Abstract
In diffusion tensor imaging (DTI), interpreting changes in terms of fractional anisotropy (FA) and mean diffusivity or axial (D||) and radial (D⊥) diffusivity can be ambiguous. The main objective of this study was to gain insight into the heterogeneity of age-related diffusion changes in human brain white matter by analyzing relationships between the diffusion measures in terms of concordance and discordance instead of evaluating them separately, which is difficult to interpret. Fifty-one cognitively normal subjects (22–79 years old) were studied with DTI at 4 Tesla. Age was associated with widespread concordant changes of decreased FA and increased MD but in some regions significant FA reductions occurred discordant to MD changes. Prominent age-related FA reductions were primarily related to greater radial (D⊥) than axial (D||) diffusivity changes, potentially reflecting processes of demyelination. In conclusion, concordant/discordant changes of DTI indices provide additional characterization of white matter alterations that accompany normal aging.
Keywords: Diffusion tensor imaging, Aging, Non-parametric analysis, Concordance, Discordance, Fractional anisotropy, Mean diffusivity, Axial diffusivity, Radial diffusivity, Demyelination
1. Introduction
Diffusion tensor imaging (DTI) is a MRI modality, which has been of increasing interest to measure changes in brain white matter. DTI measures the directional variability of random water motion and there is considerable evidence that DTI reflects white matter ultrastructural integrity (Basser and Pierpaoli, 1996; Pierpaoli et al., 1996; Mori and Barker, 1999; Beaulieu, 2002; Sullivan and Pfefferbaum, 2003). DTI measurements are usually reported as spatially invariant summary parameters such as mean diffusivity (MD) and fractional anisotropy (FA). MD is defined as the average of the three eigenvalues of the diffusion tensor, measuring the magnitude of diffusion (Basser and Pierpaoli, 1996). Fractional anisotrophy is defined as a coefficient of variation of the eigenvalues, capturing the directionally of diffusion (Klingberg et al., 2000). It has been recognized that an interpretation of MD and FA changes can be ambiguous and may lead to erroneous conclusions. In particular, contributions to FA changes could be due to changes of diffusion which are either parallel or perpendicular to the principle direction of the tensor, but the mechanisms underlying a change in one or the other direction may differ. Moreover, since FA is normalized to MD, the absolute change is not measured. To avoid some of the ambiguities, especially as related to FA, other investigators have avoided summary measures all together and analyzed diffusion in terms of axial (D||) diffusivity, which is equivalent to the largest eigenvalues of the tensor and radial (D⊥) diffusivity, which is the average of the two other eigenvalues (Alexander et al., 2007; Budde et al., 2007). DTI studies have used one or the other of these diffusion variables for analysis, but no study before has considered them and their relations together for studying diffusion changes in aging.
DTI studies of humans have consistently shown that DTI is able to detect white matter changes in aging (Abe et al., 2008; Salat et al., 2005; Pfefferbaum et al., 2005; Head et al., 2004; Sullivan et al., 2006). In the majority of these investigations, the interpretation of white matter changes has been based on FA alone or on separate analyses of FA and MD. DTI investigations in humans showed that large FA reductions can occur in regions where fibers are arranged in isolated bundles whereas MD changes can be moderate (Pierpaoli et al., 2001). This has been interpreted as an indication for Wallerian degeneration, a sequence of axonal and myelin degeneration distal to the nerve body that leads to fragmented axons without shrinkage of fiber volumes in the early stages. In contrast, axonal loss is expected to cause large concordant FA decrease and MD increase. Variability among the DTI indices raises the possibility that analyzing relationships between FA and MD changes may provide a differential assessment of the underlying morphological white matter changes. Moreover, studies in animals indicate that demyelination, a process that leads to the loss of the myelin sheath insulating the axons is characterized by increased D⊥ and only moderate changes of D|| (Song et al., 2002; Harsan et al., 2007). Although it is difficult to extend these animal studies, some DTI studies in infants (Qiu et al., 2008; Partridge et al., 2004) attempted to explain myelin generation in the developing brain by analyzing D⊥. In this study, we hypothesized that comparisons between D|| and D⊥ may provide further insight into underlying processes that lead to FA changes.
The overall goal of this project was to characterize white matter changes associated with aging by correlating FA and MD changes as well as D|| and D⊥ changes, employing a non-parametric statistical framework of voxel-wise analysis of concordance/discordance that has previously been reported (Hayasaka et al., 2006). Specifically we tested the following hypotheses: (1) Normal aging is associated with a regional pattern of simultaneous (concordant) FA decrease and MD increase involving frontal white matter, in agreement with previous DTI studies. (2) Normal aging is also associated with a pattern of discordant FA decrease without significant MD change, suggesting Wallerian degeneration. (3) Normal aging is associated with greater increase of D⊥ than D||, consistent with demyelination and Wallerian degeneration.
2. Methods
2.1. Subjects
Fifty-one cognitively normal subjects 28 men, 23 women (mean age 49.3 ± 16.7, range 22–79 years) were enrolled in this study and had DTI scans at 4 Tesla. Forty-five subjects were right-handedness, 4 were left-handedness and 2 were ambidextrous (Table 1). Normal cognitive functioning was assessed by a comprehensive battery of neuropsychological tests that included the mini mental state examination (MMSE) (Folstein et al., 1975), California verbal learning (short form) test (Delis et al., 2000), Rey-Osterrieth complex figure test (Bennett-Levy, 1984), verbal fluency test (Mungas et al., 2004), and the Wechsler adult intelligence score (digit symbol, digit span, and trails B) (Wechsler, 1997). Subjects older than 50 years had also assessments of their emotional state and functioning in daily living evaluated using the geriatric depression scale and the functional activities questionnaire (O’Hara and Yesavage, 2001), respectively. Only subjects, who scored not worse than one unit below the norm on a standardized scale (z-scores), but also not better than 2 units above the norm were included. Exclusion criteria included also any poorly controlled illness, use of medication or recreational drugs that could affect brain function, a history of brain trauma, brain surgery, ischemic events, or skull defects. Subjects with focal white matter lesions on MRI including lacunas and white matter hyperintensity lesions of grade 2 or more by Schelten’s rating scale (Scheltens et al., 1993) were also excluded. Signed informed consent approved by the Committees of Human Research at UCSF and the VA Medical Center was obtained from all participates prior to participating in the study.
