Abstract
Recent studies suggest that Stat3, a transcription factor that mediates cytokine signaling, plays a critical role in the pathogenesis of diabetic nephropathy. Complete Stat3 gene knockout is embryonic lethal; therefore, we crossed Stat3+/– mice with Stat3 mutant mice (SA/SA) that lack full Stat3 activity. This strategy generated Stat3SA/– mice (25% activity) and Stat3SA/+ mice (75% activity), which were made diabetic using streptozotocin in order to define the role of Stat3 in diabetic kidney disease. While the glomerular number was not different between these two groups of mice, the diabetic SA/– mice had significantly less proteinuria, mesangial expansion, glomerular cell proliferation, and macrophage infiltration than the diabetic SA/+ mice. The reduction in Stat3 activity did not affect glomerular hyperfiltration seen after the induction of diabetes, as it was increased to the same degree in both groups of mice. Phosphorylation of Stat3 was markedly increased in the glomeruli of diabetic SA/+ mice compared to diabetic SA/– mice. The expression of inflammatory markers, IL-6, MCP-1, and activated NF-κB; type IV collagen, TGF-β, and ICAM-1 mRNA; or type IV collagen and TGF-β protein, were all found to be significantly less in glomeruli isolated from diabetic SA/– mice, as compared with diabetic SA/+ mice. Our study shows that Stat3 plays a critical role in the regulation of inflammation and abnormal matrix synthesis at an early stage of DN.
Keywords: collagen IV, diabetic nephropathy, glomerulosclerosis, mesangial cells, Stat3
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease in the United States.1 Although our understanding of the disease pathogenesis is expanding, specific therapy for DN is still missing. The kidney in diabetes is exposed to a milieu of high serum glucose, oxidant stress, and advanced glycation end products (AGE), all of which contribute to the development of nephropathy. At the cellular level, this leads to mesangial cell activation, podocyte apoptosis, and epithelial-mesenchymal transition of renal tubular epithelial cell.2,3 Inflammatory cell infiltration into the kidney also plays a critical role in the pathogenesis of DN.4
Multiple cell signaling pathways, including MAPK, PKC, and Smad, are activated in the diabetic kidney.2 Several recent studies suggest that activation of the Janus kinase/signal transducers and activator of transcription (JAK/STAT) pathway is also important. A recent study suggests that a haplotype of interleukin 6 (IL-6), a classic upstream activator of the JAK/Stat3 pathway, is associated with reduced glomerular filtration rate (GFR) in type 2 diabetic patients.5 Furthermore, microarray analysis of gene expression in kidneys of diabetic patients showed that multiple genes in the JAK/Stat pathways were highly upregulated in glomeruli at the early stage of DN and in tubular area at the later stage of disease.6 Angiotensin II has been shown to induce the phosphorylation and activation of JAK2, STAT1, and STAT3, which are critical for angiotensin-II-induced proliferation of glomerular mesangial cells and the production of transforming growth factor-β (TGF-β), collagen IV, and fibronectin (reviewed by Marrero et al.7). Hyperglycemia is also known to activate the JAK/STAT pathway in mesangial cells, presumably due to hyperglycemia-mediated increase in the production of reactive oxidant species.8 However, the specific role of Stat in the development and progression of DN has never been confirmed in vivo using a gene knockout approach.
There are seven mammalian STAT proteins.9 STAT3 is a known transducer of signals from IL-6, which is a proinflammatory cytokine thought to have a culprit role in the development of diabetic complications including DN.10–12 Therefore, we decided to examine the role of STAT3 in the DN. STAT3 is the only member of the STAT family that leads to embryonic lethality when deleted.13 To study the role of STAT3 in DN, we generated transgenic animals with reduced STAT3 activity (SA/+ and SA/–) and characterized the renal findings of these animals with streptozotocin (STZ)-induced diabetes mellitus.
RESULTS
Transgenic mice with reduced STAT3 activity
Mice with a homozygous serine to alanine mutation in the 727 residue (SA/SA) were described earlier.14 SA/SA mice have approximately 50% of the STAT3 activity of wild-type mice. SA/SA mice were crossed with heterozygous STAT3 knockout mice (Stat3+/–) to generate SA/+ and SA/– mice 75 and 25% STAT3 activity, respectively. Mice with more than 50% Stat3 transcriptional activity have normal phenotypes, whereas SA/– mice have a lower initial body and kidney weight.15
Induction of diabetes increases Stat3 phosphorylation
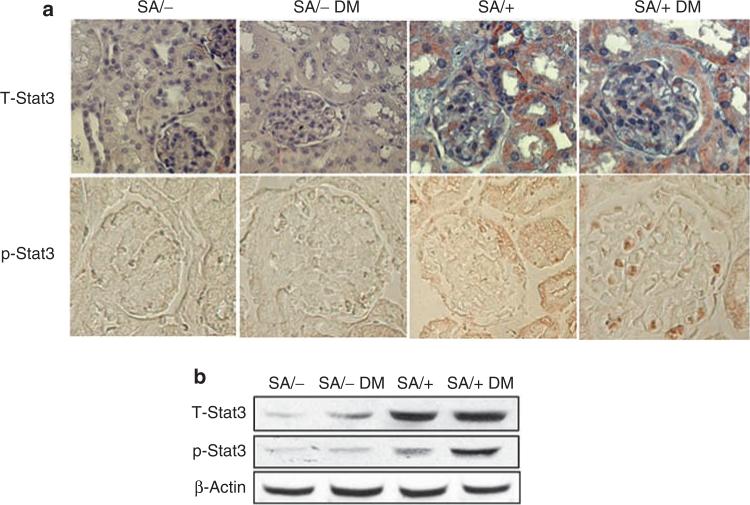
The kidney sections were obtained for immunostaining to confirm the reduction in Stat3 expression in SA/– as compared with SA/+ mice (Figure 1a). Diabetic SA/+ mice demonstrated significantly increased Stat3 phosphorylation as compared with non-diabetic SA/+ mice (Figure 1a). Staining of phosphorylated Stat3 (p-Stat3) localized predominantly to glomerular cells. Staining of p-Stat3 was not increased in diabetic SA/– mice. These findings were also confirmed in isolated glomeruli by Western blot analysis. As shown in Figure 1b, total Stat3 expression was reduced in both diabetic and non-diabetic SA/– mice as compared with SA/+ mice. Furthermore, an increase of p-Stat3 was observed in glomeruli of diabetic SA/+ mice as compared with non-diabetic SA/+ mice. However, no change in Stat3 phosphorylation was observed in glomeruli of SA/– mice with or without diabetes.
