Abstract
Purkinje cell dendrites are excitable structures with intrinsic and synaptic conductances contributing to the generation and propagation of electrical activity. Voltage-gated potassium channel subunit Kv3.3 is expressed in the distal dendrites of Purkinje cells. However, the functional relevance of this dendritic distribution is not understood. Moreover, mutations in Kv3.3 cause movement disorders in mice and cerebellar atrophy and ataxia in humans, emphasizing the importance of understanding the role of these channels. In this study, we explore functional implications of this dendritic channel expression and compare Purkinje cell dendritic excitability in wild-type and Kv3.3 knockout mice. We demonstrate enhanced excitability of Purkinje cell dendrites in Kv3.3 knockout mice, despite normal resting membrane properties. Combined data from local application pharmacology, voltage clamp analysis of ionic currents, and assessment of dendritic Ca2+ spike threshold in Purkinje cells suggest a role for Kv3.3 channels in opposing Ca2+ spike initiation. To study the physiological relevance of altered dendritic excitability, we measured [Ca2+]i changes throughout the dendritic tree in response to climbing fiber activation. Ca2+ signals were specifically enhanced in distal dendrites of Kv3.3 knockout Purkinje cells, suggesting a role for dendritic Kv3.3 channels in regulating propagation of electrical activity and Ca2+ influx in distal dendrites. These findings characterize unique roles of Kv3.3 channels in dendrites, with implications for synaptic integration, plasticity, and human disease.
INTRODUCTION
Decades of recordings from cerebellar Purkinje cells (PCs) have revealed highly organized patterns of intrinsic excitability (Khavandgar et al. 2005; Llinas and Sugimori 1980a,b; McKay and Turner 2004; Raman and Bean 1997; Stuart and Häusser 1994; Swensen and Bean 2003; Womack and Khodakhah 2002, 2004). PC somata and axons express voltage-gated Na+ channels that generate fast action potentials that are transmitted out of the cerebellar cortex. The dendritic tree lacks Na+ channels yet expresses voltage-gated Ca2+ channels that mediate dendritic spiking. Dendritic Ca2+ influx is important for both electrical and chemical signaling within PCs; dendritic Ca2+ spikes influence axonal firing and therefore the output of PCs (Davie et al. 2008; Llinas and Sugimori 1980a,b), and Ca2+ as a second messenger affects synaptic plasticity of parallel fiber inputs (Konnerth et al. 1992; Linden and Connor 1993). Whereas synaptic inhibition mediated by molecular layer interneurons has been shown to play a prominent role in determining the spatial extent of dendritic Ca2+ influx (Callaway et al. 1995; Llinas et al. 1968), less is known about the contributions of intrinsic dendritic conductances (Rancz and Häusser 2006).
The importance of voltage-gated potassium channel subunit Kv3.3 to cerebellar function is supported by observations in humans and mice. It was recently demonstrated that mutations in the Kv3.3 coding sequence are a cause of spinocerebellar ataxia (SCA13) in humans (Figueroa et al. 2010; Waters et al. 2006). Individuals with these mutations experience childhood- or adult-onset ataxia, and MRI scans from ataxic patients show gross cerebellar atrophy (Herman-Bert et al. 2000; Waters et al. 2006). Kv3.3 KO mice have been generated and show altered motor phenotypes (Joho et al. 2006). It is not yet known how altered Kv3.3 activity causes cerebellar atrophy and ataxia in humans or motor deficiencies in mice (Joho and Hurlock 2009). Altered Ca2+ homeostasis in PCs is implicated in the pathophysiology of other spinocerebellar ataxias (Duenas et al. 2006; Lin et al. 2000), highlighting the importance of understanding the contributions of Kv3.3 channels to PC Ca2+ signaling.
Kv3.3 subunits are expressed throughout mouse brain yet are dominantly expressed in cerebellar PCs (Chang et al. 2007). Moreover, PCs are one of the few cells types in which Kv3.3 subunits are expressed without significant expression of other Kv3 subunits [(Chang et al. 2007; Weiser et al. 1994; but see Martina et al. 2003; Sacco et al. 2006)], suggesting a potential lack of molecular redundancy in these cells. Kv3.3 subunits are expressed robustly in PC somata, axons, and distal dendrites (Chang et al. 2007; Martina et al. 2003; McMahon et al. 2004). PCs lacking Kv3.3 subunits display altered tonic Na+ spiking and complex spike waveforms (Akemann and Knopfel 2006; Hurlock et al. 2008; McMahon et al. 2004; Zagha et al. 2008). Dendritic excitability in Kv3.3 KO PCs has not been studied and may be important for understanding mechanisms of electrical signaling in PC dendrites as well as providing insights into cerebellar dysfunction in humans and mice with Kv3.3 mutations.
In this paper, we compare electrical properties of wild-type and Kv3.3 KO PCs. We find that Kv3.3 KO PCs have enhanced dendritic excitability due to lack of perithreshold-operating dendritic Kv3.3 channels. Moreover, using Ca2+ imaging we show that the spatial dynamics of Ca2+ influx is altered in Kv3.3 KO PCs with enhanced climbing fiber induced Ca2+ transients in distal dendrites. These data indicate that Kv3.3 proteins are important modulators of Ca2+ signaling in PC dendrites and suggest additional cellular mechanisms for how altered Kv3.3 function can cause human and mouse movement disorders.
METHODS
Acute slice preparation
Slice preparation and recordings were carried out as previously described (Zagha et al. 2008). Wild-type and Kv3.3 KO littermates, postnatal days 15–21, were anesthetized with pentobarbital and decapitated following loss of pain reflexes. Brains were rapidly removed and bathed in ice-cold artificial cerebrospinal fluid (ACSF) (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 MgSO4, 2 CaCl2, and 20 glucose, pH 7.4, saturated with 95% O2-5% CO2. Sagittal slices (350 μm thick) of cerebellar vermis were prepared using a Vibratome 3000 and incubated in a submerged holding chamber for ≥1 h before transfer to a submersion-type recording chamber. The chamber was superfused at a rate of 2–3 ml/min with ACSF. Recordings were conducted at room temperature (voltage clamp, dual soma-dendrite recordings) or 32°C (current clamp and Ca2+ imaging), controlled by an inline solution heater (Warner Instruments).
Preparation of Chinese hamster ovary (CHO) cells expressing Kv3.3 proteins
Heterologous cells were prepared according to previously described protocols (Zagha et al. 2005). The Kv3.3 cDNA used is the mouse homologue of the rat splice variant called Kv3.3El [AY822674]. The same recording conditions were established for heterologous cells as for acute brain slices.
Electrophysiological recordings
Recording pipettes of 2–5 MΩ were made from borosilicate glass using a horizontal pipette puller (Sutter Instruments, Model P-97) and filled with a K+-gluconate based internal solution (in mM): 144 K+-gluconate, 0.2 EGTA, 3 MgCl2, 10 HEPES, 4 MgATP, and 0.5 NaGTP (pH adjusted to 7.4 with KOH). For Ca2+ imaging experiments, EGTA was replaced with 0.15 bis-fura-2 (see following text). Recordings in whole cell configuration were obtained from visually targeted PC somata or dendrites. For somatic patches, a stable whole cell recording was established, and then the electrode was slowly retracted from the slice. Capacitance of outside-out patches were ∼400 fF as determined by on-line analysis of transient currents (pClamp9.2 software). Currents were recorded with an Axopatch 200B amplifier (Molecular Devices) in voltage clamp mode, low-pass-filtered at 2 kHz using an eight-pole Bessel filter (Frequency Devices), and digitized at 2.5 kHz. Series resistance was partially compensated (60–70%). Voltage clamp records were analyzed if access resistance was <15 MΩ and holding current did not change >10% during the course of the experiment.