Table 1.
Demographic and clinical characteristics of the subjects.
| Subjects | |
|---|---|
| Number | 51 |
| Age | 49.3 ± 16.7 |
| Age range | 22–79 |
| Men:women | 28:23 |
| Handedness | 45 right, 4 left, 2 ambidextrous |
| MMSE | 29.6 ± 0.6 |
MMSE = mini-mental state examination with maximal score of 30 for normal cognition.
2.2. Diffusion tensor-MRI acquisitions
DTI was preformed on a 4 Tesla (Bruker/Siemens) MRI system, equipped with a birdcage transmit and 8 channel receive coil. DTI was based on a dual spin-echo echo-planar imaging (EPI) sequence, augmented by parallel imaging acceleration (GRAPPA) (Griswold et al., 2002) by a factor 2 to reduce susceptibility distortions. Other imaging parameters were: TR/TE = 6000/77 ms; field of view 256 cm × 224 cm; 128 × 112 matrix size, yielding 2 mm × 2 mm in-plane resolution; 40 continuous slices, each 3 mm thick. A diffusion reference image (no diffusion gradient b = 0) and six diffusion-weighted images (b = 800 s/mm2 along 6 non-collinear directions) were acquired. Four DTI scans were acquired and averaged after motion correction to boost signal-to-noise. The total acquisition time of DTI was 4 min. Test-retest studies showed that measurement reproducibility of this DTI protocol yielded intraclass correlation coefficients of 0.8 and higher in the vast majority of brain regions.
2.3. DTI processing
Maps of FA, MD and tensor eigenvalues (λ1, λ2 and λ3) were generated using DTIstudio software (http://www.mristudio.org). Radial (D⊥) and axial (D||) diffusivity images were calculated on a voxel-by-voxel basis by first ranking the size of the eigenvalues (λ1 > λ2 > λ3) and then computing D|| = λ1 and D⊥ = (λ2 + λ3)/2. Further processing of the diffusion maps and voxel-wise statistics was done using SPM2 software (http://www.fil.ion.ucl.ac.uk/spm).
2.4. DTI spatial normalization protocol
Spatial normalization of the FA, MD, D⊥ and D|| maps to a common anatomical space was performed in following steps. First, the diffusion reference image (from now on termed b0-image) of each subject was transformed into the EPI-derived MNI (Montreal Neurological Institute) template in SPM by iterative non-linear transformation. The normalized b0-images were than averaged to create a customized b0-template of the study population. Second, the b0-image of each subject was then non-linearly normalized again—but now to the customized population template that was created in the first step. This reduces bias that the MNI template from a single subject might have introduced. The same transformations were then applied to the FA, MD, D⊥ and D|| maps.
2.5. Individual voxel-by-voxel analysis of DTI measures
For a voxel-by-voxel statistical analysis, the normalized images were smoothed with an 8-mm3 Gaussian kernel to approximate a normal distribution for regional variations. In order to reduce noise bias towards anisotropy in regions with small FA values as well as partial volume effects from CSF and gray matter, a threshold FA > 0.2 was used. This effectively limits the analysis to regions containing primarily white matter, since diffusion in CSF and gray matter is predominantly isotropic (Ma et al., 2004). Regional variations of FA, MD, D⊥ and D|| maps were modeled separately as a function of age using a general linear model. Since neither handedness nor gender showed significant effects on the FA and MD patterns, these factors were not included as covariates in the rest of the analysis.
2.6. Voxel-by-voxel correlation analyses between DTI measures
We performed a non-parametric correlation analysis to identify relationships between DTI measures without explicitly modeling the correlations between the measures, as previously described in (Hayasaka et al., 2006) for structural and perfusion MRI. Specifically, we assessed concordance/discordance between FA and MD changes as a function of aging and compared D⊥ versus D|| changes as a function of age.
For FA and MD, concordance means that FA and MD vary simultaneously, while discordance means that FA changes without a significant MD change or vice versa. The degree of concordance or discordance is summarized by using combining functions (described in the next paragraph) and performing a statistical inference on the resulting combining functions with a permutation test framework in the Statistical non-Parametric Mapping (SnPM) package (http://www.sph.umich.edu/ni-stat/SnPM). For D⊥ and D||, we compared changes between these measures by examining the difference between them. Similar to the analysis for FA and MD, the degree of difference is summarized using a combining function and a non-parametric framework.
