Abstract
The principal aim of this study was to gain a better understanding of where lead (Pb) accumulates and how it is distributed, within the bones of dosed goats. Adult goats were periodically dosed with Pb over a number of years for the primary purpose of producing blood pools containing endogenously bound Pb, for the New York State Blood Lead Proficiency Testing Program. Bone samples (e.g., primarily tibia, femur, humerus, and radius) were collected post-mortem from 11 animals and were analyzed for Pb content by acid digestion and electrothermal atomic absorption spectrometry (ETAAS or GFAAS). Average tibia-Pb levels were found to correlate strongly with the cumulative Pb dose (r2 = 0.81). However, the concentration of Pb in different bones and even within a small area of the same bone varied tremendously. Blood-rich trabecular (spongy) bone, such as the patella and calcaneus, were much more enriched in Pb than was cortical (compact) bone. In some dosed animals, the Pb concentration in the tibia was markedly higher at the proximal and distal ends of the bone compared to the mid-shaft. The implications of these findings with regard to the non-invasive measurement of lead in bone by XRF methods are discussed.
Keywords: Bone, lead, goat, tibia, cortical, trabecular
1. Introduction
Over the last three decades, environmental Pb concentrations have fallen considerably, due to removal of Pb from gasoline, household paint, solder, and other consumer products (CDC (Centers for Disease Control and Prevention), 2005). Despite this progress, human populations are still exposed to low levels of Pb via contaminated food, water, dust, and soil, and occupational activities. Children are especially vulnerable to the adverse health effects of Pb exposure. Several recent studies have shown that blood Pb levels <10 μg/dL in young children are associated with poorer performance on end-of-grade tests (Miranda et al., 2007) and with impaired intellectual functioning as revealed in neurobehavioral testing (Jusko et al., 2008). Therefore, further reductions in exposure to Pb remain a major public health priority (CDC (Centers for Disease Control and Prevention), 2005).
Once Pb enters the blood compartment, it distributes throughout the body, and accumulates in bone. The residence time of Pb in blood is around 4–6 weeks, so blood Pb reflects recent exposure; however, the residence time in bone ranges from several years to decades (Brito et al., 2005). Bone Pb accounts for more than 90% of the adult total body burden of Pb (50% in infants, and 70% in older children) (Barry, 1975). While blood Pb remains the best and most widely used indicator of recent exposure, bone Pb is considered a good biomarker of long-term or cumulative exposure (Barbosa F. et al., 2005; Hu et al., 1998; Shih et al., 2007).
The distribution of Pb within the human skeleton has been reviewed and summarized previously (Aufderheide and Wittmers, 1992; Shih et al., 2007). In some of the earliest studies (Barry, 1975; Barry and Mossman, 1970), it was reported that over 70% of all skeletal Pb is concentrated in dense (compact or cortical) bone. Wittmers et al. (1988) reported that the Pb content of a single bone sample depended largely on the mass ratio of spongy to compact bone at the sampling site. The latter workers carried out a detailed study of Pb distribution in the human skeleton, measuring the Pb content of tibia, skull, ribs, ilium, and vertebrae post-mortem from 134 subjects ranging in age from 1 to 98 years, using a dry ashing method followed by atomic absorption spectrometry (AAS). Results were reported in units of ppm (μg/g) ash weight. They found that Pb was unequally distributed among the different bones in distinct patterns that were age-dependent. Todd et al. (2001) measured Pb concentrations in nine post-mortem human tibia samples, using a wet ashing method followed by AAS with electrothermal atomization and Zeeman background correction (ETAAS). Results were reported in units of ppm (μg/g) dry weight. They found that Pb concentrations were enriched at the tibia surface compared to the core, regardless of the average concentration. Interestingly, they observed a statistically significantly locational trend, in which the Pb concentration at the mid-point of the tibia shaft was slightly higher than concentrations at the proximal and distal ends. This trend appeared to fit a polynomial function. However, they did not analyze any bone samples from the epiphyses (i.e., joints areas) of the tibiae. Microheterogeneity of Pb distribution within bone is less well characterized. Studies using synchrotron X-ray microscopy (SXRM) and proton-induced X-ray emission (PIXE) microanalysis (Jones et al., 1992; Jones et al., 1990; Schidlovsky et al., 1990) have shown that Pb is enriched at the periosteal surfaces, and sometimes the endosteal surfaces, of human tibiae.
Over the last 30 years, bone-Pb measurements using noninvasive in vivo x-ray fluorescence (XRF) methods have become well established in epidemiological studies, where estimates of cumulative Pb exposure are desired (Barbosa F. et al., 2005; Chettle, 2005). However, the traceability, repeatability, and reproducibility of these methods have been the subject of some debate (Preiss and Washington, 1995; Rosen and Slatkin, 1993; Todd et al., 2002a; Todd and Chettle, 1994; Todd et al., 1993; Todd et al., 2002b).