Transmembrane voltage was recorded in bridge mode using an Axoclamp 2B or fast current clamp mode using an Axopatch 200B (Molecular Devices), sampled at 20 kHz, and filtered at 10 kHz. Access resistance was monitored on break-in and bridge balance was maintained throughout the experiment. All PCs were spontaneously active in acute slice recordings, so negative current was applied through the somatic recording electrode to silence the cells and maintain a resting membrane potential near −65 mV. Holding currents were similar between wild-type (WT) and Kv3.3 KO PCs (WT 576 ± 136 pA, Kv3.3 KO 520 ± 118 pA, P = 0.46). pClamp9.2 software was used for data acquisition and analysis was performed with Clampfit9.2 and OriginPro7.0 software.
Ca2+ measurements
Ca2+ measurements from different regions of Purkinje cells were made using previously described techniques, e.g., (Nakamura et al. 1999). Briefly, a Photometrics (Tucson, AZ) Quantix cooled CCD camera, operated in the frame transfer mode, was mounted on the camera port of the microscope. Custom software (original version described in Lasser-Ross et al. 1991) controlled readout parameters and synchronization with electrical recordings. A second custom program was used to analyze and display the data. Pixels were binned in the camera to allow frame rates of 66 Hz. Cells were injected with the Ca2+ indicator bis-fura-2 via the recording pipette. The internal solution contained (mM): 145 potassium gluconate, 4 NaCl, 4 Mg-ATP, 0.3 Na-GTP, 14 Na-phosphocreatine, 0.15 bis-fura-2, and 10 HEPES, pH adjusted to 7.3 with KOH. Final osmolarity was 297 mOsm. Fluorescence changes of bis-fura-2 were measured with single wavelength excitation (382 ± 10 nm) and emission, 455 nm. The fluorescence changes resulted from CF synaptic stimulation and were induced by 200 μs pulses with glass electrodes placed on the granule cell layer of the slice ∼10–50 μm from the Purkinje cell layer. These stimulating electrodes were low resistance patch pipettes (<10 MΩ) filled with ACSF. [Ca2+]i changes are expressed as ΔF/F where F is the fluorescence intensity when the cell is at rest and ΔF is the change in fluorescence during activity. In the Purkinje cells, [Ca2+]i increases were examined over a range of 140 μm with a ×60 lens used in these experiments. Increases in different parts of the cell are displayed using either selected regions of interest (ROIs) or a pseudo “line scan” display.
Drug application
Unless otherwise noted, drugs were applied by superfusion of the recording chamber (bath). Current clamp recordings of intrinsic excitability were carried out in the presence of synaptic blockers 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 10 μM) and gabazine (10 μM). For selective drug perfusion, glass micropipette pressure pulses controlled by Picospritzer III (Parker Hannifin) locally applied 1 mM TEA to the soma or dendrites of PCs for 2 s prior to somatic current injection, continuing for 1 s into the stimulation. For further methods and validation of this local perfusion technique, see (Zagha et al. 2008). CNQX, gabazine, TTX, and ZD7288 were purchased from Tocris Cookson, iberiotoxin was from Alomone Labs, and all other salts and reagents were from Sigma-Aldrich.
Impedance protocol and data analysis
To measure subthreshold resonance, a Zap protocol was implemented (Pike et al. 2000). The injected current consisted of repeating sinusoids of constant amplitude, linearly increasing in frequency between 0 and 25 Hz over 20 s. To determine impedance values, the fast Fourier transform (FFT) of the voltage response was divided by the FFT of the injected current using the power spectrum analysis procedures included in pClamp9.2 software. The square root of the amplitude of the real component yielded the impedance in MΩ. For individual PCs, the impedance-frequency profile as it approached 0 Hz well matched the steady-state apparent membrane resistance for square pulse current injections. Steepness of frequency preference was determined by dividing the impedance at the preferred frequency by the impedance at 0.5 Hz, which is referred to as Q value.
Ca2+ spike height was measured from the plateau potential to peak of spike, whereas spike amplitude was measured from spike peak to the bottom of the AHP. Both measurements were obtained from spikes in response to just suprathreshold current injections using square pulse protocols. Voltage threshold of Ca2+ spiking was determined both by visual inspection of the inflection point and as the voltage at which the first derivative crossed 2 mV/ms; both approaches produced similar results. Na+ spike voltage threshold was determined as the voltage at which the first derivative crossed 20 mV/ms. For analysis of ionic currents, activation curves were fit with Boltzmann functions: 1 − 1/{1 + exp[(Vm − V1/2)/k]}, where Vm is the membrane potential, V1/2 is the potential at which the function is 0.5, and k is the slope factor.
Mean values are reported with their SD. Student's t-test, paired or unpaired as appropriate, was used for comparisons of population means.
RESULTS
Specific changes in dendritic excitability and Ca2+ spiking in Kv3.3 KO PCs
Prolonged, large amplitude current injections into PC somata induce repetitive Na+ spiking generated at the axosomatic region (somatic spikes) followed by repetitive Na+-Ca2+ spike complexes (spike-bursts) initiated as Ca2+ spikes in dendrites (Llinas and Sugimori 1980a,b). We conducted whole cell recordings in current clamp configuration from PC somata in acute slices from the mouse cerebellar vermis. To isolate the effects of Kv3.3 genetic manipulation on dendritic excitability, somatic spiking was eliminated using the Na+ channel blocker TTX (100 nM). Dendritic spiking was evoked by square pulse and slow ramp somatic current injections (Fig. 1), and Ca2+ spike properties were compared between WT and Kv3.3 KO PC recordings.
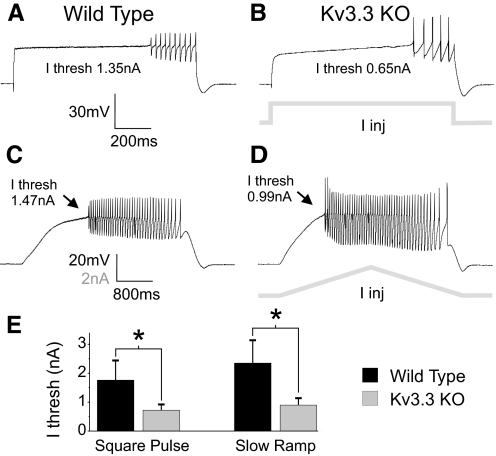
Fig. 1.
