Abstract
Monoamine oxidase A (MAOA) and the transporters for serotonin (5-HTT) and norepinephrine (NET) may play important roles in regulating maternal monoamine neurotransmitters transferred across the placenta to the fetus. We investigated whether promoter polymorphisms in MAOA (uVNTR), 5-HTT (5-HTTLPR), and NET (NETpPR AAGG4) could influence gene expression and protein activity in human placentas. Normal term human placentas (n = 73) were collected, and placental MAOA, 5-HTT, and NET mRNA levels and protein activity were determined. The mRNA levels or protein activities were compared between different genotype groups. Placentas hemizygous (male fetus) or homozygous (female fetus) for MAOA uVNTR 4-repeat allele had significantly higher MAOA mRNA levels than those hemizygous or homozygous for the 3-repeat allele (P = 0.001). However, no significant difference in MAOA enzyme activity was found for these two groups of genotypes (P = 0.161). Placentas with the 5-HTTLPR short (S)-allele (S/S+S/L) had significantly lower 5-HTT mRNA levels and serotonin uptake rate than those homozygous for the long (L)-allele (L/L) (mRNA: P < 0.001; serotonin transporting activity: P < 0.001). Placentas homozygous for the NET AAGG4 L4 allele had significantly higher NET mRNA levels, as well as dopamine and norepinephrine uptake rates, than those with the S4/L4 genotype (mRNA: P < 0.001; dopamine transporting activity: P = 0.012; norepinephrine transporting activity: P = 0.011). These findings suggest that the three promoter polymorphisms of MAOA, 5-HTT, and NET influence gene expression levels and protein activity of these genes in human placentas, potentially leading to different fetal levels of maternal monoamine neurotransmitters, which may have an impact on fetal neurodevelopment.
Keywords: monoamine oxidase A, serotonin transporter, norepinephrine transporter, neurotransmitters, mRNA levels, transporter activity, fetal neuro-development
monoamine oxidase a (MAOA) and the transporters for serotonin (5-HTT) and norepinephrine (NET) are expressed in both the nervous system and peripheral tissues, including the human placenta. They participate in the degradation or transport of monoamine neurotransmitters [e.g., dopamine (DA), serotonin (5HT), and norepinephrine (NE)] (4, 6, 49). Placental MAOA, 5-HTT, and NET may modulate the transfer of maternal monoamine neurotransmitters to the fetus. MAOA is expressed in the outer mitochondrial membrane of trophoblast cells and preferentially deaminates monoamines (48). 5-HTT and NET are expressed on the maternal-facing brush border membrane of trophoblasts (4, 35). They mediate the uptake of monoamines from the maternal blood into the syncytiotrophoblast. Although the dopamine transporter (DAT) is not found in placental tissue, NET appears to function as the transporter for both DA and NE (35). Since the placenta does not have vesicular monoamine transporters (32), maternal monoamines transferred to the syncytiotrophoblast must be either degraded or transported across the fetal-facing basal membrane into the fetal circulation. We propose that changed placental expression levels of MAOA, 5-HTT, or NET, due to variation in their genes, may result in different fetal levels of maternal monoamine neurotransmitters, leading to altered fetal neurodevelopment.
The human MAOA gene maps to chromosome Xp11.23–11.4 (29). A variable number of tandem repeat (uVNTR) polymorphism, consisting of a 30-bp repeat sequence present in 3, 3.5, 4, or 5 copies, is located 1.2 kb upstream of the MAOA coding region. The 3.5-repeat and 5-repeat alleles are rare. In vitro transfection experiments demonstrated that 3.5- and 4-repeat alleles are transcribed 2 to 10 times more efficiently than 3- and 5-repeat alleles (38). Measurements of MAOA activity in skin fibroblast cultures revealed similar results (14). Jonsson et al. (23) reported that women with 3.5- or 4-repeat alleles had higher cerebral spinal fluid concentrations of monoamine metabolites than women with other alleles, indicating that the 3.5- or 4-repeat alleles are associated with higher levels of MAOA enzyme activity. Additionally, Balciuniene et al. (3) investigated the functional effect of polymorphisms in the MAOA and MAOB genes and found that males carrying a two-locus haplotype (containing MAOA uVNTR 3-repeat allele) had significantly lower MAOA activity than those without this haplotype. These studies provide evidence that an optimal length of MAOA uVNTR is important for MAOA transcription.
The human 5-HTT gene (also referred to as SERT or SLC6A4) maps to chromosome 17q11.1–12 (27). A 44-bp insertion/deletion polymorphism (5-HTTLPR) in the promoter region creates both a short (S) and a long (L) allele. In vitro transfection experiments demonstrated that the basal activity of the L-allele is about threefold higher than that of the S-allele (19). Similar results were obtained from lymphoblast cell lines (28). Measuring 5-HTT transport activity in platelets of individuals with autism showed that the L/L genotype was associated with a 1.5-fold higher uptake rate than the S/L and S/S genotypes (2). In the above studies, the influences of S/S and S/L genotypes on 5-HTT expression were not found to be significantly different. Thus, the 5-HTTLPR polymorphism could regulate 5-HTT transcription and the S-allele may be dominant in its effect on inhibiting of 5-HTT expression.
The human NET gene (SLC6A2) is located on chromosome 16q13–21 (17). Six AAGG-repeat islands (AAGG1–6) were identified in NET promoter polymorphic region (NETpPR) (43). Among them, island AAGG4 is polymorphic. Querying the NETpPR DNA sequence against the transcription factor database TRANSFAC by the MatInspector program (33) indicated that transcription factor Elk-1 potentially binds to the region where island AAGG4 is located. The 4-bp AAGG deletion may result in the net loss of the binding site for Elk-1. However, the effect of the 4-bp AAGG deletion/insertion polymorphism in island AAGG4 on NET expression is as yet unknown.
