Abstract
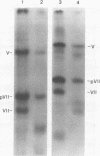
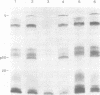
We examined acetylation of the histone-like adenovirus core proteins VII and V and the precursor of the major core protein, pVII, by measuring the incorporation of [14C]acetate. Adenovirus proteins pVII and V appeared to be acetylated, whereas protein VII was not. Label incorporated into these viral proteins in the form of acetate was metabolically stable, and labeling was not enhanced by treatment with sodium butyrate, an inhibitor of histone deacetylases. Viral protein acetylation therefore differs from the reversible acetylation of histones that has been implicated in transient alterations of chromatin structure. Inhibition of protein synthesis in infected cells resulted in a proportional reduction in [14C]acetate uptake into pVII and V, suggesting that these proteins undergo acetylation during protein synthesis and not as a post-translational modification. Therefore, these viral proteins are probably acetylated amino-terminally, a characteristic shared by three of the five major histone classes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Allfrey V. G. Changes in chromosomal proteins at times of gene activation. Fed Proc. 1970 Jul-Aug;29(4):1447–1460. [PubMed] [Google Scholar]
- Amin M., Mirza A., Weber J. Genetic analysis of adenovirus type 2. VII. Cleavage-modified affinity for DNA of internal virion proteins. Virology. 1977 Jul 1;80(1):83–97. doi: 10.1016/0042-6822(77)90382-8. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Trout testis cells. 3. Acetylation of histones in different cell types from developing trout testis. J Biol Chem. 1972 Sep 10;247(17):5506–5510. [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Ustacelebi S., Williams J., Philipson L. Assembly intermediates among adenovirus type 5 temperature-sensitive mutants. J Virol. 1978 Feb;25(2):641–651. doi: 10.1128/jvi.25.2.641-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Organ specificity of histone Acetyltransferases. FEBS Lett. 1971 Mar 22;13(5):306–308. doi: 10.1016/0014-5793(71)80247-8. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Mellor A., Smith A. E., Waterfield M. D. Partial amino-terminal sequences of the polyoma nonhistone proteins VP1, VP2, and VP3 synthesized in vitro. J Virol. 1980 Feb;33(2):631–636. doi: 10.1128/jvi.33.2.631-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Fujimoto D. Histone deacetylase from calf thymus. Biochim Biophys Acta. 1970 Nov 11;220(2):307–316. doi: 10.1016/0005-2744(70)90015-x. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Jackson V., Shires A., Chalkley R., Granner D. K. Studies on highly metabolically active acetylation and phosphorylation of histones. J Biol Chem. 1975 Jul 10;250(13):4856–4863. [PubMed] [Google Scholar]
- Jörnvall H., Ohlsson H., Philipson L. An acetylated N-terminus of adenovirus type 2 hexon protein. Biochem Biophys Res Commun. 1974 Jan 23;56(2):304–310. doi: 10.1016/0006-291x(74)90842-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Fujimoto D. Multiplicity of histone deacetylase from calf thymus. FEBS Lett. 1973 Feb 1;29(3):280–282. doi: 10.1016/0014-5793(73)80038-9. [DOI] [PubMed] [Google Scholar]
- Kit G., Daniell E. Adenovirus core protein synthesis in the absence of viral DNA synthesis late in infection. J Virol. 1978 Jul;27(1):74–80. doi: 10.1128/jvi.27.1.74-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Suriano J. R., Green M. Adenovirus proteins. II. N-terminal amino acid analysis. J Virol. 1967 Aug;1(4):723–728. doi: 10.1128/jvi.1.4.723-728.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew C. C., Haslett G. W., Allfrey V. G. N-acetyl-seryl-tRNA and polypeptide chain initiation during histone biosynthesis. Nature. 1970 May 2;226(5244):414–417. doi: 10.1038/226414a0. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Sung M. T. A histone-like protein from adenovirus chromatin. Nature. 1977 Jun 9;267(5611):552–554. doi: 10.1038/267552a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Uncoating of adenovirus type 2. J Virol. 1979 May;30(2):462–471. doi: 10.1128/jvi.30.2.462-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D. Histone acetylation during meiosis in Lilium microsporocytes. Exp Cell Res. 1976 Sep;101(2):283–292. doi: 10.1016/0014-4827(76)90379-7. [DOI] [PubMed] [Google Scholar]
- PHILLIPS D. M., JOHNS E. W. A FRACTIONATION OF THE HISTONES OF GROUP F2A FROM CALF THYMUS. Biochem J. 1965 Jan;94:127–130. doi: 10.1042/bj0940127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Paucha E., Mellor A., Harvey R., Smith A. E., Hewick R. M., Waterfield M. D. Large and small tumor antigens from simian virus 40 have identical amino termini mapping at 0.65 map units. Proc Natl Acad Sci U S A. 1978 May;75(5):2165–2169. doi: 10.1073/pnas.75.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]