Abstract
The aim of this study was to characterize the responses of individual tissues to high-fat feeding as a function of mass, fat composition, and transcript abundance. We examined a panel of eight tissues [5 white adipose tissues (WAT), brown adipose tissue (BAT), liver, muscle] obtained from DBA/2J mice on either a standard breeding diet (SBD) or a high-fat diet (HFD). HFD led to weight gain, decreased insulin sensitivity, and tissue-specific responses, including inflammation, in these mice. The dietary fatty acids were partially metabolized and converted in both liver and fat tissues. Saturated fatty acids (SFA) were converted in the liver to monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA), and oleic acid (C18:1) was the preferred MUFA for storage of excess energy in all tissues of HFD-fed mice. Transcriptional changes largely reflected the tissue-specific fat deposition. SFA were negatively correlated with genes in the collagen family and processes involving the extracellular matrix. We propose a novel role of the tryptophan hydroxylase 2 (Tph2) gene in adipose tissues of diet-induced obesity. Tissue-specific responses to HFD were identified. Liver steatosis was evident in HFD-fed mice. Gonadal, retroperitoneal and subcutaneous adipose tissue and BAT exhibited severe inflammatory and immune responses. Mesenteric adipose tissue was the most metabolically active adipose tissue. Gluteal adipose tissue had the highest mass gain but was sluggish in its metabolism. In HFD conditions, BAT functioned largely like WAT in its role as a depot for excess energy, whereas WAT played a role in thermogenesis.
Keywords: tissue panel, gene expression, insulin, obesity, steatosis, palmitic acid, stearic acid, palmitoleic acid, tryptophan hydroxylase, serum amyloid A3, regenerating islet family
obesity is a well-recognized risk factor for metabolic syndrome and disease that is often accompanied by low-grade chronic inflammation (20, 27). Pioneering work of McGarry (36) posed that insulin resistance and hyperglycemia may be better understood if viewed in the context of lipid metabolism. Alterations to the fat composition and distribution across different tissues can have whole body metabolic consequences that lead to disease states. Lipids are not only storage molecules for excess energy; they are also major components of cell membranes and act as signaling molecules that regulate systemic biological processes. As such, they can alter transcription locally or act as information carriers to alter function in other cells, tissues, or organs.
White adipose tissue (WAT) and brown adipose tissue (BAT) play complementary roles in energy balance and body weight control. In most mammals, BAT is the primary mediator of adaptive thermogenesis and therefore plays a critical role in energy expenditure. WAT serves as a depot for energy storage and signals information to peripheral organs and tissues about energy reserves. During weight gain, fat depots are not utilized equally, and they differ in their architecture and function (20, 34, 35). It has become increasingly evident that the region of fat accumulation and the availability of specific fatty acids play a pivotal role in disease susceptibility. Central obesity, characterized by high levels of visceral fat, has been shown to correlate to type 2 diabetes, dyslipidemia, and cardiovascular diseases (10, 55, 58). Alternatively, peripheral obesity, increased amounts of subcutaneous fat, can have protective effects that improve insulin sensitivity and lower risk factors for diseases (44, 51). In addition, ectopic storage of fat in liver and muscle has been linked to liver steatosis and insulin resistance in muscle (5, 23).
Nutritional genomic studies have analyzed responses of tissues to different diets and nutrients (1, 14, 18). However, results vary across species and tissues as well as experimental protocols (48, 53). The extent and manner in which different tissues respond to increased fat storage is not well understood. Therefore, the aim of this study was to link diet-induced fat deposition and fatty acid profiles with tissue-specific functional adaptations in response to fat storage. We expected that differences in tissue mass and fatty acid composition would lead to differential changes in specific transcripts involved in pathways that lead to obesity and insulin resistance.
DBA/2J mice showed the highest weight gain in response to a high-fat diet (HFD) among 43 inbred strains (49). Therefore, we exposed young DBA/2J mice to 6 wk of HFD feeding [rich in saturated fatty acids (SFA)] and examined tissue-specific mass gain, fat composition, and transcript abundances. We studied the responses of BAT, WAT (gonadal, gluteal, mesenteric, retroperitoneal, subcutaneous), liver, and muscle at 12 wk of age, when mice on HFD continued to gain weight and had an overall slowed metabolic rate.
MATERIALS AND METHODS
Animals, diets, and housing.
Twenty-four 5-wk-old male DBA/2J mice were obtained from The Jackson Laboratory. The average body weight at delivery was 14.8 ± 1.5 g. All animals were fed standard breeding diet (SBD) until 6 wk of age. Animals were then divided into two feeding groups with the same average body weight per group. In the 6 wk that followed, one group was fed a HFD and the other kept on a SBD. Animals were fed ad libitum and had free access to water. Mice were housed in pairs in a specific pathogen-free room with a 12:12-h light-dark cycle. All experimental procedures were approved by the Animal Care and Use Committee for experiments at the Jackson Laboratory (approval no. A3268-01) and by the German Animal Welfare Authorities for the experiments at the Animal Facility at Humboldt-University (approval no. G0301/08).
The HFD was a Purified TestDiet 58G8 with 45% energy from fat, which is based on TestDiet Basal Diet 5755 (Purina Mills, Richmond, IN). The diet consisted of 21.9% proteins, 23.5% fat, 4.9% fibers, and 42.7% carbohydrates, minerals, and vitamins (values are in wt/wt). The energy content was 4.70 kcal/g (19.68 kJ/g); fat contributed 45%, proteins 18.6%, and carbohydrates 36.4%. The SBD was standard Rat and Mouse/Auto 4F breeding LabDiet 5K54 (Purina Mills). The diet consisted of 18% protein, 4% fat, and 5% fibers. The gross energy of SBD was 3.97 kcal/g (16.62 kJ/g); 22% of the energy was provided by proteins, 11% by fat, and 67% by carbohydrates. The physiological fuel value was 3.28 kcal/g (13.73 kJ/g), and the metabolizable energy was 3.09 kcal/g (12.94 kJ/g). The fat in HFD was primarily lard, with some corn oil, while the fat source in the SBD was soy oil. One production batch of diet was fed over the whole experimental period. The producer provided the data on the food composition. The chemical analysis of the diets showed that HFD and SBD contained 21.8 and 4.6 g of fat per 100 g of diet, respectively. Diets were sterilized before use.
Mouse phenotyping and tissue sampling.
Mice were weighed weekly between 5 and 12 wk of age. Food intake was estimated as the difference between the offered and the remnant amount of food at 7-day intervals from week 8 onward. The food was provided as pressed pellets for low spillage, and residual spillage was not considered here. Energy intake, determined from the energy content in each diet and mass consumed, was calculated on a per-day basis. Feed efficiency was determined as the ratio of body weight gain in grams to consumed energy in kilojoules. For comparison between animals, energy intake, feed efficiency, and weekly measurements of food intake were averaged over the period between 8 and 12 wk.
After the 6-wk feeding period with different diets, blood and tissue samples were collected from 12-wk-old mice for the determination of serum factors, gene expression analyses, and fatty acid profiles. Mice were individually fasted for 2 h before dissection, which was carried out between 1 PM and 3 PM on three successive days. After the fasting period, blood was withdrawn from the retroorbital plexus of each mouse through a heparin-coated hematocrit tube into a 1.5-ml tube and placed at room temperature until all samples were centrifuged at 600 g for 10–15 min to obtain serum for the analysis of lipids, glucose, insulin, and leptin. Subsequently, animals were killed by cervical dislocation. After bleeding, the subcutaneous fat (mainly inguinal fat pads), the gluteal fat pad (which is subcutaneous upon the gluteal musculus maximus between the legs left and right of the tail), the quadriceps (musculus rectus femulus, musculus vastus intermedius, musculus vastus lateralis, musculus vastus medialis), the gonadal fat pads (surrounding gonads), the retroperitoneal fat pads (below the kidney), the liver, the mesenteric fat pad (hanging at the intestine), and the BAT (surrounded by WAT) were carefully dissected in the given order. Tissues from six animals in each feeding group were collected in RNAlater (Ambion, Austin, TX) for gene expression analysis. The tissues of the other six animals per feeding group were shock frozen and stored under nitrogen at −80°C before determination of the fatty acid profile.
Serum lipids [total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), free (nonesterified) fatty acids (NEFA)] and glucose were measured on the day of dissection with a Beckman Coulter Synchron CX5 Delta Chemistry Analyzer (Beckman Coulter, Fullerton, CA) according to the manufacturer's instructions (details at http://pga.jax.org/protocols). Total cholesterol and HDL cholesterol were measured directly. An estimate of non-HDL cholesterol, which in the mouse consists of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) cholesterol, may be obtained by subtracting HDL cholesterol from total cholesterol. Serum insulin was determined in 5-μl samples with the commercial Insulin Mouse Ultrasensitive ELISA Kit from DRG Instruments (Marburg, Germany). As described above, leptin was determined with the m/rLeptin ELISA kit by Mediagnost (Reutlingen, Germany). For the comparison of phenotypic values between feeding groups, Student's t-test for two-tailed distributions with equal variances was applied. The influence of diet and tissue on the variance of fatty acid distribution pattern was analyzed with the statistical package SAS (SAS 1989).
