Abstract
Background
Antiretroviral therapy (ART) in early HIV infection may enhance outcome. ART during acute HIV-1 infection (AI) might better control HIV than ART in recent HIV-1 infection (RI) 24 weeks after treatment interruption.
Methods
A prospective ACTG trial of ART stratified by AI vs. RI. If HIV RNA plasma concentration <50 copies/mL) after >= 52 weeks, ART was interrupted. If viremia rebounded ART and interruption was repeated. The primary endpoint was maintaining plasma HIV RNA concentration < 5,000 copies/mL for 24 weeks following either treatment interruption.
Results
121 subjects were enrolled at 15 sites. The trial was closed in mid-2007. 95% were men, median age was 34 years; 69% were white, 94% reported no injection drug use. Median baseline plasma HIV RNA concentration was higher in AI (210,000 copies/mL) than RI (43,000 copies/mL). The 73 primary endpoint subjects (28 AI, 45 RI) had significantly higher baseline CD4+ T cell counts (p=0.044) and lower HIV RNA plasma concentrations (p=0.016) but did not differ in acuity of infection from those not entering treatment interruption. The primary endpoint was achieved in 29 (40%) of the 73 and in 24% of the 121 enrolled overall. There was no significant outcome difference (p=0.81) between the AI (43%, 95% CI: 24–63%) and RI (38%, 95% CI: 24–53%) groups. Differences after longer follow up can not be ascertained by this trial. Baseline viral load <100,000/mL 22/46 (48%) compared with >100,000/mL, 7/27 (26%) and higher baseline CD4+ immune activation predicted success.
Conclusion
40% of subjects treated during AI or RI sustained an HIV RNA plasma concentration <5,000 copies/mL after 24 weeks of treatment interruption.
Introduction
Studies of acute HIV infection may provide crucial information for strategies of prevention or of treatment capable of improving disease outcome. Primary or acute HIV infection is often associated with clinical illness along with laboratory evidence of high levels of viral replication and rapid CD4+ T cell depletion1–4. High titer viremia declines over a period of weeks to months and then usually stabilizes at what has become known as the virus set point5–7. Massive depletion of CD4+ T cells occurs in gut-associated lymphoid tissues, and these CD4+ T cells do not recover as fully as those in peripheral blood8, 9. The virus set point following primary HIV infection has been shown to be highly predictive of the rate of disease progression and death5, 10, 11. The extent of immune damage during primary HIV infection has been hypothesized to be a key determinant of the virus set point and disease progression possibly by allowing a higher level of chronic immune activation12, 13. Whether early initiation of antiretroviral therapy can ameliorate the immune damage associated with primary HIV infection and attenuate the course of subsequent disease is unclear.
A preliminary report of an uncontrolled series of 14 cases suggested that a time-limited use of antiretroviral therapy and intentional treatment interruption in primary HIV infection may be beneficial14. In that study, patients with acute or recent HIV infection were treated with a variety of potent antiretroviral regimens. After HIV suppression was achieved, this therapy was interrupted for a varying duration. Each treatment interruption was quickly followed by an increase in the plasma HIV RNA concentration. It was postulated that this intentionally permitted viremia would trigger immune recognition (essentially an “auto-vaccination”) possibly leading to durable replication control. In this trial, therapy was reinitiated for one to two short periods and was later permanently discontinued. Ninety days after treatment was stopped, 5/8 patients had low concentrations of plasma HIV RNA, <5,000 copies/mL, well below the expected set point of 30–50,000 copies/mL. This was a small series, however, and it was unclear how predictable and durable such observed benefits may be and whether these were limited only to certain patients, for example those with the shortest interval between infection and treatment initiation. Such cases of acute infection may have had better intrinsic immune control and an improved outcome compared to those with longer standing infection with more immune damage15.
To address these questions, the Adult AIDS Clinical Trials Group (ACTG) designed a prospective single-arm stratified clinical trial. To explore the effect of the duration of infection prior to treatment, the trial compared subjects with “acute” HIV infection, carefully defined but essentially those with HIV viremia before the appearance of serum HIV antibodies or with a partly developed Western blot, to those with “recent” infection, after such antibodies had developed but with evidence that infection occurred within the preceding six months. All subjects were similarly treated with multi-agent protease-inhibitor based ART until durable viral load suppression was achieved. Those meeting this treatment goal were then offered a period of treatment interruption until plasma HIV RNA concentration again rose to a pre-defined level. Following this, treatment was reinstated for up to one more treatment/interruption cycle before study completion. The aim of this trial was to establish the frequency of host control of HIV replication as reflected by a non-detectable or low (<5,000 copies/mL) plasma HIV RNA concentration at least 24 weeks after all antiretroviral therapy was discontinued and to compare this rate in acute versus recent HIV infection. We hypothesized that earlier ART would result in enhanced control of HIV-1 replication after treatment interruption.