Enhanced dendritic excitability in Kv3.3 KO Purkinje cells (PCs). A and B: in the presence of Na+ channel blocker TTX (100 nM) square pulse somatic current injections were applied to evoke dendritic Ca2+ spikes. Recordings display Ca2+ spiking at threshold for a wild-type (A) and Kv3.3 KO (B) PCs. In these examples, current threshold was 1.35 nA in the wild-type recording and 0.65 nA in the Kv3.3 KO recording. C and D: Ca2+ spikes elicited by long duration (4 s) current ramps. Current threshold was determined as the current amplitude at the time of spiking onset and was 1.47 nA for wild-type (C) and 0.99 nA for Kv3.3 KO (D) recordings in these examples. Protocols are demonstrated in gray beneath voltage traces in B and D. E: population data comparing Ca2+ spike current threshold from wild-type (n = 7) and Kv3.3 KO (n = 7) PCs for square pulse and slow ramp protocols. Asterisks denotes statistical significance (P < 0.01); error bars are SD. I thresh, current threshold; I inj, current injection.
While PCs of both genetic backgrounds generated repetitive Ca2+ spikes, much less current was required to induce spiking in Kv3.3 KO (n = 7) compared with WT (n = 7) PCs. Significant reductions in current threshold were found for both square pulse (WT 1.76 ± 0.68 nA, Kv3.3 KO 0.72 ± 0.20 nA, 59% reduction, P = 0.002) and slow ramp protocols (WT 2.35 ± 0.79 nA, Kv3.3 KO 0.90 ± 0.24 nA, 62% reduction, P = 0.002; Fig. 1E). This >1 nA reduction in current threshold for dendritic spiking suggests a significant increase in dendritic excitability in Kv3.3 KO PCs.
Additional Ca2+ spike properties were compared between WT and Kv3.3 KO PCs. Ca2+ spike height (from plateau to peak) and spike amplitude (from peak to AHP) were both significantly larger in Kv3.3 KO PCs (height: WT 9.2 ± 1.8 mV, Kv3.3 KO 20.4 ± 3.8 mV, P < 0.001; amplitude: WT 21.0 ± 4.5 mV, Kv3.3 KO 42.2 ± 5.1 mV, P < 0.001; n = 7 for WT and Kv3.3 KO), suggesting that Kv3.3 channels have a role in Ca2+ spike repolarization.
While there were quantitative differences in dendritic spike properties, PC dendrites of both genetic backgrounds shared a similar excitability structure. As demonstrated in response to slow ramp protocols (Fig. 1, C and D), WT and Kv3.3 KO PC Ca2+ spiking displayed type 2 behavior in that spiking initiated at a nonzero frequency (Rinzel and Ermentrout 1989) and showed hysteresis such that much lower firing frequencies were observed on the downward slope of ramp current injection. Such responses to current ramps are suggestive of a region of bistablility between spiking and nonspiking regimes (Izhikevich 2007), and this spiking behavior was evident in all WT and Kv3.3 KO recordings.
Global subthreshold properties are not altered in Kv3.3 KO PCs
Enhanced dendritic excitability in Kv3.3 KO PCs was surprising because it is widely considered that Kv3 channels selectively contribute to suprathreshold events in spiking neurons due to their depolarized voltage dependence (Rudy and McBain 2001). One possible explanation for the observed changes is an increase in global excitability secondary to genetic manipulation. To test this, various protocols were employed to compare subthreshold properties of WT and Kv3.3 KO PCs (Fig. 2). Apparent membrane resistance, which is sensitive to somatic and dendritic membrane properties (Bekkers and Häusser 2007; Rall 1959, 1969), was determined for both populations. From a resting membrane potential of −65 mV, small hyperpolarizing and depolarizing current steps were applied. A large sag in Vm was observed in both WT and Kv3.3 KO PCs (Fig. 2, A and B). The sag was eliminated by the hyperpolarization-activated cation current (Ih) blocker ZD 7288 (20 μM; n = 2; data not shown), consistent with previous studies of Ih in PCs (Crepel and Penit-Soria 1986; Roth and Häusser 2001). Linear fits of peak and steady state Vm deflections versus injected current were similar for Kv3.3 KO and WT controls (peak: WT 34.1 ± 5.9 MΩ, Kv3.3 KO 36.5 ± 3.5 MΩ, P = 0.4; steady state: WT 17.5 ± 6.5 MΩ, Kv3.3 KO 22.1 ± 4.1 MΩ, P = 0.17; Fig. 2, C–E), indicating similar apparent membrane resistance for both cell types.
Fig. 2.
Apparent membrane resistance and subthreshold resonance unaltered in Kv3.3 KO PCs. A and B: from a resting membrane potential of −65 mV, current steps were applied from −500 to +200 pA in 100 pA increments to determine apparent membrane resistance (Rm) in wild-type (A) and Kv3.3 KO (B) PCs. A large sag was evident on onset and offset of current injections in PCs from both genetic backgrounds. C and D: voltage deflections for both peak and steady-state (SS) responses were plotted against magnitude of current injection. Linear fits of the voltage-current relationship were used to generate peak and SS apparent Rm. For this pair, wild-type peak Rm was 30.8 MΩ and SS Rm was 14.8 MΩ; Kv3.3 KO peak Rm was 32.7 MΩ and SS Rm was 18.8 MΩ. E: population data, demonstrating no significant difference in apparent peak or SS Rm between wild-type (n = 5) and Kv3.3 KO (n = 7) PCs. F and G: sinusoidal current injections of constant amplitude and increasing frequency (Zap protocol) were applied to the soma to determine subthreshold resonance properties. Both wild-type (F) and Kv3.3 KO (G) PCs displayed robust nonzero frequency preference to the sinusoidal inputs. H: impedance profiles for the wild-type and Kv3.3 KO recordings presented above. The wild-type PC had a preferred frequency of 3.9 Hz and Q value of 2.44, whereas the Kv3.3 KO PC had a preferred frequency of 4.4 Hz and a Q value of 2.8. I: average impedance profiles for wild-type (n = 5) and Kv3.3 KO (n = 7) PCs.
Impedance at subthreshold potentials was also measured for WT and Kv3.3 KO PCs using the Zap function (Hutcheon and Yarom 2000; Pike et al. 2000) (Fig. 2, F and G). Impedance profile has been shown to be sensitive to cable structure (Eisenberg 1967; Moore and Christensen 1985; Moore et al. 1993) and samples somato-dendritic passive membrane properties and voltage-dependent conductances that are active at these subthreshold potentials. WT PCs showed robust subthreshold resonance near 4 Hz (peak frequency: 4.0 ± 0.4 Hz; Q value: 2.4 ± 0.5; n = 5). Kv3.3 KO PCs also showed robust resonance with a similar preferred frequency (peak frequency: 3.8 ± 0.6 Hz, P = 0.52; Q value: 2.0 ± 0.4, P = 0.14; n = 7) and similar impedance profile (Fig. 2I), suggesting lack of significant changes in dendritic structure in Kv3.3 KO PCs. We also found that there were no changes in Na+ spike current threshold (data not shown) or voltage threshold (WT: −44.5 ± 3.5 mV, n = 5; Kv3.3 KO: −42.6 ± 7.5 mV, n = 6; P = 0.6) between WT and Kv3.3 KO PCs. The preceding findings indicate that subthreshold somato-dendritic properties are similar between WT and Kv3.3 KO PCs, and thus global changes in excitability cannot explain enhanced dendritic excitability in Kv3.3 KO PCs.