For chemical analysis of the fatty acid profiles, diet samples and different tissues of three mice per diet group were collected separately in 15-ml tubes, which were filled with nitrogen gas. All tissues were weighed, transferred into tubes, and overlaid with nitrogen gas again to prevent oxidization of fatty acids during storage at −18°C. For analysis in the serum, six sera per group were pooled to obtain enough material for the analysis. The determination of fatty acid profiles comprised quantification of the SFA C10:0, C12:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C21:0, and C24:0, the monounsaturated fatty acids (MUFA) C14:1, C15:1, C16:1, C17:1, C18:1, C18:1, C20:1, C22:1, and C24:1, and the polyunsaturated fatty acids (PUFA) C18:2 n–6, C18:3 n–3, C18–4 n–3, C20:2, C20:3 n–6, C20:4 n–6, C20:5 n–3, C22:2, C22–5, and C22–6 n–3. The accuracy of measurements for fatty acid components per 100 g was as follows: >5 g/100 g fat ± 10%, 1–5 g/100 g fat ± 10–15%, 0.5–1 g/100 g fat ± 20%, and <0.5 g/100 g fat ± 100%. Since the fatty acid amounts of C10–C15, C17, C18:4 n–3, and C20–C24 were below 0.5 g/100 g fat, and thus not reliably detectable, these fatty acids were not further considered. The chemical analyses were performed at Bilacon (Berlin, Germany) according to a standardized method that used trimethyl sulfonium hydroxide for esterification of fatty acids to fatty acid methyl ester (21) with a gas chromatograph (Shimadzu, Japan) and a flame ionization detector.
In a separate experiment, an intraperitoneal glucose tolerance test (IPGTT) and measurement of TG in the liver and fatty acid composition in serum were obtained for six DBA/2J male mice per diet group. Feeding and management conditions were the same as described above, except that housing was conventional, not pathogen free. To assess glucose tolerance, 12-wk-old animals were fasted for 9 h before the experiment. After a baseline blood sample was taken for fasting glucose and fasting insulin determination, each animal received a single intraperitoneal injection of glucose (B. Braun, Melsungen, Germany) at a dose of 2 g/kg body wt as described previously (8). For glucose concentrations, blood was obtained at 15, 30, 60, and 120 min after injection from the tail tip. Blood glucose concentration was measured with the glucose analyzer Ascensia Elite (Bayer HealthCare, Leverkusen, Germany). To minimize distress, mice were kept in their accustomed cages and had free access to water. To assess glucose tolerance, the area under glucose curves (AUC) was calculated between 0 and 120 min after glucose injection and expressed as millimoles per liter × 120 min. Lower AUC values reflect more efficient glucose clearance. Serum insulin was determined in duplicate as described above.
Liver tissue was collected from these mice to determine TG content for Oil Red O staining. The liver TG extraction procedure was adapted from Ref. 29. The content of extracted TG was determined from the supernatant with a TG kit from Sigma (St. Louis, MO). TG content was correlated to the total protein content in the liver homogenates, determined with the BCA kit from Thermo Scientific (Pierce, Rockford, IL). For histological examination, parts of livers were immediately fixed upon dissection in 4% buffered formaldehyde solution (pH 7.4) for 24 h. Cryosections (10 μm) were incubated with 0.18% Oil Red O for 10 min, washed with 60% isopropanol, and counterstained with hematoxylin. Sections were photographed at ×10 magnification (digital camera).
Microarray processing and analysis.
Total RNA was isolated by TRIzol Plus after tissue homogenization (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols. RNA quality was assessed with an Agilent 2100 Bioanalyzer instrument and the RNA 6000 Nano LabChip assay (Agilent Technologies, Palo Alto, CA). Total RNA was then reverse transcribed, followed by second-strand cDNA synthesis. An in vitro transcription (IVT) reaction was carried out incorporating biotinylated nucleotides according to the manufacturer's protocol for the Illumina Totalprep RNA amplification kit (Ambion). Biotin-labeled cRNA (1.5 μg) was hybridized onto Mouse-6 Expression BeadChips (Illumina, San Diego, CA) for 16 h at 55°C.
The tissue samples were randomized over 16 BeadChips. Posthybridization staining and washing were performed according to Illumina's protocols. BeadChips were scanned with Illumina's BeadStation 500 scanner. Images were checked for grid alignment and quantified with BeadStudio v3.1 software, and control summary graphs were generated. Probe set data, mean pixel intensities by bead type, were created with BeadStudio v3.1 and processed within the R/beadarray package (17). Data quality was assessed with histograms of raw signal intensities, MA plots, and analysis of the control probe sets. A log2 transformation was applied to the data, and quantile normalization was performed (6). The 90th quantile was calculated from the negative controls as 7.68 and used as an approximation of the gene expression background intensity.
Analysis of variance (ANOVA) was used to determine gene expression differences (30). In an ANOVA model, the expression measures are described as the sum of components that contribute to the overall intensity of each probe Yi on the array. For each tissue, the following model was fit and tested to determine differences in gene expression due to diet:
where μ is the mean for each array, Diet is a two-level factor for the two diet groups and εi captures the random error. In addition, the following ANOVA model was fit and tested:
where Tissue:Diet is a 16-level factor describing the possible interactions between the 8 tissues and 2 diet groups.
Statistical tests were performed with a modified F statistic, Fs, which incorporates shrinkage estimates of variance components (13). P values were obtained by permuting the model residuals 1,000 times (Supplemental Fig. S1).1 Calculations were performed with the R/MAANOVA package (56). The false discovery rate (FDR) for statistically significant probes was estimated with q values (46).
Correlation analysis and principal component analysis.
Pairwise correlations between variance components of differentially expressed genes from the tissue-by-diet interaction ANOVA model and fatty acid components were calculated as the Pearson correlation coefficient.
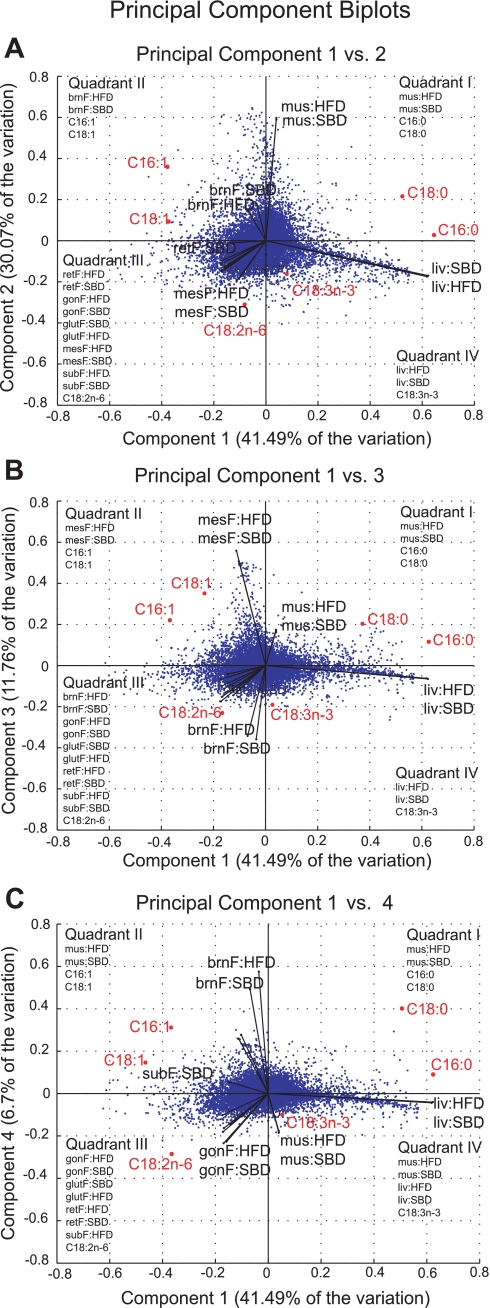
Principal component analysis (PCA) applied to individual variance components in an ANOVA model has been shown to provide insight into the effects of treatments on gene expression data (15). We applied PCA to the variance components from the ANOVA tissue-by-diet interaction model together with the fatty acid components C16:0, C16:1, C18:0, C18:1, C18:2 n–6, and C18:3 n–3 in different tissues and dietary conditions. The variance components from the tissue-by-diet interaction model were combined with the standardized fatty acid composition measurements for each tissue and diet condition for the PCA analysis. The first four principal components explained 90% of the variation. A biplot of the first four principal components is shown in Fig. 4. The Hotelling T2 statistic was calculated for each gene and fatty acid component as
where u is the score of the ith observation on the kth principal component and su2 is the variance of scores. A critical value was calculated at the α = 0.01 level as
which yielded 2,508 probe sets with significant diet-by-tissue interaction.
Fig. 4.
Principal component analysis (PCA) biplots for the first 4 principal components (PCs) (PC1 vs. PC2, A; PC1 vs. PC3, B; PC1 vs. PC4, C), which account for 90% of the variability in the PCA analysis of fatty acid composition data and variance components from the ANOVA interaction model Yi = μ + Diet + Tissue + Diet:Tissue + εi. Points represent the scores for the probes (blue) and for the fatty acid components (red) PCA loadings are shown as black lines. A list of genes that correlate with adipose tissue contents of palmitic (C16:0), stearic (C18:0), palmitoleic (C16:1), and oleic (C18:1) acids is given in Supplemental Table S6.
Pathway enrichment.
The Gene Ontology Consortium has established a controlled vocabulary, Gene Ontology (GO), to describe gene and gene product relationships (26). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways have been developed to describe relationships over metabolic, signaling, and disease pathways (28). We applied two types of pathway analyses that aim to capture different aspects of the enrichment signal to detect overrepresented KEGG and GO categories.
Hypergeometric tests were performed with the GOstats package to detect enriched biological categories (19). The gene universe (background) was defined as the 12,656 unique Entrez Gene Identifiers present in the data set. Gene sets were tested for significance at the threshold level P value < 0.01 in KEGG and GO categories.