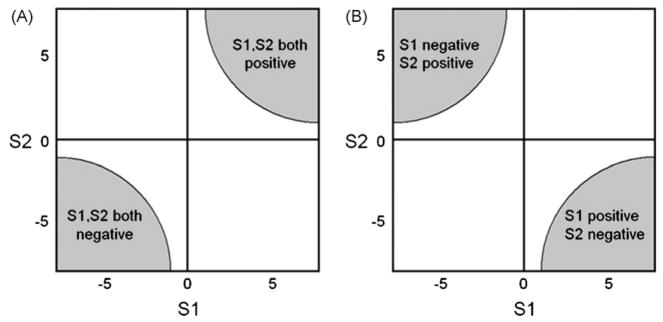
Combining functions are functions of T-statistic values from the different DTI measures. For illustration, Fig. 1 depicts two combining functions S1 and S2 that define critical regions of concordant changes of two diffusion measures such as FA and MD in the same direction, i.e. when both measures simultaneously increase or decrease, these regions are located in the upper right and lower left quadrant of the two dimensional map in Fig. 1A. Similarly, concordant changes of measures in the opposite direction, i.e. when one measure decreases while the other increases or vice versa, can be represented by S1 and S2 selecting critical regions in the upper left and lower right quadrant in Fig. 1B. In our case, combining functions were calculated based on T-statistic maps SFA and SMD or SD⊥ and SD|| from the separate statistical tests of an association of age with FA, MD, D⊥, or D||, respectively. In particular, combining functions used in our analyses were:
Fig. 1.
Illustration of combining functions (S1 and S2) that define critical regions of concordant changes between two DTI measures, such as fractional anistropy (FA) and mean diffusivity (MD) on a map of T-statistic values. (A) Simultaneous changes of DTI measures in the same directions (i.e. both measures increase or decrease); (B) Simultaneous changes of DTI measures in opposite direction (i.e. one measure increases whereas the other decreases).
Concordance functions: WCON = SFA × SMD, to identify the regions of age-related changes in both FA and MD. For statistical inference of the concordance functions, the critical threshold of T was set to Tconcordance = TFA × TMD, so that the probability of observing values larger than Tconcordance is the same with the joint probability of the individual tests (Hayasaka and Nichols, 2004).
Discordance functions: WDIS = SFA − (λ × SMD)2η, where λ defines the width and η the base of these parabolic functions (see Hayasaka et al., 2006), to identify the regions of age-related changes in FA, but not MD. Conversely, WDIS = SMD − (λ × SFA)2η to identify the regions of age-related changes in MD, but not FA. We choose parabolic functions to impose smooth boundaries for critical regions of discordance. Furthermore, we choose λ = 0.5 so that critical regions of discordance roughly span a range from −2 ≤ T ≥ 2 and a typical t-test fails to reject the null hypothesis at p = 0.05 in such regions. Also we choose η = 2 to be wider than a typical parabola (i.e. η = 1) to increase the sensitivity in the critical regions. More details about these functions and settings have been provided in previous publication (Hayasaka et al., 2006).
Comparing functions: WCOMP = SD⊥ − SD||, to identify the regions of greater D⊥ changes than D||, or WCOMP = SD|| − SD⊥ to identify regions of greater D|| changes than D⊥.
For all tests, we used in general an FWE (family wise error)-corrected threshold of p = 0.05 to identify significant differences. Significant values were then projected back into the normalized space to relate them to their anatomical locations.
To validate that the results from voxel-wise tests are not simply spurious artifacts of spatial normalization, we also performed region of interest (ROI) measurements in the native space of each subject in those regions that appeared significant in voxel-wise tests.
3. Results
3.1. Separate tests of age-related FA and MD alterations
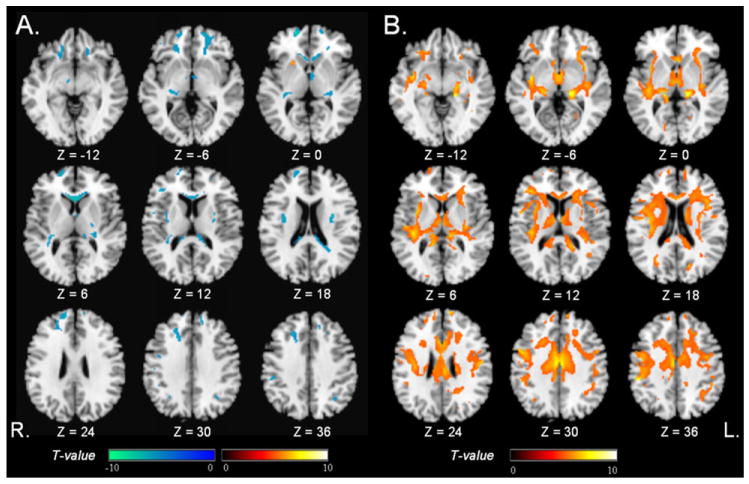
For comparison, we first performed a conventional voxel-wise analysis by testing separately correlations between age and FA or MD. The pattern of significant FA changes with increasing age is shown in Fig. 2A. Voxels with a strong negative correlation between FA and age (in cool colors) were observed predominantly in the frontal lobes, including the genu of the corpus callosum, bilateral inferior, middle and superior frontal white matter, also in the left posterior limb of internal capsule, external capsule, and posterior pericallosal regions; Voxels with a strong positive correlation between FA and age were observed in the left putamen (in warm colors).
Fig. 2.
Regions of age-related DTI changes, evaluated separately for FA (T = 5, PFWE = 0.05) and MD (T = 5, PFWE = 0.05). (A) Significant negative correlations between FA and age (cool colors); and a positive correlations (warm colors); (B) Significant positive correlation between MD and age (warm colors). Negative correlations between MD and age were not found.
The pattern of significant MD changes with increasing age is shown in Fig. 2B. Voxels with a strong positive correlation between MD and age appeared widespread in all major lobes (in warm colors). There were no regions with a significant negative correlation between MD and age.
3.2. Determination of co-analysis functions
In the scatter plot shown in Fig. 3, the T-scores of the correlation MD versus age (SMD) are plotted against the T-scores of FA versus age (SFA) for all voxels. Note, negative T-scores reflect negative correlations, i.e. reduced FA with increasing age, and positive T-scores the opposite. Furthermore, the T-scores center of gravity is heavily shifted towards negative values for FA and positive values for MD, as expected.