Figure 1. Phosphorylation of Stat3 in the kidney.
(a) Immunostaining of total and phosphorylated Stat3 in kidney sections. Immunohistochemistry was performed on paraffin sections of kidneys from SA/+ and SA/– mice with and without STZ-induced diabetes (SA/+, SA/+ DM, SA/–, and SA/– DM) using rabbit polyclonal anti-total Stat3 (T-Stat3) and anti-phospho-Stat3 antibodies (p-Stat3). The staining for T-Stat3 in SA/+ was more than in SA/– mice. Representative immunostaining results are shown. (b) Western blot of T-Stat3 and p-Stat3 in isolated glomeruli. Isolated glomeruli from diabetic and non-diabetic SA/– and SA/+ mice were lysed in lysis buffer containing protease and phosphatase inhibitors. Western blot was performed using anti-total Stat3, anti-phospho-Stat3 (Cell Signaling Technology), and anti-β-actin (Sigma) antibodies. Representative blots of three independent experiments are shown.
Stat3 deficiency prevented diabetes-induced increase in kidney-to-body weight ratio and albumin excretion
Data on mouse body and kidney weights, blood glucose and urine albumin/creatinine ratio were summarized in Table 1. Consistent with earlier studies, SA/– mice were significantly smaller than SA/+ mice and kidney size was also smaller in SA/– mice, although there was no significant difference in kidney-to-body weight ratio between non-diabetic SA/– and SA/+ mice. To determine whether the reduced kidney weight in SA/– mice was due to a reduction in nephron number, we determined the total glomerular number of SA/– and SA/+ mice. We did not find a significant difference in the total number of glomeruli between SA/– and SA/+ mice (16,306±1096 vs 15,896±1120, respectively, P>0.05). Induction of diabetes did not alter the body weight of either SA/+ or SA/– mice significantly. However, kidney-to-body weight ratio was increased in diabetic SA/+ mice as compared with non-diabetic SA/+ mice, whereas no significant increase in kidney-to-body weight ratio was observed between diabetic and non-diabetic SA/– mice. This suggests that diabetes-induced kidney hypertrophy was prevented in diabetic SA/– mice. Diabetic SA/– mice developed significantly less albuminuria than diabetic SA/+ mice. Blood glucose levels did not differ between diabetic SA/+ and diabetic SA/– mice.
Table 1.
Characteristics of mice
| BW (g) | KW (mg) | KW/BW (mg/g) | Serum glucose | Urine albumin/creatinine | |
|---|---|---|---|---|---|
| SA/+ | 25.2 ± 2.9 | 222 ± 36 | 8.9 ± 1.2 | 175 ± 18 | 0.08 ± 0.07 |
| SA/+ DM | 24.2 ± 3.8 | 243 ±45** | 10.1 ± 0.8** | 404 ± 89 | 0.31 ± 0.10 |
| SA/– | 21.4 ± 0.9+ | 190 ± 35+ | 9.1 ± 0.86 | 164 ± 12 | 0.08 ± 0.09 |
| SA/– DM | 20.1 ± 0.8 | 193 ± 13 | 9.6 ± 1.41 | 393 ± 42 | 0.12 ± 0.10* |
BW, body weight; KW, kidney weight; KW/BW, kidney-to-body weight ratio.
Characteristics of mice: mouse body weight, kidney weight, serum glucose, and urinary protein excretion (urine albumin/creatinine ratio) are summarized.
N=6
P < 0.05 as compared with SA/+ with DM (SA+DM)
P < 0.05 as compared with SA/+ mice
P < 0.05 as compared with SA/+.