Random set enrichment analysis was applied to assess which pathways and processes were most significantly changed in the HFD condition within each of the tissues (41). Briefly, the method calculates category scores from gene-level evidence, which is a quantitative measure for each gene in the expression data set. In the case at hand, we considered the genes, g ∈ {g1, g2, . . . , gG}, and their gene level scores, sg ∈ { s1, s2, . . . , sG}, as the t statistics calculated from the Fs statistic. In this analysis, a biological category of genes is scored for enrichment by averaging the gene level evidence across the category
and computing the standardized category enrichment score Z = (X̄ minus μ)/σ, where μ and σ are the first two moments of the underlying distribution. Enrichment scores were calculated for each tissue and adjusted to account for the effects of category size and multiple probes per gene. All computations were done with the R function allez.R (http://www.stat.wisc.edu/∼newton/), with mappings from the Illumina annotation package lumiMouseV1 in Bioconductor (http://www.bioconductor.org/).
RESULTS
Physiological changes due to high-fat feeding.
Mice on a HFD gained 17.5 g body wt compared with a 8.3-g weight gain in mice on SBD (Supplemental Table S1). Increased weight on HFD was observed in all examined tissues except muscle (Table 1). The increased liver weight represents ectopic fat storage and is consistent with elevated TG content (Fig. 1, A and B), which was about three times higher in HFD- compared with SBD-fed mice (Fig. 1C). Adipose tissues showed markedly different mass distribution patterns, gluteal fat pads showed the highest gain (HFD 3.7 times heavier), and mesenteric adipose tissue showed the lowest (HFD 2.0 times heavier). Supporting this, the fatty acid binding proteins (Fabp3 and Fabp5) that transport fatty acids into the cell were activated in most adipose tissues (Table 2). Increased fat mass was accompanied by high serum leptin levels (17.0 ± 5.7 ng/ml on HFD vs. 2.4 ± 0.9 ng/ml on SBD) (Table 3) but did not lead to a reduction of consumed energy per day (Supplemental Table S1). Leptin expression was high and activated under HFD conditions in nearly all adipose tissues and was strongest in BAT (3.7-fold) and muscle (3.5-fold) (Table 2). Gluteal adipose tissue had the highest leptin expression levels (with no difference between diet groups) and the highest activation of fatty acid binding proteins in response to HFD, which is consistent with it having the largest relative mass gain under HFD conditions.
Table 1.
Fat deposition in tissues
| Tissue | HFD | SBD | HFD/SBD | P Value |
|---|---|---|---|---|
| BAT | 0.39 ± 0.02 | 0.14 ± 0.02 | 2.38 | <0.001 |
| GonF | 1.08 ± 0.18 | 0.45 ± 0.11 | 2.41 | 0.002 |
| GlutF | 0.35 ± 0.03 | 0.09 ± 0.03 | 3.73 | <0.001 |
| MesF | 0.5 ± 0.02 | 0.24 ± 0.04 | 2.04 | <0.001 |
| RetF | 0.42 ± 0.01 | 0.16 ± 0.03 | 2.74 | <0.001 |
| SubF | 1.51 ± 0.16 | 0.54 ± 0.13 | 2.81 | <0.001 |
| Liver | 1.21 ± 0.04 | 1.07 ± 0.03 | 1.13 | 0.003 |
| Muscle | 0.24 ± 0.04 | 0.25 ± 0.02 | 1.09 | 0.398 |
Values (in g) are mean ± SD weights of the surveyed tissues [brown adipose tissue (BAT); gonadal (GonF), gluteal (GlutF), mesenteric (MesF), retroperitoneal (RetF), and subcutaneous (SubF) white adipose tissues; liver; and muscle] in male mice at 12 wk after a 6-wk feeding period with standard breeding diet (SBD) or high-fat diet (HFD).
Fig. 1.
Oil Red O staining showing steatosis in mice on a high-fat diet (HFD). A: fat deposition in liver tissue of mice on a standard breeding diet (SBD). B: high fat deposition in mice on HFD. C: triglyceride (TG) content in liver of SBD- and HFD-fed mice. **P < 0.01.
Table 2.
Fold changes of selected genes influenced by diet
| Symbol | BAT | GonF | GlutF | MesF | RetF | SubF | Liver | Muscle |
|---|---|---|---|---|---|---|---|---|
| Highly upregulated genes | ||||||||
| Ccdc3 | 1.5 | 1.7 | 1.6 | 1.7 | 1.5 | 1.4 | −1.0 | 1.0 |
| Col6a1 | 1.6 | 2.0 | 1.4 | 1.5 | 1.9 | 1.8 | −1.0 | −1.1 |
| Degs1 | 1.6 | 1.4 | 1.5 | 1.3 | 1.7 | 1.7 | −1.0 | −1.0 |
| Ear2 | 3.7 | 2.3 | 2.1 | 1.6 | 3.9 | 2.6 | 1.0 | 1.4 |
| Fgf13 | 1.9 | 2.9 | 2.2 | 2.2 | 1.8 | 3.0 | 1.1 | 1.1 |
| Hp | 1.4 | 1.7 | 1.3 | 2.0 | 2.4 | 1.7 | 1.2 | 1.5 |
| Lbp | 2.4 | 1.8 | 1.4 | 2.3 | 1.8 | 2.1 | −1.3 | 1.2 |
| Lrg1 | 1.5 | 1.4 | 1.0 | 2.0 | 1.9 | 1.3 | 1.1 | 1.3 |
| Mest | 3.9 | 2.0 | 2.3 | 3.4 | 1.7 | 4.2 | −1.0 | 1.4 |
| Mrc2 | 1.6 | 3.8 | 1.5 | 2.0 | 2.5 | 3.0 | −1.1 | 1.1 |
| Saa3 | 2.4 | 3.3 | 4.8 | 2.0 | 2.3 | 5.1 | −1.2 | 1.3 |
| Slc5a7 | 1.2 | 3.0 | 2.7 | 1.4 | 2.1 | 2.9 | −1.0 | 1.0 |
| Tnfrsf12a | 2.4 | 1.8 | 2.8 | 1.7 | 1.7 | 2.2 | −1.0 | −1.3 |
| Tph2 | 4.6 | 20.7 | 13.6 | 5.2 | 24.7 | 16.2 | −1.1 | 1.3 |
| Trem2 | 2.3 | 2.5 | 2.8 | 1.8 | 7.1 | 2.7 | 1.0 | 1.1 |
| Ubd | 4.6 | 9.4 | 7.7 | 1.8 | 9.6 | 4.0 | 1.2 | 1.6 |
| Highly downregulated genes | ||||||||
| Aacs | −1.7 | −1.7 | −1.5 | −1.7 | −1.9 | −1.7 | 1.5 | −1.4 |
| Acaca | −1.2 | −2.2 | −1.6 | −2.7 | −3.0 | −2.5 | 1.1 | −1.8 |
| Acacb | −1.5 | −2.2 | −1.8 | −2.4 | −3.1 | −3.9 | 1.7 | 1.1 |
| Acly | −1.1 | −2.1 | −1.5 | −2.5 | −2.5 | −2.9 | 1.3 | −1.7 |
| Acsm5 | −1.2 | −2.4 | −2.3 | −1.4 | −2.0 | −1.6 | −1.1 | −1.1 |
| AU018778 | −1.7 | −2.6 | −1.6 | −1.7 | −2.9 | −1.6 | −1.2 | 1.0 |
| Cldn22 | −1.6 | −2.6 | −1.7 | −1.5 | −2.5 | −1.9 | −1.0 | 1.0 |
| Cyp2e1 | −7.0 | −11.4 | −5.4 | −6.2 | −8.2 | −7.6 | 1.1 | −3.3 |
| Dlc1 | −1.5 | −1.4 | −1.6 | −1.2 | −1.6 | −1.2 | −1.0 | −1.1 |
| Elovl6 | −1.5 | −2.7 | −1.9 | −4.4 | −5.1 | −6.4 | 1.2 | −1.8 |
| Gcat | −1.5 | −1.5 | −1.3 | 1.2 | −1.6 | −1.6 | −1.3 | 1.0 |
| Gchfr | −1.5 | −2.7 | −1.4 | −1.6 | −2.2 | −2.8 | 1.1 | −1.0 |
| Gpr81 | −2.2 | −1.6 | −1.4 | −1.8 | −2.1 | −2.0 | 1.0 | −1.1 |
| Gstp1 | −1.3 | −1.6 | −1.5 | −1.3 | −1.5 | −1.4 | −1.3 | 1.1 |
| Hsd11b1 | −1.5 | −2.0 | −1.9 | −1.9 | −2.3 | −2.5 | −1.0 | −1.3 |