Methods
ACTG Protocol 371 was a single-arm prospective, stratified trial of four-drug intentionally interrupted ART in acute or recent HIV infection. Each subject signed an informed consent approved by their institutional review board and the National Institute of Allergy and Infectious Diseases (NIAID). The study was sponsored by NIAID. Study drugs were supplied by manufacturers including GlaxoSmithKline, Bristol Myers Squibb, Agouron/Pfizer.
ACTG 371 was first opened in 1999 to evaluate an induction-maintenance strategy of antiretroviral therapy in subjects with acute or recent HIV infection, with a single treatment interruption at week 88. Because of slow accrual and the information regarding the potential benefit of the treatment/treatment interruption strategy discussed above, in 2001 the study was redesigned to evaluate structured treatment interruption with a smaller sample size. Further refinements to the design were introduced over time including treatment regimen reconfiguration, addition of an entry criterion for recent infection using the newly developed “detuned” ELISA described below, prolonging the duration of follow up and requiring confirmation of transient “blips” in HIV viremia.
In the final study (version 5.0), subjects were stratified by predetermined criteria into acute infection (AI) or recent infection (RI) groups. AI was defined as having a plasma HIV RNA concentration >2,000 copies/mL within 14 days of study entry and either (1) a negative ELISA or (2) a positive ELISA but a negative or indeterminate Western blot (CDC/ASTPHLD criteria) or a positive ELISA and Western blot in conjunction with either a negative ELISA or a plasma HIV RNA concentration <2,000 copies/mL in the 30 days prior to entry. The general intention of these criteria was to identify cases in which HIV infection had occurred within the four weeks prior to study entry. RI was defined as (1) a positive ELISA and Western blot within the 14 days prior to entry but a negative ELISA or plasma HIV RNA concentration <2,000 copies/mL within the 31–90 days before entry or (2) a positive ELISA and Western blot plus a non-reactive detuned ELISA in subjects with >200 CD4+ cells/mm3 all within the 21 days before study entry. The general intention of these criteria was to define cases in which HIV infection had occurred > 14 days to as much as approximately six months prior to entry.
All subjects were required to meet laboratory safety criteria of hematologic, renal and hepatic systems. Co-infection with Hepatitis C or B viruses (HCV or HBV) was not excluded. All subjects were >= 16 years old. Women and men were enrolled. Women were non-pregnant at entry. All subjects were instructed on effective contraception including the use of barrier contraception methods during the trial. Subjects were excluded if there was prior antiretroviral therapy treatment (apart from post-exposure prophylaxis completed more than 6 months prior to study entry), recent pancreatitis or acute hepatitis, and were not allowed to use medications with a risk of adverse interaction with study medication.
Induction phase (step 1)
Subjects were treated with a protease inhibitor containing antiretroviral regimen for >=52 weeks. The standard regimen included stavudine, lamivudine, abacavir, ritonavir and amprenavir. Several subjects in the first protocol version received unboosted amprenavir but in later versions, after March 2000, all received ritonavir in a boosting dose. This induction regimen, no longer recommended, was considered appropriate at the time of trial design for aggressive HIV suppression. Substitution of any drug for intolerance was approved on an individual basis by the study leaders. Didanosine was allowed for abacavir intolerance, nelfinavir for amprenavir/ritonavir intolerance, and other nucleoside or nucleotide reverse transcriptase inhibitors could be substituted for study drugs in this class. All subjects were instructed in the appropriate methods of taking study drugs in each class. Medication adherence was reinforced and measured in the trial. Patients were monitored every 4 weeks for side effects and laboratory safety parameters and dose modifications or drug discontinuation criteria were predefined. Toxicities were graded according to standard ACTG criteria. Abacavir hypersensitivity reactions were defined and managed according to accepted standards and were reported to the manufacturer. HLA screening for abacavir hypersensitivity was not available during this trial. All subjects were treated with the initial goal of a reduction of plasma HIV RNA concentration below detection limits.
Step 2 was a maintenance phase of treatment in the first protocol version. It was eliminated when the study was redesigned in 2001 to evaluate treatment interruption. The five subjects then in step 2 were allowed to enter step 3.