Acute block of dendritic Kv3 channels enhances dendritic excitability
If changes in dendritic excitability in Kv3.3 KO PCs are due to reduced Kv3 conductance, then acute block of Kv3 channel activity should similarly affect dendritic spiking in WT mice. In normal PCs, current threshold for evoking Ca2+ spikes was measured before and after bath application of 1 mM TEA. At this concentration, TEA blocks 80–90% of Kv3 channel activity (Rudy et al. 1999). Current threshold was highly reduced in the presence of TEA (wt: 2.08±0.81 nA, TEA: 0.47±0.16 nA, 74% reduction, P < 0.05, n = 3), comparable to the effects of genetic ablation, suggesting that acute block of Kv3 channels also reduces current threshold for evoking Ca2+ spikes. As BK Ca2+-activated K+ channels are also sensitive to TEA, we tested whether the effect of TEA was due to the block of these channels. Bath application of BK channel blocker iberiotoxin (100 nM) (Womack and Khodakhah 2002), while causing a depolarization of the plateau potential (8.7 ± 6.1 mV, P < 0.05, n = 4), did not significantly affect current threshold (P = 0.55). Moreover, subsequent bath application of 1 mM TEA in the presence of iberiotoxin significantly reduced current threshold (65 ± 9% reduction, P = 0.001, n = 4), suggesting that the large effect of TEA is unlikely due to the manipulation of BK channel activity.
We then used a selective drug targeting approach to determine the specific contribution of dendritically located Kv3 channels to PC dendritic excitability (Fig. 3). Prolonged, moderate amplitude current injections (3 s, 0.8 nA) were applied that were below threshold for dendritic spiking. TEA (1 mM) was locally applied to the molecular layer of the cerebellar cortex, to target PC dendrites, prior to current injection (see methods). Dendritic perfusion of TEA resulted in generation of repetitive spike-burst activity (n = 5; Fig. 3). Selective somatic perfusion of TEA significantly altered Na+ spike properties but did not induce spike-burst firing (data not shown). These data suggest that acute block of Kv3 channel activity, specifically in dendrites, results in enhanced dendritic excitability similar to what is observed in Kv3.3 KO PCs.
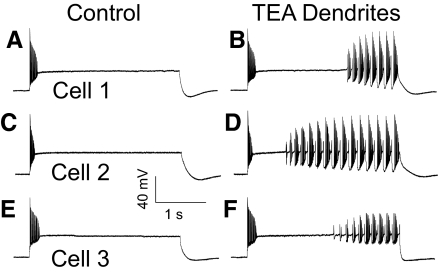
Fig. 3.
Acute block of dendritic Kv3 activity increases dendritic excitability. A: square pulse somatic current injections were applied to wild-type PCs below threshold to induce dendritic Ca2+ spikes and spike bursts. In contrast to experiments in Fig. 1, TTX was not used in these recordings. In the control trace, without drug application, there was onset of repetitive somatic spikes followed by somatic spike failure and a plateau potential (McKay et al. 2005). B: same cell as in A with same amplitude current injection, in response to local dendritic application of 1 mM TEA. This pharmacological approach resulted in the emergence of repetitive dendritic spiking during the plateau. C and E: control responses from 2 additional cells, also demonstrating the generation of dendritic spiking (D and F) following local dendritic application of TEA. Scale bar applies to all panels.
Reduced outward currents in Kv3.3 KO PCs
If enhanced dendritic excitability in Kv3.3 KO PCs is due to lack of a perithreshold operating Kv3 conductance, one would expect a reduction in voltage-dependent outward currents near Ca2+ spike threshold. To test this hypothesis, we recorded ionic currents from WT and Kv3.3 KO patches from acute cerebellar slices to compare current magnitudes and properties from these two cells (Fig. 4). Outside-out patches from WT PCs displayed large outward, voltage-dependent currents (Fig. 4A). TEA at 1 mM concentration blocked 87% of this current, consistent with the pharmacological properties of Kv3 channels (Rudy et al. 1999). Recordings from Kv3.3 KO patches showed a marked reduction in current amplitude (Fig. 4D). On average, this corresponded to a 69% reduction in voltage-dependent outward currents (WT: 1.93 ± 0.60 nA at +40 mV; Kv3.3 KO: 0.59 ± 0.31 nA: P = 0.001; Fig. 4E).
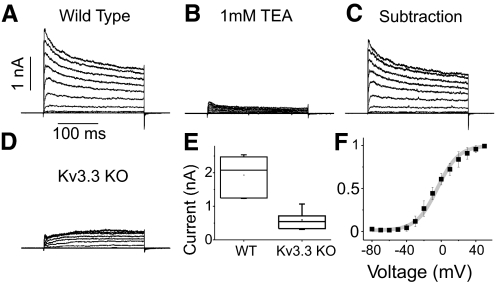
Fig. 4.
Reduction of outward currents in Kv3.3 KO patches. A–D: voltage-dependent currents in response to 10 mV step depolarizations from −80 to +40 mV from outside-out somatic patches from wild-type and Kv3.3 KO PCs. Large outward currents in patches from wild-type PCs (A) were significantly reduced on application of 1 mM TEA (B). C: subtraction of B from A shows currents sensitive to 1 mM TEA. D: voltage clamp recordings from a Kv3.3 KO somatic outside-out patch showing highly reduced outward currents. Scale bars in A refer to traces in A-D. E: box plot of peak currents at +40 mV from wild-type (n = 7) and Kv3.3 KO (n = 5) recordings. F: normalized conductance-voltage curve for TEA-sensitive currents from wild-type PCs (n = 6). Gray line is a Boltzmann fit: V1/2, −4.3 mV; k, 12.2. Error bars are SD.
TEA-sensitive Kv3-like currents in WT PCs activated robustly at voltage steps to −30 or −20 mV (Fig. 4F). G-V plots of these currents were well fit with Boltzmann functions (V1/2, −4.3 ± 2.1 mV; k, 12.2 ± 1.8; n = 6). Outward currents had fast activation and deactivation rates [deactivation time constant (tau) was 1 ms at −70 mV; Supplementary Fig. S11], consistent with kinetic properties of Kv3 channels (Rudy and McBain 2001). The voltage-dependence and kinetic properties reported here are within the range of previous measurements of Kv3-like currents in PCs (Martina et al. 2003, 2007; McKay and Turner 2004; Sacco et al. 2006).
Voltage threshold for Ca2+ spikes were between −35 and −30 mV as measured at the soma for WT and Kv3.3 KO PCs, determined from both square pulse and slow ramp protocols (square pulse: WT −33.3 ± 3.8 mV, Kv3.3 KO −31.7 ± 1.6 mV, P = 0.34; slow ramp: WT −34.2 ± 4.9 mV, Kv3.3 KO −30.0 ± 2.9 mV, P = 0.1). These voltages are just at the onset of robust Kv3 activation as measured in voltage clamp recordings. However, the membrane potential is likely to be more depolarized in the dendrites where Ca2+ spikes originate, as dendritic plateau potentials and spikes are supported by depolarizing Ca2+ currents in that region, with anticipated voltage attenuation into the soma where this current is less prominent (De Schutter and Bower 1994; Lev-Ram et al. 1992; Llinas and Sugimori 1980a).