| Immp2l | −1.4 | −2.0 | −1.3 | −1.2 | −1.9 | −2.0 | 1.2 | −1.0 |
| Irs1 | −1.7 | −2.0 | −2.4 | −1.5 | −2.6 | −1.9 | −1.3 | −1.3 |
| Lman2l | −1.4 | −1.4 | −1.4 | −1.3 | −1.6 | −1.7 | −1.2 | −1.1 |
| Ndufa12 | −1.3 | −1.7 | −1.4 | −1.3 | −1.7 | −1.5 | −1.1 | −1.0 |
| Prkrir | −1.3 | −1.4 | −1.5 | −1.3 | −1.7 | −1.3 | −1.1 | 1.1 |
| Pth1r | −2.5 | −1.7 | −1.8 | −1.7 | −2.0 | −2.5 | −1.0 | −1.0 |
| Rorc | −1.5 | −1.9 | −2.3 | −1.7 | −3.1 | −3.5 | 1.2 | −1.1 |
| Slc1a3 | −1.6 | −2.1 | −2.2 | −1.6 | −2.0 | −2.0 | −1.0 | −1.3 |
| Tmem79 | −1.4 | −2.1 | −1.4 | −2.0 | −3.5 | −2.9 | −1.0 | −1.1 |
| Signature genes associated with obesity | ||||||||
| Acadl | 1.2 | 1.2 | 1.7 | 1.1 | 1.1 | 1.1 | 1.1 | 1.3 |
| Acadm | 1.3 | 1.2 | 1.7 | 1.5 | 1.3 | 1.4 | 1.0 | 1.3 |
| Acot3 | −1.1 | 1.1 | 1.1 | 1.1 | 1.0 | −1.1 | 2.0 | 1.1 |
| Acsl1 | 1.4 | 1.7 | 2.3 | 1.5 | 1.4 | 1.5 | −1.1 | 1.1 |
| Fabp3 | 1.2 | 1.0 | 4.3 | 2.8 | −1.1 | 1.3 | −1.1 | 1.2 |
| Fabp5 | 1.1 | 1.7 | 2.3 | 1.1 | 1.5 | 1.4 | −1.2 | −1.1 |
| Fads1 | −1.2 | −1.2 | −1.5 | −1.1 | 1.0 | −1.3 | 1.5 | −1.3 |
| Fads2 | −1.1 | −1.2 | −1.1 | −1.0 | −1.2 | −1.1 | 2.0 | −1.1 |
| Hmgcs1 | 1.5 | 1.1 | 1.1 | 1.2 | 1.1 | 1.3 | 1.5 | 1.0 |
| Lep | 3.7 | 1.4 | 1.0 | 2.8 | 1.4 | 1.9 | −1.1 | 3.5 |
| Retn | −1.1 | −2.1 | −1.1 | −1.2 | −1.5 | 1.0 | −1.0 | 1.0 |
| Slc2a3 | 1.0 | −1.9 | −1.6 | −1.0 | −1.5 | −1.4 | 1.0 | −1.1 |
| Sqle | −1.8 | −1.1 | 1.2 | −1.0 | −1.1 | −1.3 | 1.4 | −1.2 |
| Ucp1 | 1.3 | 4.8 | 4.6 | 4.1 | 2.2 | 1.1 | −1.1 | 1.2 |
| Ucp2 | −1.1 | 1.6 | 1.8 | 1.1 | 1.9 | 1.5 | 1.3 | 1.2 |
| Genes associated with extracellular matrix | ||||||||
| Col1a1 | 1.3 | 2.1 | −1.1 | 1.2 | 2.0 | 1.3 | −1.1 | −1.2 |
| Col3a1 | 1.8 | 1.6 | 1.5 | 1.3 | 1.9 | 1.5 | −1.0 | −1.2 |
| Col4a1 | 1.5 | 1.4 | 1.0 | 1.3 | 1.2 | 1.6 | −1.3 | −1.2 |
| Col4a6 | −1.0 | −1.5 | −1.6 | −1.4 | −1.6 | −1.2 | 1.0 | −1.1 |
| Col5a1 | 1.4 | 1.4 | −1.1 | 1.2 | 1.4 | 1.3 | −1.0 | −1.2 |
| Col5a2 | 1.3 | 1.8 | 1.1 | 1.2 | 1.7 | 1.5 | −1.1 | −1.2 |
| Col6a1 | 1.6 | 2.0 | 1.4 | 1.5 | 1.9 | 1.8 | −1.0 | −1.1 |
| Col6a2 | 1.4 | 1.9 | 1.5 | 1.4 | 1.9 | 1.7 | 1.0 | −1.0 |
| Col8a1 | 1.5 | 1.6 | 1.3 | 1.4 | 1.4 | 1.6 | −1.1 | 1.1 |
| Col15a1 | 1.5 | 1.2 | 1.2 | 1.5 | 1.1 | 1.8 | 1.1 | −1.0 |
| Col16a1 | 1.5 | 2.0 | 1.7 | 1.4 | 2.3 | 2.1 | −1.0 | −1.1 |
| Col17a1 | 1.1 | −1.2 | −1.2 | −1.1 | −1.2 | 1.2 | −1.0 | −1.0 |
| Col18a1 | 1.7 | 1.3 | 1.1 | 1.4 | 1.5 | 1.8 | −1.0 | −1.1 |
| Col20a1 | −1.6 | −1.1 | −1.3 | −1.2 | −1.3 | −1.4 | −1.1 | 1.0 |
| Mmp12 | 3.2 | 3.3 | 3.5 | 2.1 | 7.5 | 4.4 | −1.0 | 1.1 |
| Mmp9 | −1.3 | −1.4 | −1.9 | −1.1 | −1.4 | −1.8 | −1.0 | −1.1 |
| Mrc2 | 1.6 | 3.8 | 1.5 | 2.0 | 2.5 | 3.0 | −1.1 | 1.1 |
| Pcolce2 | 1.7 | 1.7 | 1.7 | 1.6 | 1.6 | 1.8 | −1.2 | 1.2 |
Values are fold change (HFD vs. SBD). In cases where >1 transcript represents a gene, the fold change corresponding to the most significant transcript (smallest P value) is represented. A full list of absolute normalized expression values of the transcripts of all genes differentially expressed in at least 1 tissue in response to diet is given in Supplemental Table S5. Normalized expression data are also available through the Gene Expression Omnibus (GEO) database (accession no. 15822). Boldface, significantly upregulated; italics, significantly downregulated.
Table 3.
Serum measurements
| HFD (n = 10) | SBD (n = 11) | HFD/SBD | P Value | |
|---|---|---|---|---|
| Leptin, ng/ml | 17.03 ± 5.70 | 2.37 ± 0.87 | 7.18 | <0.001 |
| Insulin, ng/ml | 8.57 ± 5.16 | 1.73 ± 0.76 | 4.95 | <0.001 |
| Glucose, mg/dl | 160.9 ± 16.29 | 118.9 ± 8.84 | 1.35 | <0.001 |
| Triglycerides, mg/dl | 112.9 ± 27.05 | 139.6 ± 13.72 | 0.81 | 0.009 |
| NEFA, meq/l | 1.82 ± 0.25 | 2.03 ± 0.19 | 0.90 | 0.044 |
| Total cholesterol, mg/dl | 120.8 ± 9.29 | 73.45 ± 5.85 | 1.65 | <0.001 |
| HDL cholesterol, mg/dl | 116.5 ± 10.95 | 52.48 ± 4.90 | 2.22 | <0.001 |
| non-HDL cholesterol, mg/dl | 3.59 ± 4.78 | 19.23 ± 6.74 | 0.19 | <0.001 |
| T4 thyroid hormone, μg/dl | 4.95 ± 0.68 | 7.23 ± 1.19 | 0.69 | <0.001 |
Serum values are means ± SD for n mice. Measurements were done on 12-wk-old males after feeding of either HFD or SBD between 6 and 12 wk of age. Serum was collected 2 h after fasting at 1:00 PM. NEFA, nonesterified fatty acids; HDL, high-density lipoprotein.
A decline in feed efficiency and a decline in weight gain was observed over time in HFD-fed mice (Supplemental Table S1), indicating an adaptation to the obese state. This could be caused in part by increased thermogenesis. Members of the uncoupling protein family (Ucp1 and Ucp2) that enhance thermogenesis were up to fourfold increased in WAT of HFD-fed mice (Table 2). BAT had the highest Ucp levels, but no significant difference was detected between diet groups.
Total cholesterol and HDL levels were elevated in HFD-fed mice; the non-HDL cholesterol portion was reduced 10-fold. The high cholesterol level is supported by the 1.4-fold upregulation of squalene epoxidase (Sqle) in the liver—a critical mediator of cholesterol biosynthesis (Table 2). Mice fed a HFD had increased levels of serum insulin and glucose (Table 3) and reduced glucose clearance, indicating reduced insulin sensitivity (Fig. 2). Serum TG and NEFA decreased, likely because of a decrease in energy demand and their clearance through tissues. Thyroid hormone T4 levels fell, mirroring a decline in the basal metabolic rate. Transcriptional changes in mice fed HFD support these observations. Insulin receptor substrate 1 (Irs1), glucose transporter 3 (Slc2a3), and hydroxysteroid 11-β dehydrogenase 1 (Hsd11b1) were significantly reduced in nearly all tissues of HFD-fed mice, reflecting the onset of insulin resistance, reduced glucose clearance, and a decline in the metabolic rate (Table 2).
Fig. 2.
A: glucose clearance in mice on SBD or HFD after intraperitoneal glucose injection after 9-h fasting. B: area under the curve (AUC). *P < 0.05.