Stat3 deficiency reduced glomerular volume and mesangial expansion in diabetes
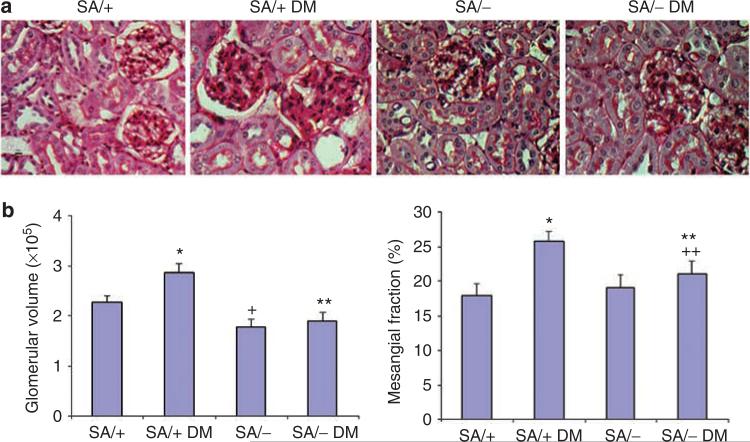
Diabetic SA/+ mice had increased glomerular volume and developed significant mesangial expansion as characterized by increased mesangial/glomerular area fraction (Figure 2a and b). However, diabetic SA/– mice did not have significantly increased glomerular volume and mesangial/glomerular fraction as compared with non-diabetic SA/– mice (Figure 2a and b). Non-diabetic SA/– and SA/+ mice did not differ significantly. The smaller glomerular volume of non-diabetic SA/– as compared with non-diabetic SA/+ mice suggests that the smaller average kidney size of SA/– mice is most likely due to a reduction in nephron size.
Figure 2. Analysis of kidney histology.
(a) Kidney histology. Periodic acid-Schiff (PAS) staining was performed in kidneys of SA/+ and SA/– mice with or without diabetes (SA/+, SA/+ DM, SA/–, and SA/– DM). An increase in mesangial area was observed in SA/+ DM mice as compared with SA/– DM mice. Representative pictures are shown here. (b) Morphometric analysis of glomerular volume and PAS-positive area was performed in kidney sections of all mice. The glomerular volume and mesangial area per glomerular cross-section area (% of mesangial fraction) is shown (n = 6, *P<0.01 compared with SA/+, **P>0.05 compared with SA/–, +P<0.05 compared with SA/+, and + +P<0.05 compared with SA/+ DM).
Reduction in Stat3 activity does not prevent diabetes-induced glomerular hyperfiltration in SA/– mice
Glomerular filtration rate was determined by the measurement of fluorescein isothiocyanate (FITC)-inulin clearance before and after the induction of diabetes in SA/+ and SA/– mice. We found that the induction of diabetes increased GFR to the same extent in both SA/+ and SA/– mice (SA/+: 201 ± 28, diabetic SA/+: *253 ± 34, SA/–: 198 ± 25, diabetic SA/–: *246 ± 34, *P<0.05 as compared with non-diabetic mice, n = 6). These data suggest that reduction of Stat3 activity in SA/– mice prevented the early diabetic kidney disease without affecting glomerular hyperfiltration. Mice with myeloid cell-specific deficiency of Stat3 are known to develop diarrhea due to spontaneous colitis.16 However, we did not observe any diarrhea in our SA/– mice, which is most likely due to the fact that our SA/– mice preserved a baseline Stat3 activity (25%), and the mice with myeloid cell-specific deficiency of Stat3 have a completely absence of Stat3 expression. We also measured urinary osmolarity randomly in SA/+ and SA/– mice as a surrogate marker of volume status to ensure that SA/– mice were not hypovolemic. We did not find any difference between the urine osmolarity of SA/+ and SA/– mice (1023 ± 112 vs 987 ± 84 mOsm/kg, n = 6).
Reduction of Stat3 reduces glomerular type IV collagen accumulation and TGF-β expression
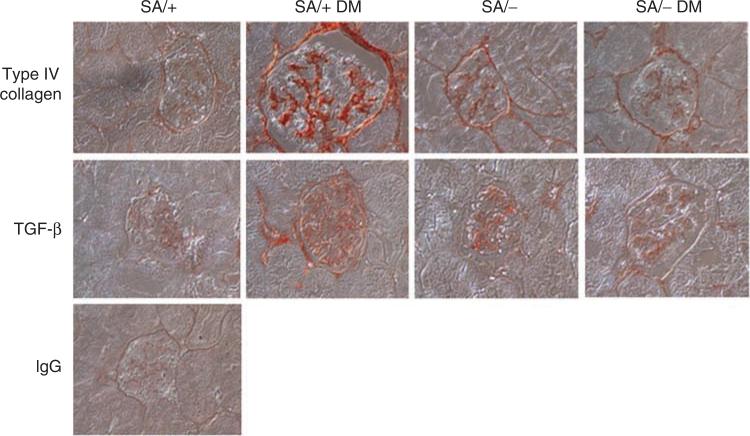
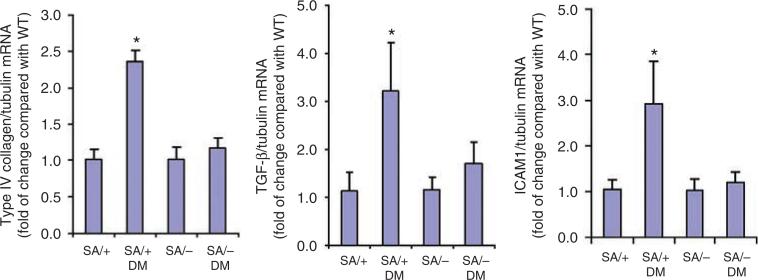
To further examine the development of histological changes in SA/+ and SA/– mice with diabetes, we examined the molecular pathology that is classically associated with DN. As it has been shown earlier that Stat3 mediated hyperglycemia-induced type IV collagen and TGF-β expression in mesangial cells in vitro,17 we determined the glomerular expression of type IV collagen and TGF-β in SA/+ and SA/– mice with and without STZ-induced diabetes. Diabetic SA/+ mice had significantly more glomerular expression of type IV collagen and TGF-β as compared with diabetic SA/– mice (Figure 3 and Table 2). As immunostaining is a semiquantitative assay, we confirmed these findings by quantitative real-time PCR of RNA extracted from isolated glomeruli. As expected, we found that the expression of type IV collagen and TGF-β was significantly attenuated in diabetic SA/– mice as compared with diabetic SA/+ mice (Figure 4).