There is evidence that the promoter polymorphisms MAOA uVNTR, 5-HTT 5-HTTLPR, and NET AAGG4 are associated with vulnerability to a number of neuropsychiatric disorders. MAOA uVNTR has been associated with impulsive/antisocial behavior (8, 12, 30, 50), autism (9, 53), affective disorders (1, 54), substance dependence (12), and posttraumatic stress disorder (51). 5-HTT 5-HTTLPR may influence anxiety-related personality traits (28, 39), affective disorders (10, 20, 25, 45), Alzheimer's disease (34, 42), autism (5, 11, 24), sudden infant death syndrome (47), alcohol dependence (15, 31), obsessive-compulsive disorder (21), attention-deficit/hyperactivity disorder (36), and type 2 diabetes (22). NET AAGG4 has not been well studied, though a positive association between NET AAGG4 and anorexia nervosa has been reported (43). Additionally, an interactive effect of NET AAGG4 and MAOA uVNTR on anorexia nervosa was observed (44).
Since MAOA, 5-HTT, and NET are present in the placenta, altered expression and activity of these proteins due to promoter polymorphisms (MAOA uVNTR, 5-HTT 5-HTTLPR, and NET AAGG4) in their genes may influence fetal brain development. Animal studies have demonstrated that placental 5-HTT is involved in the transfer of maternal 5-HT to the fetus during the early stages of fetal development (52). The placental transporter may also participate in the localized clearance of vasoconstrictive biogenic amines such as 5-HT from the intervillous space. Insufficient clearance may lead to impaired placental blood flow and intrauterine growth retardation (16). Furthermore, studies have shown that placental transporters regulate catecholamine levels in fetal circulation by reuptake of catecholamines from the fetal plasma compartment (6). Therefore, it is important to determine whether these promoter-associated polymorphisms affect gene transcription and protein activity of the respective genes and gene products in human placentas.
MATERIALS AND METHODS
Placental tissues.
Normal term (37–42 wk gestation) human placentas (n = 73) were obtained from uncomplicated pregnancies with normal outcomes from a primarily Caucasian population at the Kingston General Hospital (Ontario, Canada). None of the pregnancies were complicated (i.e., pre-eclampsia, preterm birth, intrauterine growth restriction, chorioamnionitis, stillbirth/early neonatal death). Placental tissue was processed immediately following delivery. After removal of the basal plate (including maternal decidua), the placental villous tissue (central portion of the placenta) was dissected from the chorionic plate for brush-border membrane vesicle (n = 21) and mitochondrial (n = 73) preparations and for DNA (n = 73) and total RNA (n = 73) extractions. The study protocol was approved by the institutional review boards of Queen's University and the Kingston General Hospital.
DNA extraction and genotyping.
Genomic DNA was extracted from placental tissue using QIAamp Tissue Kits (QIAGEN, Valencia, CA). The DNA fragment containing promoter polymorphism MAOA uVNTR was amplified by polymerase chain reaction (PCR) as described by Sabol et al. (38) using the following primers: forward 5′-ACAGCCTGACCGTGGAGAAG-3′ and reverse 5′-GAACGGACGCTCCATTCGGA-3′. The number of 30-bp repeats was deduced from the allele size. Fetal sex was examined by amplifying two loci (ZFX and ZFY) that resulted in X-specific and Y-specific products of 488 and 340 bp, respectively (26). Based on sex information, MAOA genotypes of hemizygous male and homozygous female fetuses were distinguished.
Genotyping of 5-HTTLPR 44-bp deletion/insertion polymorphism was performed as previously described (13). 5-HTTLPR alleles were denoted as S or L. To genotype NET AAGG4, the following primers were designed: forward, 5′-GATGGAAGGAAGGTGGGAAA-3′ (position −4225 to −4206), and reverse, 5′-TCCTCCCTTTCTTTCTCTCTCC-3′ (position −4024 to −4003). Labeling PCR reactions were carried out in 10-μl volumes containing 25 ng genomic DNA, 200 μM dNTPs, 10 pmol of each primer, 0.5 U Taq DNA polymerase (Invitrogen, Carlsbad, CA), 1× PCR reaction buffer, 1.0 mM MgCl2, and 0.1 pmol α-32P dCTP. The PCR program was set as: 95°C for 5 min; 30 cycles of 95°C for 1 min, 67°C for 1 min, 72°C for 1 min; and finally 72°C for 10 min. PCR products were separated by electrophoresis on a 6% denaturing acrylamide gel. The 4-bp AAGG deletion allele (or L4) was 219 bp long and the 4-bp AAGG insertion allele (or S4) was 223 bp long.
Determination of mRNA levels by quantitative duplex PCR.
Total RNA was isolated from placental tissue using TRIzol Reagent (Invitrogen). The first-strand cDNA was synthesized from 1 μg of total RNA in a 20-μl reaction with the Reverse Transcription System (Promega, Madison, WI). Relative transcription levels of MAOA, 5-HTT, and NET were determined by quantitative duplex PCRs using the β-actin gene (ACTB) as an internal control. To show unambiguously that the PCR products were derived from cDNA and not from genomic DNA, the forward and reverse primers were located in different exons, separated by at least one intron. Sequences of primers and sizes of PCR products from both genomic DNA and cDNA are given in Table 1.
Table 1.