Fig. 3.
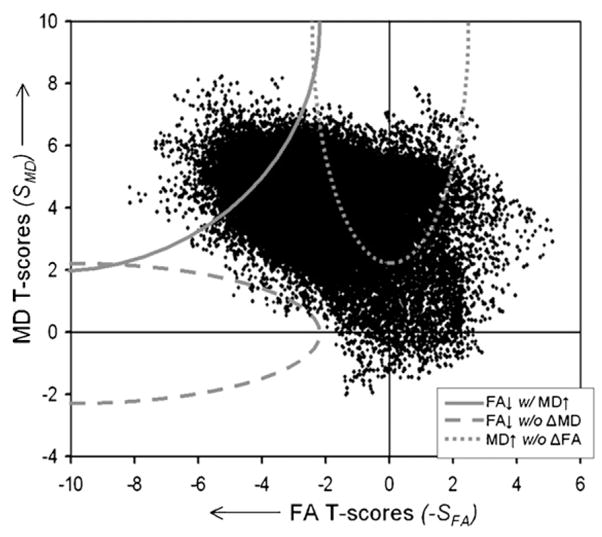
Scatter plot of FA and MD T-scores from a voxel-by-voxel analysis of FA or MD changes as a function of age. Positive T-scores indicate positive correlations between increasing FA or MD values and age. The hyperbolic curves indicate lower thresholds of significance for combining functions (see definition in the text) of concordant FA decrease and MD increase with age (solid line), discordance between FA decrease but no substantial MD change (broken line, stretching along the horizontal axis), and discordant MD increase without substantial FA change (punctuated line, stretching along the vertical axis).
We started the co-analysis between FA and MD by constructing a combining function of concordance WCON = −SFA × SMD for the upper left quadrant of the scatter plot to identify the range of concordant negative correlations of FA (negative SFA) and positive correlations of MD (positive SMD) with age. The hyperbolic curve WCON = −SFA × SMD in the upper left corner of the scatter plot in Fig. 3, indicates the lower threshold at which concordant FA and MD changes were significant. Similarly, a concordant function can be constructed for the opposite scenario (a hypothetical positive correlation of FA and a negative correlation of MD with age), in which the hyperbolic curve appears in the lower right corner of the scatter plot. However, since the center of gravity of the T-scores is heavily shifted towards the upper left corner, no T-scores appear larger than the threshold of significant concordance, as expected for this unlikely scenario.
Next, we tested discordance between FA and MD changes by constructing the discordance function WDIS = −SFA − (0.5 × SMD)4, which identifies negative correlations between FA and age in absence of significant MD changes and stretches in the scatter plot horizontally along the axis towards the left.
The broken hyperbolic line indicates the lower threshold at which values are significant. Similarly, we constructed the discordance function WDIS = −SMD − (0.5 × SFA)4, which identifies positive correlations between MD and age in absence of FA changes and stretches vertically towards the top. The lower threshold of significance for this function is indicated as punctuated line in Fig. 3. The two other potential discordances (either a positive correlation of FA or negative correlation of MD with age) are hypothetical and have no biological basis. This is also demonstrated by the lack of T-scores in this range.
In the scatter plot shown in Fig. 4, the T-scores of the correlation SD|| versus age are plotted against SD⊥ versus age. The comparison function WCOMP = SD⊥ − SD||, which tests if positive correlations of D⊥ with age are greater than those for D|| is simply a diagonal line from the lower left to the upper right corner in the plot and the corresponding shaded area indicates the range of T-scores for which this difference is significant. Lastly, the comparison function WCOMP = SD|| − SD⊥, which tests if positive correlations between D|| and age are greater than those for D⊥ is the other diagonal line and corresponding shaded area shown in Fig. 4.
Fig. 4.
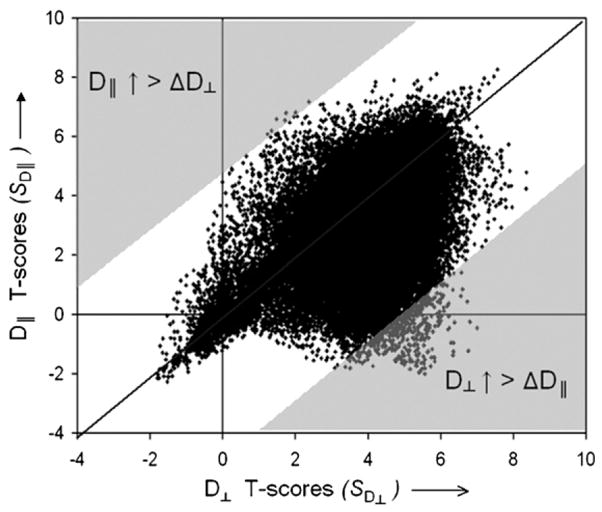
Scatter plot of T-scores from a voxel-by-voxel analysis of axial diffusivity (D||) or radial diffusivity (D⊥) changes with age. Positive T-scores indicate positive correlations between increasing D|| or D⊥ with age. The diagonal lines represent the combining functions of comparisons between D|| and D⊥ changes. The shaded areas indicate the range of T-scores with significant comparisons, including greater D|| than D⊥ changes (upper left) and vise versa (lower right).
