | ||
| 9 | 2.39 (1.69, 3.39) | ||
| Reduced risk drinking | Unadjusted | 3 | 1.69 (1.22, 2.32) |
| 6 | 1.55 (1.12, 2.14) | ||
| 9 | 1.46 (1.06, 2.01) | ||
| After adjusting for confounders* | 3 | 1.79 (1.28, 2.51) | |
| 6 | 1.64 (1.15, 2.33) | ||
| 9 | 1.54 (1.09, 2.18) |
Confounders adjusted for were the number of male intercourse partners and scores on the AUDIT, Readiness for Change for Contraception, Processes of Change for Alcohol, Decision Balance Scale for Alcohol, and Temptation for Alcohol (BSCQ-T).
AEP, alcohol-exposed pregnancy; CI, confidence interval; OR, odds ratio.
The average number of binge-drinking episodes in the intervention group was reduced from 30.1 at base line to 7.1 episodes at 9 months follow-up. In comparison, women in the control group changed from 29.1 binge episodes at baseline to 9.8 at 9 months follow-up. The median number of drinks per week at baseline was reduced from 36 drinks to 2.3 drinks at 9 months for intervention women, compared to 38 drinks at baseline and 3.1 drinks at the 9-month follow-up for the control group. Women in the intervention group at the 9-month follow-up were more likely to reduce alcohol consumption to below risk levels at an OR of 1.5 (95% CI 1.1–2.2), and were also more likely to use effective contraception at an OR of 2.4 (95% CI 1.7–3.4). The effect of the brief motivational intervention was consistent at each phase of follow-up with no significant interactions between intervention and phase of follow-up.
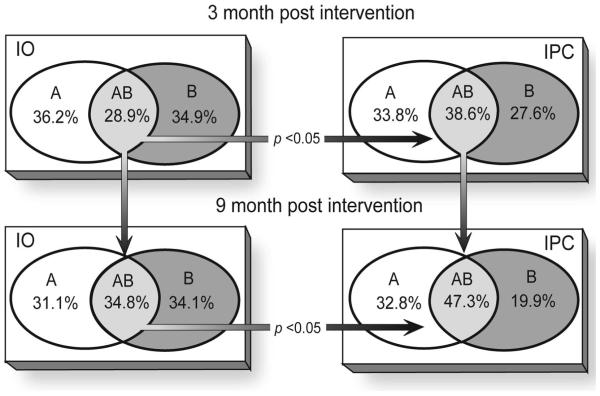
Percent differences in reduced risk for AEP in the intervention versus the control group were 18%, 17%, and 14.8% at 3, 6, and 9 months, respectively, with an overall average of 16.6%. Although significantly more women in the intervention group reduced their risk for an AEP, many women in the control group also reduced their risk for an AEP over the course of the study (Table 4). In both study groups the number of women at reduced risk for each outcome measure increased from 3 to 9 months. Figure 2 shows the participants’ methods of reducing risk for AEP by reducing alcohol use alone, using effective contraception alone, or doing both. At the 3-month follow-up, 10% more women in the intervention group versus the control group reduced both risk behaviors (p <0.05). At the 9-month follow-up this difference increased to 13% (p <0.05).
Table 4.
Proportion of participants meeting risk reduction thresholds for targeted behaviors and reduced risk for AEP
| Risk outcomes (%) | 3 months |
6 months |
9 months |
|||
|---|---|---|---|---|---|---|
| IO* n=333† |
IPC** n=332† |
IO n=305 |
IPC n=299 |
IO n=302 |
IPC n=291 |
|
| Alcohol use less than 8 drinks per week | 36.3% | 46.4% | 37.3% | 46.0% | 44.0% | 54.9% |
| No binge drinking | 38.1% | 52.1% | 41.4% | 52.0% | 46.8% | 57.9% |
| Reduced risk drinking ‡ | 30.3% | 42.2% | 32.5% | 42.4% | 40.4% | 48.8% |
| Effective contraception | 28.4% | 45.8% | 32.8% | 47.7% | 38.7% | 56.3% |
| Reduced risk for AEP | 45.6% | 63.6% | 46.9% | 63.9% | 54.3% | 69.1% |
IO=Information Only (Control Group).
