Abstract
Signalling through the IFNαR (interferon-α receptor) and TCR (T-cell receptor) in Jurkat T lymphocytes results in distinct immune responses. Despite this both receptors elicit ERK (extracellular-signal-regulated kinase)/MAPK (mitogen-activated protein kinase) phosphorylation. Vav and Slp76 are shown to be required for IFNα (interferon-α)-stimulated ERK activity. These form a subset of proteins which behave identically on stimulation of both receptors. TCR deletion abrogates IFNαR-stimulated MAPK activity, whereas the canonical JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathway is unaffected. Thus recruitment of the intact TCR ESC (early signalling complex) is necessary for this downstream MAPK response. Despite using a common ESC, stimulation of the IFNαR does not produce the transcriptional response associated with TCR. Up-regulation of the MAPK pathway by IFNαR might be important to ensure that the cell responds to only one stimulant.
Keywords: interferon-α receptor (IFNαR), Janus kinase/signal transducer and activator of transcription pathway (JAK/STAT pathway), mitogen-activated protein kinase pathway (MAPK pathway), Slp76, T-cell receptor early signalling complex (TCR ESC), Vav
Abbreviations: ERK, extracellular-signal-regulated kinase; ESC, early signalling complex; FBS, fetal bovine serum; IFNα, interferon-α; IFNαR, IFNα receptor; IFNR, interferon receptor; IL, interleukin; JAK, Janus kinase; LAT, linker for activation of T-cells; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; NFAT, nuclear factor of activated T-cells; NF-κB, nuclear factor κB; NP-40, Nonidet P40; PBMC, peripheral blood mononuclear cell; RFP, red fluorescent protein; RLU, relative light unit; STAT, signal transducer and activator of transcription; TCR, T-cell receptor
INTRODUCTION
Lymphocytic class I IFNR (interferon receptor) and TCR (T-cell receptor) have distinct responses to extracellular stimulation. The former is a member of the cytokine family of receptors and signals primarily through the JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathway resulting in antiviral and growth inhibitory effects. In contrast, activation of the TCR through antigen presentation during an adaptive immune response leads to cell proliferation and secretion of cytokines. These discrete cellular responses are derived from the distinct downstream gene transcriptional activity. Despite the contrasting cellular outcomes arising from their activation, both receptors invoke up-regulation of the ERK (extracellular-signal-regulated kinase)/MAPK (mitogen-activated protein kinase) pathway [1–3].
The different cellular responses to these cytokine- and antigen-stimulated receptors have been shown to involve recruitment of a subset of common proteins. These proteins have all previously been identified as being involved in the ESC (early signalling complex) formed at the TCR within minutes of stimulation [4,5]. For example, the proto-oncogene product Vav, which is phosphorylated upon stimulation of the TCR in human peripheral blood lymphocytes and human leukaemic T-cells [6], also interacts with the IFNR-associated tyrosine kinase, Tyk2 [7,8]. Similarly, the tyrosine kinases Zap70 and Lck, and the phosphatase CD45, which are all fundamental components of the TCR signal response, are also recruited to the IFNαR (interferon-α receptor) in Jurkat cells and primary lymphocytes [9]. The MAPK responses elicited from both IFNαR- and TCR-stimulated signals are dependent on the presence of both Zap70 and Lck [2,3,10] and the resultant tyrosine phosphorylation of Zap70 occurs by the same mechanism upon IFNαR stimulation as when it is activated as part of the TCR signalling pathway [11]. Furthermore, both Lck and Zap70 were shown to play an integral role in IFNα signalling as stimulated cells where these proteins were deleted were unable to demonstrate the typical anti-proliferative effects of this cytokine [9]. The absence of Lck and Zap70 in these cells did not however affect IFNαR signalling through JAK or offer protection to cells from viral infection [9].
The utilization of common proteins in transducing two signals with distinct cellular outcomes suggests a potential cross-talk between these pathways in lymphocytes. In the present study we show further that IFNα stimulation of cells recruits the TCR ESC protein machinery, and requires a functional TCR to produce the MAPK response in both lymphoma and ex vivo in healthy human primary CD4+ T-cells. This response has a more limited time course than the sustained signalling at the TCR and leads to a different gene expression profile. The activation of the MAPK pathway via TCR after IFNR stimulation may be a way of committing the lymphocyte to a single course of action by occupying/blocking a subsequent TCR response to extracellular antigen presentation.
EXPERIMENTAL
DNA constructs and mutagenesis
The SLP76 construct used in this paper was cloned, in frame, into the pcDNAhygro3.1-mRFP fusion vector [3], between the NheI and XhoI sites of the multiple cloning site so that Slp76 was C-terminally tagged with monomeric RFP (red fluorescent protein). The Slp76-Y3F–RFP mutant was generated through single A→T base pair mutations using the Stratagene mutagenesis kit according to the manufacturer's instructions. These mutations resulted in replacement of tyrosine residues with phenylalanine residues at positions 112, 128 and 145 of Slp76. The integrity of all three point mutations was confirmed by gene sequencing.
Cell culture
Jurkat E6.1 cells were purchased from the ECACC (European Collection of Animal Cell Cultures), the J14 (Slp76-deficient) and PF2.4 (TCRβ-reconstituted) cell lines were a gift from A. Weiss (Dept. of Medicine and HHMI, University of California San Franciso, San Franciso, CA, U.S.A.), the J.Vav1 cell line and clone 15-11 reconstituted J.Vav1 cell line were kindly provided by R. Abraham (Program in Signal Transduction Research, The Burham Insitute, La Jolla, CA, U.S.A.), the JRT3-T3.5 (TCRβ-deficient) cell line was purchased from A.T.C.C. The cell lines above and primary human peripheral CD4+ T-cells (purification of these is described below) were all cultured in RPMI-1640 containing L-glutamine (Cambrex). This medium was additionally supplemented with 10% (v/v) heat-inactivated, γ-radiated FBS (fetal bovine serum) (Biosera) and antimycotic/antibiotic (BioWhittaker). Cells were maintained in a humidified incubator at 5% (v/v) CO2 and 37 °C. J.Vav1 cells and reconstituted J.Vav1 cells were cultured in the above medium, supplemented with 500 μg/ml G418.