3.3. Regional pattern of concordant FA and MD changes with age
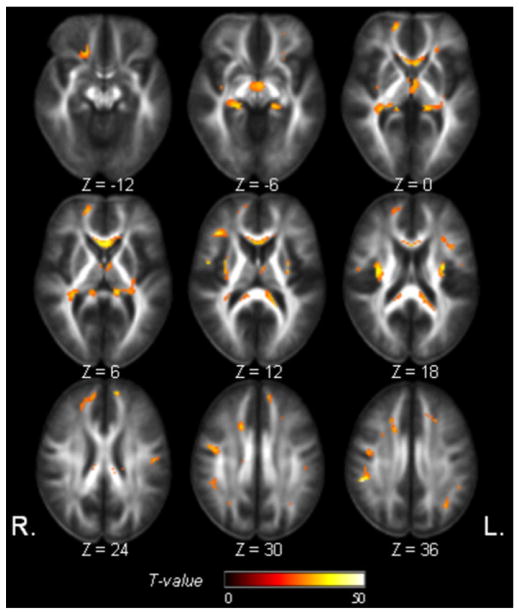
Concordant FA decrease and MD increase, as a function of age was widespread in all major lobes, as seen in Fig. 5. In particular, regions in the corpus callosum (especially the frontal part), bilateral frontal white matter, external capsule, and the periventricular white matter, and brain stem were involved. Although regions of concordant FA and MD changes are – as expected as – generally much smaller than those of separate FA and MD changes, in a few regions concordance appears where there were FA but only marginal MD changes before, or vice versa.
Fig. 5.
Regions of significant concordant FA decrease and MD increase with age (Tconcordance = 25, PFWE = 0.002).
3.4. Regional pattern of discordant FA and MD changes with age
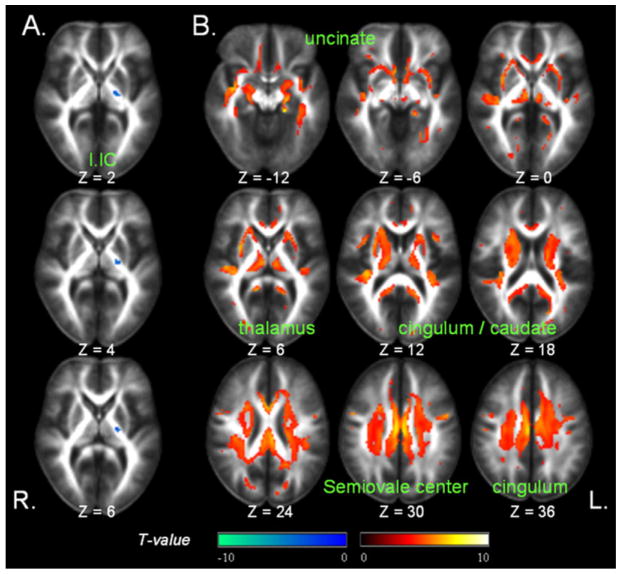
FA decrease without significant MD increase as a function of age was found in only few voxels in the left posterior limb of internal capsule, as shown in Fig. 6A. In contrast, MD increase as a function of age without significant FA changes was predominantly found in bilateral cingulum, thalami, caudate nuclei, medial temporal white matter, and the semiovale center area as shown in Fig. 6B.
Fig. 6.
Regions of significant discordances between age-related FA (Tdiscordance = 4.3, PFWE = 0.05) and MD (Tdiscordance = 3.8, PFWE = 0.05) changes. (A) Significant age-related FA decreases without major MD changes (in cool colors); (B) Significant MD increase without major FA changes as a function of age (warm colors). l.IC = Left internal capsule.
3.5. Regional pattern of different D⊥ and D|| changes with age
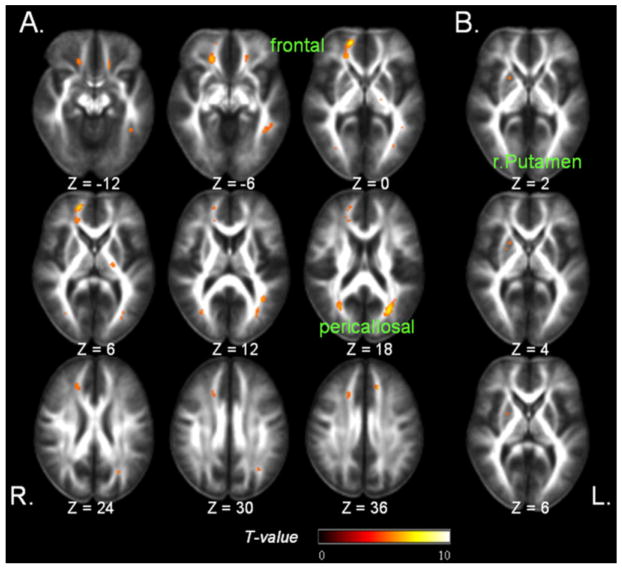
The regional pattern of a significantly greater positive correlation between D⊥ and age than between D|| and age is depicted in Fig. 7A. The regions include bilateral frontal white matter, posterior pericallosal (superior temporal) areas, and the left posterior limb of the internal capsule.
Fig. 7.
Regions of significant differences between age-related D⊥ and D|| changes (Tcomparison = 5, PFWE = 0.05). (A) Regions of greater correlation of age with D⊥ than with D||. (B) Regions of greater correlation of age with D|| and with D⊥.
The regional pattern of a significant greater positive correlation between D|| and age than between D⊥ and age involves the right putamen (Fig. 7B). This is the only region that also showed a significant positive correlation between FA and age.