To measure local voltage threshold for Ca2+ spikes in dendrites more directly, we recorded in whole cell configuration simultaneously from the soma and dendrite of a PC. Dendritic spikes were activated in response to square pulse current injections (Supplementary Fig. S2). With somatic or dendritic stimulation, the somatic voltage recordings were approximately −35 mV during the plateau and −30 mV at Ca2+ spike threshold, similar to values obtained from somatic recordings alone (see preceding text). However, in the same sets of experiments, the dendritic voltages were approximately −24 mV during the plateau and −17 mV at spike threshold. These dendritic voltages are considerably more depolarized than measurements from somatic recordings, and well within the range of robust activation of Kv3 channels in PCs (Fig. 4F).
Enhanced spatial dynamics of climbing fiber evoked Ca2+ signaling in Kv3.3 KO PCs
The preceding experiments demonstrate enhanced intrinsic excitability of PC dendrites in Kv3.3 KO mice due to lack of Ca2+ spike perithreshold operating dendritic Kv3.3 channels. As PCs physiologically generate dendritic Ca2+ spikes in response to climbing fiber (CF) activation, we hypothesized that dendritic Ca2+ signaling may be augmented in Kv3.3 KO PCs in response to this synaptic input. To test this, we filled PCs with the Ca2+ indicator bis-fura 2 and measured changes in fluorescence in response to CF stimulation. Stimulation produced all-or-none supra-threshold electrical responses. Figure 5 shows representative Ca2+ responses from a WT and Kv3.3 KO PC in response to CF stimulation. WT PCs displayed large Ca2+ transients localized to proximal dendritic regions, consistent with previous measurements in guinea pig PCs (Miyakawa et al. 1992). In contrast, Ca2+ transients in Kv3.3 KO PCs were consistently robust in both proximal and distal dendritic regions throughout the dendritic tree (Fig. 5B).
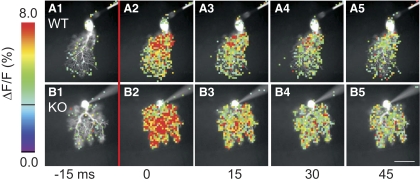
Fig. 5.
Enhanced synaptically evoked Ca2+ transients in Kv3.3 KO PC dendrites. Ca2+ signals from wild-type (A) and Kv3.3 KO (B) PCs captured in 15 ms frames before and after climbing fiber stimulation. Time indicators below images are in reference to CF stimulation with A2 and B2 acquired immediately after stimulation. Signals are represented as percent change in fluorescence over total fluorescence (ΔF/F) with warmer colors indicating larger signals (left). In these images, signals were masked out from locations where the resting fluorescence intensity (F) was <3.5% of the maximum fluorescence in the cell and from all locations where ΔF/F was below a minimum threshold of 3% (black line on color scale). In both cells, climbing fiber stimulation produced Ca2+ transients extending throughout the dendritic tree. However, in the wild-type PC, prominent Ca2+ signals were mostly limited to proximal dendritic regions, whereas robust signals in the Kv3.3 KO PC appeared to extend throughout the dendritic tree. Scale bar in (B5) is 50 μm.
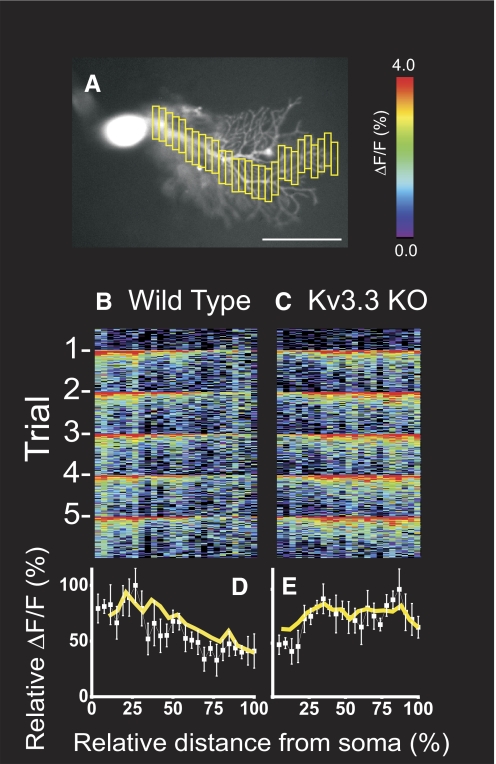
Line scan analyses were conducted from the fluorescence imaging data through the long axis of the dendritic tree following contiguous dendritic branches from proximal to terminal dendrites (Fig. 6). At each dendritic location, peak Ca2+ signals were averaged from five climbing fiber responses and normalized to the overall largest Ca2+ signal within the cell. In WT PCs, Ca2+ signals peaked proximally and substantially tapered in distal dendrites (Fig. 6D). In contrast, in Kv3.3 KO PCs robust Ca2+ signals were maintained throughout the dendritic arborization (Fig. 6E).
Fig. 6.
Line scan analysis of Ca2+ signals in proximal and distal dendrites of Kv3.3 KO PCs. A: example of line scan analysis in which relative fluorescence changes were quantified within successive dendritic segments (yellow boxes) extending from the junction with the soma to terminal dendrites. Scale bar is 50 μm. B and C: line scan analysis of Ca2+ signals from PCs oriented from proximal to distal dendrites. Abscissa is relative position along a continuous dendritic projection from junction with the soma (0) to most distal branches (100); ordinate is time in 15 ms measurements progressing from top to bottom of the panel. Five stimuli at 1 Hz were given over the course of the experiment. The warmer pixels occurred at the times of CF stimulation. D and E: plots of Ca2+ signals vs. distance along the dendrites showing the average of trials for the PCs depicted above (white boxes with SD error bars) and population averages (yellow lines; n = 4 wild-type, n = 6 Kv3.3 KO). Ordinate is relative Ca2+ signal normalized to maximum ΔF/F for each cell. These data demonstrate reduction of CF evoked Ca2+ signals from proximal to distal dendrites in wild-type PCs and sustained or augmented signals in distal dendrites of Kv3.3 KO PCs.
To compare amplitude of Ca2+ transients, ROIs were demarcated in proximal and distal dendritic fields within each PC (Fig. 7). Ca2+ signals from climbing fiber responses were averaged within ROIs. Consistent with line scan data, WT PCs displayed large Ca2+ signals at proximal dendritic ROIs and lesser signals at distal dendritic ROIs. Kv3.3 KO PCs produced similar amplitude Ca2+ signals as WT PCs in proximal dendritic ROIs (WT: 5.5 ± 0.8% n = 4; Kv3.3 KO: 5.7 ± 1.7% n = 6; P = 0.83). In contrast, Kv3.3 KO PC Ca2+ signals were significantly enhanced in distal dendritic ROIs (WT: 4.1 ± 0.7%; Kv3.3 KO: 6.7 ± 1.8%; P = 0.025) and in distal regions in comparison to proximal signals within the same trial (WT: 75.0 ± 7.9%; Kv3.3 KO: 121.3 ± 32.9%; P = 0.028). Both sets of measurements indicate a 1.6-fold increase in Ca2+ activity in distal dendrites of Kv3.3 KO PCs compared with WT. These data indicate that spatial dynamics of synaptically evoked Ca2+ signaling is altered in Kv3.3 KO PCs and augmented specifically in distal dendrites, suggesting a role for dendritic Kv3.3 channels in dampening excitability in distal dendritic segments during physiologic stimuli.
Fig. 7.