Purification of primary CD4+ T-cells
PBMCs (peripheral blood mononuclear cells) were isolated from buffy coats purchased from the Blood Bank (St. Georges Hospital, Tooting, London U.K.). The PBMCs were isolated through Ficoll density-gradient centrifugation separation according to the supplier's instructions (Amersham Biosciences). PBMCs were collected and washed and subjected to negative selection in order to isolate CD4+ T-cells. This was achieved using MACS (magnetic cell sorting) in accordance with the protocol described by the manufacturer (Miltenyi Biotec). FACS analysis showed that purity of the resultant CD4+ T-cells was above 97% in all experiments. Following purification, cells were incubated overnight in RPMI 1640 medium supplemented with 10% (v/v) FBS and antimycotic/antibiotic. The following day 5×106 cells per time point were placed into 2-cm-diameter dishes and stimulated with 1 mg/ml UCHT1 monoclonal antibody or 6000 units/ml IFNα (Roferon-α; Roche). Cells were incubated for the time period required at 37 °C. Where an inhibitor was used, cells were pre-incubated for 2 h with 20 nm Lck II inhibitor (Calbiochem) or 15 nM JAK inhibitor I (Calbiochem) before being stimulated with 1 mg/ml UCHT1 or 6000 units/ml IFNα. The medium was then aspirated and cells were washed with 1 ml of 1× Dulbecco's PBS (Biowhittaker) and centrifuged at 900 g for 5 min. Finally cells were lysed in 50–100 μl ice-cold NP-40 (Nonidet P40)-containing lysis buffer and centrifuged at 22000 g for 20 min. Protein concentration was then determined and Western blotting was performed as described below.
Western blot analysis
Cells were grown to approx. 50% confluency and 5×106 cells were then used per time point. Cells were serum-starved for 2 h before being stimulated with either 1 mg/ml OKT3 monoclonal antibody (eBioscience) or 6000 units/ml of Roferon-α and incubated at 37 °C for the time periods indicated. Cells were then lysed in NP-40-containing lysis buffer [50 mM Hepes, pH 7.5, 1% NP-40, 1 mM sodium pervanadate, 10 mM sodium fluoride, 10% (v/v) glycerol, 50 mM NaCl and 1 mM EDTA] supplemented with 1% (v/v) protease inhibitor cocktail III (Calbiochem). The protein concentration of the whole-cell extracts obtained was determined through use of Bradford assay reagents (BioRad Laboratories). For Western blot analysis, 50 μg of cell lysates were separated by SDS/PAGE (12% gels). Proteins were then transferred on to nitrocellulose membranes (Millipore). Membranes were then incubated with specific antibodies in 3% (w/v) BSA or 5% (w/v) dried low-fat milk. β-Actin was used as a loading control. Protein bands were visualized by enhanced chemiluminescent detection (Cell Signaling Technology). Images were scanned using an Alpha Innotech Densitometer.
Polyclonal antibodies specific for phospho(Tyr1054/Tyr1055)-Tyk2, phospho(Tyr701)-STAT1, phospho(Tyr705)-STAT3, phospho(Tyr694)-STAT3, STAT5, phospho(Ser217/Ser221)-MEK (MAPK/ERK kinase) 1/2, and phospho(Thr202/Tyr204)-ERK1/2 and ERK1/2 were all purchased from Cell Signaling Technology. Polyclonal antibodies against phospho(Tyr1022/Tyr1023)-JAK1, phospho(Tyr174)-Vav, Vav (H-211), Slp76 (H-300) and pTyr (phosphotyrosine) were purchased from Santa Cruz Biotechnology.
Immunoprecipitation of protein complexes
Cells were grown at a concentration of 10×103 cells per ml prior to stimulation, 10×106 cells were then used for each time point. Cells were stimulated either with 1 mg/ml OKT3 or 6000 units/ml Roferon-α and incubated at 37 °C for the amount of time indicated before being transferred on to ice and lysed immediately with 1 ml of ice-cold lysis buffer as above. Nuclei and unbroken cells were pelleted through centrifugation at 22000 g for 20 min and the supernatant was decanted into a fresh tube. The lysate concentration was determined through use of Bradford Assay reagents (BioRad Laboratories). Antibody (2 μg) was then added to 2 mg of lysate and placed in continuous rotation at 4 °C overnight. After 18 h, 80 μl of hydrated Protein A–agarose beads (in a 1:1 slurry in 1× phosphate-buffered solution) were added to each sample and returned to continuous rotation for a further 4 h. Cells were then spun for 1 min at 22000 g and washed with ice-cold lysis buffer. This wash step was repeated four times. The beads were then dried and protein was eluted from the beads through addition of an equal amount of 2× Laemmli buffer followed by boiling at 95 °C for 5 min. The supernatant was then resolved via SDS/PAGE (10% gels). The proteins were then transferred on to a nitrocellulose membrane for 2 h at 250 mA, blocked with 3% (w/v) BSA and probed with specific antibodies.
Transfection of cells and generation of stable cell lines
Cells (20×106–30×106) were washed with unsupplemented RPMI 1640 medium, resuspended in 350 μl RPMI 1640 medium and transferred into an electroporation chamber. Plasmid DNA (50 μg) was added to the cells and mixed gently. The chamber containing the cells plus DNA was then pulsed, using a BioRad gene pulser, at a charging pulse of 0.27 V and a capacitance of 960 μF. Cells were resuspended in RPMI 1640 medium supplemented with 10% (v/v) FBS. After 48 h, 400 μg/ml hygromycin was added and the cells were maintained, with regular medium changes, in the presence of antibiotic for 2–3 weeks in order to select for transfected cells. Antibiotic-resistant cells were then dilution-cloned in 96-well plates. Individual clones were selected for expression of the construct through Western blot analysis and on the basis of visualization of fluorescence intensity using fluorescence microscopy.
Luciferase assays
Jurkat cells stably transfected with a construct containing the gene encoding firefly luciferase under the control of a trimer of the NFAT (nuclear factor of activated T-cells)-binding site from the IL (interleukin)-2 gene promoter were activated by immobilized anti-CD3 monoclonal antibody alone, Roferon-α alone (6000 units/ml) or a combination of Roferon-α and anti-CD3 antibody as described previously [12]. Cells were collected 2–24 h after activation and processed for luciferase assays as described previously [13]. Light emission was quantified in a luminometer (Berthold technologies Junior LB 9509) and expressed in RLUs (relative light units).