First treatment interruption phase (step 3)
Subjects who maintained plasma HIV RNA concentration <50 copies/mL after at least 52 weeks of treatment and had a CD4+T cell count >= 200 cells/mm3 were instructed to discontinue study drug treatment with close clinical and laboratory monitoring. Plasma HIV RNA concentrations and CD4+ T cells were measured weekly for weeks 0–4 of interruption, biweekly for weeks 4–8, and every 4–8 weeks thereafter for 96 weeks or to study week 160 whichever was later.
First reinduction phase (step 4)
Subjects who experienced a confirmed plasma HIV RNA concentration >= 50,000 copies/mL or three consecutive concentrations >= 5,000 copies/mL or had a confirmed fall in CD4 cell count during step 3 of > 50% from the start of that study step (and also <350 cells/mm3 in the last study version) or to <200 cells/mm3 had study treatment reinitiated in study step 4. The goal of this retreatment was again to achieve a plasma HIV RNA concentration below detection limits. HIV and CD4+ cell monitoring followed the same schedule and duration as in study step 1.
Second treatment interruption phase (step 5)
Subjects who in study step 4 achieved a plasma HIV RNA concentration <50 copies/mL and maintained <400 copies/mL for at least 8 weeks and with a CD4+ T cell count >= 200 cells/mm3 were again offered a period of treatment interruption in study step 5. Subjects were monitored during this second interruption as outlined in study step 3
Second reinduction phase (step 6)
Treatment was again reinitiated based on plasma HIV RNA concentration and CD4+ cell counts as described in study step 4.
Statistical methods
The primary efficacy endpoint of this trial was to compare, between subjects in the AI and RI cohorts, the probability that potent antiretroviral therapy early in the course of HIV infection led to controlled viremia, specifically a plasma HIV RNA concentration <5,000 copies/mL at 24 weeks of continuous treatment interruption. A subject could be a success for this primary endpoint during either the first (step 3) or second (step 5) treatment interruption. The sample size goal was 120 subjects stratified by predetermined criteria into AI and RI groups. The study also was designed to assess the safety and tolerance of the selected drug regimen.
The main secondary efficacy endpoints were a plasma HIV RNA concentration < 50 copies/mL at week 48 of first induction therapy (step 1) and time to loss of viral control (first plasma HIV RNA concentration >5,000 copies/mL)
The sample size was designed to detect with 80% power a 35% difference in virologic control following treatment interruption between the AI and RI groups. A total of 74 subjects were estimated to be required to undergo treatment interruption, approximately equally divided by study stratum. Specifically, it was expected that at least 30 subjects in the AI stratum would enter treatment interruption. Primary efficacy analysis comparing strata employed Fisher’s exact test of proportions and exact confidence intervals; logistic regression models examined baseline factors associated with primary endpoint success. Time to event analyses used the logrank test and continuous measurements were analyzed by the Wilcoxon ranksum test.
Results
Between July 1999 and September 2003, 121 subjects were enrolled in ACTG Protocol 371 at 15 sites in the United States. The leading sites of accrual were the Bellevue Hospital Center (24 subjects, 20%) and the University of California San Diego Medical Center (23 subjects, 19%). The total sample included 50 entered in the AI stratum and 71 in the RI stratum. All subjects began treatment within three days of enrollment. A total of 73 subjects (28 AI and 45 RI) entered the first treatment interruption (step 3). Of these, two did not register for this step but met all criteria including treatment discontinuation and were included in data analysis.
Baseline characteristics of the 121 subjects enrolled are described in Table 1. Overall, 115 (95%) were male. The median age was 34 years. Most (84, 69%) were white and most (114, 94%) had no history of injection drug use. The median overall CD4 cell count was 535 cells/mm3 with a range of 87 to 1835 cells/mm3 and not significantly different between the AI and RI groups. The overall median plasma HIV RNA concentration was five-fold higher for the AI stratum (210,000 copies/mL) compared to the RI stratum (43,000 copies/mL). Table 2 displays the baseline characteristics for the 73 subjects who entered the first treatment interruption and who comprise the analysis group for the primary efficacy endpoint. The median CD4+ T cells prior to treatment interruption were 893 for AI and 829 for RI. The 48 subjects not in the primary analysis had lower baseline CD4+ T cells than the 73 subjects included in the primary analysis (p=0.044) and higher baseline plasma HIV RNA concentration (p=0.016), and these trends were similar when assessed separately for the AI and RI strata.
Table 1.