3.6. ROI analysis
The results of DTI alterations with age from ROI measurements in each subject’s native space are listed in Table 2. Overall, the ROI measurements in native space yielded similar results than the voxel-wise tests in normalized space for every region that was tested. In detail, the ROI tests in native space showed: (1). In the genu of the corpus callosum, FA decreased (p < 0.001) and MD increased (p = 0.005) with age. (2). In the left internal capsule, FA decreased with age (p < 0.001), whereas MD changes were not significant (p = 0.77); Furthermore, D⊥ in this region increased substantially by 1.11 × 10−6 mm2/s/year (p = 0.002) with age, greater than the change of D|| (−0.62 × 10−6 mm2/s/year and p = 0.36). (3). In the left cingulum MD increased with age (p = 0.009), whereas FA changes were not significant (p = 0.42). (4). In the right frontal white matter region, D⊥ increased by 1.97 × 10−6 mm2/s/year (p < 0.001) with age, while D|| slightly decreased by −1.29 × 10−6 mm2/s/year (p = 0.07); The ROI analysis in this region also showed significant concordance of FA (p < 0.001) and MD (p = 0.007) changes, consistent with the results of voxel-wise tests. (5). In the right putamen D|| increased by 1.78 × 10−6 mm2/s/year (p < 0.001) with age, while D⊥ did not significantly increased (p = 0.49). Furthermore, FA in this region increased (p < 0.001) with age in agreement with the finding from the voxel-wise analysis.
Table 2.
Associations between DTI indices and age based on region-of-interest (ROI) tests in native space of each subject. Findings from voxel-wise tests in normalized space are also listed, for comparison.
| Selected ROIs | ROI analysisap-value (coefficientb) |
Voxel-wise analysis | |||
|---|---|---|---|---|---|
| FA | MD | D⊥ | D|| | ||
| Genu of corpus callosum | <0.001 (−1.45) | 0.005 (1.57) | <0.001 (4.19) | n.s. (1.72) | Concordant FA decrease and MD increase |
| Left internal capsule | <0.001 (−1.16) | n.s. (−0.06) | 0.002 (1.11) | n.s (−0.62) | FA decrease without MD change |
| Left cingulum | n.s. (−0.41) | 0.009 (1.08) | 0.03 (1.50) | n.s. (1.64) | MD increase without FA change |
| Right frontal white matter | <0.001 (−2.31) | 0.007 (1.58) | <0.001 (1.97) | n.s. (−1.29) | Greater increase of D⊥ than D|| |
| Right putamen | <0.001 (1.29) | n.s. (0.68) | n.s. (0.28) | <0.001 (1.78) | Greater increase of D|| than D⊥ |
n.s.: no significant association with age.
ROI analysis by ANOVA with threshold for significant of p ≤ 0.05.
Coefficient from linear regression: a positive value indicates increased diffusion with age. The unit of coefficient for MD, D⊥ and D|| is: ×10−6 mm2/s/year.
4. Discussion
The main findings are: (1) concordant age-related changes of FA decrease and MD increase are widespread in the brain, consistent with other DTI studies (Kochunov et al., 2007; Camara et al., 2007; Abe et al., 2008; Salat et al., 2005; Head et al., 2004). (2) Discordant age-related FA reductions without MD changes are found in regions of isolated bundles, such as the internal capsule. This new finding may indicate Wallerian degeneration accompanies normal aging. (3) The prominent FA reductions were mostly explained by increased radial diffusivity D⊥. This finding may be interpreted to indicate processes of demyelination and Wallerian degeneration; since animal studies showed demyelination leads to increase of D⊥ without major D|| change (Song et al., 2002; Harsan et al., 2006). The findings and interpretation are summarized in Table 3.
Table 3.
Summary of findings and interpretation.a.
| DTI changes with age | Interpretation | Findings |
|---|---|---|
| FA decrease | Demyelination, Axonal loss, Wallerian D | Frontal white matter, genu of corpus callosum, left posterior limb of internal capsule, external capsule, posterior pericallosum (Fig. 2A) |
| MD increase | Demyelination, Axonal loss | Widespread (Fig. 2B) |
| Concordant FA decrease and MD increase | Demyelination, Axonal loss | Frontal white matter, corpus callosum, external capsule, periventricular area (Fig. 5) |
| FA decrease without MD change | Wallerian D | Left posterior limb of internal capsule (Fig. 6A) |
| MD increase without FA change | Inconclusive | Cingulum, thalami, caudate nuclei, medial temporal white matter, centrum semiovale (Fig. 6B) |
| Greater increase of D⊥ than D|| | Demyelination, Wallerian D | Frontal white matter, left posterior limb of the internal capsule, posterior pericallosum (Fig. 7A) |
| Greater increase of D|| than D⊥ | Inconclusive | Putamen (Fig. 7B) |
Wallerian D = Wallerian degeneration.
The ‘interpretation’ is based on previous opinions about the meaning of DTI changes (Kochunov et al., 2007; Salat et al., 2005; Bronge et al., 2002; Meier-Ruge et al., 1992; Pierpaoli et al., 2001; Song et al., 2002) and not in anyway conclusive.
The first finding of widespread concordant changes of decreased FA and increased MD with age is consistent with previous DTI aging studies (Kochunov et al., 2007; Camara et al., 2007; Abe et al., 2008; Salat et al., 2005; Head et al., 2004). However, these previous studies analyzed FA and MD separately but did not compare the relationship between FA and MD. The regions of concordant FA and MD changes in our analysis include bilaterally frontal white matter regions, the corpus callosum, external capsule, and periventricular areas, in agreement with these DTI studies where have found both FA and MD changes with age. Histological studies reported wide spectrum of degenerative processes in the aging brain, including high incidence of hyperintensity white matter lesions due to ischemic changes (Horsfield and Jones, 2002), shrinkage of white matter volumes (Mrak et al., 1997; Meier-Ruge et al., 1992), retrogenic white matter maturational pattern of myelination (Bronge et al., 2002; Meier-Ruge et al., 1992), and inflammatory changes such as gliosis (Grafton et al., 1991; Takao et al., 1999). Furthermore, some regions of concordant FA and MD changes appeared where individual tests had indicated FA but only marginal MD changes, or vice versa. This implies that the combined analysis of MD and FA can be more sensitive than individual tests of MD or FA changes with age.