RESULTS
Vav1 is required for MAPK activation upon IFNαR stimulation
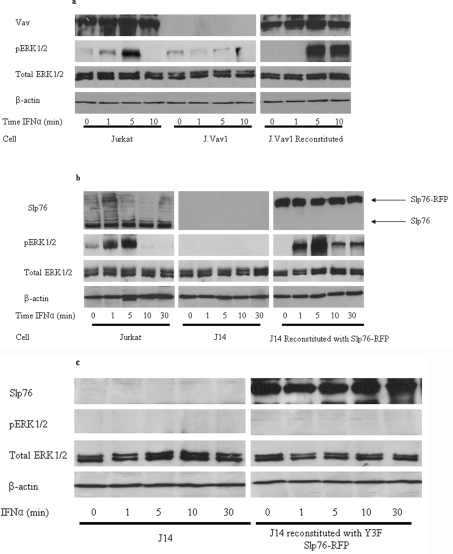
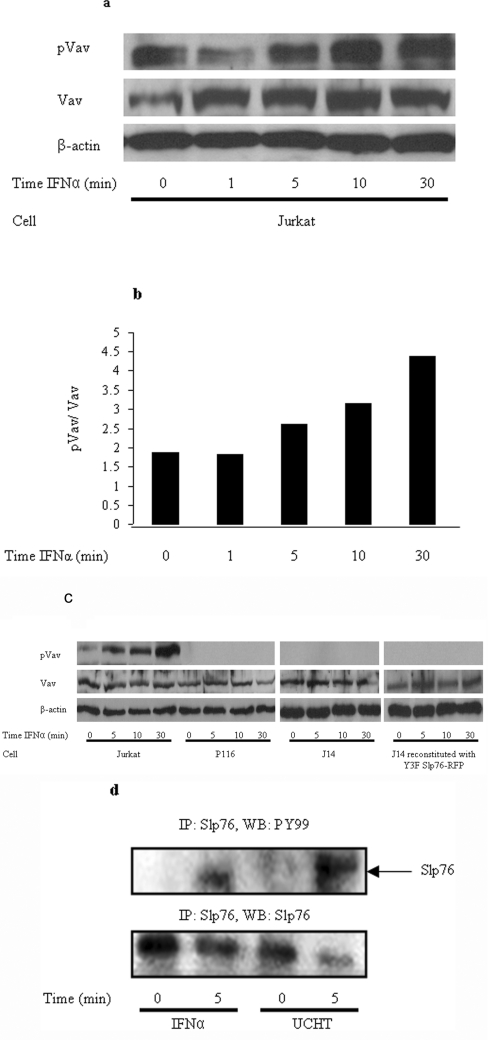
The stimulation of Jurkat T lymphocytes by IFNα results in increased activity of the MAPK pathway shown by elevated levels of ERK1/2 phosphorylation for up to approx. 5 min [3]. This is abrogated in the absence of Lck or Zap70. We considered whether two other known proteins of the TCR ESC, namely Vav1 and Slp76, are also implicated in an IFNαR-stimulated response. J.Vav1 cells, in which Vav expression is completely suppressed (through somatic cell gene targeting), show multiple signalling defects including the virtual abolition of ERK1/2 phosphorylation in response to TCR stimulation [14]. These cells also exhibited a significant reduction in ERK1/2 phosphorylation upon stimulation with IFNα (Figure 1a). This loss of ERK phosphorylation is reversed in reconstituted J.Vav1 cells, in which the Vav1 gene is restored (Figure 1a). The elevation of MAPK activity in Jurkat cells and reconstituted J.Vav1 cells was maximal at 5 min, mirroring the time course observed previously in IFNα-stimulated cells [3]. In resting T-cells Vav1 adopts an inactive conformation whereby the N-terminus forms an inhibitory loop that occludes the catalytic GEF (guanine-nucleotide-exchange factor) domain from accessing its substrates. TCR-stimulated phosphorylation of the key tyrosine residue, Tyr174, by upstream kinases, such as Zap70, releases this loop allowing full catalytic activation [15,16]. Western blots using an antibody against phospho(Tyr174)-Vav1 showed increasing levels of phosphorylation over a 30 min time course post-stimulation by IFNα (Figures 2a and 2b). To demonstrate that this effect was based upon Zap70 kinase activity we showed that Vav1 was not phosphorylated upon stimulation by IFNα in Zap70-deficient P116 cells (Figure 2c). Thus our results suggest that the MAPK response, elicited by IFNα, requires Vav1 function in an identical manner to that reported for TCR activation.
Figure 1. Absence of Vav or Slp76 abolishes downstream ERK1/2 phosphorylation in response to IFNα in Jurkat cells.
Protein lysates were obtained from (a) Jurkat cells, Vav-deficient J.Vav1 cells and J.Vav1 cells reconstituted with a Vav-expressing construct, (b) Jurkat cells, Slp76-deficient J14 cells and J14 cells reconstituted with Slp76–RFP or (c) Slp76-deficient J14 cells and J14 cells reconstituted with the Slp76-Y3F–RFP mutant. Cells were stimulated with IFNα over the time course indicated. Western blotting was used to determine the expression levels of phospho(Thr202/Tyr204)-ERK1/2, ERK1/2 and to determine Vav and Slp76 expression levels, to ensure the presence or absence of each protein in the respective cell lines. β-Actin was used as a loading control.
Figure 2. Activation of both Vav and Slp76 by IFNα mirrors the TCR ESC response in Jurkat and primary T-cells.
(a) A Western blot was performed to determine the expression levels of phospho(Tyr174)-Vav and Vav in IFNα-stimulated Jurkat cells over the indicated time course. β-Actin was used as a loading control. (b) Phospho(Tyr174)-Vav levels normalized to Vav expression by densitometric analysis. (c) Western blotting was performed to determine the expression levels of phospho(Tyr174)-Vav and Vav in IFNα-stimulated Jurkat, Zap70-deficient P116 cells and Slp76-deficient J14 cells over the time periods indicated. β-actin was used as a loading control. (d) Immunoprecipitation (IP) of Vav and Slp76 from primary T-cell lysate. Probing with anti-phosphotyrosine antibody (pY99) reveals that Slp76 and Vav are tyrosine-phosphorylated following 5 min of IFNα stimulation. As a control, the TCR was also stimulated for the same time period and the UCHT1 antibody was used to show the expected protein phosphorylation.