Baseline Characteristics
| Acute Infection (N = 50) | Recent Infection (N = 71) | Total (N = 121) | |
|---|---|---|---|
| Male, N (%) | 48 (96%) | 67 (94%) | 115 (95%) |
| Age, median | 34 | 35 | 34 |
| Race/Ethnicity, N (%) | |||
| White Non-Hispanic | 34 (68%) | 50 (70%) | 84 (69%) |
| Black Non-Hispanic | 9 (18%) | 6 (8%) | 15 (12%) |
| Hispanic (Regardless of Race) | 6 (12%) | 13 (18%) | 19 (16%) |
| Asian, Pacific Islander | 1 (2%) | 1 (1%) | 2 (2%) |
| More than one race | 0 (0%) | 1 (1%) | 1 (1%) |
| IV drug use, N (%) | |||
| Never | 49 (98%) | 65 (92%) | 114 (94%) |
| Currently | 1 (2%) | 1 (1%) | 2 (2%) |
| Previously | 0 (0%) | 5 (7%) | 5 (4%) |
| CD4 cells/mm3, median (Q1 – Q3) | 561 (409 – 753) | 528 (425 – 756) | 535 (422 – 753) |
| CD8 cells/mm3, median (Q1 – Q3) | 1,269 (926 – 1,874) | 976 (676 – 1,135) | 1,093 (733 – 1,471) |
| HIV RNA copies/mL, median (Q1 – Q3) | 210,300 (37,000 – 881,400) | 42,700 (14,800 – 436,100) | 58,000 (24,200 – 505,000) |
Table 2.
Baseline Characteristics for the 73 subjects in the primary efficacy analysis
| Acute Infection (N = 28) | Recent Infection (N = 45) | Total (N = 73) | |
|---|---|---|---|
| Male, N (%) | 27 (96%) | 42 (93%) | 69 (95%) |
| Age, median | 35 | 36 | 35 |
| Race/Ethnicity, N (%) | |||
| White Non-Hispanic | 20 (71%) | 34 (76%) | 54 (74%) |
| Black Non-Hispanic | 5 (18%) | 2 (4%) | 7 (10%) |
| Hispanic (Regardless of Race) | 3 (11%) | 7 (16%) | 10 (14%) |
| Asian, Pacific Islander | 0 (0%) | 1 (2%) | 1 (1%) |
| More than one race | 0 (0%) | 1 (2%) | 1 (1%) |
| IV drug use, N (%) | |||
| Never | 28 (100%) | 43 (96%) | 71 (97%) |
| Currently | 0 (0%) | 1 (2%) | 1 (1%) |
| Previously | 0 (0%) | 1 (2%) | 1 (1%) |
| CD4 cells/mm3, median (Q1 – Q3) | 593 (503 – 696) | 551 (457 – 768) | 581 (467 – 756) |
| CD8 cells/mm3, median (Q1 – Q3) | 1,312 (1,028 – 1,799) | 997 (719 – 1,309) | 1,093 (843 – 1,416) |
| HIV RNA copies/mL, median (Q1 – Q3) | 109,000 (34,400 – 634,900) | 37,000 (15,500 – 162,000) | 47,200 (22,300 – 227,900) |
Treatment toxicity was as expected for the drug regimen employed and did not differ between study strata. Abacavir hypersensitivity was seen in nine subjects, 6/73 (8%, 2 AI, 4 RI) of those in the primary analysis and 3/48 (6%, 0 AI, 3 RI) of the remaining subjects. No myocardial infarctions were reported.
During the first 52 weeks after initiating treatment, clinical symptoms associated with drug toxicity of grade two or three were reported in 71 of the overall 121 enrolled subjects, 26/50 (52%) in the AI stratum and 45/71 (63%) in the RI stratum. There were no grade 4 signs or symptoms reported during the first 52 weeks and only one grade 4 chest pain in the AI stratum in the subsequent weeks. Laboratory toxicities of grade 2 and above were reported in 44 of the 121 enrolled subjects in the first 52 weeks (18/50 AI, 26/71 RI). Lipoatrophy was not systematically defined in this trial but no cases were reported as reason for treatment discontinuation. The safety of treatment interruption was examined after the SMART trial results were presented and there was no evidence detected of accelerated disease progression or clinical events.
The disposition of subjects registered into ACTG 371 are shown in Table 3. Study treatment was discontinued before week 52 in 24 subjects (11 AI, 13 RI). In 7 (4 AI, 3 RI), toxicity was the stated cause, 8 subjects (4 AI, 4 RI) discontinued treatment for other reasons including intolerance and pill burden, in 3 (1 AI, 2 RI) treatment was stopped for virologic failure and 6 (2 AI, 4 RI) subjects were lost to follow up.
Table 3.