The second finding is that normal aging is associated with decreased FA in the absence of significant changes of MD; these changes were only detected in regions composed highly coherent structures (with high FA values), such as the internal capsule. Decreased FA without MD increases may indicate loss of coherent fiber structures without tissue loss. A previous DTI study (Pierpaoli et al., 2001) found severely reduced FA with only mild MD changes in a number of healthy populations with small chronic lacuna infarctions in the motor pathway. This severe FA reduction, in absence of MD changes has been interpreted as an indication of Wallerian degeneration involving axonal loss accompanied by reactive gliosis (Pierpaoli et al., 1996, 2001; Pierpaoli and Basser, 1996).
The third finding of greater radial than axial diffusivity in regions of large age-related FA reductions agrees with findings of demyelination in animal studies (Harsan et al., 2006, 2007; Kim et al., 2006; Song et al., 2002, 2003). Several DTI studies in animals (Harsan et al., 2007; Song et al., 2002) reported increased D⊥ without major changes of D|| reflect demyelination whereas axonal loss generally involves a decrease of D||. In addition to the internal capsule, we found greater D⊥ than D|| also in bilateral frontal white matter tracts and posterior pericallosal (superior temporal) areas. Our results in frontal white matter are also consistent with histopathological studies of age-related alternations in these regions that recapitulate developmental myelogenesis, that is, areas last myelinated during development are the first affected by aging (Kemper, 1994; Bartzokis, 2004; Bartzokis et al., 2004). We recognize, however, that a distinction between demyelination and Wallerian degeneration based on comparisons between D⊥, D|| and FA is difficult.
Our analysis yielded several additional results. First, we found increased MD without significant FA changes in the bilateral cingulum, thalami, caudate nuclei, medial temporal white matter, and the semiovale center area. One explanation is that some degenerative brain changes in aging other than demyelination, such as tissue damage due to focal ischemia, decreased tissue density which increases water diffusivity while the underlying directional structure is maintained. Second, our analysis of D⊥ and D|| comparisons identified greater age-related D|| than D⊥ changes in the right putamen. The structural basis for this result is currently unclear.
The image analysis approach used in this report is based upon our previously reported methods which summarize the degree of concordance or discordance between measures by using combining functions and performing a statistical inference on the resulting combining function with a permutation test framework. Previously we employed this approach to explore the concordance/discordance between structural and perfusion changes in patients with Alzheimer’s disease and mild cognitive impairment (Hayasaka et al., 2006). For this study, we adapted the statistical concept to evaluate the concordance and discordance between FA and MD changes in aging as well as comparisons between radial and axial diffusivity changes. However, note since FA and MD changes are derived from the same DTI measurement they form a different class of concordance/discordance analyses than stuctural and perfusion changes, which are derived from different measuremts. Nonetheless, our results demonstrate the feasbiltiliy and new informaton provided by this approach. We suggest that methods similar to these could be employed to study concordance and discordance between FA and MD in neurodegenertaive disseaseas such as Alzheimer’s and frontotemporal dementia. Furthermore, such methods could potentially be used to explore the relationship between various imaging modalities, such as comparing tensor based morphometry measures of local contraction, expension or shape changes in the brain with DTI measures.
4.1. Limitations
Several limitations of this study should be mentioned: because of intrinsic limitations of DTI, we cannot exclude that crossing fibers and voxel compartmentation mimicked age-related alterations of diffusion measures. Moreover, the voxel-wise analysis, relying on accurate morphing of brains from different subjects into a common space, may have been confounded by misregistrations between brains that induce artifacts and further may have suffered reduced sensitivity due to spatial smoothing. Other methods which can improve image registration without spatial smoothing, such as Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006) and techniques that are less confounded by crossing fibers, such as high angular resolution diffusion imaging (HARDI) (Anderson, 2005), Q-ball diffusion imaging (Poupon et al., 2007), and Diffusion spectrum imaging (DSI) (Wedeen et al., 2005), may therefore lead to different results. In addition, other approaches to generate FA templates, such as a true average intensity and shape FA template (Park et al., 2003) might improve accuracy. Since the resolution of DTI was anisotropic (2 mm × 2 mm × 3 mm), it is also possible that this feature introduced a selective measurement bias on the different diffusion parameters, which may have caused spurious discordance for technical reasons. Another limitation is that the ability to detect significant reductions of FA require that the brain structure must be sufficiently coherent, and thus to have a sufficiently high FA value, to begin with. The consequence of this is that we only could detect evidence of age-related FA changes in those brain regions with high coherence in the young subjects. Yet another limitation is that we did not differentiate in our DTI analysis between normal appearing white matter and white matter lesions, although we screened the subjects for absence of major cerebrovascular disease and major lesions. Nonetheless, mild white matter lesions rather than aging may have induced some regional findings of DTI alterations. Finally, our statistical approach of concordance/discordance may be less sensitive than multivariate statistics, which permits to assess simultaneously all elements of the diffusion tensor (Whitcher et al., 2007). In addition, the choice of combining functions is somewhat arbitrary and subjective, although the sensitivity and robustness of the combining functions can be evaluated by simulations and permutation tests, allowing interpretation of these functions as we previously showed (Hayasaka and Nichols, 2004). Further investigations will be necessary to compare the sensitivity of the two statistical approaches.