Location and sequence of primers for quantitative duplex PCRs and PCR product size
| PCR Product Size, bp |
|||
|---|---|---|---|
| Gene | Primer Location and Sequence | From Genomic DNA | From cDNA |
| ACTB | F: 5′-AGC ACA GAG CCT CGC CTT T-3′ (exon 1) | 1,343 | 346 |
| R: 5′-CAC ACG CAG CTC ATT GTA GA-3′ (exon 3) | |||
| MAOA | F: 5′-TGC TGC CAA ACT CTT GAC TG-3′ (exon 2) | 26,370 | 437 |
| R: 5′-ACC GCC TAG CAG TCT TTG TC-3′(exon 6) | |||
| 5-HTT | F: 5′-AGC TGC AAG AAC TCC TGG AA-3′ (exon 3) | 2,519 | 404 |
| R: 5′-ATC TGA GCG GCT GCA TCT AT-3′ (exon 6) | |||
| NET | F: 5′-TTT GGA AAA TCT GCC CAT TC-3′ (exon 2) | 2,467 | 226 |
| R: 5′-TAC TTG GTG TGG TTG CCA AG-3′ (exon 3) | |||
Duplex-PCR amplification of MAOA and ACTB cDNAs was carried out in 10 μl of reaction mix containing 2% of the cDNA synthesized from 1 μg total RNA, 1× PCR reaction buffer, 1 mM MgCl2, 200 μM dNTPs, 0.5 U Taq DNA polymerase (Invitrogen), 0.5 pmol MAOA forward primer, 0.49 pmol MAOA reverse primer, 0.01 pmol γ-32P end-labeled MAOA reverse primer, 0.5 pmol ACTB forward primer, 0.49 pmol ACTB reverse primer, and 0.01 pmol γ-32P end-labeled ACTB reverse primer. PCR was performed as follows: denaturation at 95°C for 5 min, followed by 20 cycles (optimized) of 95°C for 1 min, 65°C for 1 min, and 72°C for 1 min. Final extension was at 72°C for 10 min.
Duplex-PCR amplification of 5-HTT and ACTB cDNAs was carried out in 10 μl of reaction mix, containing 2% the cDNA synthesized from 1 μg total RNA, 1× PCR reaction buffer, 1 mM MgCl2, 200 μM dNTPs, 0.5 U Taq DNA polymerase (Invitrogen), 1 pmol 5-HTT forward primer, 0.98 pmol 5-HTT reverse primer, 0.02 pmol γ-32P end-labeled 5-HTT reverse primer, 0.5 pmol ACTB forward primer, 0.49 pmol ACTB reverse primer, and 0.01 pmol γ-32P end-labeled ACTB reverse primer. Successful amplification was achieved using an initial denaturation step at 95°C for 5 min, followed by 20 cycles (optimized) of 95, 62, and 72°C for 1 min each, and a final extension step at 72°C for 10 min. Duplex-PCR amplification of NET and ACTB cDNAs was performed using the same conditions as for 5-HTT cDNA, except 0.5 pmol NET forward primer, 0.45 pmol NET reverse primer, 0.05 pmol γ-32P end-labeled NET reverse primer were used in the reaction.
PCR products were separated on 6% denaturing acrylamide gels. Gels were dried and exposed to a Storage Phosphor Screen and quantified on a Molecular Dynamics Phosphor Imager (Amersham Pharmacia Biotech, Piscataway, NJ). Counts per minute of each band in uniformly sized sections was measured. Incorporation ratios (MAOA/ACTB, 5-HTT/ACTB, and NET/ACTB) for each cDNA sample were calculated and viewed as relative transcription levels for each of the three tested genes. The assays were done in duplicate for each sample.
Mitochondria isolation and assays of MAOA enzyme activity.
Isolation of mitochondria from placental tissue and the assay of MAOA activity were based on the method described by Weyler and Salach (49). The measurement of MAOA enzyme activity was carried out in 50 mM sodium phosphate buffer, pH 7.2, containing 0.2% Triton X-100 (monoamine oxidase assay buffer), plus 2 mM of kynuramine (substrate for MAOA), and an appropriate amount of the mitochondrial supernatant (optimized to be 100 ng of protein in the supernatant). The mixture was incubated at room temperature for 30 min, and the OD value at 314 nm (specific absorbency peak for the product, 4-hydroxyquinoline) was recorded. One unit of active enzyme equals to the amount of enzyme protein forming 1 μmol of product/min under these assay conditions. Enzyme assay was performed in duplicate for each sample.
Brush-border membrane vesicle preparation and 5-HTT and NET activity assay.
Brush-border membrane vesicles from 21 placentas were prepared according to the method described previously (4). The activity of 5-HTT and NET in brush border membrane vesicles was determined by measuring the uptake of H3-labeled monoamines (5-HT, DA, or NE) in the presence of an inwardly directed sodium chloride gradient. Uptake measurements were made by a rapid filtration technique as previously described (35). Briefly, uptake was initiated by rapidly mixing 40 μl of the membrane suspension (0.24 mg of protein) with 160 μl of uptake buffer (20 mM MES/Tris, 140 mM NaCl, pH 6.5) containing H3-labeled monoamines. After 15 s incubation at room temperature, uptake was terminated by adding 3 ml of ice-cold stop buffer (5 mM MES/Tris, 160 mM KCl, pH 6.5), and the mixture was filtered. Membrane vesicles retained in the filter were washed three times with 3 ml of the stop buffer. The filter was then placed in a 5-ml counting vial containing 2.5 ml of liquid scintillation cocktail CytoScint ES (ICN Pharmaceuticals, Costa Mesa, CA). The radioactivity associated with the filter was measured using a Beckman Model LS-230 liquid scintillation spectrometer (Beckman Coulter, Brea, CA). The experiments were performed in triplicate.