The fatty acid composition of the diets was not directly reflected in serum and tissues (Fig. 3, Table 4). The fatty acid composition of the liver for both diet groups was primarily SFA (41–42%), whereas the fatty acids in muscle and adipose tissues were mainly MUFA. SFA was reduced in all adipose tissues and muscle (23–28% in HFD-fed mice, 26–34% in SBD-fed mice) as well as in serum (39% in HFD-fed mice compared with 63% on SBD), whereas MUFA was increased (50–53% in HFD-fed mice, 37–47% in SBD fed mice, and 23% compared with 10% in serum). Oleic acid (C18:1) was the most abundant MUFA in the HFD group (Table 4). In contrast to the total MUFA fraction, the portion of palmitoleic acid (C16:1) was reduced in all tissues. However, the proportion of C16:1 was higher in tissues compared with diet and serum, suggesting its synthesis within tissues, but this synthesis is reduced under HFD as fatty acid biosynthesis is repressed in general (Table 5). The expression level of stearyl-coenzyme A desaturase 1 (Scd1) was not affected (Supplemental Table S5). HFD-fed mice had lower PUFA percentages (C18:2 n–6 and C18:3 n–3) in WAT and liver compared with SBD-fed mice (22–25% vs. 28–36%). This is primarily due to the lower PUFA content in the HFD. The percentage of PUFA in all tissues and serum was higher than in the HFD itself (16% in HFD vs. 21–25% in tissues and 40% in serum). This may be due in part to the accelerated conversion and degradation of SFA in the liver, facilitated by fatty acid desaturases 1 and 2 and acyl-CoA thioesterase 3 (Fads1, Fads2, and Acot3), which were activated (1.5- to 2.0-fold) in HFD conditions (Table 2). Activation of 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1 (Hmgcs1) in the liver (1.5-fold) is further evidence of hepatic SFA breakdown for the synthesis of ketone bodies in HFD mice. The high proportion of MUFA and PUFA in the serum of HFD-fed mice suggests the transport to WAT following conversion.
Fig. 3.
Percentage of saturated fatty acid (SFA), polyunsaturated fat (PUFA), and monounsaturated fat (MUFA) in the diet, serum, and tissues of mice fed SBD (A) and HFD (B). BAT, brown adipose tissue; Glut, Gon, Mes, Ret, Sub, gonadal, gluteal, mesenteric, retroperitoneal, and subcutaneous white adipose tissues.
Table 4.
Fatty acid composition in diets, serum, adipose tissues, liver, and muscle
| Tissues |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAT |
GonF |
GluF |
MesF |
RetF |
SubF |
Liver |
Muscle |
||||||||||||
| Diet | Fatty Acid | Diet | Serum | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| HFD | C16:0 | 25.4 | 21.7 | 19.6 | 0.9 | 20.4 | 0.4 | 18.7 | 0.3 | 20.8 | 0.2 | 19.5 | 0.2 | 18.9 | 0.1 | 27.7 | 1.1 | 22.0 | 0.4 |
| C16:1 | 1.7 | 1.9 | 4.3 | 0.3 | 6.2 | 0.2 | 5.9 | 0.2 | 5.7 | 0.1 | 6.3 | 0.2 | 6.6 | 0.3 | 2.4 | 0.8 | 7.9 | 0.5 | |
| C18:0 | 16.0 | 9.5 | 6.2 | 1.1 | 2.7 | 0.1 | 2.7 | 0.0 | 3.1 | 0.0 | 2.9 | 0.6 | 3.1 | 0.4 | 5.3 | 0.5 | 4.0 | 1.3 | |
| C18:1 | 33.9 | 19.7 | 45.0 | 2.0 | 43.9 | 0.5 | 45.5 | 0.7 | 46.4 | 0.5 | 45.6 | 0.7 | 45.6 | 1.7 | 30.0 | 1.2 | 41.2 | 2.4 | |
| C18:2n-6 | 15.5 | 32.9 | 20.7 | 0.7 | 24.0 | 0.4 | 24.3 | 0.3 | 21.2 | 0.4 | 23.0 | 0.5 | 22.3 | 0.2 | 23.7 | 2.6 | 20.3 | 1.1 | |
| SBD | C16:0 | 17.3 | 43.3 | 24.5 | 1.7 | 22.7 | 2.0 | 20.8 | 1.4 | 24.6 | 0.7 | 22.7 | 0.9 | 21.2 | 0.5 | 29.4 | 2.7 | 24.0 | 0.6 |
| C16:1 | 1.7 | 1.4 | 5.1 | 1.7 | 7.9 | 0.5 | 7.6 | 0.8 | 7.1 | 0.2 | 8.0 | 0.5 | 7.3 | 0.5 | 4.2 | 1.1 | 10.6 | 1.4 | |
| C18:0 | 3.8 | 14.4 | 5.9 | 0.7 | 2.1 | 0.2 | 2.4 | 0.6 | 3.4 | 0.6 | 2.6 | 0.7 | 3.0 | 0.2 | 6.3 | 1.8 | 4.2 | 1.3 | |
| C18:1 | 21.8 | 8.5 | 37.3 | 1.4 | 28.1 | 0.2 | 28.4 | 0.6 | 33.4 | 0.4 | 29.7 | 0.5 | 34.4 | 0.5 | 21.1 | 4.0 | 35.6 | 0.6 | |
| C18:2n-6 | 43.4 | 14.6 | 22.7 | 2.1 | 34.0 | 0.3 | 34.7 | 2.4 | 26.8 | 0.9 | 31.7 | 2.3 | 29.7 | 0.6 | 26.9 | 4.6 | 20.2 | 2.7 | |
| C18:3n-3 | 4.2 | 1.05 | 0.9 | 0.1 | 2.1 | 0.1 | 2.2 | 0.2 | 1.4 | 0.1 | 1.7 | 0.2 | 1.4 | 0.1 | 1.5 | 0.2 | 1.1 | 0.2 | |
Serum measurements were derived from pooled serum from 6 animals. Fatty acid compositions in tissues are based on 3 separate measurements from 3 individual animals. Values are expressed as gram of specific fatty acid per 100 g of total fatty acids. Diet had effect on the proportion of all fatty acids, except for stearic acid (C18:0). A tissue effect was evident for all fatty acids. C16:0, palmitic acid; C16:1, palmitoleic acid; C18:1, oleic acid; C18:2 n–6, linoleic acid; C18:3 n–3, α-linolenic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Table 5.
Enrichment scores for KEGG pathways associated with high-fat diet
| Major category | Subcategory | KEGG Pathway | BAT | GonF | GlutF | MesF | RetF | SubF | Liver | Muscle |
|---|---|---|---|---|---|---|---|---|---|---|
| Pathways downregulated in most adipose tissues | ||||||||||
| Metabolism | Carbohydrate metabolism | Glycolysis/gluconeogenesis | −2.76 | −1.79 | 0.78 | −2.42 | −2.37 | −3.19 | 1.84 | 2.06 |
| Propanoate metabolism | −2.77 | −3.76 | −1.70 | −2.65 | −3.16 | −4.08 | 2.97 | 2.94 | ||
| Butanoate metabolism | −2.96 | −3.06 | −0.73 | −2.10 | −2.43 | −2.97 | 3.25 | 0.36 | ||
| Pyruvate metabolism | −4.12 | −4.06 | −0.21 | −4.36 | −5.54 | −5.81 | 3.98 | 1.63 | ||
| Lipid metabolism | Fatty acid biosynthesis | −2.80 | −2.81 | −2.23 | −4.87 | −3.03 | −3.99 | 1.87 | −1.13 | |
| Arachidonic acid metabolism | −0.96 | −3.68 | −1.32 | −1.26 | −2.34 | −2.30 | 0.83 | −1.51 | ||
| Linoleic acid metabolism | −1.76 | −2.40 | 0.82 | −1.19 | −1.76 | −0.89 | −1.86 | −2.63 | ||
| Amino acid metabolism | Alanine and aspartate metabolism | −2.90 | −4.29 | −0.37 | −2.95 | −4.78 | −3.94 | 2.31 | −0.22 | |
| Lysine degradation | −2.75 | −2.18 | −0.71 | −1.78 | −1.45 | −1.63 | 0.95 | 0.01 | ||
| Oxidative phosphorylation | Oxidative phosphorylation | −2.57 | −6.59 | 1.96 | −1.21 | −5.62 | −4.83 | −2.01 | 1.73 | |
| Xenobiotics biodegradation and metabolism | Benzoate degradation via CoA ligation | −2.37 | −2.33 | −0.57 | −1.08 | −2.23 | −1.63 | 4.02 | 0.60 | |
| Glutathione metabolism | −2.38 | −4.73 | −6.21 | −1.28 | −3.97 | −3.44 | −6.67 | −0.59 | ||
| Metabolism of xenobiotics by cytochrome P-450 | −2.71 | −6.26 | −5.01 | −1.45 | −5.22 | −3.17 | −6.39 | −1.86 | ||
| Tetrachloroethene degradation | −1.96 | −2.02 | 0.15 | −2.30 | −1.16 | −2.57 | 1.13 | 0.00 | ||
| Glucan biosynthesis and metabolism | Keratan sulfate biosynthesis | −0.98 | −2.46 | −2.48 | −2.31 | −2.49 | −1.76 | −0.50 | 1.06 | |
| Environmental inform. processing | Signal transduction | Hedgehog signaling pathway | −1.19 | −1.55 | −2.99 | −1.04 | −2.65 | −0.85 | 0.58 | 1.40 |
| Cellular processes | Endocrine system | Insulin signaling pathway | −3.25 | −2.41 | −1.51 | −1.29 | −2.99 | −2.55 | 0.83 | −0.56 |
| Adipocytokine signaling pathway | −2.17 | −0.30 | −1.14 | −1.35 | −1.89 | −2.22 | 0.00 | 1.85 | ||
| Pathways upregulated in most adipose tissues | ||||||||||
| Environment inform. processing | Signaling molecules and interaction | ECM-receptor interaction | 0.55 | 2.04 | −3.36 | −0.48 | 0.16 | 2.15 | −0.87 | −1.24 |
| Cell adhesion molecules (CAMs) | 3.30 | 3.47 | −1.23 | −1.59 | 1.76 | −1.15 | −0.64 | 1.23 | ||
| Cytokine-cytokine receptor interaction | 4.86 | 2.89 | −0.48 | 1.13 | 3.33 | −0.77 | 0.46 | 3.08 | ||
| Genetic inform. processing | Translation | Ribosome | 2.84 | −0.52 | −0.15 | 2.94 | 6.50 | −0.89 | −8.39 | −4.80 |
| Cellular processes | Development | Dorso-ventral axis formation | 1.74 | 1.70 | 0.14 | 2.33 | 0.75 | 3.22 | −2.34 | −2.25 |
| Cell motility | Regulation of actin cytoskeleton | 2.71 | 3.01 | 1.82 | −0.08 | 3.46 | 2.87 | −1.20 | −2.45 | |
| Cell growth and death | Cell cycle | 3.70 | 2.81 | 3.15 | 3.32 | 3.84 | 4.30 | −0.27 | −0.69 | |
| Cell communication | Focal adhesion | 2.05 | 3.02 | −1.55 | 1.24 | 0.96 | 3.63 | −1.13 | −1.63 | |
| Immune system | Complement and coagulation cascades | 3.84 | 2.00 | 0.57 | 2.21 | 1.38 | 3.56 | −2.69 | −3.21 | |
| Antigen processing and presentation | 2.74 | 4.97 | 2.16 | 0.01 | 4.55 | −0.54 | 0.65 | 2.50 | ||
| Toll-like receptor signaling pathway | 2.80 | 3.33 | 3.08 | 1.92 | 4.06 | 1.77 | −0.21 | −0.10 | ||