Study Subject Disposition
| Study Component | Total number (number in primary analysis group) | Acute Infection (AI) | Recent Infection (RI |
|---|---|---|---|
| Entered Step 1 (treatment induction) | 121 | 50 | 71 |
| Did not complete 52 weeks suppressive treatment | 24 | 11 | 13 |
| Did not enter Step 3 (treatment interruption) | 24 | 11 | 13 |
| Entered Step 3 (Primary analysis group) | 73 | 28 | 45 |
| Entered Step 4 (re-induction) | 49 | 17 | 32 |
| Finished Step 5 (second interruption) | 36 | 15 | 21 |
| Entered Step 6 (second re-induction) | 21 | 8 | 13 |
Twenty-four subjects who were on study for at least 52 weeks did not undergo treatment interruption. Of these, 8 subjects (2 AI, 6 RI) completed an early study version, 3 (1 AI, 2 RI) had toxicities, 6 (2 AI, 4 RI) did not interrupt due to patient preference, 4 (3 AI, 1 RI) had virologic failure, and 3 (3 AI) were lost to follow up.
The primary efficacy endpoint, plasma HIV RNA concentration <5000 copies/mL after 24 weeks of treatment interruption, was achieved in 40% of the 73 subjects who underwent treatment interruption and 24% of the 121 subjects enrolled overall. Virologic success was achieved in 12/28 (43%: 95% CI: 24% – 63%) in the AI stratum and in 17/45 (38%, 95% CI: 24% – 53%) in the RI stratum who underwent treatment interruption. Most (25) primary endpoint successes were achieved in the first treatment interruption. There was no statistically significant difference between the two groups (p=0.81; 95% CI on the difference between groups: −31% to 19%). Considering the first treatment interruption, 34 (47%) subjects re-initiated treatment prior to week 24. We also found no differences between the AI and RI strata in having average plasma HIV RNA concentration <5,000 or <10,000 copies/mL between weeks 18–30 of treatment interruption (AI: 13/28, 46%, RI:18/45, 40% p=0.63; AI: 16/28, 57%, RI: 21/45, 47% p=0.47 respectively), or plasma HIV RNA concentration <10,000 copies/mL at week 24 of treatment interruption (AI: 14/28, 50%, RI: 22/45, 49% p=1.0).
Secondary efficacy analysis for the first treatment interruption also revealed no statistically significant difference between the two strata in terms of:
Time to three consecutive plasma HIV RNA concentrations >=5,000 copies/ml or two >= 50,000 copies/ml (median, weeks AI: 10, RI: 14, p=0.76);
Peak (maximum) plasma HIV RNA concentration (median, 28,224 copies/ml AI, 36,241 copies/ml RI; p=0.67);
Time to peak plasma HIV RNA concentration (median weeks AI: 8, RI: 8, p=0.35).
Rate of initial increase in plasma HIV RNA concentration (median, log10/week AI: 0.29, RI: 0.50 p=0.46).
Changes in CD4+ T cells between those immediately preceding treatment interruption (median, 893 cells/mm3 AI, 829 cells/mm3 RI) and the average of all measurements between weeks 18–30 of continuous treatment interruption were not significantly different (median changes, −165 cells/mm3 AI, −90 cells/mm3 RI; p=0.25).
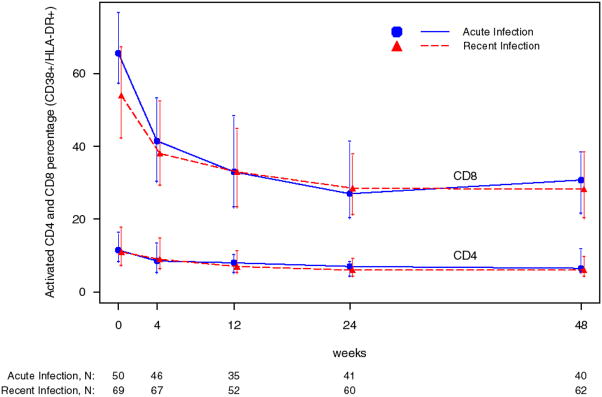
Immune activation markers were elevated at baseline, especially in the AI stratum. These markers declined with treatment in both strata in the study population overall (figure 1) and similarly in the 73 subjects analyzed for primary efficacy endpoint (data not shown).
Figure 1.
Percentage of activated (CD38+/HLA-DR+) CD4+ and CD8+ T cells during the induction phase.