Slp76 is recruited to a TCR-like ESC on IFNαR stimulation
On stimulation of the TCR, Vav1 and Slp76 physically associate and co-operate to induce sustained ERK1/2 signalling [17–19]. J14 cells, derived from parental Jurkat cells that completely lack Slp76 expression, elicit diminished ERK1/2 phosphorylation in response to TCR activation [20]. Similarly, the IFNα stimulation of J14 cells, as with the Vav1-deficient cells above, showed no ERK1/2 response over a 30 min time course (Figure 1b). Wild-type and J14 cells reconstituted with RFP-tagged Slp76 show recovery of MAPK activity (Figure 1b). Slp76 is implicated in the recruitment of Vav1 to the TCR ESC prior to phosphorylation [21,22]. Figure 2(c) shows that in J14 cells lacking Slp76, no Vav1 phosphorylation was apparent on IFNα stimulation suggesting that Slp76 is behaving in a similar way on cytokine stimulation. Furthermore, it is known that as part of the TCR ESC, Slp76 is phosphorylated at three key tyrosine residues located at positions 112, 128 and 145. All three of these residues are required for optimal downstream ERK activation and NFAT activity [21,22]. J14 cells reconstituted with Slp76–RFP in which the three tyrosine sites are replaced with phenylalanine (Slp76-Y3F–RFP) failed to rescue ERK1/2 activation (Figure 1c). These results are consistent with the notion that the function of Slp76 in IFNαR signalling is similar to that observed on TCR stimulation [23–25].
Slp76 is phosphorylated in primary CD4+ T-cells stimulated by IFNα
Although Jurkat cells provide an excellent model for studying signal transduction, as they are a leukaemic cell line, they bear several gene mutations and may not completely represent signalling in healthy T-cells in vivo. For example, Jurkat cells are known not to express the phosphatases PTEN (phosphatase and tensin homologue deleted on chromosome 10) and SHIP [SH2 (Src homology 2)-domain-containing inositol phosphatase], which are involved in phospholipid metabolism (reviewed in [26]). To corroborate our Jurkat cell results peripheral primary CD4+ T-cells were isolated from fresh blood donors and cells were stimulated in a similar manner to Jurkat cells. Unstimulated cells, or cells stimulated with IFNα for 5 min, were lysed and immunoprecipitated with Slp76. The lysates were then subjected to Western blotting and probed with anti-phosphotyrosine antibodies. Figure 2(d) illustrates that Slp76 was tyrosine-phosphorylated following 5 min of IFNα stimulation. As a control, cells were also stimulated through the TCR with the UCHT1 antibody for the same period of time. This blot demonstrates for the first time that Slp76 undergoes IFNα-stimulated phosphorylation both in Jurkat cells and primary CD4+ primary T-cells in response to IFNα stimulation.
An intact TCR is required for IFNα-stimulated MAPK activity
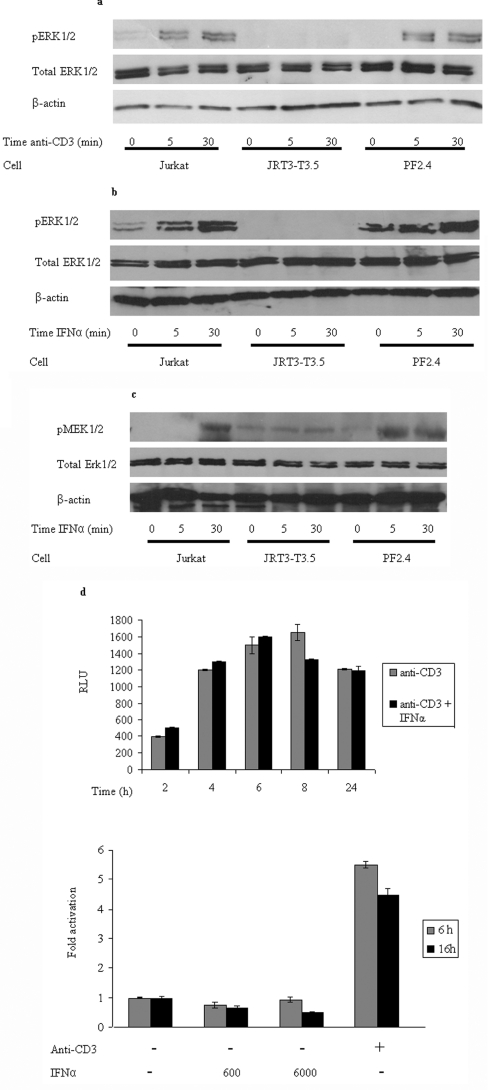
It has been demonstrated previously that Lck, Zap70 and CD45 all play a role in IFNα signalling [3,9,27]. In the present study we show that, in addition, both Vav1 and Slp76 are also intimately involved in cytokine-induced signalling and respond in a way that is similar to their response to TCR stimulation. Thus five of the proteins known to be recruited to the ESC at the TCR on antigen presentation are also associated with producing a MAPK response on IFNαR stimulation. This suggests that either these proteins act as independent entities in the two distinct signalling pathways, or that IFNαR and TCR signalling are inextricably linked, i.e. the ERK1/2 activation via IFNα stimulation requires the recruitment and assembly of the TCR ESC. To test this we asked whether the TCR itself is involved in the IFNα-stimulated response. The JRT3-T3.5 cell line does not express the β-subunit of the TCR and consequently proper assembly of a complete functional receptor on the cell surface cannot occur [28]. As predicted, only basal levels of MAPK activity were exhibited upon stimulation of the cells with OKT3 compared with the parental Jurkat cell line (Figure 3a). The MAPK response was rescued in PF2.4 cells, which are reconstituted with the β-subunit and hence have a functional TCR [29]. Stimulation of JRT3-T3.5 cells with IFNα failed to produce the elevated ERK1/2 phosphorylation over the time course seen in wild-type Jurkat cells (Figure 3b). This activity can be restored in the PF2.4 cells. In addition to reduced phosphorylation of ERK1/2, IFNα stimulation of cells lacking the intact TCR also failed to effect a MEK1/2 response (Figure 3c). As MEK is the serine/threonine kinase that phosphorylates ERK this suggests that the loss of ERK1/2 activity reflects a defect in the pathway upstream of these kinases. Therefore our results show that class I interferon signalling appropriates at least part of the TCR ESC and stimulates a MAPK response via the usual mechanism associated with active TCR signalling.
Figure 3. IFNαR-induced MAPK requires a functional TCR, but, despite this, does not induce up-regulation of the same genes as TCR-induced MAPK signalling.