Ca2+ transients are specifically elevated in distal dendrites of Kv3.3 KO PCs. A and B: resting fluorescence images of wild-type and Kv3.3 KO PCs, with regions of interest (ROIs) demarcated corresponding to proximal (■) and distal ( ) dendritic regions. Scale bar in B is 50 μm. C and D: Ca2+ signals averaged within each ROI vs. time for 5 successive climbing fiber stimulations at 1 Hz. Signals in both ROIs peaked simultaneously; however, for purpose of presentation, distal ROI signals are temporally offset slightly in these plots. In the wild-type example, the peak proximal and distal dendritic fluorescence changes are 6.2 ± 1 and 4.1 ± 0.5%, respectively; for the Kv3.3 KO example, 4.9 ± 0.4 and 5.8 ± 0.6%. E: peak ΔF/F signals were determined for each cell and compared between wild-type (n = 4) and Kv3.3 KO (n = 6) PCs. There was no statistically significant difference between amplitudes of proximal signals; however, distal dendritic signals and distal relative to proximal signals within individual trials were significantly larger for Kv3.3 KO PCs (*, P < 0.05). Errors bars are SD.
) dendritic regions. Scale bar in B is 50 μm. C and D: Ca2+ signals averaged within each ROI vs. time for 5 successive climbing fiber stimulations at 1 Hz. Signals in both ROIs peaked simultaneously; however, for purpose of presentation, distal ROI signals are temporally offset slightly in these plots. In the wild-type example, the peak proximal and distal dendritic fluorescence changes are 6.2 ± 1 and 4.1 ± 0.5%, respectively; for the Kv3.3 KO example, 4.9 ± 0.4 and 5.8 ± 0.6%. E: peak ΔF/F signals were determined for each cell and compared between wild-type (n = 4) and Kv3.3 KO (n = 6) PCs. There was no statistically significant difference between amplitudes of proximal signals; however, distal dendritic signals and distal relative to proximal signals within individual trials were significantly larger for Kv3.3 KO PCs (*, P < 0.05). Errors bars are SD.
DISCUSSION
In this paper we assess the unique contributions of Kv3.3 potassium channels to dendritic physiology in cerebellar PCs. We found that PCs in Kv3.3 KO mice displayed increased dendritic excitability. This altered excitability was manifested as reduced current threshold for intrinsic Ca2+ spiking, suggesting that Kv3.3 channels regulate PC Ca2+ spike generation. Moreover, following climbing fiber stimulation, Kv3.3 KO PCs displayed enhanced Ca2+ transients specifically in distal dendrites, suggesting that dendritic Kv3.3 channels may be particularly important in regulating spatial dynamics of Ca2+ spikes during physiologic activity.
A series of experiments supports the hypothesis that enhanced dendritic excitability in Kv3.3 KO PCs may be attributed to a reduction in Kv3-mediated outward currents. Resting membrane parameters and subthreshold excitability were not altered in Kv3.3 KO PCs, as apparent membrane resistance and subthreshold resonance properties were similar to measurements in PCs from WT littermates. Consistent with these measurements, there did not appear to be gross differences in PC morphology at the ages tested in this study (as example Fig. 5, A1 and B1). Acute block of Kv3 channels specifically in dendrites of WT PCs also reduced current threshold for Ca2+ spiking. Moreover, voltage-dependent outward currents were remarkably reduced in Kv3.3 KO PCs. Last, voltage threshold of Ca2+ spikes as measured directly in PC dendrites overlapped with the onset of robust Kv3-like current activity in PCs. Together, these data strongly argue that enhanced dendritic excitability in Kv3.3 KO PCs is due to lack of perithreshold operating voltage-dependent outward currents mediated by dendritic Kv3.3 channels.
Kv3.3 mediated currents in somatic and dendritic PC membranes
Dendritic expression of Kv3.3 subunits has been described using immunohistochemistry (Chang et al. 2007; Martina et al. 2003; McMahon et al. 2004). Kv3.3 immunoreactivity throughout mouse brain was strongest in the cerebellar cortex, which we determined by light and electron microscopy to be largely localized to PC membranes and eliminated in Kv3.3 KO mice (Chang et al. 2007). Robust somatic Kv3.3 expression tapers considerably within proximal PC dendrites (Chang et al. 2007), similar to expression patterns of Kv3 subunits in other high-frequency firing neurons (Rudy and McBain 2001). However, unlike in these other neuronal populations, strong expression was again observed in PC distal dendrites (Chang et al. 2007). The possible functional relevance of this enhanced distal dendritic distribution is discussed in the following text. Dendritic Kv3-like currents have been recorded directly from patches of rat PCs (Martina et al. 2003) with reported properties similar to somatic currents.
A limitation of the current study is that we were unable to obtain measurements of currents from isolated distal dendritic membranes, which is particularly difficult in mouse, and therefore we could not directly compare current properties in WT and KO PC dendrites. We expect, however, that dendritic currents are significantly reduced in Kv3.3 KO PCs considering the nearly 70% reduction in ionic currents from somatic patches (Fig. 4) and the well-characterized distribution of Kv3.3 subunits in PC dendrites (Chang et al. 2007). Moreover, the observation that altered dendritic excitability in Kv3.3 KO PCs was similar to the effects of acute block of dendritic Kv3 channels in WT PCs is consistent with a reduction of outward currents in Kv3.3 KO PC dendrites.
Kv3-like currents sensitive to 1 mM TEA became noticeable at −30 or −20 mV from PC patches (Fig. 4), and current magnitude at these voltages was significantly reduced in Kv3.3 KO PCs. This activity differs from Kv3 channels as previously studied in heterologous cells (Rudy et al. 1999), which display a somewhat more depolarized voltage dependence. This hyperpolarized shift in Kv3 channel properties in PCs was also observed by others (Martina et al. 2003; McKay and Turner 2004; Sacco et al. 2006). To directly compare Kv3.3 channel properties in native membranes and heterologous cells, we recorded Kv3.3-mediated currents in heterologous cells under identical recording conditions as those used in acute brain slices. This revealed robust activation of currents at −10 mV (data not shown) similar to previous reports (Rudy and McBain 2001; Rudy et al. 1999). The cause of this 10–15 mV observed difference in voltage dependence of native currents is unknown. Possible causes include incorporation of accessory subunits or differences in phosphorylation pattern, both of which have been shown to alter gating properties for multiple Kv channels (Jerng et al. 2004; Nadal et al. 2003; Park et al. 2008; Pongs et al. 1999; Zagha et al. 2005).
Kv3.3 channel regulation of dendritic Ca2+ spike generation
Specific properties of dendritic Ca2+ spikes were altered in Kv3.3 KO PCs. Based on studies using acute block of Kv3 channels with TEA, McKay and Turner (2004) proposed that dendritic Kv3 channels repolarize PC Ca2+ spikes. We observed increased Ca2+ spike amplitude in Kv3.3 KO PCs, further supporting a role of dendritic Kv3 channels in regulating Ca2+ spike repolarization. A major finding of this study is that, in addition to effects on spike repolarization, dendritic Kv3.3 channels appear to regulate dendritic excitability.