| Hematopoietic cell lineage | 3.06 | 2.95 | 0.25 | 0.56 | 3.98 | −1.03 | 0.10 | 0.67 | ||
| Natural killer cell-mediated cytotoxicity | 3.22 | 4.34 | 2.71 | 0.99 | 5.00 | 0.13 | −0.36 | 0.45 | ||
| T-cell receptor signaling pathway | 2.61 | 3.89 | 1.84 | −0.27 | 4.60 | −1.96 | 0.17 | −0.40 | ||
| B-cell receptor signaling pathway | 3.18 | 4.73 | 2.36 | 0.60 | 6.08 | −0.05 | −0.13 | −0.31 | ||
| FcεRI signaling pathway | 2.52 | 4.09 | 2.90 | 0.89 | 3.90 | 1.22 | −0.39 | −0.48 | ||
| Leukocyte transendothelial migration | 3.97 | 2.92 | −0.84 | 0.33 | 2.16 | 1.36 | −1.36 | −0.71 | ||
| Human diseases | Metabolic disorder | Type 1 diabetes mellitus | 2.84 | 3.41 | 1.75 | 0.41 | 3.72 | −0.52 | 1.22 | 1.92 |
| Cancers | Colorectal cancer | 3.10 | 2.32 | 1.51 | 1.56 | 2.42 | 3.20 | −0.18 | 0.23 | |
| Pancreatic cancer | 2.98 | 2.23 | 1.07 | 1.23 | 2.48 | 2.43 | 0.21 | −1.27 | ||
| Glioma | 2.49 | 3.10 | 3.20 | 1.92 | 2.99 | 2.80 | 0.08 | −0.84 | ||
| Chronic myeloid leukemia | 2.08 | 1.91 | 1.78 | 1.93 | 2.32 | 2.04 | −0.46 | −1.75 | ||
| Melanoma | 2.20 | 1.06 | 3.12 | 0.70 | 1.94 | 3.09 | −0.25 | −1.09 | ||
Thresholds of +2 and −2 were used to determine significant up- and downregulation in a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, respectively. The t statistic was used as gene-level evidence for the calculations. Boldface, significantly upregulated; italics, significantly downregulated.
Consistent with the high availability of fatty acids and excessive storage of fat, genes that encode enzymes for fatty acid biosynthesis (e.g., Aacs, Acaca, Acacb, Acly) and elongation of fatty acids (Elovl6) were strongly downregulated across tissues and among the top 10 most highly up-/downregulated genes (Table 2). In WAT, while most metabolic pathways were repressed (Table 5), the facilitators of beta-oxidation (Acadm, Acadl, Acsl1) were activated in most WATs in HFD conditions (Table 2). Thus the relative reduction of SFA compared with MUFA may also be due to preferred oxidation of SFA in HFD-fed mice.
Fatty acid profiles associate with transcriptional changes.
Eight hundred sixty genes were significantly correlated (P value < 0.01) to at least one of the fatty acid components (Supplemental Table S2). Saturated fatty acids C16:0 and C18:0 were correlated with a large number of transcripts that showed tissue-specific responses to diet, and there was a considerable overlap of genes correlated to both (Supplemental Table S2, Supplemental Fig. S2). These genes are enriched for regulation of cellular growth, cell adhesion, anatomic and extracellular structure development and organization, as well as transport of phosphate, inorganic ions, and electrons (P value < 0.01). C18:0 is positively correlated with the activation of NF-κB-induced signaling, while C16:0 is associated with Ras and Rho protein signal transduction. The strongest negative correlations were observed between C16:0 and C18:0 and genes in the collagen family (Col16a1, Col1a1, Col4a1, Col4a2, Col4a6, Col5a1, Col5a2, Col16a1, Col16a2, Col16a2, Col16a3, Col18a1) and genes related to collagen binding and processing (Mrc2, Mmp12). Many of these genes, along with others critical for regulation of extracellular matrix (ECM), were activated by HFD in adipose tissues but not in liver and muscle (Table 2, Supplemental Fig. S3). Leptin (Lep) and resistin (Retn) transcript amounts were negatively correlated with C18:0 (r = −0.70 and r = −0.71, respectively).
The MUFA C16:1 also showed strong correlation with transcripts. In contrast with the SFA, it is negatively correlated to transcripts that are enriched for metabolic processes, including biosynthesis of steroids, nutrient turnover, and energy partitioning. Despite an abundance of C18:1 in tissues of HFD-fed mice, there was only a weak association with transcripts.
PCA analysis was performed over fatty acid composition data together with variance components from the ANOVA tissue-by-diet interaction model. The first four principal components explained 90% of the variation and revealed genes that are significantly affected by the interaction of tissue and diet. These genes are highly enriched (P value < 0.001) for processes that utilize fat, including Ppar signaling pathways, fatty acid metabolism, arachidonic acid metabolism, and biosynthesis of steroids. The first two components (41% and 30% of the variation, respectively) separate the tissue groups, liver, muscle, WAT, and BAT (Fig. 4). The major signature of the transcripts highlights differences in gene expression changes between liver and WAT, particularly differences in the metabolic roles of liver and fat. The collagen genes and processes involved in the ECM were also overrepresented and positively correlated with loadings for WAT and negatively correlated with SFA components C16:0 and C18:0.
Effects of HFD on genes and pathways are tissue specific.
ANOVA models were used to determine differences due to HFD within each tissue. Differential expression was determined at the threshold level P value <0.001, which yielded an estimated FDR <0.15 for each test (Supplemental Table S3). Among the adipose tissues, retroperitoneal adipose tissue had the largest number of significant changes (1,790 genes) and mesenteric adipose tissue had the smallest number of significant changes (245 genes). Mesenteric adipose tissue samples were highly variable across individuals and most different from other WAT (Supplemental Table S4, Fig. 4). This reduces our ability to detect statistically significant differences between diet groups in the mesenteric adipose tissue. Aside from the mesenteric tissue, there was a high concordance in the differentially expressed genes across WAT, ranging from 31% to 71% pairwise overlap (Supplemental Table S4). Among the genes that were differentially expressed in BAT, 68% were also differentially expressed in at least one WAT. Liver and muscle had limited transcriptional responses to HFD and only a small overlap with other tissue types (Supplemental Fig. S4).
Additionally, we identified differentially expressed genes that were consistently up- or downregulated (Table 2). Tryptophan hydroxylase 2 (Tph2), ubiquitin D (Ubd), and serum amyloid A3 (Saa3) were strongly activated across all adipose tissues and muscle. Mesoderm-specific transcript (Mest), fibroblast growth factor 13 (Fgf13), and collagen type VI α1 (Col6a1) were highly activated across adipose tissues. Interestingly, many of the upregulated genes play a role in cell communication as they encode transmembrane proteins (Tph2, Degs1, Mrc2, Slc5a7) or are secreted into the extracellular space (Col6a1, Saa3, Trem2). The corresponding pathway for environmental information processing (including the ECM-receptor interaction pathway) is also upregulated in most adipose tissues (Table 5).
Genes of the regenerating islet family (Reg2, Reg3a, Reg3b, Reg3g) had the largest transcriptional responses to diet in mesenteric tissue (Supplemental Fig. S5). However, we cannot completely rule out the possibility of contamination of mesenteric fat with nearby pancreatic tissue, despite careful and complete separation of the pancreas before mesenteric adipose tissue was collected.
Pathway analysis distinguished diet-induced responses in KEGG pathways (Table 5). Pathways contributing to the processing of environmental information, including ECM-receptor interaction, cell adhesion, and cytokine-cytokine interaction, were significantly activated across most adipose tissues. Cellular processes that drive cell motility by regulation of the actin cytoskeleton and cell growth and death and that contribute to the communication between cells via focal adhesion are upregulated across most adipose tissues. Pathways related to immune response and diseases, including cancers and type 1 diabetes mellitus, are significantly upregulated in gonadal and retroperitoneal adipose tissues and BAT. Consistent with its reputation as the “good” adipose tissue, the subcutaneous depot was not significantly activated for immune response.