Figure 3. Immunostaining of type IV collagen and TGF-β in kidneys of mice.
Immunohistochemistry was performed in paraffin sections of kidneys from diabetic and non-diabetic SA/+ and SA/– mice using anti-type IV collagen and anti-TGF-β antibodies. There are significant increases in type IV collagen and TGF-β expression in SA/+ DM mice compared with SA/– DM mice. The representative pictures are shown here. Rabbit IgG was used as a negative control. Semiquantification of intensity of staining is summarized in Table 2.
Table 2.
Semiquantitative assessment of staining
| Type IV collagen (%) | TGF-β (%) | Ki67 | CD68 | |
|---|---|---|---|---|
| SA/+ | 7.8 ± 1.3 | 4.4 ± 1.1 | 1.34 ± 2.46 | 0.35 ± 0.50 |
| SA/+ DM | 26.2 ± 3.2 | 15.3 ± 3.5 | 7.87 ± 2.52 | 3.55 ± 0.68 |
| SA/– | 8.2 ± 1.4 | 5.1 ± 1.6 | 1.25 ± 1.56 | 0.18 ± 0.12 |
| SA/– DM | 9.3 ± 2.4* | 5.5 ± 1.2* | 3.00 ± 0.36* | 0.52 ± 0.11* |
TGF-β, transforming growth factor-β.
Semiquantitative assessment of staining: intensity of immunostaining was quantified as described in the Materials and Methods section. For type IV collagen and TGF-β, % of positive staining area per glomerular cross-section was measured by MetaMorph software. For Ki67 and CD68, number of positive cells per glomerular cross-section was counted.
N=6
P < 0.01 as compared with SA/+ DM.
Figure 4. Real-time PCR for type IV collagen, TGF-β, and ICAM-1.
Total RNA was extracted from the glomerular isolates. Real-time PCR was performed as described in the Materials and Methods. The ratio of type IV collagen, TGF-β, and ICAM-1 to tubulin mRNA levels was obtained and the fold of change for each as compared to the pooled glomeruli from wild-type (WT) mice is shown. *P<0.05 compared with SA/+ and SA/– DM mice, n = 6.
Stat3 reduction prevented mesangial cell proliferation in diabetes
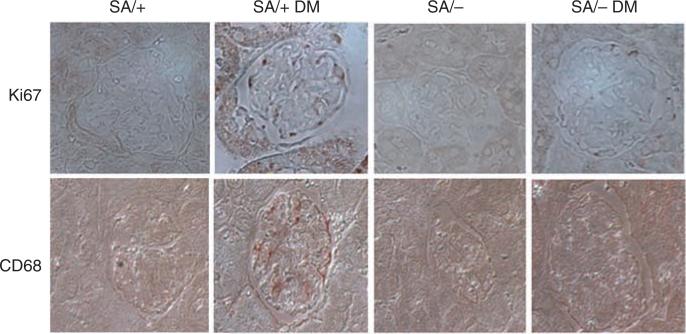
We also examined the expression of Ki67 as a marker for cell proliferation. Diabetic SA/+ mice had increased Ki67 staining following a mesangial distribution. However, this increase in Ki67 expression was not seen in diabetic SA/– mice, indicating that Stat3 mediated diabetes-induced mesangial cell proliferation (Figure 5 and Table 2).
Figure 5. Immunostaining of Ki67 and CD68 in kidneys of mice.
Immunohistochemistry was performed in paraffin sections of kidneys from diabetic and non-diabetic SA/+ and SA/– mice using anti-Ki67 and anti-CD68 antibodies. There is a significant increase of Ki67 and CD68 staining in kidneys of SA/+ DM mice as compared with SA/– DM mice. Semiquantitative measurement of the staining intensity is summarized in Table 2.
Reduction of Stat3 reduced macrophage infiltration in diabetes
As Stat3 is known to mediate inflammatory response, we also examined the expression of CD68, a macrophage marker. We found that CD68 expression was increased in diabetic SA/+ mice as compared with non-diabetic SA/+, but absent in diabetic SA/– mice. This suggested that a reduction in Stat3 activity prevented macrophage infiltration in diabetes (Figure 5 and Table 2). Furthermore, the expression of intercellular adhesion molecule-1 (ICAM-1), a monocyte-macrophage infiltration marker,18 was also found to be suppressed in diabetic SA/– mice as compared with diabetic SA/– mice (Figure 4).