The Jurkat, JRT3-T3.5 (TCRβ−/−) and PF2.4 (stably reconstituted with the TCRβ chain) cell lines were stimulated with (a) OKT3, which activates the TCR or (b) IFNα for the indicated time periods. Western blotting was then performed to determine the expression levels of phospho(Thr202/Tyr204)-ERK1/2 and ERK1/2. β-actin was used as a loading control. (c) Western blotting was used to determine the expression levels of phospho(Ser217/Ser221)-MEK1/2 and ERK1/2 in Jurkat, JRT3-T3.5 and PF2.4 cells that were stimulated with IFNα for the indicated time periods. β-Actin was used as a loading control. (d) Relative luciferase activity from an NFAT–luciferase reporter in Jurkat cells activated for the indicated times by immobilized anti-CD3 monoclonal antibody, either alone or in combination with IFNα. The results are expressed as RLUs (upper panel) or as relative luciferase activity in treated compared with untreated samples (fold activation; lower panel).
Although our results reveal that the IFNαR appears to employ the TCR ESC to elicit MAPK activity, this does not result in the transcriptional response derived from anti-CD3 antibody TCR stimulation. A luciferase-based assay was used to assess whether IFNα stimulation of Jurkat cells resulted in the transcription of NFAT-dependent genes, which are characteristic of anti-CD3 antibody TCR activation. Whereas anti-CD3 antibody elicited the expected, and previously reported, transcription of NFAT, this did not occur in response to different concentrations of IFNα, over time (Figure 3d, lower panel). The effect of IFNαR stimulation in combination with anti-CD3 antibody was to moderately enhance NFAT activation at earlier time points and blunt it after 6 h (Figure 3d, upper panel). This effect on the transient NFAT activation might mean that IFNα is able to reduce the time required for anti-CD3 antibody to activate transcription. Thus although interferon stimulation elicits TCR ESC assembly and MAPK activity, the clear difference in transcriptional response suggests that the signal is subject to downstream modulation/modification.
IFNαR-induced activation of MAPK is independent of JAK/STAT signalling
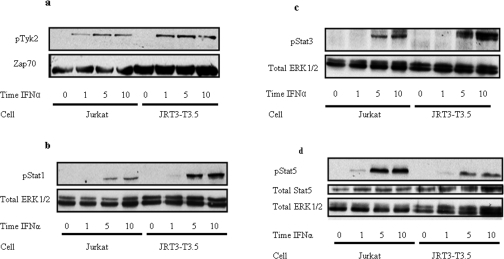
Stimulation-dependent phosphorylation of JAK, and subsequent phosphorylation of the STAT proteins, is essential for mediating many of the anti-viral responses brought about by stimulation of the IFNαR. To assess whether the IFNα-stimulated signal, which uses the TCR ESC, affects the JAK/STAT pathway, cells deleted for the TCR were again used. IFNα stimulation of JRT3-T3.5 cells had no effect on Tyk2 activity (Figure 4a). This confirms that TCR ESC formation does not affect the normal JAK/STAT signalling from the IFNαR. To exemplify this further Figures 4(b)–4(d) show that STAT1, STAT3 and STAT5 respectively were tyrosine-phosphorylated in response to IFNα stimulation in wild-type Jurkat and in the JRT3-T3.5 cells. Thus one can conclude that the JAK/STAT and MAPK responses derived from IFNαR are entirely independent.
Figure 4. Absence of the TCRβ chain does not affect phosphorylation of components of the JAK/STAT signalling pathway.
Western blotting on cell lysates obtained from IFNα-stimulated Jurkat and JRT3-T3.5 cells using (a) anti-phospho(Tyr1054/Tyr1055)-Tyk2 antibody, (b) anti-phospho(Tyr701)-STAT1 antibody, (c) anti-phospho(Tyr705)-STAT3 antibody and (d) anti-phospho(Tyr694)-STAT5 and anti-STAT5 antibodies. Total ERK1/2 was used to demonstrate equal loading
MAPK is phosphorylated in response to IFNα in primary human CD4+ cells and the TCR machinery is required
The results presented above show that the T-cell ESC machinery is mandatory for ERK MAPK signalling in response to IFNα. In order to check whether ERK phosphorylation also occurs in healthy human CD4+ T-cells downstream of the IFNαR, cells were stimulated ex vivo with IFNα. In agreement with previous findings in Jurkat cell lines [3], ERK phosphorylation was also seen over a 30 min time period (Figures 5a and 5b). The levels of ERK phosphorylation mirrored those seen in wild-type Jurkat cells (Figure 1).
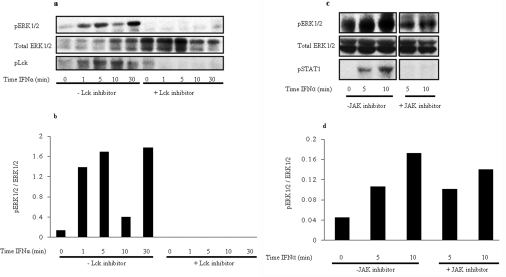
Figure 5. ERK is phosphorylated in response to IFNα in primary human CD4+ T-cells: components of the TCR machinery are required for this ERK phosophorylation.
(a) Freshly isolated human peripheral CD4+ T-cells were stimulated for up to 30 min in the presence and absence of Lck inhibitor I. UCHT1 was used to stimulate cells as a control for 5 min in the presence and absence of inhibitor. Lysates were immunoblotted with anti-phospho-ERK1/2 antibody (pERK1/2; top panel), anti-phospho-Lck antibody (pLck; bottom panel) or anti-ERK1/2 antibody (total ERK 1/2; middle panel). (b) Normalized densitometric analysis of ERK phosphorylation. The level of increased ERK activity is relative to the level of total protein. (c) Human peripheral CD4+ T-cells were stimulated for the time periods indicated without (left-hand side) or with 15 nm (right-hand side) of JAK-1 inhibitor for 5 and 10 min on the same gel. The same basal time point (no IFNα added) was used, as shown in the left-most lane. Lysates were used to immunoblot with either anti-phospho-ERK1/2 antibody (top panel), anti-phospho-STAT1 antibody (bottom panel) or anti-ERK1/2 antibody (total ERK 1/2; middle panel). (d) Densitometric analysis of ERK phosphorylation taking into account level of protein present in each lane. The level of ERK phosphorylation is shown in arbitrary units.