IPC=Information Plus Counseling (Intervention Group).
Sample sizes per cell may vary slightly from the overall (n) due to missing data for some cells at a given follow-up point.
Reduced risk drinking includes both alcohol use less than 8 drinks per week and no binge drinking.
AEP, alcohol-exposed pregnancy.
Figure 2.
Distribution of choices selected by women who achieved reduced AEP risk by control (IO)* and intervention (IPC) groups. A, women who used effective contraception only; B, women who reduced risk-drinking only; AB, women who both used effective contraception and reduced risk drinking. *IO, information only; IPC, information plus counseling. Only women who provided information on both contraception and alcohol behavior are included in these counts.
No significant differences in sociodemographic variables were found between women who completed the study and those lost to follow-up with the exception that more women lost to follow-up had less than a high school education. At the 9-month follow-up considerably more of these women were in the control group. Of the 82 participants lost to follow-up after 3 months (n=46 in the intervention and n=36 in the control group), 53% of the control group and 70% of the intervention group had reduced their risk for AEP at 3 months. In the second intent-to-treat analysis, in which all participants lost to follow-up were treated as treatment failures, the ORs were found to be lower across all phases (after controlling for confounders) but still significant: 3 months (OR=1.8, 95% CI 1.3–2.4); 6 months (OR=1.65, 95% CI 1.2–2.2); and 9 months (OR=1.45, 95% CI 1.1–1.9). Taken together, these findings do not suggest that the loss to follow-up caused a major bias to the study findings.
Comments
This randomized trial found that a brief motivational intervention considerably decreased the risk of AEP in high-risk women by altering the targeted behaviors of risky drinking and ineffective contraception use. Although women in both intervention and control groups reduced their risk for an AEP by instituting changes in the targeted risk behaviors over the 9-month follow-up, the odds of being at reduced risk for AEP were more than double in the group that received the Project CHOICES intervention compared to the control group. Further, women receiving the intervention were more likely to adopt changes in both targeted behaviors simultaneously, thereby maximizing the likelihood of avoiding an AEP.
The efficacy of brief interventions for reducing risky drinking has been well established in previous clinical trials,13,45-47 but few have addressed both drinking and effective contraception use in one intervention directed at reducing AEP in high-risk women. Project CHOICES intervention participants reduced weekly drinking over levels reported by control participants and binge drinking levels as well. Binge drinking was substantially reduced by intervention participants from 30 episodes in the past 3 months at baseline to 7 episodes in the past 3 months at the 9-month follow-up. Women in the control group also reduced binge drinking over time, but at 9 months 57.9% of the intervention group reported no binge episodes versus 46.8% in the control group (Table 4).
One clinical study targeting contraception use as a component of treatment for substance-abusing women reported that at 6-months post-treatment 73% of the intervention group regularly used birth control compared with 52% of the control group.48 In the present study, at 9 months, 56.3% of the intervention group was using effective contraception as compared with 38.7% of the control group. Project BALANCE,14 an RCT using a one-session (2.5 hours) adaptation of the Project CHOICES feasibility intervention and targeting college-aged women, found at 1-month follow-up that 74% of the intervention women and 54% of the control women were no longer at risk for AEP with the odds of being at continued risk for AEP greater in the control group (OR, 2.9). Risky drinking, ineffective contraception, and AEP risk were considerably reduced in both the intervention and control groups, but notably more so in the intervention group. Reductions in risk behaviors among control group participants are not uncommon in women’s alcohol studies.14,49 Previous researchers have cited reactivity to research protocols, regression to the mean, reporting bias,50 assessment effects,51 and a placebo effect52 as possible explanations that could have similarly affected this study.