Expression of IL-6 and MCP-1 was reduced in diabetic SA/– mice
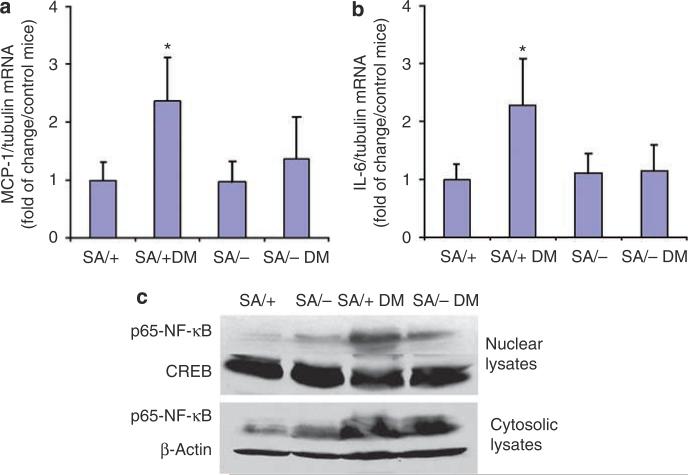
As our data suggested a role of Stat3 in mediating the inflammatory response in the diabetic kidney, we examined the expression of IL-6 and MCP-1 in the glomeruli of these mice. We found that IL-6 and MCP-1 mRNA expression was increased in diabetic SA/+ mice as compared with non-diabetic SA/+ (Figure 6a and b). However, no significant change in the expression of MCP-1 and IL-6 was observed between diabetic SA/– and non-diabetic SA/– mice. As it is known that Stat3 interacts with the nuclear factor (NF)-κB pathway,19,20 we assessed NF-κB activity in kidneys of these mice by determining its expression in the cytoplasm and nucleus. By Western blotting (Figure 6c), we found that the expression of NF-κB increased significantly in the cytosolic fraction of the glomerular lysates from both diabetic SA/+ and SA/– mice as compared with their non-diabetic counterparts. However, a marked increase in NF-κB was observed in diabetic SA/+ mice but not in diabetic SA/– mice. These data demonstrate that STZ-induced diabetes increases the expression of NF-κB, which is independent of Stat3 activity. However, a reduction of Stat3 activity is associated with a decrease in NF-κB nuclear shuttling and presumably its activation. cAMP response element binding and β-actin were used here as the loading controls for nuclear and cytosolic proteins, respectively.
Figure 6. Analysis of inflammatory markers in the kidneys.
(a and b) Real-time PCR for MCP-1 and IL-6: total RNA was isolated from glomeruli of these mice. Real-time PCR was performed as described in the Material and Methods section. The ratio of MCP-1 and IL-6 to tubulin mRNA levels was obtained and the fold of change for each as compared to the pooled glomeruli from wild-type (WT) mice is shown. *P<0.05 compared with SA/+ and SA/– DM mice, n = 6. (c) Western blot of p65-NF-κB: both cytosolic and nuclear proteins were isolated as described. Western blot was performed using rabbit anti-p65-NF-κB antibody, anti-CREB (loading control of nuclear protein), and anti-β-actin (loading control of cytosolic protein) antibodies. Representative blots of four independent experiments are shown.
DISCUSSION
Multiple in vitro studies have suggested that Stat3 is an important mediator of DN pathogenesis. In this study, we demonstrate a significant role for Stat3 in the development of DN in vivo. Although SA/+ and SA/– mice developed similar degrees of hyperglycemia during the study, SA/– mice were less susceptible to the development of proteinuria and pathological changes associated with DN.
In our study, SA/+ (75%) and SA/– (25%) littermates were used for comparison, because it has been shown previously that a reduction in Stat3 activity to 25% causes phenotypic changes.15 In a preliminary study, we compared the severity of DN between SA/+ and wild-type mice and did not find any difference in proteinuria and mesangial expansion (data not shown). SA/– mice are known to have lower body and kidney weight. Our findings suggest that the number of glomeruli did not differ between SA/+ and SA/– mice. Therefore, the reduction in kidney weight ismost likely due to a reduction in nephron size.
Earlier studies reported that mice with myeloid cell-specific deletion of Stat3 developed spontaneous colitis.16 However, our SA/– mice with 25% of Stat3 activity did not have any watery stools. To rule out volume depletion in SA/– mice, urinary osmolarity was determined and no difference was observed between SA/+ and SA/– mice. Furthermore, we confirmed that both SA/+ and SA/– mice developed similar degree of hyperfiltration at the early stage of DN. Therefore, reduction of Stat3 activity to 25% did not prevent the development of hyperfiltration at the early stage of diabetes in SA/– mice.
Of interest, Stat3 is a known mediator of insulin-like growth factor 1 (IGF-1) signaling.21 Earlier studies indicate that SA/– mice have decreased circulating levels of IGF-1,15 which may contribute to the small body and kidney size. IGF-1 has been implicated in the pathogenesis of DN.22 It has been also shown that dwarf mice are protected from DN.23 The reduction in IGF-1 level and body weight are potential confounders for the development of DN. In future studies, we will need to delineate the relative contribution of reduced IGF-1 level and small body weight to the protection of DN in SA/– mice. To address this issue, we are developing a podocyte-specific Stat3 knockout mice, in which Stat3 activity will be reduced only in podocytes.