The role of Kv3 channels in regulating Ca2+ spike threshold was unexpected, as Kv3 channels are traditionally believed to selectively activate near spike peak due to their voltage dependence and therefore contribute mainly to spike repolarizaton (Rudy and McBain 2001). Indeed in multiple cell types, it has been shown that block of Kv3 channels does not affect threshold properties for Na+ spikes (Rudy and McBain 2001), and we also observed that Na+ spike threshold properties were unaltered in Kv3.3 KO PCs. The unique contribution of Kv3 channels in opposing Ca2+ spike generation is likely due to differences in axosomatic Na+ versus dendritic Ca2+ electrogenesis. Voltage threshold for Ca2+ spiking as measured in dendrites was approximately −17 mV (Supplementary Fig. S2), consistent with measurements of voltage-dependent Ca2+ currents from PC dendritic membranes, which were found to activate robustly at potentials positive to −30 mV (Usowicz et al. 1992). These potentials are considerably more depolarized than Na+ spike voltage threshold in central neurons (Bean 2007) (approximately −45 mV in this study of PCs) and yet match well to the onset of robust activation of Kv3-like currents measured from WT PCs (Fig. 4).
There are a few possible mechanisms by which dendritic Kv3 channels regulate Ca2+ spike properties. First, Kv3 channels may shunt somatic or dendritic axial currents prior to spike onset. Second, Kv3 channels at the site of spike generation may increase current threshold by opposing regenerative currents. For this latter mechanism, one may expect a depolarized shift in dendritic voltage threshold for Ca2+ spikes in Kv3.3 KO PCs, and further dendritic recordings in these mice may enhance our understanding of Kv3.3 regulation of Ca2+ spikes. Third, dendritic Kv3 channels are likely to reduce the length constant of the dendritic membrane in a voltage-dependent manner, which we hypothesize is an essential mechanism in dampening Ca2+ spike propagation throughout the dendritic tree (see following text).
Kv3.3 channel regulation of spatial profiles of dendritic Ca2+ signaling
To study physiological implications of dendritic hyperexcitability in Kv3.3 KO PCs, we optically measured Ca2+ signals throughout the dendritic tree following climbing fiber stimulation. It has been previously observed that Ca2+ signals from climbing fiber responses are consistently robust in proximal dendritic regions and reduced (though variable) in distal dendrites (Miyakawa et al. 1992). As climbing fiber synaptic inputs are targeted to proximal dendrites (Larramendi and Victor 1967), this finding is consistent with Ca2+ spike generation at proximal dendritic membranes with variable propagation into distal dendrites.
Our measurements in WT PCs also showed robust climbing fiber induced Ca2+ signals in proximal dendrites that taper distally. In Kv3.3 KO PCs, the amplitudes of Ca2+ signals in proximal dendrites were similar to measurements from WT PCs. However, in Kv3.3 KO PCs, Ca2+ signals tended to be elevated throughout distal regions, such that Ca2+ signals in distal dendrites were significantly larger in Kv3.3 KO compared with WT PCs (Fig. 7). These differences in the spatial profile of Ca2+ signaling suggest either that Ca2+ spikes generated in proximal dendrites propagated more effectively into distal dendritic segments or that the site of Ca2+ spike initiation shifted to distal dendrites; the temporal resolution of our imaging methods was not able to distinguish between these possibilities.
The finding of enhanced synaptically evoked Ca2+ signals in Kv3.3 KO PC distal dendrites is consistent with our findings concerning channel localization and channel properties. Enrichment of Kv3.3 channels in distal dendrites and activation near Ca2+ spike threshold enables these channels to preferentially regulate Ca2+ spike generation and propagation in distal, rather than proximal, dendrites. We propose that regulation of spatial dynamics of Ca2+ signaling by modulation of distal dendritic excitability is a key function of Kv3.3 channels in PC dendrites and is dependent on the unique spatial distribution of dendritic Kv3.3 channels.
Previous studies have identified other cellular and network properties as important regulators of PC dendritic Ca2+ signaling. Synaptic inhibition from molecular layer interneurons may dynamically alter the spatial profile of Ca2+ transients by electrically isolating distal from proximal dendritic branches (Callaway et al. 1995). Kv1 channels appear to be important regulators of PC dendritic excitability as blockers of Kv1 channels reduce Ca2+ spike current threshold (McKay et al. 2005) and enable spontaneous dendritic Ca2+ spiking (Khavandgar et al. 2005). PC dendrites also express BK Ca2+-activated K+ channels (Sausbier et al. 2006), and these channels may also regulate Ca2+ spike propagation (Edgerton and Reinhart 2003; Rancz and Häusser 2006).
Implications of altered dendritic excitability for mouse phenotypes and human disease
Dendritic Kv3.3 channels do not appear to have a prominent effect on shaping axosomatic Na+ spike waveforms (Zagha et al. 2008). However, the regulation of dendritic Ca2+ signaling explored in this study has important implications for dendritic integration and plasticity. Synchronous parallel fiber inputs produce regenerative Ca2+ transients in isolated PC dendritic branches (Eilers et al. 1995; Miyakawa et al. 1992). Increased dendritic excitability in Kv3.3 KO PCs may facilitate local dendritic spiking and thereby enhance the effect of synchronous parallel fiber inputs on axonal spiking. Moreover, dendritic Ca2+ activity causes synaptic plasticity at short time scales by retrograde release of cannabinoids (Kreitzer and Regehr 2001) and at longer time scales through postsynaptic receptor trafficking (Chung et al. 2003; Linden and Connor 1993). Consequently, enhanced Ca2+ signaling in Kv3.3 KO PCs may alter the normal synaptic requirements for inducing plasticity, further disturbing input-output properties of the cerebellar cortex.
Kv3.3 KO mice display altered motor coordination (Joho et al. 2006). Motor deficits are largely reversed in mice with PC-specific rescue of Kv3.3 expression (Hurlock et al. 2008), providing strong evidence that motor phenotypes are at least in part mediated by altered PC activity. It is still unknown, however, how altered cellular properties of Kv3.3 KO PCs cause motor dysfunction. Dendritic excitability in Kv3.3 KO PCs has not been previously explored, and the changes described here suggest possible cellular processes that may contribute to motor phenotypes in Kv3.3 KO mice.