To determine the uptake rate by 5-HTT, 5-[1,2-3H(N)]-hydroxytryptamine creatinine (H3-5-HT) (PerkinElmer, Waltham, MA) was used as substrate (final concentration in the mixture: 25 nM). To adjust for nonspecific binding of the radio-label to membrane vesicles and filters, uptake was also measured in the presence of 100 μM imipramine, a 5-HTT inhibitor (4). Two other substrates, 3,4-[7-3H]-dihydroxyphenylethylamine (H3-DA) and levo-[7-3H]-norepinephrine (H3-NE) (PerkinElmer), were used to determine NET transport activity. Their final concentrations were 50 and 200 nM, respectively. To adjust for nonspecific binding of the radio-label to membrane vesicles and the filter, uptake was also measured in the presence of 100 μM desipramine, a NET inhibitor (35).
Statistical analyses.
The relationship between genotypes and transcription levels or protein activities was analyzed by unpaired t-test (two-tailed) program incorporated in software GraphPad Prism 5.02 (GraphPad Software, La Jolla, CA). The comparisons were between two groups of MAOA uVNTR genotypes [3-repeat (males) + 3-repeat/3-repeat (females) vs. 4-repeat (males) + 4-repeat/4-repeat (females)], two groups of 5-HTT 5-HTTLPR genotypes (L/L vs. S/L+S/S), and two groups of NET AAGG4 genotypes (L4/L4 vs. S4/L4).
RESULTS
MAOA uVNTR genotypes and MAOA transcription level/enzyme activity.
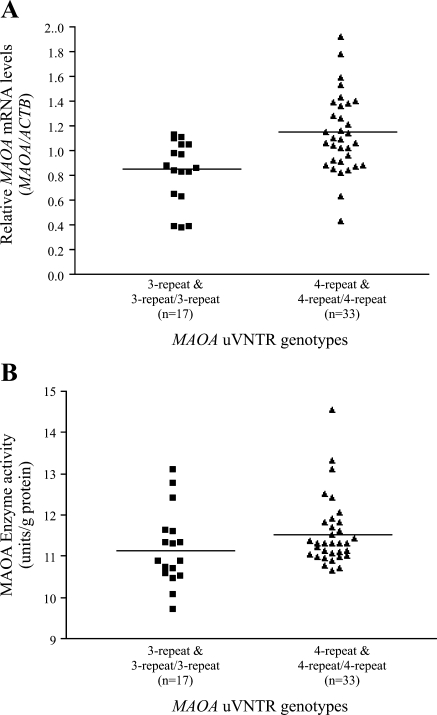
Based on the genotype of ZFX and ZFY, 34 fetuses were identified to be males and 39 fetuses were identified to be females. The 3.5-repeat allele appeared in only three placentas (two with the genotype 3-repeat/3.5-repeat and one with genotype 3.5-repeat/4-repeat) and the 5-repeat allele was found in a single placenta (genotype 3-repeat/5-repeat). The remaining 69 placentas had genotypes consisting of the two common alleles (3-repeat and/or 4-repeat). MAOA transcription levels and MAOA enzyme activity were compared between the two groups of placentas [hemizygous (male) or homozygous (female) for the 3-repeat allele vs. hemizygous (male) or homozygous (female) for the 4-repeat allele]. A significant difference in relative MAOA mRNA levels (MAOA/ACTB) was found between these two groups of placentas (t = 3.44, df = 48, P = 0.001). The 4-repeat allele was associated with significantly higher mRNA levels (mean ratio ± SE: 1.14 ± 0.05) than the 3-repeat allele (mean ratio ± SE: 0.84 ± 0.06) (Table 2 and Fig. 1A). However, MAOA enzyme activity in the two groups of placentas was not significantly different (t = 1.42, df = 48, P = 0.161) (Table 2 and Fig. 1B).
Table 2.
Association of MAOA, 5-HTT, and NET genotypes with transcription levels and protein activities in human placentas
| Genotypes | Relative mRNA Levels, mean ± SE | Protein Activities, mean ± SE |
|---|---|---|
| MAOA uVNTR | MAOA/ACTB | Enzyme activity, units/g protein |
| 3-repeat | 0.85 ± 0.07 (n = 12 males) | 11.20 ± 0.28 (n = 12 males) |
| 3-repeat/3-repeat | 0.80 ± 0.12 (n = 5 females) | 11.17 ± 0.41 (n = 5 females) |
| 4-repeat | 1.21 ± 0.05 (n = 22 males) | 11.78 ± 0.20 (n = 22 males) |
| 4-repeat/4-repeat | 1.01 ± 0.12 (n = 11 females) | 11.12 ± 0.11 (n = 11 females) |
| 3-repeat +3-repeat/3-repeat | 0.84 ± 0.06 (n = 17) | 11.19 ± 0.22 (n = 17) |
| vs. | ||
| 4-repeat +4-repeat/4-repeat | 1.14 ± 0.05 (n = 33) | 11.56 ± 0.14 (n = 33) |
| t = 3.44, df = 48, P = 0.001 | t = 1.42, df = 48, P = 0.161 | |
| 5-HTT 5-HTTLPR | 5-HTT /ACTB | 5-HT uptake rate, pmol/mg protein/15 s |
| S/S | 0.28 ± 0.01 (n = 20) | 1.53 ± 0.02 (n = 8) |
| vs. | ||
| S/l | 0.31 ± 0.02 (n = 28) | 1.55 ± 0.02 (n = 9) |
| t = 1.25, df = 46, P = 0.218 | t = 0.77, df = 15, P = 0.450 | |
| L/l | 0.65 ± 0.03 (n = 25) | 1.85 ± 0.01 (n = 4) |
| vs. | ||
| S/S+S/l | 0.29 ± 0.01 (n = 48) | 1.54 ± 0.01 (n = 17) |
| t = 14.29, df = 71, P < 0.001 | t = 9.66, df = 19, P < 0.001 | |
| NET AAGG4 | NET/ACTB | DA or NE uptake rate, pmol/mg protein/15 s |
| S4/S4 | 0.09 (n = 1) | N/A |
| S4/l4 | 0.22 ± 0.02 (n = 25) | 2.18 ± 0.05 (n = 7, DA uptake) |
| 4.00 ± 0.10 (n = 7, NE uptake) | ||
| L4/l4 | 0.53 ± 0.02 (n = 47) | 2.34 ± 0.03 (n = 14, DA uptake) |
| 4.29 ± 0.06 (n = 14, NE uptake) | ||
| S4/l4 vs. L4/l4 | t = 8.46, df = 70, P < 0.001 | t = 2.77, df = 19, P = 0.012 (DA) |
| t = 2.80, df = 19, P = 0.011 (NE) |
Fig. 1.