The most important predictor of success in meeting the primary efficacy endpoint was baseline plasma HIV RNA concentration (p=0.003 for log 10 RNA) as summarized in Table 4. Successful outcomes were more common in the group with baseline plasma HIV RNA concentration < 100,000 copies/mL (22/46, 48%) than in those with baseline plasma HIV RNA concentrations above 100,000 copies/mL (7/27, 26%)). A higher percentage of activated CD4+ T cells at baseline (HLA-DR+/CD38+, overall median: 11%, Q1–Q3: 7%–16%) was also associated with primary endpoint success in a model that controlled for baseline plasma HIV RNA concentration and AI/RI strata (odds ratio = 3.3 comparing CD4+ activation above versus below the median, 95% CI: 1.03–10.7, p=0.036); without controlling for baseline plasma HIV RNA concentration, baseline activated CD4+ T cell percentage showed no association (p>0.2). Baseline percentages of activated CD8+ T cells, naïve and memory CD4+ and CD8+ T cells, and absolute CD4+ and CD8+ T cell counts were not associated with primary endpoint success in models adjusted for baseline plasma HIV RNA concentration.
Table 4.
Primary Endpoint Success: Effect of Baseline Plasma HIV RNA Concentration
| Baseline HIV RNA copies/mL | Total (%) | Acute Infection (AI) (%) | Recent Infection (RI) (%) |
|---|---|---|---|
| </= 100,000 | 22/46 (48%) | 8/14 (57%) | 14/32 (44%) |
| > 100,000 | 7/27 (26%) | 4/14 (29%) | 3/13 (23%) |
| Total | 29/73 (40%) | 12/28 (43%) | 17/45 (38%) |
Discussion
This was an ambitious and complex trial of potent antiretroviral therapy in patients with acute and recent HIV infection with up to two cycles of intentional treatment interruption. This trial of 121 subjects with acute and recent HIV infection is the largest trial to date to evaluate the frequency of HIV viral load suppression after structured treatment interruption and was based on an earlier report of 14 subjects that had suggested this approach would allow a high frequency of prolonged viral control after treatment discontinuation. Additionally, it tested the hypothesis that the benefits of this strategy would be most apparent in those with acute as opposed to recent HIV infection based on the supposition that a more intact immune system at study entry would be more responsive to the antigenic stimulation provided by structured treatment interruptions. Indeed, approximately 40% of subjects in the primary endpoint analysis group had plasma HIV RNA concentrations <5,000 copies/mL after 24 weeks and remained off all antiretroviral therapy. Among all those initially enrolled, control of viremia was seen in 12/50 (24%) AI and in 17/71 (24%) RI. Contrary to our initial hypothesis, however, there was no evidence of differences in the rate of viral control between individuals treated during acute and recent infection. Baseline tests for genomic predictors of viral suppression were not anticipated in the trial design and therefore not included. Also, the relatively wide confidence intervals of viral suppression rates leave open the possibility of a clinically relevant difference between the treatment groups. The high number of enrolled subjects not meeting criteria for primary endpoint analysis and the baseline differences between these groups was concerning and probably reflected the trial’s complexity. While the rate of viral suppression was lower overall than in the primary endpoint group, there was still no difference between the AI and RI strata.
Overall, the study was safe although the rates of adverse effects and drug discontinuations were almost certainly higher than expected with more current antiretroviral regimes. While the regimen used in this trial is not currently recommended for initial treatment, it resulted in prompt HIV viral load suppression with high rates of medication adherence. Relatively few subjects discontinued study treatment for toxicity or virologic failure, and rates of toxicity were similar to those in chronically infected individuals treated with these medications. Lipoatrophy commonly associated with the use of one of the study drugs, stavudine, may not have been a common or severe problem because of the relatively short duration of therapy in this trial although it was not systematically monitored, a reflection of the limited knowledge of this problem when the study was designed.
Most studies of ART treatment interruption in chronic HIV-1 infection have found no virologic benefit16, 17, 18, 19 with one exception where a modest benefit from two interruptions was reported20, nor did the addition of hydroxyurea add benefit21. The strategy of antiretroviral treatment interruption has fallen into disfavor based upon recent findings in chronically HIV-1-infected individuals that interruption of antiretroviral therapy is associated with increased morbidity and mortality22. The subjects in the present study were distinct from those in the SMART study in that they were recently infected and most would not have met standard guidelines for initiation of antiretroviral therapy. When findings of the SMART Study were released, a systematic review of ACTG 371 occurred and it was concluded that there was no evidence of harm from treatment interruption in these subjects and the study was allowed to complete enrollment. Importantly, however, subjects in ACTG 371 were more frequently monitored during treatment interruptions than those in the SMART Study, and therefore the interruption strategies were not truly comparable.