Missense mutations in Kv3.3 coding sequence are responsible for human spinocerebellar ataxia (SCA13) (Figueroa et al. 2010; Waters et al. 2006). Ataxia in these patients is associated with gross cerebellar atrophy (Waters et al. 2006). Altered Ca2+ homeostasis is a well known cause of cellular degeneration (Orrenius et al. 2003), and in other spinocerebellar ataxias, there is evidence of altered Ca2+ handling in PCs (Duenas et al. 2006; Lin et al. 2000). We demonstrated enhanced synaptically evoked Ca2+ signaling in Kv3.3 KO PC dendrites, which may lead to disrupted Ca2+ homeostasis and PC pathology over long periods. Kv3.3 KO mice did not develop overt cerebellar degeneration at the ages tested in this study, possibly reflecting the chronic nature of a disease requiring many years of altered physiology for full expression. A second possibility is that the missense mutations identified in SCA13 generate altered channels that even more dramatically disturb dendritic excitability and Ca2+ homeostasis. Better mouse models of SCA13 which replicate the human missense mutations are required to test the relationships between Kv3.3 regulation of dendritic excitability, Ca2+ homeostasis, and cellular degeneration.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-30989 and NS-045217 to B. Rudy and NS-016295 to W. N. Ross, National Science Foundation Grant IBN-0314645 to B. Rudy, and National Research Service Award F31 NS-59124 to E. Zagha.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Nathaniel Heintz (Rockefeller University) for providing the Kv3.3 knockout mice. We thank Dr. Kamran Khodakhah for insightful discussion and J. Maffie for preparation of heterologous cells.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Akemann W, Knopfel T. Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons. J Neurosci 26: 4602–4612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Häusser M. Targeted dendrotomy reveals active and passive contributions of the dendritic tree to synaptic integration and neuronal output. Proc Natl Acad Sci USA 104: 11447–11452, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci 15: 2777–2787, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, Ellisman MH, Heintz N, Rudy B. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol 502: 953–972, 2007 [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300: 1751–1755, 2003 [DOI] [PubMed] [Google Scholar]
- Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol 372: 1–23, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JT, Clark BA, Häusser M. The origin of the complex spike in cerebellar Purkinje cells. J Neurosci 28: 7599–7609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E, Bower JM. An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J Neurophysiol 71: 375–400, 1994 [DOI] [PubMed] [Google Scholar]
- Duenas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain 129: 1357–1370, 2006 [DOI] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol 548: 53–69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers J, Augustine GJ, Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature 373: 155–158, 1995 [DOI] [PubMed] [Google Scholar]
- Eisenberg RS. The equivalent circuit of single crab muscle fibers as determined by impedance measurements with intracellular electrodes. J Gen Physiol 50: 1785–1806, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa KP, Minassian NA, Stevanin G, Waters M, Garibyan V, Forlani S, Strzelczyk A, Burk K, Brice A, Durr A, Papazian DM, Pulst SM. KCNC3: phenotype, mutations, channel biophysics-a study of 260 familial ataxia patients. Hum Mutat 31: 191–196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Bert A, Stevanin G, Netter JC, Rascol O, Brassat D, Calvas P, Camuzat A, Yuan Q, Schalling M, Durr A, Brice A. Mapping of spinocerebellar ataxia 13 to chromosome 19q13.3-q13.4 in a family with autosomal dominant cerebellar ataxia and mental retardation. Am J Hum Genet 67: 229–235, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci 28: 4640–4648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci 23: 216–222, 2000 [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Dynamical Systems in Neuroscience Cambridge, MA: MIT Press, 2007 [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci 27: 343–369, 2004 [DOI] [PubMed] [Google Scholar]
- Joho RH, Hurlock EC. The role of Kv3-type potassium channels in cerebellar physiology and behavior. Cerebellum 8: 323–333, 2009 [DOI] [PubMed] [Google Scholar]
- Joho RH, Street C, Matsushita S, Knopfel T. Behavioral motor dysfunction in Kv3-type potassium channel-deficient mice. Genes Brain Behav 5: 472–482, 2006 [DOI] [PubMed] [Google Scholar]
- Khavandgar S, Walter JT, Sageser K, Khodakhah K. Kv1 channels selectively prevent dendritic hyperexcitability in rat Purkinje cells. J Physiol 569: 545–557, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Dreessen J, Augustine GJ. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc Natl Acad Sci USA 89: 7051–7055, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001 [DOI] [PubMed] [Google Scholar]
- Larramendi EM, Victor T. Synapses on the Purkinje cell spines in the mouse. An electronmicroscopic study. Brain Res 5: 15–30, 1967 [DOI] [PubMed] [Google Scholar]
- Lasser-Ross N, Miyakawa H, Lev-Ram V, Young SR, Ross WN. High time resolution fluorescence imaging with a CCD camera. J Neurosci Methods 36: 253–261, 1991 [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Miyakawa H, Lasser-Ross N, Ross WN. Calcium transients in cerebellar Purkinje neurons evoked by intracellular stimulation. J Neurophysiol 68: 1167–1177, 1992 [DOI] [PubMed] [Google Scholar]
- Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci 3: 157–163, 2000 [DOI] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Cellular mechanisms of long-term depression in the cerebellum. Curr Opin Neurobiol 3: 401–406, 1993 [DOI] [PubMed] [Google Scholar]
- Llinas R, Nicholson C, Freeman JA, Hillman DE. Dendritic spikes and their inhibition in alligator Purkinje cells. Science 160: 1132–1135, 1968 [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305: 197–213, 1980a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 305: 171–195, 1980b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Metz AE, Bean BP. Voltage-dependent potassium currents during fast spikes of rat cerebellar Purkinje neurons: inhibition by BDS-I toxin. J Neurophysiol 97: 563–571, 2007 [DOI] [PubMed] [Google Scholar]
- Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci 23: 5698–5707, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Molineux ML, Mehaffey WH, Turner RW. Kv1 K+ channels control Purkinje cell output to facilitate postsynaptic rebound discharge in deep cerebellar neurons. J Neurosci 25: 1481–1492, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Turner RW. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci 20: 729–739, 2004 [DOI] [PubMed] [Google Scholar]
- McMahon A, Fowler SC, Perney TM, Akemann W, Knopfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci 19: 3317–3327, 2004 [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Lev-Ram V, Lasser-Ross N, Ross WN. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J Neurophysiol 68: 1178–1189, 1992 [DOI] [PubMed] [Google Scholar]
- Moore LE, Christensen BN. White noise analysis of cable properties of neuroblastoma cells and lamprey central neurons. J Neurophysiol 53: 636–651, 1985 [DOI] [PubMed] [Google Scholar]
- Moore LE, Hill RH, Grillner S. Voltage-clamp frequency domain analysis of NMDA-activated neurons. J Exp Biol 175: 59–87, 1993 [DOI] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37: 449–461, 2003 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron 24: 727–737, 1999 [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology 23: 49–57, 2008 [DOI] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol 529: 205–213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese KP, Silva AJ, Storm JF. Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann NY Acad Sci 868: 344–355, 1999 [DOI] [PubMed] [Google Scholar]
- Rall W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol 1: 491–527, 1959 [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J 9: 1483–1508, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Häusser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci 26: 5428–5437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinzel J, Ermentrout GB. Analysis of Neural Excitability and Oscillations Cambridge, MA: The MIT Press, 1989 [Google Scholar]
- Roth A, Häusser M. Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J Physiol 535: 445–472, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contributions of Kv3 channels to neuronal excitability. Ann NY Acad Sci 868: 304–343, 1999 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24: 517–526, 2001 [DOI] [PubMed] [Google Scholar]
- Sacco T, De Luca A, Tempia F. Properties and expression of Kv3 channels in cerebellar Purkinje cells. Mol Cell Neurosci 33: 170–179, 2006 [DOI] [PubMed] [Google Scholar]
- Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem Cell Biol 125: 725–741, 2006 [DOI] [PubMed] [Google Scholar]
- Stuart G, Häusser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 13: 703–712, 1994 [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci 23: 9650–9663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz MM, Sugimori M, Cherksey B, Llinas R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron 9: 1185–1199, 1992 [DOI] [PubMed] [Google Scholar]
- Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 38: 447–451, 2006 [DOI] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci 14: 949–972, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci 16: 1214–1222, 2002. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci 24: 3511–3521, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Lang EJ, Rudy B. Kv3.3 channels at the Purkinje celsoma are necessary for generation of the classical complex spike waveform. J Neurosci 28: 1291–1300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, McCormack T, Akinsanya KO, Qi SY, Rudy B. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem 280: 18853–18861, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.