Association of MAOA uVNTR genotypes and MAOA mRNA level/enzyme activity. ■, Relative MAOA mRNA level/enzyme activity in placentas with only the 3-repeat allele (n = 17). ▲, Relative MAOA mRNA level/enzyme activity in placentas with only with the 4-repeat allele (n = 33). Horizontal lines represent mean values.
5-HTTLPR genotypes and 5-HTT transcription level/transport activity.
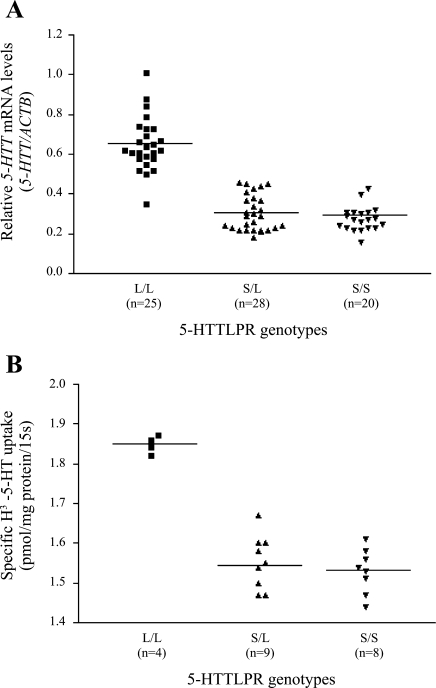
Among 73 placental DNAs, 25 had the L/L genotype, 28 had the S/L genotype, and 20 had the S/S genotype. Relative transcription levels (5-HTT/ACTB, mean ratio ± SE) associated with the L/L, S/L, and S/S genotypes were 0.65 ± 0.03 (n = 25), 0.31 ± 0.02 (n = 28), and 0.28 ± 0.01 (n = 20), respectively. Placentas with the 44-bp deletion allele (S/S and S/L) had significantly lower 5-HTT mRNA levels than those homozygous for the 44-bp insertion allele (L/L) (t = 14.29, df = 71, P < 0.001). No significant difference in 5-HTT mRNA levels was found between placentas with S/S and S/L genotypes (t = 1.25, df = 46, P = 0.218) (Table 2 and Fig. 2A).
Fig. 2.
Association of 5-HTTLPR genotypes and 5-HTT mRNA level/transport activity. Each square indicates relative 5-HTT mRNA level/transport activity in placentas with the L/L genotype (n = 25 for mRNA, n = 4 for protein). ▲, Relative 5-HTT mRNA level/transport activity in placentas with the S/L genotype (n = 28 for mRNA, n = 9 for protein). ▼, Relative 5-HTT mRNA level/transport activity in placentas with the S/S genotype (n = 20 for mRNA, n = 8 for protein). 5-HTT transport activity was measured in 21 placentas by uptake experiments with substrate H3-5-HT. The horizontal lines represent mean values.
5-HTT activity in 21 placentas was determined by uptake measurements using H3-5-HT as the substrate. The mean (± SE) uptake rates associated with the three genotypic groups were 1.85 ± 0.01 (L/L, n = 4), 1.55 ± 0.02 (S/L, n = 9), and 1.53 ± 0.02 (S/S, n = 8) pmol/mg protein/15 s, respectively. Placentas with the L/L genotype had a significantly higher uptake rate than those with the 44-bp deletion allele (S/L and S/S genotypes) (t = 9.66, df = 19, P < 0.001). However, there was no significant difference in the uptake rate between placentas with S/S and S/L genotypes (t = 0.77, df = 15, P = 0.450) (Table 2 and Fig. 2B).
NET AAGG4 genotypes and NET transcription level/transport activity.
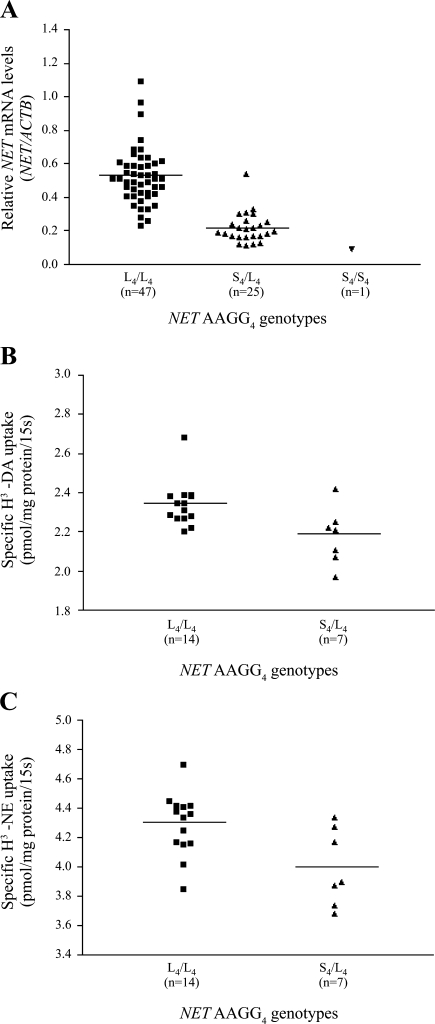
Genotyping the NET AAGG4 polymorphism indicated that 47 placentas were homozygous for the 4-bp AAGG insertion allele (L4/L4), 25 were heterozygous (S4/L4), and 1 was homozygous for the 4-bp AAGG deletion allele (S4/S4). Relative NET mRNA levels (NET/ACTB) (mean ratio ± SE) corresponding to the three groups of genotypes were 0.53 ± 0.02 (47 L4/L4), 0.22 ± 0.02 (25 S4/L4), and 0.09 (1 S4/S4), respectively. NET mRNA levels were significantly higher in placentas with the L4/L4 genotype than in those with the S4/L4 genotype (t = 8.46, df = 70, P < 0.001) (Table 2 and Fig. 3A). Since only one placenta was homozygous for the deletion allele (S4), genotype S4/S4 was not included in the analysis.