As there are no TCR-deficient primary T-cells available, to assess the requirement of one of the TCR ESC proteins Lck inhibitor was utilized to pretreat CD4+ cells before stimulation. Lck is an essential component of the TCR ESC and signalling through ERK is blocked in its absence at the TCR. Previous studies in Jurkat cell lines have shown that Lck is also imperative for IFNα-induced ERK phosphorylation [2,3]. Figures 5(a) and 5(b) show that when peripheral CD4+ T-cells were stimulated in the presence of 20 nM Lck inhibitor, ERK phosphorylation was blocked downstream of the TCR when compared with CD4+ T-cells where no inhibitor had been added. To check that Lck was blocked by the inhibitor, the blot was also probed with anti-phospho-Lck antibody. In lanes containing Lck inhibitor-treated lysates, no phosphorylation was seen, confirming that this is an effective inhibitor. The total levels of Lck were also unaffected by this inhibitor, proving that it is effective in binding to Lck in a manner that prevents phosphorylation but does not cause degradation.
Figures 5(c) and (d) also provide confirmation that, as in Jurkat cells, ERK phosphorylation was predominantly independent of JAK/STAT signalling. When primary CD4+ T-cells were pre-treated with the JAK1 inhibitor, ERK phosphorylation was still seen at 5 and 10 min. STAT1, which is a downstream component of the IFNαR-induced JAK/STAT signalling pathway, was not phosphorylated in the presence of the JAK1 inhibitor. Collectively these results obtained from healthy human T-cells show that ERK phosphorylation occurs downstream of the IFNαR and that this is largely independent of JAK/STAT but dependent on functional TCR ESC.
DISCUSSION
The well characterized signalling events immediately following TCR ligation involve recruitment and assembly of a carefully orchestrated complex of enzymes and adaptor proteins. This leads to sustained signalling through the ERK/MAPK pathway, as well as a calcium flux, increased phospholipid metabolism and activation and nuclear translocation of various transcription factors such as NF-κB (nuclear factor κB) and NFAT. These pathways control the transcription of genes that promote cytoskeletal reorganization and polarization towards the antigen-presenting cell and cellular proliferation. On the other hand, IFNα is a cytokine that is produced by cells in response to viral dsRNA (double-stranded RNA) and bacterial lipopolysaccharides and glycoproteins as part of the innate immune response. The IFNαR signals primarily through the JAK/STAT pathway, but additional signalling through MAPKs (including p38 and c-Jun N-terminal kinase), PI3K (phosphoinositide 3-kinase)/Akt, activation of the NF-κB transcription factor and CrkL/C3G are implicated in the overall cellular response. IFNα binding to the IFNαR induces growth inhibition and apoptosis. Thus the cellular response to TCR and IFNαR activation are fundamentally different.
Despite the two receptors eliciting opposing cellular responses, it is evident that they share similarities in their modes of signalling as both receptors are able to phosphorylate ERK/MAPK. In order for this pathway to ensue the two receptors require an overlapping subset of proteins. Although these proteins were initially thought to be exclusively part of the TCR signalling machinery, they have also been shown to be utilized in signalling by other T-cell-expressed receptors, e.g. the chemokine receptor CXCR4 [30–32] and integrin receptors [33,34].
Previous work has demonstrated that the IFNαR requires TCR-associated proteins including Lck, Zap70, CD45 [3,9], Vav1 [7,35] and possibly LAT (linker for activation of T-cells) [36]. In the present study we show for the first time that Vav1 is required for ERK1/2 phosphorylation in response to IFNαR stimulation. The IFNαR-stimulated Vav1 phosphorylation appears to be identical with that observed with TCR, i.e. phosphorylation occurs upon Tyr174, located within the acidic region of the protein [15,16]. In addition, we have demonstrated that Slp76 is tyrosine-phosphorylated as a result of IFNαR stimulation and is important for the ensuing ERK1/2 MAPK response. IFNα-stimulated Slp76 phosphorylation appears to be important as reconstitution of J14 cells with the Slp76-Y3F mutant, did not restore MAPK signalling. The acidic region of Slp76 contains residues involved in interactions with Zap70, LAT, Nck, Vav and ITK (IL2-inducible T-cell kinase), resulting in a multimolecular signalling complex [20]. The fact that the Slp76-Y3F mutant ablated Vav1 phosphorylation suggests that in interferon signalling Vav1 functions downstream of Slp76. Thus the role of Vav1 and Slp76 on IFNα-stimulation appears to be identical with that seen when they are recruited to the TCR on presentation of an antigen. Furthermore, we show for the first time that Slp76 is tyrosine-phosphorylated in response to IFNα stimulation in primary human peripheral CD4+ T lymphocytes. This substantiates results obtained from the Jurkat T-cell lymphoma cell line.
The involvement of the TCR ESC proteins CD45, Lck, Zap70, Vav and Slp76 in IFNα-stimulated signalling via the MAPK pathway prompted investigation of whether the TCR itself was in some way involved. A requirement for the TCR would be indicative of cross-talk between the IFNαR and the TCR. Our results show that in the absence of the intact TCR, ERK1/2 phosphorylation is compromised. The JRT3-T3.5 cell line, lacking the TCRβ subunit, exhibited severely diminished ERK1/2 phosphorylation, which could be restored upon reconstitution with the TCRβ subunit. In addition, the JRT3-T3.5 cells displayed curtailed MEK phosphorylation and lower levels of total protein tyrosine phosphorylation, thus confirming the integrity of the TCR-activated signal through the MAPK pathway. The fact that the phosphorylation of Tyk2 and the transcription factors STAT1, STAT3 and STAT5 were detected at equivalent levels in both wild-type Jurkat and JRT3-T3.5 cells suggest that the normally observed interferon-induced JAK/STAT signalling was unaffected by the MAPK parallel pathway. However, as STAT proteins can also be serine-phosphorylated upon cytokine induction [37], our results do not exclude the possibility that activation of the parallel MAPK signalling pathway could be involved in serine phosphorylation of the IFNα-activated STAT proteins. Further evidence that the IFNαR utilizes TCR machinery in healthy human CD4+ T-cells is shown in Figure 5. IFNα-induced MAPK signalling was seen over a 30 min time course, but this was blocked in the presence of Lck inhibitor. Lck is an integral component of the TCR ESC and its absence has been shown previously to prevent proper TCR ESC formation and halt downstream ERK/MAPK signalling at the TCR [3]. We demonstrated that Lck is phosphorylated in response to IFNα in human CD4+ T-cells. Additionally, Lck was also required for ERK/MAPK signalling at the IFNαR (Figure 5a), which demonstrates cross-talk between the TCR and IFNαR for the first time in healthy human T-cells. Inhibition of Lck did not affect phosphorylation of STAT1, which corroborates the finding in Jurkat cells that cross-talk between the TCR and IFNαR is involved in ERK/MAPK activation independently of JAK/STAT signalling and does not overlap with this pathway during early signalling events. Furthermore, inhibition of JAK activation did not affect ERK phosphorylation downstream of the TCR (Figure 5c) thus JAKs are not involved in initiation of ERK/MAPK signals at the IFNαR upon IFNα stimulation. Thus, as in Jurkat cells, JAK/STAT independent TCR-IFNαR cross-talk is evident and essential for ERK/MAPK phosphorylation.