There are potential limitations to this study. Although self-reports are the major data source for clinical and research purposes, some skepticism still exists about such reports. However, numerous major reviews53-58 have concluded that retrospective self-reports of alcohol use show adequate reliability and validity when data are collected in situations to minimize bias (e.g., a clinical or research setting with voluntary, alcohol-free participants assured of the confidentiality of their reports, as was true in this study). Another potential limitation is the number of participants lost to follow-up by 9 months; however, several analyses addressing this issue found evidence that the intervention was robust, and that its effect was not likely biased by systematic patterns of lost to follow-up. Participants in the control group received health-focused literature but did not receive counselor contact time equivalent to that received by intervention participants. This is a study limitation owing to the difficulty in creating inert counselor–participant sessions that could serve as a placebo control.59 Contact time alone may have contributed to the effect observed in the intervention group. Finally, this study was conducted in targeted settings and its generalizability may extend only to similar populations at this time.
In conclusion, this study demonstrated that a brief behavioral motivational intervention produced significant reductions in risk for AEP among women who met high-risk criteria prior to the study. These findings are encouraging because the intervention was preconceptional, and although many of the participants were not planning to become pregnant, they were not aware they were at risk for an AEP. Women who are not planning to become pregnant may think they have little reason to be concerned about either their drinking or contraceptive practices. Findings from this study indicate that those women who are at risk for an AEP can be made aware of their risk, and can make subsequent changes to reduce that risk. Further research is needed to determine which components of the intervention were most effective, how minimal an intervention will retain effectiveness, and the extent to which this intervention can prove effective in other populations not included in this study. In view of these results and considering that brief interventions are cost effective,60,61 the Project CHOICES intervention appears to be a good potential candidate for large-scale implementation in public health settings to reduce the risk of AEP in high-risk populations.
Acknowledgments
The Project CHOICES Efficacy Study Group acknowledges the following team members who were responsible for this report: Primary Investigators: Louise Floyd (Chair), Mark Sobell, Mary Velasquez, Karen Ingersoll, Mary Nettleman. Co-PIs and Investigators: Linda Carter Sobell, Patricia Dolan Mullen, Sherry Ceperich, Kirk von Sternberg, Burt Bolton, and Kenneth Johnson. Data Coordinating Center: Bradley Skarpness, Jyothi Nagaraja, and Elizabeth Stone. We thank Sangeeta Argrawal and Owen Devine, who provided statistical consultation; Johnni Hutcherson, Treana Johnson-James, and Yvette Dominique, who assisted in manuscript preparation; and Coleen Boyle, who provided scientific input and ongoing support for the study.
The complete Project CHOICES Efficacy Study Group: Centers for Disease Control and Prevention, Atlanta GA: Jon Baio, EdS, Shahul Ebrahim, MD, MPH, R. Louise Floyd, DSN, Jorge Rosenthal, PhD, and Jasjeet S. Sidhu, MD, MPH. Nova Southeastern University, Ft Lauderdale FL: Burt G. Bolton, MS, G. Stephen Bowen, MD, MPH, Liane Dornheim, PhD, Kenneth E. Johnson, DO, Ian T. Kravitz, MS, Linda C. Sobell, PhD, ABPP, and Mark B. Sobell, PhD, ABPP. University of Texas-Houston Health Science Center, Houston TX: Joseph P. Carbonari, EdD, Raul Carvajal, MPH, A. Gaye Cummins, MA, Patricia Dolan Mullen, DrPH, Kirk von Sternberg, PhD, and Mary Marden Velasquez, PhD. Virginia Commonwealth University, Richmond VA: Joseph Borzelleca, Jr., MD, Sherry D. Ceperich, PhD, Karen S. Ingersoll, PhD, Mary Q. Lewis, MS, Sally Brocksen, PhD, Wendy Klein, MD, and Mary D. Nettleman, MD, MS. Battelle Institute: Bradley