Fig. 3.
Association of NET AAGG4 genotypes and NET mRNA level/transport activity. ■, Relative NET mRNA level/transport activity in placentas with the L4/L4 genotype (n = 47 for mRNA, n = 14 for protein). ▲, Relative NET mRNA level/transport activity in placentas with the S4/L4 genotype (n = 25 for mRNA, n = 7 for protein). ▼, Relative NET mRNA level in placentas with the S4/S4 genotype (n = 1). NET transport activity was measured in 21 placentas by uptake experiments with H3-DA or H3-NE as the substrate as indicated. The horizontal lines represent mean values.
Transport activity of NET in 21 placentas was determined by uptake of H3-labeled DA and NE, separately, as substrates. Mean transport activities (± SE) corresponding to genotypes L4/L4 (14 placentas) and S4/L4 (7 placentas) were 2.34 ± 0.03 and 2.18 ± 0.05 pmol/mg protein/15 s, respectively, for the uptake of H3-DA, and 4.29 ± 0.06 and 4.00 ± 0.10 pmol/mg protein/15 s, respectively, for the uptake of H3-NE. Transport was more efficient by placentas with the L4/L4 genotype compared with those with the S4/L4 genotype (uptake of H3-DA: t = 2.77, df = 19, P = 0.012; uptake of H3-NE: t = 2.80, df = 19, P = 0.011) (Table 2; Fig. 3, B and C).
DISCUSSION
Expression of MAOA, 5-HTT, and NET in placental tissue suggests that these proteins may be involved in the regulation of monoamine neurotransmitter levels in the fetal circulation. Altered activities of these proteins in placentas may thus have a direct influence on fetal growth and development. For example, reduced placental MAOA activity and elevated maternal 5-HT levels have been implicated in pre-eclampsia (7). In the present study, we examined the effect of three promoter-associated polymorphisms (MAOA uVNTR, 5-HTT 5-HTTLPR, and NET AAGG4) on gene expression and protein activity in human placentas.
Our study demonstrates that the MAOA uVNTR promoter polymorphism affects MAOA transcription in human placentas. Alleles with four copies of the 30-bp repeat were transcribed more efficiently than alleles with three copies of the repeat. This result is consistent with that obtained from in vitro transfection experiments (14, 38), and indicates that 4-repeat alleles have an optimal length for maintaining MAOA promoter activity. Despite the transcription difference, placentas with the 4-repeat allele did not show significantly higher MAOA enzyme activity than those with the 3-repeat allele. First, this may be due to the limited sample size, which did not have enough power to detect a small but significant difference between the two groups of genotypes. Second, proteins go through a series of post-translational modifications (e.g., NH2-terminal signal peptide cleavage, disulfide bond generation, glycosylation, and steric structure formation, etc) before they attain biological function. There may be limits to any of these processes that affect the amount of functional protein even if the amount of translated products differed in placentas with different genotypes. Third, endogenous enzyme inhibitors may affect MAOA enzyme activity. Sivasubramaniam et al. (40) examined MAOA expression and activity in human placentas from pre-eclamptic and normotensive pregnancies and found that the catalytic efficiency of MAOA enzyme in pre-eclamptic placentas is significantly reduced, but MAOA mRNA levels and MAOA protein/mg total protein are not changed. Decreased MAOA enzyme activity may result from the presence of endogenous enzyme inhibitors that are activated in conditions such as pre-eclampsia. Fourth, placental MAOA expression and activity may vary based on the stage of fetal development. Developmental expression of MAOA has been observed in mouse brain. Strong MAOA expression can be detected in serotonergic neurons of the raphe nuclei from E12 to P7. During postnatal life, MAOA expression declines progressively to reach a minimal stable level by P21 (46). This may happen in the placenta as well. During later stages of pregnancy, the fetus may start to produce its own monoamine neurotransmitters. The maternal monoamine neurotransmitters would then no longer be as important as in the early stages of pregnancy. Correspondingly, the regulatory effect of gene variation on placental MAOA expression and activity may become unnoticeable. Finally, in addition to MAOA, other enzymes (e.g., catechol-O-methyltransferase or COMT) metabolize monoamines. A study by Wang et al. (46a) showed that COMT was more important than MAO in metabolizing intrarenal dopamine, suggesting that the enzyme activity of MAOA may be compensated for by other enzymes. Therefore, future studies should examine whether the interaction of monoamine metabolizing enzymes in the placenta regulates the transfer of maternal monoamines to the fetus.
With regard to the promoter polymorphism 5-HTTLPR, the 44-bp deletion allele (or S-allele) was associated with a decreased transcription level of 5-HTT and a low level of 5-HTT transport activity in human placentas. The dominant effect of the S-allele on 5-HTT expression was also found for human platelets and lymphoblasts (2, 18, 28). However, it is unknown why the influence of the L-allele on 5-HTT promoter activity is suppressed in heterozygous (S/L) subjects. A possible reason is that the function of the L allele is silenced due to genomic imprinting, in which DNA methylation may play a key role (37). Further studies are warranted.