In summary, our DTI study of normal brain aging demonstrates heterogeneous changes of FA, MD, and radial and axial diffusivity with variable concordance/discordance of these findings. The results are consistent with both Wallerian degeneration and demyelination. We conclude that coanalysis of DTI indices provides additional characterization of the heterogeneous changes of white matter occurring during normal aging. This approach could also be utilized to analyze DTI studies of brain disordered in aging, such as Alzheimer’s disease.
Acknowledgments
We thank Mr. Shannon Buckley for the help in DTI image processing, and thank Dr. Susanne Mueller for the neuropsychological assessment. This work was supported in part by the NIH grant AG010897, a grant from department of defense W81XWH-05-2-0094, and the San Francisco Veterans Affairs Medical Center.
Footnotes
Disclosure statement
We have no conflicts of interest to disclose. None of the authors has commercial or financial involvements in connection with the submitted article. There are no agreements that involve any financial interest in our work.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29 (1):102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4 (3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AW. Measurement of fiber orientation distributions using high angular resolution diffusion imaging. Magn Reson Med. 2005;54 (5):1194–1206. doi: 10.1002/mrm.20667. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25 (1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: novel therapeutic targets for preventing cognitive decline and Alzheimer’s disease. J Alzheimers Dis. 2004;6 (6 Suppl):S53–S59. doi: 10.3233/jad-2004-6s604. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111 (3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15 (7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett-Levy J. Determinants of performance on the Rey-Osterrieth Complex Figure Test: an analysis, and a new technique for single-case assessment. Br J Clin Psychol. 1984;23 (Pt 2):109–119. doi: 10.1111/j.2044-8260.1984.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord. 2002;13 (4):205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57 (4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Camara E, Bodammer N, RodrÃguez-Fornells A, Tempelmann C. Age-related water diffusion changes in human brain: a voxel-based approach. Neuroimage. 2007;34 (4):1588–1599. doi: 10.1016/j.neuroimage.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. (2) 2000 doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12 (3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Sumi SM, Stimac GK, Alvord EC, Jr, Shaw CM, Nochlin D. Comparison of postmortem magnetic resonance imaging and neuropathologic findings in the cerebral white matter. Arch Neurol. 1991;48 (3):293–298. doi: 10.1001/archneur.1991.00530150061019. [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47 (6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Parizel N, Skoff RP, Ghandour MS. Astrocytic hypertrophy in dysmyelination influences the diffusion anisotropy of white matter. J Neurosci Res. 2007;85 (5):935–944. doi: 10.1002/jnr.21201. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Parizel N, Skoff RP, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83 (3):392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Du AT, Duarte A, Kornak J, Jahng GH, Weiner MW, Schuff N. A non-parametric approach for co-analysis of multi-modal brain imaging data: application to Alzheimer’s disease. Neuroimage. 2006;30 (3):768–779. doi: 10.1016/j.neuroimage.2005.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. Neuroimage. 2004;23 (1):54–63. doi: 10.1016/j.neuroimage.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14 (4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases—A review. NMR Biomed. 2002;15 (7–8):570–577. doi: 10.1002/nbm.787. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert ML, Kusefel J, editors. Clinical Neurology of Aging. Oxford; New York: 1994. pp. 3–67. [Google Scholar]
- Kim JH, Budde MD, Liang HF, Klein RS, Russell JH, Cross AH, Song SK. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis. 2006;21 (3):626–632. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25 (2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35 (2):478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Ma X, Kadah YM, LaConte SM, Hu X. Enhancing measured diffusion anisotropy in gray matter by eliminating CSF contamination with FLAIR. Magn Reson Med. 2004;51 (2):423–427. doi: 10.1002/mrm.10703. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anat Rec. 1999;257 (3):102–109. doi: 10.1002/(SICI)1097-0185(19990615)257:3<102::AID-AR7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin ST, Graham DI. Aging-associated changes in human brain. Review J Neuropathol Exp Neurol. 1997;56 (12):1269–1275. doi: 10.1097/00005072-199712000-00001. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16 (4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- O’Hara R, Yesavage JA. The Geriatric Depression Scale: its development and recent application. Principles Pract Geriatr Psychiatry 2001 [Google Scholar]
- Park HJ, Kubicki M, Shenton ME, Guimond A, McCarley RW, Maier SE, Kikinis R, Jolesz FA, Westin CF. Spatial normalization of diffusion tensor MRI using multiple channels. Neuroimage. 2003;20 (4):1995–2009. doi: 10.1016/j.neuroimage.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22 (3):1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: evidence from diffusion tensor imaging. Neuroimage. 2005;26 (3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13 (6 Pt 1):1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201 (3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson, Med. 1996;36 (6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Poupon C, Poupon F, Roche A, Cointepas Y, Dubois J, Mangin JF. Real-time MR diffusion tensor and Q-ball imaging using Kalman filtering. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10 (Pt 1):27–35. doi: 10.1007/978-3-540-75757-3_4. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41 (2):223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26 (8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. Semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114 (1):7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31 (4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20 (3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17 (3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45 (3):244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16 (7):1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Takao M, Koto A, Tanahashi N, Fukuuchi Y, Takagi M, Morinaga S. Pathologic findings of silent hyperintense white matter lesions on MRI. J Neurol Sci. 1999;167 (2):127–131. doi: 10.1016/s0022-510x(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Adult Intelligence Scale. (3) 1997 [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54 (6):1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Whitcher B, Wisco JJ, Hadjikhani N, Tuch DS. Statistical group comparison of diffusion tensors via multivariate hypothesis testing. Magn Reson Med. 2007;57 (6):1065–1074. doi: 10.1002/mrm.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]