The IL-2 gene is transcribed upon TCR stimulation. The secreted cytokine binds to its receptor to augment the G1/S-phase transition of the cell cycle in TCR-activated T-cells resulting in clonal expansion and cellular differentiation [38,39]. It is known that at the TCR, both Vav1 and Slp76 co-operate to induce IL-2 transcription [40,41]. Given, as we have demonstrated, that both of these proteins are involved in IFNα signalling, it is plausible that they could mediate transcription of this gene. This would, however, not be consistent with the growth inhibitory events normally mediated by the IFNαR. The lack of NFAT transcriptional activity and hence absence of IL-2 up-regulation show that IFNα is unable to effect an entirely normal TCR-like transcriptional response. Thus the cell is not compromised between two distinct, opposing responses.
Although the assembly of the ESC for signalling through both IFNαR and TCR appears to be highly similar, the downstream effects of the resulting MAPK activation are different. The IFNαR signal results in maximal ERK1/2 phosphorylation at approx. 5 min (Figures 1a and 1b), whereas the TCR response is prolonged [3]. In PC12 cells it has been proposed that transient activation precludes nuclear translocation of ERK2, which occurs with a sustained signal [42,43]. It is thus possible that the IFNα-induced short-lived phosphorylation of ERK2 abrogates its translocation into the nucleus. Cytoplasmically localized ERK1/2 has been implicated in the regulatory function of IFNα signalling. Phosphorylated ERK1/2 has been shown to have serine/threonine kinase activity towards STAT1, enabling this protein to activate transcription [44]. In addition, direct binding of phosphorylated ERK2 to the IFNR further suggests a role in modulation of this pathway.
Our results are consistent with the previously reported mechanism for the involvement of TCR ESC proteins in IFNαR signalling being based on regulation by Lck and/or CD45 [9]. The physical association of these proteins with the IFNαR is able to activate the kinase domain of membrane-localized Lck. Once activated Lck can act on the ζ-subunit of the TCR and, in a similar way to that observed on antigen presentation, the TCR is able to recruit the ESC.
In conclusion, the present study highlights that signalling from cell membrane-bound receptors is not based on isolated mutually exclusive pathways, but rather that these pathways are in some way networking to allow greater communication in the cell. We speculate that networking of this type may be essential to allow the cell to discern which course to commit to in the case of exposure to multiple external stimuli. For example, a cell committed to an interferon-stimulated response needs to ensure that a signal from another cell-surface receptor is not able to corrupt this. Thus signalling through a common pathway, such as MAPK in the present study, may serve to alert the cell to down-regulate/block signalling from other receptors. Engaging the MAPK pathway via the TCR ESC in transducing one response (non-proliferation from IFNαR) disables this pathway and halts any possibility that the cell will transduce a simultaneous signal from the TCR invoking proliferation. Engaging the TCR machinery in order to prevent signalling through the TCR while the cell is committed to an alternative response is a fail-safe method to stop the cell from responding to two opposing instructions.
AUTHOR CONTRIBUTION
Claire Stevens performed most of the experimental work and conceived many of the experiments. Ann-Marie Simeone repeated some of the original Western blots and prepared several of the Figures. Susan John supervized Claire Stevens in the primary cell work and performed repeat blots with inhibitors in primary cells. Zamal Ahmed oversaw Claire Stevens and provided useful input for the cell-based assays and also provided useful comment at the manuscript write-up stage. Orso Lucherini performed the transcriptional analysis experiments. Tatiana Baldari supervised and provided reagents for the transcriptional analysis experiments. John Ladbury conceived the project and supervised throughout and also had a major role in manuscript writing and production.
FUNDING
This work was supported in part by a Wellcome Trust Senior Research Fellowship [number 054191 (awarded to J. E. L.)]; a U.K. Biotechnology and Biological Sciences Research Council graduate scholarship (awarded to C. N. S.); the Associazione Italiana per la Ricerca sul Cancro (C. T. B.); and by the U.K. Medical Research Council [grant number G0400197 (S. J.)].
References
- 1.David M., Petricoin E., Benjamin C., Pine R., Weber M. J., Larner A. C. Requirement for MAP kinase (ERK2) activity in interferon α- and interferon β-stimulated gene expression through STAT proteins. Nature. 1995;269:1721–1723. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 2.Lund T. C., Medveczky M. M., Medveczky P. G. Interferon-αinduction of STATs1, -3 DNA binding and growth arrest is independent of Lck and active mitogen-activated kinase in T cells. Cell. Immunol. 1999;192:133–139. doi: 10.1006/cimm.1999.1466. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed Z., Beeton C. A., Williams M. A., Clements D., Baldari C. T., Ladbury J. E. Distinct spatial and temporal distribution of ZAP70 and Lck following stimulation of Interferon and T-cell receptors. J. Mol. Biol. 2005;353:1001–1010. doi: 10.1016/j.jmb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Pacini S., Valensin S., Telford J. L., Ladbury J., Baldari C. T. Temporally regulated assembly of a dynamic signalling complex associated with the activated TCR. Eur. J. Immunol. 2000;30:2620–2631. doi: 10.1002/1521-4141(200009)30:9<2620::AID-IMMU2620>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke L., Ladbury J. E. Specificity is complex and time consuming: mutual exclusivity in tyrosine kinase-mediated signalling. Acc. Chem. Res. 2003;36:410–416. doi: 10.1021/ar020167s. [DOI] [PubMed] [Google Scholar]
- 6.Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 7.Uddin S., Sweet M., Colamonici O. R., Krolewski J. J., Platanias L. C. The vav proto-oncogene product (p95vav) interacts with the Tyk-2 protein tyrosine kinase. FEBS Lett. 1997;403:31–34. doi: 10.1016/s0014-5793(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 8.Tybulewicz V. L. J. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Petricoi E. F., III, Ito S., Williams B. L., Audet S., Stancato L. F., Gamero A., Clouse K., Grimley P., Weiss A., Beeler J., et al. Antiproliferative action of interferon-α requires components of T-cell-receptor signalling. Nature. 1997;390:629–632. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]