The present study provides the first evidence that the NET AAGG4 promoter polymorphism is a functional variant or in close linkage disequilibrium with a functional variant. The AAGG4 4-bp deletion allele (S4) is associated with low transcription levels and low transport activity for both DA and NE. However, it is uncertain whether the S4-allele exerts a dominant effect on NET expression because only one placenta was homozygous for the deletion allele (S4/S4). Nevertheless, the transcription level in that placental tissue (0.09) was much lower than that associated with heterozygous genotype S4/L4 (0.22 ± 0.09). This may be because the deletion of the 4-bp AAGG on both alleles leads to the loss of a binding site for a putative transcription factor Elk-1, thus substantially decreasing NET transcription. Stober et al. (41) investigated a highly polymorphic silent polymorphism 1287G/A in NET to distinguish the expression of maternal and paternal alleles in adult human brain. Their results suggest that NET is not imprinted in brain tissues and is thus biallelic in terms of expression. If biallelic expression of NET is indeed shown in placental tissue, then it can be deduced that genotypes S4/S4, S4/L4, and L4/L4 are associated with the lowest, moderate, and highest transcription levels, respectively.
There are several strengths to our experimental design. First, the present study is the first to address the relationship between MAOA, 5-HTT, and NET genotypes and transcription levels and protein activities in human placentas obtained following uncomplicated term pregnancies. Second, the quantitative duplex PCR method applied in this study is more accurate than that used by Sivasubramaniam et al. (40). In their study, amplification of MAOA and the internal control, ACTB, were performed in separate reactions using different amounts of template cDNA and different numbers of PCR cycles. Small variations in cDNA template may lead to biased transcription levels of the target gene. Lastly, our study, for the first time, demonstrated that the NET AAGG4 promoter polymorphism is likely functional and that the 4-bp AAGG deletion may result in decreased NET promoter activity.
This study demonstrated that transcription levels of MAOA, and both transcription and protein activities of 5-HTT and NET, in the placenta are regulated by sequence variation in the promoter region of the three genes. In future studies, the effects of other polymorphisms in MAOA, 5-HTT, and NET should be studied as they may be in close linkage disequilibrium with the above promoter polymorphisms or they may be the true variants that affect gene transcription and protein activity.
GRANTS
This work was supported by research grants from the Canadian Institutes of Health Research through the Interdisciplinary Health Research Team Program (#43580) to the Autism Spectrum Disorders-Canadian-American Research Consortium (ASD-CARC) (J. J. A. Holden, PI; www.autismresearch.ca) and the Ontario Mental Health Foundation (OMHF) to J. J. A. Holden. H. Zhang was supported by a scholarship from the OMHF and National Institute of Health Grant DA-022891.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Anne E. Farley and the Kingston General Hospital staff for help in collecting placental samples. We are also very grateful to those women who participated in this study.
Current address for H. Zhang: Dept. of Psychiatry, Yale Univ. School of Medicine, New Haven, CT.
REFERENCES
- 1.Aklillu E, Karlsson S, Zachrisson OO, Ozdemir V, Agren H. Association of MAOA gene functional promoter polymorphism with CSF dopamine turnover and atypical depression. Pharmacogenet Genomics 19: 267–275, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, Ferrari P, Roubertoux PL, Tordjman S. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Mol Psychiatry 7: 831–836, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin E. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 110: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem 264: 2195–2198, 1989 [PubMed] [Google Scholar]
- 5.Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry 163: 2148–2156, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bzoskie L, Blount L, Kashiwai K, Humme J, Padbury JF. The contribution of transporter-dependent uptake to fetal catecholamine clearance. Biol Neonate 71: 102–110, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Carrasco G, Cruz MA, Dominguez A, Gallardo V, Miguel P, Gonzalez C. The expression and activity of monoamine oxidase A, but not of the serotonin transporter, is decreased in human placenta from pre-eclamptic pregnancies. Life Sci 67: 2961–2969, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science 297: 851–854, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cohen IL, Liu X, Schutz C, White BN, Jenkins EC, Brown WT, Holden JJ. Association of autism severity with a monoamine oxidase A functional polymorphism. Clin Genet 64: 190–197, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Collier DA, Stober G, Li T, Heils A, Catalano M, Di BD, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1: 453–460, 1996 [PubMed] [Google Scholar]
- 11.Conroy J, Meally E, Kearney G, Fitzgerald M, Gill M, Gallagher L. Serotonin transporter gene and autism: a haplotype analysis in an Irish autistic population. Mol Psychiatry 9: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Contini V, Marques FZ, Garcia CE, Hutz MH, Bau CH. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 141B: 305–308, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry 2: 247–250, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet 105: 542–551, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 133B: 79–84, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ganapathy V, Leibach FH. Human placenta: a direct target for cocaine action. Placenta 15: 785–795, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gelernter J, Kruger S, Pakstis AJ, Pacholczyk T, Sparkes RS, Kidd KK, Amara S. Assignment of the norepinephrine transporter protein (NET1) locus to chromosome 16. Genomics 18: 690–692, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet 88: 83–87, 1999 [PubMed] [Google Scholar]