Skarpness, PhD, Jyothi Nagaraja, MS, and Yvette Dominique, MS. University of Nebraska Medical Center: Sangeeta Argrawal, MS. Drs. Floyd, Sidhu, M. Sobell, L. Sobell, Velasquez, Mullen, Ingersoll, Nettleman, Ceperich, and Mr. Biao served as the primary and coprimary investigators. Interviews were conducted or facilitated at Nova Southeastern University by Rose Ajimatanrareje, Maryse Aupont, Lisa Gonzales, Elizabeth Goodwin, Heather Proctor, Elizabeth Pulliam, Monica Roy, Lisa Smith. Special thanks go to the Nova Southeastern University’s Women Health Center directed by Dr. Kenneth Johnson and the North Broward Hospital District’s primary care clinics for valuable participation in the recruitment of our participants into Project CHOICES. The University of Texas-Houston site acknowledges the assistance of Casey Barton, Carla Garza, Sonya Gonzales-Adams, Kelly Jamison, Chau Pham, Donna Rochon, and Danielle Sorelle-Miner (recruitment, data collection, and follow-up); Matiko Bivins, Stacey Bourland, Mary Elijah, and Nanette Stephens (therapists); Kimberly Lopez and Nancy Meyers (family planning education and services); Kathie Rickman, Michael Seale, and Caroline Zorn-Pickens (coordination at the Door to Recovery, the Harris County Jail, and the Houston Recovery Campus, respectively). Virginia Commonwealth University interviews were conducted by Mary Q. Lewis, and interventions were conducted by Kimberly Karanda, Mary Lee Magee, Jill Clarida, Jessye Cohen, and Tawana Olds. Data was managed by Kristina Hash and Sally Brocksen. VCU investigators thank the staff of the VCU Medical Center OB/GYN clinic for their facilitation of recruitment for the study. Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. TRIAL REGISTRATION: http://www.ClinicalTrials.gov; ID: CDC-NCBDDD-3271.
Funding for this study was provided by the Centers for Disease Control and Prevention.
Footnotes
No financial conflict of interest was reported by the authors of this paper.
References
- 1.American Academy of Pediatrics. Committee on Substance Abuse and Committee on Children with Disabilities Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106:358–61. [PubMed] [Google Scholar]
- 2.Mattson SN, Schoenfeld REP. Teratogenic effects of alcohol on brain and behavior. Alcohol Clin Exp Res. 2001;25:185–91. [PMC free article] [PubMed] [Google Scholar]
- 3.Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet C. 2004;127C:3–9. doi: 10.1002/ajmg.c.30010. [DOI] [PubMed] [Google Scholar]
- 4.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal Alcohol Spectrum Disorder. JAMA. 2003;290:2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 5.Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of Fetal Alcohol Spectrum Disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- 6.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: a summary. Alcohol Res Health. 2001;25:159–67. [PMC free article] [PubMed] [Google Scholar]
- 7.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C. 2004;127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Sociodemographic and behavioral characteristics associated with alcohol consumption during pregnancy—United States, 1988. MMWR Morb Mortal Wkly Rep. 1995;44:261–4. [PubMed] [Google Scholar]
- 9.Floyd RL, Decoufle P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;12:101–7. doi: 10.1016/s0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Alcohol consumption among women who are pregnant or who might become pregnant—United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;44:1178–80. [PubMed] [Google Scholar]
- 11.Henshaw SK. Unintended pregnancy in the United States. Fam Plan Perspect. 1998;30:24–29. 46. [PubMed] [Google Scholar]
- 12.Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plan Perspect. 1999;31:64–72. 93. [PubMed] [Google Scholar]
- 13.Manwell LB, Fleming MF, Mundt MP, Stauffacher EA, Barry KL. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24:1517–24. [PubMed] [Google Scholar]
- 14.Ingersoll KS, Ceperich SD, Nettleman MD, Karanda K, Brocksen S, Johnson BA. Reducing alcohol-exposed pregnancy risk in college women: initial outcomes of a clinical trial of a motivational intervention. Subst Abuse Treat. 2005;29:173–89. doi: 10.1016/j.jsat.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Project CHOICES Intervention Research Group Reducing the risk of alcohol-exposed pregnancies: a study of a motivational intervention in community settings. Pediatrics. 2003;111:1131–5. [PubMed] [Google Scholar]