As expected, acute infection cases had higher baseline plasma HIV RNA concentrations than those with recent infection; however, when adjusted for baseline viremia titer, suppression was seen equally commonly in the two study strata. Baseline plasma HIV RNA concentration was also the most significant negative predictor of ability to control viremia in subjects who underwent treatment interruption. Thus, subjects with high baseline plasma HIV RNA concentrations were least likely to meet the endpoint success definition in this interruption trial.
Results from cohort studies of untreated primary HIV infection suggest a higher plasma HIV RNA concentration at set point than in the current trial5–7, 23, but selection differences make such comparisons hazardous. Thus, it is not possible to firmly conclude from the present study that early treatment enhances virologic suppression. The results of the present study are nonetheless important in that they establish the expected outcomes for potent antiretroviral therapy in acute and recent infection, and response rates following one or two cycles of intentional treatment interruption. As such, this trial should serve as an important point of reference for future interventions in this vitally important patient population so much now a focus on questions of the kinetics of viral replication and dissemination after infection and of the rate and specific nature of early immune damage.
We had hypothesized that the earlier antiretroviral therapy would limit immune damage thereby enhancing acutely infected individuals’ ability to control virus replication compared to recently infected individuals. The failure to observe this, however, does not necessarily disprove the hypothesis that early intervention confers greater benefit, as it is unclear whether the acutely and recently infected individuals were comparable. Previous studies have demonstrated that highly symptomatic seroconverters who are identified during acute HIV-1 infection have a worse prognosis than other HIV-1-infected individuals1–4, 7. Thus, the finding that acutely infected and recently infected individuals had similar rates of virologic control could mask the true immune benefit of treatment in acute infection. It is possible, as well, that immune benefits may take longer than 24 weeks of treatment interruption to be appreciated. Hecht et al. in a nonrandomized study compared plasma HIV RNA concentrations among acute and recent HIV-1 seroconverters who received a minimum of 12 weeks of antiretroviral therapy and subsequently interrupted therapy for at least 6 months with concentrations in untreated seroconverters, after controlling for baseline viral load and CD4+ T cell counts. They concluded that both acute and recent seroconverters who received treatment during acute infection had significantly lower viral loads and higher CD4+ T cell counts at 24 weeks, but the effects seemed to wane in the recent group after longer followup23. In contrast, longer term follow-up of the same cohort of subjects who initially prompted the ACTG 371 study design at three years and comparison of their outcomes with those of historical controls within the MACS suggested a loss of apparent immune benefit from early treatment and structured treatment interruptions 15. Clearly, the lack of randomized, controlled trials has impaired ability to achieve conclusive results on this issue. Nevertheless, collectively, these data suggest that early antiretroviral therapy followed by treatment interruption does not produce the profound alterations in disease course that were initially suggested by uncontrolled studies.
The findings that higher percentages of activated CD4+ T cells at baseline were associated with greater likelihood of virologic success upon treatment interruption, after adjusting for baseline viral load, and that baseline CD8+ T cell activation was unrelated to virologic success were unexpected. Previous studies have found a negative association between T cell immune activation and the ability to suppress virus replication during antiretroviral treatment24–26 and we had anticipated that similar patterns would be observed during treatment interruption. To our knowledge, this is the first study to report a direct relationship between CD4+ T cell activation prior to treatment and ability to control viremia following treatment interruption. It is conceivable that high levels of CD4+ T cell immune activation in early disease signify a more competent immediate host immune response. Further studies are needed to confirm whether higher levels of CD4+ T cell immune activation in early HIV-1 disease after adjusting for plasma HIV RNA concentrations indeed signal a better prognosis.
The results of this trial do not prove that immediate treatment is superior to deferred treatment or that there is a benefit to the strategy of treatment interruption, a popular concept when this trial was designed. The lack of difference in the acute versus recent infection cases is worth further reflection. It seems highly unlikely that any trial will enroll patients any closer to the actual moment of infection than in ACTG 371. Most of the AI stratum had plasma viremia with no antibody response and were almost certainly within the first four weeks of infection at study screening. Treatment strategies or trials relying on finding even earlier cases do not address the reality of primary infection as seen in the clinical setting. Even with the very early infection entered in the AI stratum of ACTG 371, we now know that immune damage had already occurred. While much more is known in this regard than when ACTG 371 was designed it remains as difficult now to study these issues in human subjects. Recent data from simian infection in particular suggests that initial infection dramatically affects T-lymphocyte populations – particularly the CD4+ CCR5+ memory cells in the gut lymphoid tissue-within one to several weeks of exposure.27
Further insight into the appropriate care of acute or recent infection could be provided by a comparison to a concurrent untreated control group, especially if randomization between strategies was possible. While not feasible when ACTG 371 was launched, these data should help provide the equipoise needed to conduct controlled trials and, in fact, several prospective trials with a randomized control are now in progress. Treatment interruption may not be needed in future trials, especially given evidence of adverse outcomes of a similar strategy in chronic infection. An obvious question is whether antiretroviral therapy, if initiated in primary infection, should ever be discontinued. While ACTG 371 does not directly answer this question, it does provide evidence of the safety of treatment discontinuation followed in 40% of cases by prolonged viral control.