- 19.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hoefgen B, Schulze TG, Ohlraun S, von Widdern O, Höfels S, Gross M, Heidmann V, Kovalenko S, Eckermann A, Kölsch H, Metten M, Zobel A, Becker T, Nöthen MM, Propping P, Heun R, Maier W, Rietschel M. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry 57: 247–251, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 78: 815–826, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iordanidou M, Tavridou A, Petridis I, Arvanitidis KI, Christakidis D, Vargemezis V, Manolopoulos VG. The serotonin transporter promoter polymorphism (5-HTTLPR) is associated with type 2 diabetes. Clin Chim Acta 411: 167–171, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Jonsson EG, Norton N, Gustavsson JP, Oreland L, Owen MJ, Sedvall GC. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatr Res 34: 239–244, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Cox N, Courchesne R, Lord C, Corsello C, Akshoomoff N, Guter S, Leventhal BL, Courchesne E, Cook EH., Jr Transmission disequilibrium mapping at the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry 7: 278–288, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet 133B: 110–115, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lau YF, Chan KM. The putative testis-determining factor and related genes are expressed as discrete-sized transcripts in adult gonadal and somatic tissues. Am J Hum Genet 45: 942–952, 1989 [PMC free article] [PubMed] [Google Scholar]
- 27.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect 95: 157–162, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Levy ER, Powell JF, Buckle VJ, Hsu YP, Breakefield XO, Craig IW. Localization of human monoamine oxidase-A gene to Xp11.23–114 by in situ hybridization: implications for Norrie disease. Genomics 5: 368–370, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri R, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103: 6269–6274, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, Ansseau M. The short allele of the serotonin transporter promoter polymorphism influences relapse in alcohol dependence. Alcohol Alcohol 43: 398–400, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Prasad PD, Hoffmans BJ, Moe AJ, Smith CH, Leibach FH, Ganapathy V. Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells. Placenta 17: 201–207, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878–4884, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quaranta D, Bizzarro A, Marra C, Vita MG, Seripa D, Pilotto A, Sebastiani V, Mecocci P, Masullo C. Psychotic symptoms in Alzheimer's disease and 5-HTTLPR polymorphism of the serotonin transporter gene: evidence for an association. J Alzheimers Dis 16: 173–180, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Ramamoorthy S, Leibach FH, Mahesh VB, Ganapathy V. Active transport of dopamine in human placental brush-border membrane vesicles. Am J Physiol Cell Physiol 262: C1189–C1196, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Retz W, Freitag CM, Retz-Junginger P, Wenzler D, Schneider M, Kissling C, Thome J, Rosler M. A functional serotonin transporter promoter gene polymorphism increases ADHD symptoms in delinquents: interaction with adverse childhood environment. Psychiatry Res 158: 123–131, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rothenburg S, Koch-Nolte F, Thiele HG, Haag F. DNA methylation contributes to tissue- and allele-specific expression of the T-cell differentiation marker RT6. Immunogenetics 52: 231–241, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103: 273–279, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet 127B: 85–89, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Sivasubramaniam SD, Finch CC, Billett MA, Baker PN, Billett EE. Monoamine oxidase expression and activity in human placentae from pre-eclamptic and normotensive pregnancies. Placenta 23: 163–171, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Stober G, Nothen MM, Porzgen P, Bruss M, Bonisch H, Knapp M, Beckmann H, Propping P. Systematic search for variation in the human norepinephrine transporter gene: identification of five naturally occurring missense mutations and study of association with major psychiatric disorders. Am J Med Genet 67: 523–532, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Sukonick DL, Pollock BG, Sweet RA, Mulsant BH, Rosen J, Klunk WE, Kastango KB, DeKosky ST, Ferrell RE. The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Arch Neurol 58: 1425–1428, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Urwin RE, Bennetts B, Wilcken B, Lampropoulos B, Beumont P, Clarke S, Russell J, Tanner S, Nunn KP. Anorexia nervosa (restrictive subtype) is associated with a polymorphism in the novel norepinephrine transporter gene promoter polymorphic region. Mol Psychiatry 7: 652–657, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Urwin RE, Bennetts BH, Wilcken B, Lampropoulos B, Beumont PJ, Russell JD, Tanner SL, Nunn KP. Gene-gene interaction between the monoamine oxidase A gene and solute carrier family 6 (neurotransmitter transporter, noradrenalin) member 2 gene in anorexia nervosa (restrictive subtype). Eur J Hum Genet 11: 945–950, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Verhagen M, van der Meij A, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Effect of the 5-HTTLPR polymorphism in the serotonin transporter gene on major depressive disorder and related comorbid disorders. Psychiatr Genet 19: 39–44, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Vitalis T, Fouquet C, Alvarez C, Seif I, Price D, Gaspar P, Cases O. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J Comp Neurol 442: 331–347, 2002 [DOI] [PubMed] [Google Scholar]
- 46a.Wang Y, Berndt TJ, Gross JM, Peterson MA, So MJ, Knox FG. Effect of inhibition of MAO and COMT on intrarenal dopamine and serotonin and on renal function. Am J Physiol Regul Integr Comp Physiol 280: R248–R254, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Weese-Mayer DE, Berry-Kravis EM, Maher BS, Silvestri JM, Curran ME, Marazita ML. Sudden infant death syndrome: association with a promoter polymorphism of the serotonin transporter gene. Am J Med Genet A 117A: 268–274, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Weyler W, Hsu YP, Breakefield XO. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 47: 391–417, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Weyler W, Salach JI. Purification and properties of mitochondrial monoamine oxidase type A from human placenta. J Biol Chem 260: 13199–13207, 1985 [PubMed] [Google Scholar]
- 50.Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, Costa PT, Schofield PR, Gordon E. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology 34: 1797–1809, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry 66: 1201–1209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yavarone MS, Shuey DL, Sadler TW, Lauder JM. Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta 14: 149–161, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Yoo HJ, Lee SK, Park M, Cho IH, Hyun SH, Lee JC, Yang SY, Kim SA. Family- and population-based association studies of monoamine oxidase A and autism spectrum disorders in Korean. Neurosci Res 63: 172–176, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology 30: 1719–1723, 2005 [DOI] [PubMed] [Google Scholar]