- 16.Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addictions. 1993;88:315–35. doi: 10.1111/j.1360-0443.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller WR, Rollnick S. Motivational interviewing: preparing people to change. 2nd ed. Guilford Press; New York: 2002. [Google Scholar]
- 18.Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychother Ther Res Practice Train. 1982;19:276–88. [Google Scholar]
- 19.Project CHOICES Research Group Alcohol-exposed pregnancy: characteristics associated with risk. Am J Prev Med. 2002;23:166–73. doi: 10.1016/s0749-3797(02)00495-6. [DOI] [PubMed] [Google Scholar]
- 20.Sobell MB, Sobell LC. Guided self-change treatment for substance abusers. J Cogn Psychother Int Q. 2005;19:199–210. [Google Scholar]
- 21.Streissguth AP, Sampson PD, Olson HC, et al. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring—a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–18. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson JL, Jacobson SW, Sokol RJ, Ager JW., Jr. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–51. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 23.Konovalov HV, Kovetshy NS, Bobryshev YV, Ashwell KW. Disorders of brain development in the progeny of mothers who used alcohol during pregnancy. Early Hum Dev. 1997;48:153–66. doi: 10.1016/s0378-3782(96)01848-8. [DOI] [PubMed] [Google Scholar]
- 24.Gunzerath L, Faden V, Zakhari S, Warren K. National Institute on Alcohol Abuse and Alcoholism report on moderate drinking. Alcohol Clin Exp Res. 2004;28:829–47. doi: 10.1097/01.alc.0000128382.79375.b6. [DOI] [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. Humana Press; Clifton NJ: 1992. [Google Scholar]
- 26.Sobell LC, Sobell MB. Alcohol consumption measures. 2nd ed. National Institute on Alcohol Abuse and Alcoholism; Rockville MD: 2003. [Google Scholar]
- 27.Sobell LC, Sobell MB. Alcohol timeline followback (TLFB) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 28.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–9. [PubMed] [Google Scholar]
- 29.Derogatis LR. Brief Symptom Inventory: administration, scoring and procedures manual. National Computer Systems, Inc.; Minneapolis MN: 1993. [Google Scholar]
- 30.Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. Stud Alcohol. 1997;58:365–71. doi: 10.15288/jsa.1997.58.365. [DOI] [PubMed] [Google Scholar]
- 31.Neiman L, Velasquez MM, Groff JY, Cheng L, Foxhall LE. Implementation of a smoking cessation counseling module in a preceptorship program. J Fam Med. 2005;37:105–11. [PubMed] [Google Scholar]
- 32.King TK, DiClemente CC. A decisional balance measure for not seeking treatment in a general population sample; Association for advancement of behavior therapy meeting; Atlanta GA. 1993. [Google Scholar]
- 33.Grimley DM, Prochaska GE, Prochaska JO, et al. Cross-validation of measures assessing decisional balance and self-efficacy for condom use. Am Health Behav. 1996;20:406–16. [Google Scholar]
- 34.Prochaska JO, Velicer WF, DiClemente CC, Fava J. Measuring processes of change: application to the cessation of smoking. Consult Clin Psychol. 1988;56:520–8. doi: 10.1037//0022-006x.56.4.520. [DOI] [PubMed] [Google Scholar]
- 35.Breslin FS, Sobell LC, Sobell MB, Agrawal S. A comparison of a brief and long version of the Situational Confidence Questionnaire. Behav Res Ther. 2000;38:1211–20. doi: 10.1016/s0005-7967(99)00152-7. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention Motivational intervention to reduce alcohol-exposed pregnancies—Florida, Texas, and Virginia, 1997–2001. MMWR Morb Mortal Wkly Rep. 2003;52:441–4. [PubMed] [Google Scholar]
- 37.Sobell LC, Agrawal S, Sobell MB, et al. Responding to an advertisement: a critical event in promoting self-change of drinking behavior; 37th annual meeting of the Association for Advance of Behavior Therapy; Boston MA. 2003. [Google Scholar]
- 38.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1030–45. [PubMed] [Google Scholar]