Pathways in the liver were often regulated in the opposite direction from adipose tissues, reflecting the overall flow of energy in HFD conditions. Generally, KEGG pathways contributing to the accumulation of lipids (fatty acid biosynthesis, arachidonic acid and linoleic acid metabolisms), carbohydrates (glycolysis/gluconeogenesis), and amino acids (alanine aspartate metabolism) were significantly downregulated in all adipose tissues on HFD and activated in liver. Gluteal and mesenteric metabolic pathways often show changes in the same direction as other adipose tissues, but they are not significant. This suggests that these two tissues retain their metabolic activity under diet-induced obesity. However, for mesenteric adipose tissue, this conclusion must be drawn with care, as the number of genes contributing to pathway analysis is much smaller than in gluteal adipose tissue (245 vs. 1,973). Muscle showed few significant pathway changes that were often in the same direction as adipose tissues.
DISCUSSION
DBA/2J mice responded to HFD with increased adiposity and typical features of metabolic syndrome. After 6 wk on HFD, DBA/2J mice have elevated glucose levels and reduced insulin sensitivity, despite carbohydrate reduction in HFD. Mice show ectopic fat deposition in liver and muscle. Developing liver steatosis and high hepatic and serum TG and cholesterol levels indicate impaired fatty acid utilization. Furthermore, low thyroid hormone T4 serum levels mirror lowered metabolic rate. High leptin levels correspond with the high adipose tissue mass. Both changes in leptin and thyroid hormone levels hint at imbalances of energy uptake and partitioning. The observed physiological changes are likely the result of high dietary SFA and subsequent high oleic acid (C18:1) and reduced PUFA content in tissues. The typical features of the metabolic syndrome in DBA/2J mice were also observed with a HFD rich in cholesterol in a recent comparison among 10 inbred mouse strains (43). However, the cholesterol prescription led to the inactivation of cholesterol synthesis and many differently regulated pathways in the liver compared with our study.
Patterns of pathway regulation in liver suggest the increased production and accumulation of pyruvate from glucose, alanine, and fatty acid precursors in HFD-fed mice. The accrued pyruvate was likely converted to acetyl-coenzyme A for the synthesis of fats and ketone bodies, processes that repress oxidative phosphorylation. Together with mass gain, these factors suggest the development of fatty liver in the HFD feeding group. Ketone bodies could be a necessary energy provision because of the reduction of dietary carbohydrates but also for the synthesis of cholesterol, resulting in the high total cholesterol serum level in HFD-fed mice. However, an overproduction of ketone bodies followed by hyperketonemia is seen in diabetes mellitus as glucose is not sufficiently taken up by the cells. Elevated glucose levels accompanied by reduced insulin sensitivity despite reduced carbohydrates in HFD might be explained by reduced content of PUFA, which are able to suppress liver lipogenesis and enhance glucose transport and fatty acid oxidation in muscle (1, 9). In addition, the lowered C16:1 synthesis in the tissues together with the high cellular content of C18:1 in HFD-fed DBA/2J mice may contribute to reduced transcripts of Irs1 in adipose tissues and impaired glucose uptake in muscle, which in turn leads to impaired insulin sensitivity (16). For circulating C16:1, stimulation of muscle insulin action and suppression of hepatosteatosis could be shown (9).
Fatty acid profiles and transcriptional responses in liver, where most fat is metabolized, indicated that HFD-fed mice exhibited an overall increase in the conversion of SFA to MUFA and PUFA before being taken up by target tissues. The high degree of the monounsaturated oleic acid (C18:1) in adipose tissues and muscle suggests it as the preferred fatty acid for storage, but the proportions of SFA to MUFA could also be explained by preferred oxidation of SFA. The transport of MUFA seems to play a secondary role, as the level of MUFA in serum is lower than in the tissues.
Fatty acid composition and gene transcription profiles in five WATs, BAT, liver, and muscle suggest that dietary fatty acids exert direct actions on gene transcription. However, the dietary fatty acids are further metabolized and converted, and the adipocyte itself actively contributes to these processes. Both the intracellular ratio of fatty acids and the amount of intracellular fat seem to influence the tissue-specific transcriptional regulation of genes. As a result, several proteins are produced and partially secreted to the ECM, which may change the flexibility of the cell and support the invasion of macrophages leading to enhanced immune response. Furthermore, the intracellular ratio of fatty acids and the amount of fat influence the composition of the lipid bilayer in cell membranes. This may affect not only the elasticity of the cell but also the activation of intra- and transmembrane proteins including receptors, transporters, proteinases, and peptidases.
In WAT of HFD-fed mice, SFA content was decreased and collagen gene expression was increased. A significant negative correlation between C16:0 and C18:0 with collagen gene expression and related processes involving the ECM was detected. Elevated levels of collagen genes in HFD conditions are in agreement with findings in ob/ob versus db/db and db/db versus wild-type mice (32). Among these collagen genes, recently Col6a1 has been identified as a factor that controls adipocyte size by forming a fibrinogenic net around the cell surface and suppresses cell expansion (25). Lack of Col6a1 in knockout mice resulted in reduction of lipid accumulation and thus improved carbohydrate and lipid metabolism (32). Col6a1 transcription in our experiment correlates with expression of other genes of the collagen family across tissue and diet conditions. Because adipose tissues respond differently in the activation or inactivation of genes encoding collagens and collagen processing enzyme, their influence on the ECM and subsequently the infiltration of macrophages into adipose tissues, for example, might affect distinct immune responses of adipose tissues.
Serum amyloid A3 (Saa3) is an inflammatory adipokine associated with adipose tissue mass that was activated in WAT of animals on HFD (57). Lipopolysaccharides and IL-1β were found to stimulate expression of Saa3 in a dose-dependent manner (45). Saa3 also has a role in reverse cholesterol transport process as an HDL particle. Saa3 particles contain apolipoproteins (38). The activation of Saa3 in tissues of mice fed HFD is consistent with the observed increase in transcription of apolipoprotein C-IV (Apoc4). A feedback mechanism involving Saa3 was recently found to regulate lipid metabolism in pigs (12). We suggest that Saa3 supports the fatty acid transport in serum and thus communication between tissues.
Tryptophan hydroxylase (Tph2) and ubiquitin D (Ubd) were among the most highly activated genes on HFD. Tph2 is a key enzyme in the synthesis of serotonin (39) and is expected to modify the mitochondrial transmembrane potential (4). Tph2 is a primary regulator of serotonin in the gastrointestinal tract, where it stimulates formation of precursors in development of gut microvilli (33, 40). Mice lacking Tph2 had growth retardation, altered body temperature control, and decreased blood pressure (2). These data suggest a novel role of Tph2 in autonomic pathways linking obesity with health risk factors. A causal link between perinatal ω-3 fatty acid deficiency and reduced central serotonin synthesis via reduced Tph2 transcript amounts has been shown in rat (37). Therefore, we believe that the high Tph2 transcript levels in adipose tissues in our model result from direct interaction with tissue-specific fatty acid composition.
Ubd catalyzes the ubiquitination of proteins as a key step for their proteasomal degradation. Ubiquitination also controls the stability, function, and intracellular localization of a wide variety of proteins. The high transcript levels of a variety of genes, including Saa3 and Tph2, and their translation into proteins likely require increased control of translated protein quality and levels. This cleaning routine itself requires the provision of energy and likely contributes to cellular stress, which in turn could lead to the activation of the immune response.
Given the less pronounced downregulation in metabolic pathways, it is likely that mesenteric adipose tissue is the most metabolically active adipose tissue leading to low mass gain. The strong activation of genes regenerating islets (Reg gene family) suggests their role in intestinal inflammation. The mesenteric adipose tissue-specific diet-response genes showed no significant contribution to KEGG pathways corresponding to human diseases.
Gluteal adipose tissue exhibited the highest mass gain compared with SBD and showed only a slight response in metabolic pathways. This is consistent with evidence that gluteal adipose tissue is more sluggish in its metabolism (22) and contributes less to cardiovascular disease risk (50). In some sheep breeds, gluteal adipose tissue is well known to be a major fat storage depot for the delivery of energy during seasonally restricted feed supply (3, 31).
Traditionally, BAT has been known for its role in thermogenesis. Clearly, the role of BAT in obesity has changed to mirror WAT as a major storage depot of excess energy. Not only did the majority of differentially expressed genes in BAT overlap with WAT, but there was a high correspondence in pathway regulation, particularly with gonadal and retroperitoneal adipose tissues. On the other hand, Ucp1 and Ucp2 activation together with an enhanced fatty acid oxidation in WAT suggests a role of thermogenic activity in response to sustained high-fat feeding and insulin resistance. The role of WAT in thermogenesis has been suggested in the literature, but not specific to the depot location (24). High levels of Ucp1 and Ucp2 transcripts in BAT did not change with diet. We conclude that in HFD conditions BAT maintains its function in thermogenesis and takes on additional roles in fat storage, signaling, and mobilization that are typical to WAT. The conversion of BAT to WAT and vice versa could be a strategy for the control of fat mass (52).
Muscle was the weakest responder to HFD, although it is plausible that a longer HFD feeding period may be required to see changes. Fatty acids present in muscle are mostly liberated from adipose tissues and from the breakdown of TG in pools of liver-derived LDL. Despite the mixed origin of deposited fat we found a striking similarity in fatty acid composition of muscle and adipose tissues, implicating adipose tissues as the dominant source. It is tempting to speculate that at a later time point muscle would begin to play a physiological role that parallels adipose tissue responses to HFD. Muscle tissue has been shown previously to play a role in the development of obesity (11, 42). In correspondence with physiological findings, genetic studies provided evidence that genetic factors contributing to obesity and body weight can act through mechanisms affecting muscle weight and fat weight (7).