- 10.Williams S., Couture C., Gilman J., Jascur T., Deckert M., Altman A., Mustelin T. Reconstitution of T cell antigen receptor-induced Erk kinase activation in Lck-negative JCam1 cells by Syk. Eur. J. Biochem. 1997;245:84–90. doi: 10.1111/j.1432-1033.1997.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 12.Milia E., Di Somma M. M., Baldoni F., Chiari R., Lanfrancone L., Pelicci P. G., Telford J. L., Baldari C. T. The aminoterminal phosphotyrosine binding domain of Shc associates with ZAP-70 and mediates TCR dependent gene activation. Oncogene. 1996;13:767–775. [PubMed] [Google Scholar]
- 13.Baldari C. T., Di Somma M. M., Majolini M. B., Ulivieri C., Milia E., Telford J. L. NF-AT-luciferase reporter T cell lines as tools to screen immunosuppressive drugs. Biologicals. 1998;26:1–5. doi: 10.1006/biol.1997.0116. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y., Janssen E. M., Duncan A. W., Altman A., Billadeau D. D., Abraham R. T. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 2002;21:4809–4819. doi: 10.1093/emboj/cdf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespo P., Schuebel K. E., Ostrom A. A., Gutkind J. S., Bustelo X. R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 16.Aghazadeh B., Lowry W. E., Huang X.-Y., Rosen M. K. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 17.Onodera H., Motto D. G., Koretzzky G. A, Rothstein D. M. Differential regulation of activation-induced tyrosine phosphorylatoin and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phophatase. J. Biol. Chem. 1996;271:22225–22230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 18.Tuosto L., Micheal F., Acuto O. p95Vav associates with tyrosinephosphorylated SLP-76 in antigen-stimulated T-cells. J. Exp. Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J., Zhao Q., Kurosaki T., Weiss A. The Vav binding site (Y315) in Zap-70 is critical for antigen receptor-mediated signal transuction. J. Exp. Med. 1997;185:1877–1882. doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yablonski D., Kuhne M. R., Kadlecek T., Weiss A. Uncoupling of non-receptor tyrosine kinases from PLC-γ in a Slp-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 21.Fang N., Motto D. G., Ross S. E., Koretzky G. A. Tyrosines 113, 128 and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- 22.Jordan M. S., Sadler J., Austin J. E., Finkelstein L. D., Singer A. L., Schwartzberg P. L., Koretzky G. A. Functional hierarchy of the N-terminal tyrosines of SLP-76. J. Immunol. 2006;176:2430–2438. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- 23.Bunnell S. C., Singer A. L., Hong D. I., Jacque B. H., Jordan M. S., Seminario M.-C., Barr V. A., Koretzky G. A., Samelson L. E. Persistence of cooperatively stabilised signalling clusters drive T-cell activation. Mol. Cell. Biol. 2006;26:7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hishimoto-Tane A., Tokunaga M., Dustin M. L., Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and Slp76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 25.Bunnell S. C., Hong D. J., Kardon J. R., Yamazaki T., McGlade J., Barr V. A., Samelson L. E. T cell receptor ligation induces the formation of dynamically regulated signalling assemblies. J. Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astoul E, Edmunds C., Cantrell D. A., Ward S. G. PI3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 2001;22:490–496. doi: 10.1016/s1471-4906(01)01973-1. [DOI] [PubMed] [Google Scholar]
- 27.Uddin S., Majchrzak B., Woodson J., Arunkumar P., Alsayed Y., Pine R., Young P. R., Fish E. N., Platanias L. C. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 1999;274:30127–30131. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 28.Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi T., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985;316:606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- 30.Fernandis A. Z., Cherla R. P., Ganju R. K. Diffrential regulation of CXCR4-mediated T-cell chemotaxis and Mitogen-activated protein kinase activation by membrane tyrosine phosphatase, CD45. J. Biol. Chem. 2003;278:9536–9543. doi: 10.1074/jbc.M211803200. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A., Humpreys T. D., Kremer K. N., Bramati P. S., Bradfield L., Edgar C. E., Hedin K. E. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Petrussi L., Ulivirei C., Lucherini O. M., Paccani S. R., Gamberucci A., Pelicci P. G., Baldari C. T. p52Shc is required for CXCR4-dependent signalling and chemotaxis in T-cells. Blood. 2007;110:1730–1738. doi: 10.1182/blood-2007-01-068411. [DOI] [PubMed] [Google Scholar]
- 33.Obergfell A., Judd B. A., del Pozo M., Schwart A., Koretzky G. A., Shattil S. J. The molecular adaptor Slp-76 relays signals from the platelet integrin αIIbβ3 to the actin cytoskeleton. J. Biol. Chem. 2001;276:5916. doi: 10.1074/jbc.M010639200. [DOI] [PubMed] [Google Scholar]
- 34.Del Pozo M., Schwartz M. A., Hu J., Kiosses W. B., Altman A., Villalba M. Guanine exchange-dependent and -independent effects of Vav1 on integrin-induced T cell spreading. J. Immunol. 2003;170:41–47. doi: 10.4049/jimmunol.170.1.41. [DOI] [PubMed] [Google Scholar]
- 35.Adam L., Bandyopadhyay D., Kumar R. Interferon-α signalling promotes nucleus-to-cytoplamic redistribution of p95Vav and formation of a multisubunit complex involving Vav, Ku80 and Tyk2. Biochem. Biophys. Res. Commun. 2000;267:692–696. doi: 10.1006/bbrc.1999.1978. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H., Hu P., Quinn D. F., Wang Y. K. Phosphotyrosine proteomic study of interferon signaling pathway using a combination of immunoprecipitation and immobilized metal affinity chromatography. Mol. Cell. Proteomics. 2005;4:721–730. doi: 10.1074/mcp.M400077-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Decker T., Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 38.Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 39.Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 40.Raab M., da Silva A. J., Findell P. R., Rudd C. E. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCRζ/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 41.Rudd C. E., Raab M. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol. Rev. 2003;192:32–41. doi: 10.1034/j.1600-065x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 42.Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 43.Schüller A. C., Ahmed Z., Ladbury J. E. Extracellular point mutations in FGFR2 result in elevated Erk1/2 activation and perturbation of neuronal differentiation. Biochem. J. 2008;410:205–211. doi: 10.1042/BJ20070859. [DOI] [PubMed] [Google Scholar]
- 44.Wen Z., Zhong Z., Darnell J. E., Jr Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]