- 39.Spivak K, Sanchez-Craig M, Davila R. Assisting problem drinkers to change on their own: effect of specific and non-specific advice. Addictions. 1994;89:1135–42. doi: 10.1111/j.1360-0443.1994.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 40.Borenstein M, Rothstein H, Cohen J. Development funded by the National Institutes of Health. Computer Software; Rockville MD: 1997. Power and precision, release 1.20. [Google Scholar]
- 41.Gillings D, Koch G. The application of the principle of intent-to-treat to the analysis of clinical trials. Drug Inf J. 1991;25:411–25. [Google Scholar]
- 42.Diggle PJ, Kung-Yee L, Seger SL. Analysis of longitudinal data. Oxford University Press; New York: 1994. [Google Scholar]
- 43.SAS Institute Inc. SAS users guide. 9.1 ed. SAS Institute Inc.; Cary SC: 2006. [Google Scholar]
- 44.Kleinbaum DG, Klein M. Logistic regression: a self-learning text. 2nd ed. Springer-Verlag; New York: 2002. [Google Scholar]
- 45.Miller WR, Wilbourne PL, Hettema JE. A summary of alcohol treatment research. 3rd ed. Allyn and Bacon Publishing; Boston MA: 2003. What works? [Google Scholar]
- 46.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. Consult Clin Psychol. 2003;71:843–61. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 47.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S Preventive Services Task Force. Ann Intern Med. 2004;140:557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 48.Ernest CC, Grant TM, Streissguth AP, Sampson PD. Intervention with high-risk alcohol and drug-abusing mothers: II. Three-year findings from the Seattle Model of paraprofessional advocacy. Community Psychol. 1999;12:19–38. [Google Scholar]
- 49.Chang G, Wilkins-Haug BS, Goetz MA. Brief interventions for alcohol use in pregnancy: a randomized trial. Addiction. 1999;94:1499–508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- 50.Chang G, McNamara TK, Orav EJ, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105:991–8. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richmond R, Healther N, Wodak A, Kehoe L, Webster I. Controlled evaluation of a general practice-based brief intervention for excessive drinking. Addiction. 1995;90:119–32. doi: 10.1046/j.1360-0443.1995.90111915.x. [DOI] [PubMed] [Google Scholar]
- 52.Wampold BE, Minami T, Tierney SC, Baskin TW, Ghati KS. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. Clin Psychol. 2005;61:835–54. doi: 10.1002/jclp.20129. [DOI] [PubMed] [Google Scholar]
- 53.Midanik L. The validity of self-reported alcohol consumption and alcohol problems: a literature review. Br Addict. 1982;77:357–82. doi: 10.1111/j.1360-0443.1982.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 54.Del Boca FK, Babor TF, McRee B. Reliability enhancement and estimation in multisite clinical trials. Stud Alcohol. 1994;(suppl 12):130–6. doi: 10.15288/jsas.1994.s12.130. [DOI] [PubMed] [Google Scholar]
- 55.Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: measuring drinking outcomes in clinical trials. Stud Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 56.Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(suppl 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 57.Connors GJ, Maisto SA. Drinking reports from collateral individuals. Addiction. 2003;98(suppl 2):21–9. doi: 10.1046/j.1359-6357.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- 58.Poikolainen K, Podkeltnova I, Alho H. Accuracy of quantity-frequency and graduated frequency questionnaires in measuring alcohol intake: comparison with daily diary and commonly used laboratory markers. Alcohol Alcohol. 2002;37:573–6. doi: 10.1093/alcalc/37.6.573. [DOI] [PubMed] [Google Scholar]
- 59.Kazdan AE. Research design in clinical psychology. 4th ed. Allyn and Bacon; Needham Heights MA: 2002. [Google Scholar]
- 60.Wutze SE, Sheill A, Gomel MK, Conigrave KM. Cost effectiveness of brief interventions for reducing alcohol consumption. Soc Sci Med. 2001;52:863–70. doi: 10.1016/s0277-9536(00)00189-1. [DOI] [PubMed] [Google Scholar]
- 61.Fleming MF, Mundt MP, French MT, Manwell B, Stauffacher EA, Barry KL. Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Med Care. 2000;38:7–18. doi: 10.1097/00005650-200001000-00003. [DOI] [PubMed] [Google Scholar]