Acknowledgments
This work was supported by the AIDS Clinical Trials Group, under the National Institute of Allergy and infectious Diseases grant AI-68636, AI-38858, AI 69450 and AI-38855.
Footnotes
These data were presented in part at the Conference on Retroviruses and Opportunistic Infections February 2008, Boston MA.
This clinical trial is registered in the national clinical trials database, # NCT00000940. “Five-Drug Anti-HIV Treatment Followed by Treatment Interruption in Patients Who Have Recently Been Infected With HIV”
Bibliography
- 1.Dorrucci M, Rezza G, Vlahov D, et al. Clinical characteristics and prognostic value of acute retroviral syndrome among injecting drug users. Italian Seroconversion Study. Aids. 1995;9(6):597–604. doi: 10.1097/00002030-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen C, Lindhardt BO, Jensen BL, et al. Clinical course of primary HIV infection: consequences for subsequent course of infection. Bmj. 1989;299(6692):154–7. doi: 10.1136/bmj.299.6692.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128(8):613–20. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr. 2007;45(4):445–8. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 5.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181(3):872–80. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 6.Hubert JB, Burgard M, Dussaix E, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. The SEROCO Study Group. Aids. 2000;14(2):123–31. doi: 10.1097/00002030-200001280-00007. [DOI] [PubMed] [Google Scholar]
- 7.Henrard DR, Phillips JF, Muenz LR, et al. Natural history of HIV-1 cell-free viremia. Jama. 1995;274(7):554–8. [PubMed] [Google Scholar]
- 8.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197(5):714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 9.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272(5265):1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 11.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 12.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 13.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. Aids. 2008;22(4):439–46. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann DE, Lichterfeld M, Altfeld M, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;1(2):e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163(10):1220–6. doi: 10.1001/archinte.163.10.1220. [DOI] [PubMed] [Google Scholar]
- 17.Papasavvas E, Kostman JR, Mounzer K, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1(3):e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewin SR, Murray JM, Solomon A, et al. Virologic determinants of success after structured treatment interruptions of antiretrovirals in acute HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47(2):140–47. [PubMed] [Google Scholar]
- 19.Smith DE, Kaufmann GR, Kahn JO, et al. Greater reversal of CD4+ cell abnormalities and viral load reduction after initiation of antiretroviral therapy with zidovudine, lamivudine, and nelfinavir before complete HIV type 1 seroconversion. AIDS Res Hum Retroviruses. 2003;19(3):189–99. doi: 10.1089/088922203763315696. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson JM, Pat Bucy R, Spritzler J, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis. 2006;194(5):623–32. doi: 10.1086/506364. [DOI] [PubMed] [Google Scholar]
- 21.Bloch MT, Smith DE, Quan D, et al. The role of hydroxyurea in enhancing the virologic control achieved through structured treatment interruption in primary HIV infection: final results from a randomized clinical trial (Pulse) J Acquir Immune Defic Syndr. 2006;42(2):192–202. doi: 10.1097/01.qai.0000219779.50668.e6. [DOI] [PubMed] [Google Scholar]
- 22.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 23.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194(6):725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 24.Hoen B, Cooper DA, Lampe FC, et al. Predictors of virological outcome and safety in primary HIV type 1-infected patients initiating quadruple antiretroviral therapy: QUEST GW PROB3005. Clin Infect Dis. 2007;45(3):381–90. doi: 10.1086/519428. [DOI] [PubMed] [Google Scholar]
- 25.Resino S, Bellon JM, Gurbindo MD, Munoz-Fernandez MA. CD38 expression in CD8+ T cells predicts virological failure in HIV type 1-infected children receiving antiretroviral therapy. Clin Infect Dis. 2004;38(3):412–7. doi: 10.1086/380793. [DOI] [PubMed] [Google Scholar]
- 26.Shepard BD, Loutfy MR, Raboud J, et al. Early changes in T-cell activation predict antiretroviral success in salvage therapy of HIV infection. J Acquir Immune Defic Syndr. 2008;48(2):149–55. doi: 10.1097/QAI.0b013e31816d9c3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]