Earlier in vitro studies suggest that JAK/Stat3 plays an important role in the pathogenesis of DN.7 Stat3 is activated by many components of the diabetic milieu, including high glucose, reactive oxygen species, AGE, and angiotensin II in primarily, in mesangial cells.24–26 Both angiotensin-converting enzyme inhibitor and angiotensin II receptor blockade prevent hyperglycemia-induced activation of JAK-Stat activation in diabetic kidney glomeruli.25 Reactive oxygen species plays a critical role in the pathogenesis of DN. It is well known that reactive oxygen species activate the Stat3 pathway. Recently, it has been shown that antioxidants inhibit hyperglycemia-induced Stat3 activation.27 Selective inhibitor of JAK2 (AG490) ameliorates nephrotic syndrome in adriamycin-induced nephropathy, suggesting that Stat3 plays a role in podocyte injury, which is presumably due to reactive oxygen species-induced Stat3 activation.28 The AGE and receptor for AGE (RAGE) interaction plays a key role in the pathogenesis of DN. It has been shown that Stat3 also mediates RAGE-mediated collagen synthesis.24 Furthermore, simvastatin, which has been shown to improve diabetic kidney disease, also attenuates high glucose and angiotensin II-induced Stat3 activation.29
Stat3 also plays an important role in other kidney diseases including Thy-1 glomerulonephritis model,30 Gas6 nephropathy,31 and HIV-associated nephropathy.32 Stat3 has also been shown to mediate hypoxia-induced vascular endothelial growth factor synthesis in renal carcinoma cells.33 In addition, many studies suggest that Stat3 plays an important role in acute kidney injury mediating apoptosis, epithelial-mesenchymal transitions, and inflammation in renal tubular epithelial cells.34
This is the first study to examine the role of Stat3 in DN in vivo using a knockdown animal model (75% reduction of Stat3 activity). We believe this knockdown model is better than the knockout system because a basal activity of most signaling molecules is required for normal biological function of cells. Consistent with previous findings,7 we found that diabetes-induced mesangial cell proliferation, TGF-β expression, and abnormal type IV collagen synthesis were significantly inhibited in SA/– mice, indicating that Stat3 plays a role in the regulation of extracellular matrix synthesis.
Interestingly, we found that infiltration of inflammatory cells was also significantly reduced in SA/– mice. Consistent with our findings, it has been shown that inhibition of the JAK/Stat3 pathway reduces infiltration of interstitial inflammatory cells and production of chemokines in the adriamycin-induced nephropathy model.28 Furthermore, we found that a reduction of Stat3 activity abrogated the stimulation of proinflammatory markers, including IL-6, ICAM-1, and MCP-1, and blocked nuclear translocation of NF-κB. IL-6 is a well-known proinflammatory cytokine that is activated through the Stat3 pathway.4 Recent studies suggest that an IL-6 haplotype is associated with impaired renal function in type 2 diabetic patients.5 MCP-1 and ICAM-1 are known to cause infiltration of inflammatory cells in the diabetic kidney.4 Knockout of either MCP-1 or ICAM-1 prevents kidney disease in diabetic animal models.35,36 Earlier studies suggest that NF-κB and its target genes, including IL-6 and MCP-1, are activated in diabetic kidney.4,37,38 Stat3 is known to cross-talk and activate NF-κB.19,20 NF-κB expression is known to be increased in the diabetic condition,39 which is consistent with our finding of increased NF-κB expression in the cytosolic fraction of glomerular lysates from diabetic SA/+ and SA/– mice as compared with their non-diabetic counterparts. In addition, we also observed that Stat3 mediates diabetes-induced translocation of NF-κB in glomerular cells. On the basis of these findings, we believe that Stat3 mediates inflammatory response either directly by stimulating its target genes, such as ICAM-1, or indirectly through the activation of NF-κB leading to MCP-1 and IL-6 production. This increase in IL-6 production could then further stimulate the Stat3 pathway resulting in a vicious cycle of escalating inflammation and inflammatory response in the diabetic milieu. Therefore, we believe that Stat3 mediates both fibrosis, through the TGF-β pathway, and inflammatory pathways in diabetic kidney; however, this will need to be further confirmed in the future.
A limitation of our study is that Stat3 is ubiquitously expressed and the reduction of Stat3 activity in our animal model occurs in all tissues expressing Stat3. Therefore, our study does not address the relative contribution of Stat3 inactivation in renal cells versus systemic effects. Diabetes-induced Stat3 activation in podocytes and glomerular endothelial cells has not been carefully examined in the past. Earlier studies have focused on the role of Stat3 signaling in mesangial cells.7 Our studies suggest that an increase in Stat3 phosphorylation is localized predominantly to the glomerular region of mice with early DN. Although we did not perform colocalization studies, our immunostaining data showed that Stat3 phosphorylation seems to occur mostly in podocytes. However, our studies suggest that the reduction of Stat3 activity prevents diabetes-induced mesangial activation and abnormal matrix synthesis. Further studies are required to confirm the cellular localization of pStat3 and to investigate whether and how podocytes cross-talk with mesangial cells through Stat3 activation. We propose that hyperglycemia, AGE, and/or angiotensin II may activate the Stat3 pathway in podocytes, thus promoting the synthesis and the secretion of growth factors and cytokines such as TGF-β and IL-6. These growth factors and cytokines could act on the surrounding mesangial cells in a paracrine manner to activate the synthesis of abnormal matrix by mesangial cells.
Another limitation of the study is that these mice did not develop significant glomerulosclerosis as seen in human DN most likely due to the genetic background of the experimental animal and the duration of diabetes in the study. To further confirm the results of our study, the role of Stat3 needs to be determined in these animals with longer duration of diabetes as well as in other animal models of DN. Future studies are also required to determine whether inhibition of JAK/Stat3 pathway could be a novel target of therapy for DN.
MATERIALS AND METHODS
Experimental design
All mice are in C57BL/6 background. Genotyping by PCR was performed at 3 weeks of age as described earlier.15 SA/+ and SA/– mice (six mice of each genotype) at 6 weeks of age were injected with 5 serial doses of STZ (50 mg/kg) or vehicle as described by the Animal Models of Diabetic Complications Consortium with one modification: instead of 5 consecutive injections, animals were injected on alternating days without fasting before injection. Unrestricted food and water were provided throughout the duration of the experiment. Serum glucose was monitored every 2–3 days. Diabetes was defined as a blood glucose level above 300 mg per 100 ml. All mice developed diabetes at 8–10 weeks of age and were killed at 4–5 months of age for tissue collection after 8 weeks of hyperglycemia. Blood and urine samples were collected at the end of studies. Body and kidney weight was recorded. Studies were performed according to the protocols approved by the Institutional Animal Care and Use Committee. Of note, wild-type mice were also rendered diabetic with STZ. However, there was no significant difference in the amount of proteinuria or glomerular/mesangial area fraction between SA/+ and wild-type mice with diabetes. Therefore, we used SA/+ mice as controls for SA/– mice in the study.