In summary, HFD in DBA/2 mice led to weight gain, metabolic dysregulation, and insulin sensitivity. Effects on transcriptional regulation were largely a function of the tissue-specific proportions of different fatty acids. Novel associations were found between SFA C16:0 and C18:0, which negatively correlated with genes in the collagen family and processes involving the ECM. SFA was converted in the liver to MUFA and PUFA, and oleic acid (C18:1) was the preferred MUFA for storage of excess energy in all tissues of HFD-fed mice. A role of Tph2 in adipose tissues in diet-induced obesity has been proposed. We have identified tissue-specific responses to HFD. Liver steatosis was evident, and pathways in liver and adipose tissues were often regulated in opposite directions. Gonadal, retroperitoneal, and subcutaneous adipose tissues and BAT exhibited severe inflammatory and immune responses and had activated disease pathways associated with cancers and type 1 diabetes. Mesenteric adipose tissue was the most metabolically active adipose tissue, and the least affected by the dietary condition. Gluteal adipose tissue had the highest relative mass gain but was sluggish in its metabolism. In HFD conditions, BAT functioned largely like WAT in its role as a depot for excess energy, whereas WAT played a role in thermogenesis.
Our findings support the importance of multidimensional examinations of the biology of complex systemic traits like obesity (54). Assessments of response to diet of individual tissues as a function of mass, fat composition, and genetic factors are critical steps in unraveling the pathologies of metabolic disorders and diseases. The effects found in our study are largely caused by the high proportion of fatty acids in the HFD. However, it is necessary to mention that some observed effects could also result from the influence of other food components (e.g., phytoestrogens) that might differ between the diets and that may exert independent effects on obesity or direct effects on increased expression of inflammatory genes, for example (43, 47).
GRANTS
This project was funded with grants from National Institute of General Medical Sciences Centers of Excellence in Systems Biology Program GM-076468 (G. A. Churchill), the National Genome Research Network NGFNplus 01GS0829, Germany (G. A. Brockmann, A. Wagener), National Heart, Lung, and Blood Institute NSRA Fellowship 1F32-HL-095240-01 (R. S. Hageman), and the Deutsche Forschungsgemeinschaft for the graduate college program GRK 1208 (C. Hantschel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephan Scherneck for determination of liver triglycerides.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Al-Hasani H, Joost HG. Nutrition-/diet-induced changes in gene expression in white adipose tissue. Best Pract Res Clin Endocrinol Metab 19: 589–603, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boyé P, Vilianovitch L, Sohr R, Tenner K, Hörtnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attia N, Bocquierb F, Khaldic G. Performance of the fat-tailed Barbarine sheep in its environment: adaptive capacity to alternation of underfeeding and re-feeding periods. A review. Anim Res 53: 165–176, 2004 [Google Scholar]
- 4.Basu B, Desai R, Cherkady JBR, Sriram V, Maiti S, Panicker MM. Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction 135: 657–669, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Basciano H, Adeli L. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta 368: 1–19, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bolstad BM, Irizarry RA, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brockmann GA, Tsaih SW, Neuschl C, Churchill GA, Li R. Genetic factors contributing to obesity and body weight can act through mechanisms affecting muscle weight, fat weight, or both. Physiol Genomics 36: 114–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmann J, Meyer C, Neschen S, Augustin R, Schmolz K, Kluge R, Al-Hasani H, Jürgens H, Eulenberg K, Wehr R, Dohrmann C, Joost HG, Schürmann A. Ablation of the cholesterol transporter ABCG1 reduces adipose cell size and corrects diet induced insulin resistance. Endocrinology 148: 1561–1573, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cao HG, Gerhold K, Mayers J, Wiest M, Watkins S, Hotamisligil G. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey VJ, Walters EE, Coldiz AG, Solomon GC, Willet CW, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of insulin-dependent diabetes mellitus in women. Am J Epidemiol 145: 614–619, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schürmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet 40: 1354–1359, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Wang PH, Bing H, Liu HH, Mersmann HJ, Ding ST. Serum amyloid A protein regulates the expression of porcine genes related to lipid metabolism. J Nutr 138: 674–679, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Cui X, Hwang JT, Qui J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance component estimates. Biostatistics 6: 59–75, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Daniel H, Dieck TH. Nutrient-gene interactions: a single nutrient and hundreds of target genes. J Biol Chem 385: 571–583, 2004 [DOI] [PubMed] [Google Scholar]
- 15.de Haan JR, Wehrens R, Bauerschmidt S, Piek E, van Schaik RC, Buydens LMC. Interpretation of ANOVA models for microarray data using PCA. Bioinformatics 23: 184–190, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Denechaud PD, Dentin R, Girard F, Postic C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett 582: 68–73, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Dunning MJ, Smith ML, Ritchie MF, Tavare S. R classes and methods for Illumina bead-based data. Bioinformatics 23: 2183–2184, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, Muzumdar R, Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes 54: 672–678, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Festa A, D'Agostino R, Jr, Williams K, Karter A, Mayer-Davis E, Tracey R, Haffner W. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 25: 1407–1415, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Fettforschung DGF DGF Einheitsmethoden Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft, 2009 [Google Scholar]
- 22.Frayn KN, Fielding BA, Karpe F. Adipose tissue fatty acid metabolism and cardiovascular disease. Curr Opin Lipidol 16: 409–415, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg H, Zhang Y, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 14: 41S–49S, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Grannerman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab 285: E1230–E1236, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Halberg N, Wernstedt I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am 37: 753–768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gene Ontology Consortium The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res 32: D258–D261, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotamisligil G. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katterle Y, Keipert S, Hof J, Klaus S. Dissociation of obesity and insulin resistance in transgenic mice with skeletal muscle expression of uncoupling protein 1. Physiol Genomics 32: 352–359, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kerr MK, Martin M, Churchill GA. Analysis of variance for gene expression microarray. J Comput Biol 7: 819–837, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Khachadurian AK, Adrouni B, Yacoubian H. Metabolism of adipose tissue in the fat tail of the sheep in vivo. J Lipid Res 7: 427–436, 1996 [PubMed] [Google Scholar]
- 32.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Schere PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, Hu Z, Yu W, Jonas B, Pineda R, Claderon-Gay V, Germann M, O'Neill E, Brommage R, Cullinan E, Platt K, Powell D, Sands A, Zambrowicz B, Shi ZC. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. Pharmacol Exp Ther 325: 47–55, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N. Crucial role of a long-chain fatty acid elongase, Elov16, in obesity-induced insulin resistance. Nat Med 13: 1193–1202, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc 3: 35–42, 2006 [DOI] [PubMed] [Google Scholar]
- 36.McGarry J. What if Minkowski had been ageusic? Science 30: 770–774, 1992 [DOI] [PubMed] [Google Scholar]
- 37.McNamara RK, Able J, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J Psychiatr Res 656–663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meek RL, Eriksen N, Benditt EP. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci USA 89: 7949–7952, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy KL, Zhang X, Gainetdinov RR, Beaulieu JM, Caron MG. A regulatory domain in the N terminus of tryptophan hydroxylase 2 controls enzyme expression. J Biol Chem 283: 13216–13224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K, Sato T, Ohashi A, Tsurui H, Hasegawa H. Role of a serotonin precursor in development of gut microvilli. Am J Pathol 172: 333–344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newton M, Quintana F, Boon JD, Sengupta S, Ahlquist P. Random-set methods identify distinct aspects of the enrichment signal in gene set-analysis. Ann Appl Stat 1: 85–106, 2001 [Google Scholar]
- 42.Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab 15: 110–115, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Shockley KR, Witmer D, Burgess-Herbert SL, Paigen B, Churchill GA. Effects of atherogenic diet on hepatic gene expression across mouse strains. Physiol Genomics 39: 172–182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijder M, Dekker J, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JA, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr 77: 1192–1197, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Sommer G, Weise S, Kralisch S, Scherer PE, Lössner U, Blüher M, Stumvoll M, Fasshauer M. The adipokine SAA3 is induced by interleukin-1beta in mouse adipocytes. J Cell Biochem 104: 2241–2247, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Storey JD. A direct approach to false discovery rates. J R Stat Soc B 64: 479–498, 2002 [Google Scholar]
- 47.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFurosa J, Jick Z, Greenberg AS, Olbin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56: 2910–2918, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Strolien LH, Bauer LA, Krinketos AD, Pan DA, Cooney GJ, Jenkins AB, Calvert GD, Campbell LV. Dietary fats and insulin action. Diabetologia 39: 621–631, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Svenson KL, Smith RV, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol 102: 2369–2378, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tan GD, Goossens GH, Humphreys SM, Vidal H, Karpe F. Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obes Res 12: 114–118, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation 107: 1626–1631, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Tiraby C, Langin D. Conversion from white to brown adipocytes: a strategy for the control of fat mass? Trends Endocrinol Metab 14: 439–441, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 43: 1353–1357, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Voy BH, Aronow BJ. Embrace diversity! Systems genetics-enabled discovery of disease networks. Physiol Genomics 39: 169–171, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 81: 555–563, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Kerr K, Churchill GA. MAANOVA: a software package for the analysis of spotted cDNA microarray experiments. In: The Analysis of Gene Expression Data and Overview of Methods and Software New York:Springer, 2003 [Google Scholar]
- 57.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 3: 884–894, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364: 11–17, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.