Histology
Mice were perfused with phosphate-buffered solution and the kidneys were fixed in 4% paraformaldehyde for 2 h. The kidneys were paraffin embedded by American Histolabs Inc. (Gaithersberg, MD, USA). The kidney histology was examined after periodic acid-Schiff staining.
Measurement of urine albumin and creatinine
Urine albumin concentration was measured following the manufacturer's protocol (Mouse Albumin ELISA Quantitation Kit, Bethyl Laboratory Inc., Houston, TX, USA). Urine creatinine concentration was measured using an assay based on the Jaffe's reaction according to the manufacturer's protocol (Creatinine Assay Kit, Cayman Chemical Company, Ann Arbor, MI, USA). The amount of urine albumin excretion was expressed as a ratio of albumin to creatinine.
Glomerular count
The glomerular number was determined as described earlier.40 Briefly, kidneys were decapsulated, cut into 2 mm pieces, and incubated in 5 ml of 6n HCL at 37°C for 90 min. The resultant suspension was diluted to 30 ml of sterile water and allowed to stand overnight at 4°C. The glomerular number was counted in triplicate under a phase microscope using a counting chamber.
Quantifying mesangial expansion
Glomerular volume and mesangial area were determined by examining plastic-embedded sections using a digitizing tablet and video as described earlier.41 The relative mesangial area was expressed as mesangial/glomerular surface area (%).
Immunohistochemistry
The paraffin-embedded kidney sections from SA/+ and SA/– mice were prepared in identical manner. Immunostaining was performed using anti-total or phospho-Stat3 (Cell Signaling Technology, Danvers, MA, USA), anti-type IV collagen or anti-Ki67 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-CD68 (R&D Systems, Minneapolis, MN, USA), and biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA). Slides were mounted with Aqua Poly/Mount (Polysciences Inc., Burlingame, CA, USA) and photographed under an Olympus BX60 microscope (Olympus Optical Co, Center Valley, PA, USA) with a digital camera. Quantitative analyses of type IV collagen and TGF-β expression in glomerular area were performed using a quantitative image analysis system (Metamorph, Sunnyvale, CA, USA). Twenty random glomerular cross-sections (×40 power) were chosen from each tissue section and examined. The examined area was outlined, the positive staining patterns were identified, and the percent positive staining area per glomerular cross-section was then measured. For Ki67 and CD68 staining, the number of positive cells per glomerular cross-section area in kidney sections was counted as described earlier.32 The data were presented as the number of positive cells per glomerulus per cross-section. Positive was defined as a cell that had obvious changes of intensity above baseline levels. Two investigators blinded to the samples quantified the staining independently.
Western blot
Glomeruli were isolated from the kidney cortex of these mice after perfusion with <5 mμ iron oxide solution as described earlier42 and lysed with a buffer containing 1% Triton, a protease inhibitor cocktail and tyrosine, and serine-threonine phosphorylation inhibitors. To determine NF-κB activity, both nuclear and cytosolic proteins were isolated from glomeruli. Cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotted using anti-phospho-Stat3 and anti-total Stat3 antibodies, anti-p65 NF-κB (Santa Cruz), anti-CREB (Cell Signaling Technology), and anti-β-actin antibody (Sigma, St Louis, MO, USA).
Real-time PCR
Total RNA was isolated from glomeruli of these mice using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Quantitative real-time PCR was performed using a Roche Lightcycler and the Qiagen QuantiTect One Step RT-PCR SYBR green kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Pre-designed primer sets were purchased from Qiagen, GeneGlobe for type IV collagen, TGF-β, ICAM-1, IL-6, and MCP-1. The following primer pair was used for tubulin: 5′-TGCCTTTGTGCACTGGTATG-3′ and 5′-CTGGAGCAGTTTGACGACAC-3′. LightCycler analysis software was used to determine crossing points based on the second derivative method. Data were normalized to tubulin expression and presented as fold increase relative to RNA isolated from pooled normal glomeruli of wild-type mice using the 2–ΔΔCT method.
Measurement of GFR by single bolus injection of FITC-inulin
Diabetes was induced at the age of 6 weeks by injection of STZ in both SA/+ and SA/– mice. FITC-inulin clearance was measured 6 weeks after the onset of diabetes as described.43 Briefly, dialyzed FITC-inulin (3.74 μl/g body weight) was injected retroorbitally under light anesthesia with isoflurane (Baxter Pharmaceutical Products, Deerfield, IL, USA). Approximately 20 μl of blood were collected through the tail vein at 3, 7, 10, 15, 35, 55, and 75 min post-injection of FITC-inulin, yielding 10μl of plasma for the determination of FITC-inulin concentration by measurement of fluorescence. Two-compartment clearance models were used for the calculation of GFR.
Measurement of urinary osmolarity
Fresh urine samples were collected in the tubes and immediately sealed with parafilm. Urinary osmolarity was determined using the Wescore Vapro Osmometer. All samples were measured in triplicates.
Statistical analysis
Data were expressed as mean ± s.d. (X ± s.d.). The unpaired t-test was used to analyze the data. Statistical significance will be considered when P<0.05.
ACKNOWLEDGMENTS
JC He was supported by NIH R01 DK078897. PY Chuang was supported by NIH K08 KD082760.
Footnotes
DISCLOSURE
All the authors declared no competing of interests.
REFERENCES
- 1.US Renal Data System . USRDS 2005 Annual Data Report. Atlas of End stage Renal Disease in the United States. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2005. [Google Scholar]
- 2.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 3.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 4.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 5.Ng DP, Nurbaya S, Ye SH, et al. An IL-6 haplotype on human chromosome 7p21 confers risk for impaired renal function in type 2 diabetic patients. Kidney Int. 2008;74:521–527. doi: 10.1038/ki.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of JAK-STAT pathway members in human diabetic nephropathy. Diabetes. 2009;58:469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrero MB, Banes-Berceli AK, Stern DM, et al. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F762–F768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 8.Simon AR, Rai U, Fanburg BL, et al. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 9.Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- 10.Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 11.Saraheimo M, Teppo AM, Forsblom C, et al. Diabetic nephropathy is associated with low-grade inflammation in Type 1 diabetic patients. Diabetologia. 2003;46:1402–1407. doi: 10.1007/s00125-003-1194-5. [DOI] [PubMed] [Google Scholar]
- 12.Shikano M, Sobajima H, Yoshikawa H, et al. Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Nephron. 2000;85:81–85. doi: 10.1159/000045634. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Noguchi K, Shi W, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Schlessinger K, Zhu X, et al. Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol. 2004;24:407–419. doi: 10.1128/MCB.24.1.407-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Shaw S, Amiri F, et al. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes. 2002;51:3505–3509. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto H, Shikata K, Hirata K, et al. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997;46:2075–2081. doi: 10.2337/diab.46.12.2075. [DOI] [PubMed] [Google Scholar]
- 19.Yu Z, Kone BC. The STAT3 DNA-binding domain mediates interaction with NF-kappaB p65 and iuducible nitric oxide synthase transrepression in mesangial cells. J Am Soc Nephrol. 2004;15:585–591. doi: 10.1097/01.asn.0000114556.19556.f9. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y, Kumar A, Koyama Y, et al. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004;279:1768–1776. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]
- 21.Zong CS, Chan J, Levy DE, et al. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 22.Oemar BS, Foellmer HG, Hodgdon-Anandant L, et al. Regulation of insulin-like growth factor I receptors in diabetic mesangial cells. J Biol Chem. 1991;266:2369–2373. [PubMed] [Google Scholar]
- 23.Chen NY, Chen WY, Bellush L, et al. Effects of streptozotocin treatment in growth hormone (GH) and GH antagonist transgenic mice. Endocrinology. 1995;136:660–667. doi: 10.1210/endo.136.2.7835300. [DOI] [PubMed] [Google Scholar]
- 24.Huang JS, Guh JY, Chen HC, et al. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Banes AK, Shaw S, Jenkins J, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:F653–F659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 26.Amiri F, Shaw S, Wang X, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605–1616. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang JS, Chuang LY, Guh JY, et al. Antioxidants attenuate high glucose-induced hypertrophic growth in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1072–F1082. doi: 10.1152/ajprenal.00020.2007. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Yang N, Zhang L, et al. Inhibition of Jak/STAT signaling ameliorates mice experimental nephrotic syndrome. Am J Nephrol. 2007;27:580–589. doi: 10.1159/000108102. [DOI] [PubMed] [Google Scholar]
- 29.Banes-Berceli AK, Shaw S, Ma G, et al. Effect of simvastatin on high glucose- and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol Renal Physiol. 2006;291:F116–F121. doi: 10.1152/ajprenal.00502.2005. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Abe H, Arai H, et al. Activation of STAT3/Smad1 is a key signaling pathway for progression to glomerulosclerosis in experimental glomerulonephritis. J Biol Chem. 2005;280:7100–7106. doi: 10.1074/jbc.M411064200. [DOI] [PubMed] [Google Scholar]
- 31.Yanagita M, Arai H, Nakano T, et al. Gas6 induces mesangial cell proliferation via latent transcription factor STAT3. J Biol Chem. 2001;276:42364–42369. doi: 10.1074/jbc.M107488200. [DOI] [PubMed] [Google Scholar]
- 32.He JC, Husain M, Sunamoto M, et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 34.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow FY, Nikolic-Paterson DJ, Ozols E, et al. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 36.Chow FY, Nikolic-Paterson DJ, Ozols E, et al. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- 37.Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003. doi: 10.2337/db06-0477. [DOI] [PubMed] [Google Scholar]
- 38.Mezzano S, Aros C, Droguett A, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:2505–2512. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 39.Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 40.He C, Zalups RK, Henderson DA, et al. Molecular analysis of spontaneous glomerulosclerosis in Os/+ mice, a model with reduced nephron mass. Am J Physiol. 1995;269:F266–F273. doi: 10.1152/ajprenal.1995.269.2.F266. [DOI] [PubMed] [Google Scholar]
- 41.Zheng F, Zeng YJ, Plati AR, et al. Combined AGE inhibition and ACEi decreases the progression of established diabetic nephropathy in B6db/ db mice. Kidney Int. 2006;70:507–514. doi: 10.1038/sj.ki.5001578. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Z, Whitt I, Mehta A, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]