The principal aim of the present study was to gain a better understanding of where Pb accumulates and how it is distributed within the bones of dosed goats. These data were previously presented as part of a doctoral thesis that was submitted to the University at Albany (Zong, 1996), and some results have been previously summarized (Parsons et al., 2006). Recently, our laboratory has reported some preliminary data on the microdistribution of Pb in a cross-section of a single goat tibia using prototype instrumentation based on monochromatic microbeam X-ray fluorescence (MμXRF) coupled with doubly-curved crystal optics (DCC) (Bellis et al., 2009). Here we report detailed data on the accumulation of Pb in a variety of bone samples obtained from Pb-dosed goats. Adult goats were periodically dosed with Pb over a period of several years, for the primary purpose of producing blood pools containing endogenously bound Pb, for blood lead proficiency testing purposes. Bone samples from various skeletal sites were collected post-mortem from these animals, and were analyzed for Pb content using a well established method based on wet ashing, followed by Pb determination by ETAAS with Zeeman background correction (Zong et al., 1996).
2. Methods
Source of animal bones
The New York State Department of Health’s (NYS DOH) Wadsworth Center maintains a herd of goats (Capra hircus) that are periodically dosed with lead acetate (CH3COO)2Pb), to produce whole blood pools for use in the NYS Proficiency Testing (PT) program for blood Pb. The animals are dosed orally, so that Pb is metabolically incorporated into the red blood cells. Aliquots of the blood pools are circulated to over 100 participating laboratories for PT purposes. Details of the NYS PT program, which has been in operation for more than three decades, have been provided elsewhere (Parsons et al., 2001).
Briefly, approximately 25–30 goats, ranging in age from 3 to 14 years, are assigned to the blood Pb PT program at any given time. These animals are treated in accordance with a protocol that is approved by the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC). As animals near the end of their expected lifetimes, the Center’s Facility Veterinarian will recommend euthanasia. In such cases, arrangements are made to collect various organs and bone samples post mortem. Typically, the autopsy protocol calls for removal of the brain, liver, kidneys, teeth, and samples of various bones for use in research studies. Long bones are collected, including the femur, tibia, humerus, and radius. In some cases, samples of skull, ilium, vertebrae and ribs are also collected for further studies.
In addition to bones from dosed goats, we obtained certified reference materials from the National Institute of Standards and Technology (NIST) (Gaithersburg, MD): Standard Reference Material (SRM) 1486 Bone Meal; and SRM 1400 Bone Ash (with certified Pb values of 9.07 μg/g and 1.335 μg/g respectively).
Bone sample preparation
Samples of intact bones, including the femur, tibia, skull, rib, vertebrae and ilium were collected post mortem from 11 goats (10 dosed and 1 undosed). However, for the purpose of this study the principal samples were the long bones. Fur and much of the overlying tissue were removed with stainless steel knives, while any remaining adherent tissues were removed using a custom bone scraper fabricated from 99.99 % (w/w) tantalum (Ta) metal (NRC Inc., Newton, MA). Use of a Ta, rather than stainless steel, bone scraper was deemed desirable so as to avoid contaminating the bone surface with metals (e.g., Cr, Mn, Ni) that might be of analytical interest in future studies using the same bone digests. Bone marrow was also removed from long bones after separation of the epiphysis (bone joint) from the diaphysis (bone shaft). Bare bones were soaked with ether (to remove fat content), and then hydrogen peroxide (to remove remaining blood deposits), and were washed with double-deionized water. The cleaned bone samples were stored at −70°C until analysis.
Initially, large bones were cut into cross-sections with a reciprocating stainless steel autopsy saw (Stryker Instruments, Kalamazoo, MI). A diamond disk saw was used to separate bone samples into smaller (0.5 g) cross sections for analysis. The sectioned bone samples were further washed with H2O2 (50% v/v stabilized; Fisher Scientific) to remove any remaining blood and tissue fragments. Samples of H2O2 wash solutions were analyzed for Pb content by GFAAS, to monitor potential losses/leaching during the wash procedure. Results showed that no detectable Pb (i.e., <1 μg/L) is leached from the bones following H2O2 washing. For internal quality-control (QC) purposes, some of the bone materials were pooled and homogenized in a custom Ta ball-mill (GlenMills Inc., Maywood, NJ) equipped with Ta balls.
Bone samples were digested in one of two ways. For bone sample masses >0.5 g, we used microwave-assisted heating to digest bone samples in concentrated HNO3. For smaller sample masses (< 0.5 g), we digested bones in concentrated HNO3 at room temperature over 48 h. For microwave-assisted heating, approximately 0.5 g bone was digested with 10 mL concentrated HNO3 in a closed-vessel microwave digestion system (Model 81D, CEM, Matthews, NC) using a heating program of 8 min at 100% power (650 W), followed by 10 min at 65% power (422 W) under a pressure feedback control set to 448 kPa (65 psi). The final digestate was diluted to 50 mL to give approximate 10 mg bone/mL of HNO3, a concentration that was optimal for the determination of Pb by ETAAS with Zeeman background correction.
Determination of Pb by ETAAS
We used a Perkin-Elmer Model Z5100 AAS, equipped with transverse Zeeman background correction, for determination of Pb. Aliquots of the sample digestate and standard Pb (as Pb(NO3)2) stock solutions were diluted 1+9 with a modifier solution (0.22% NH4H2PO4 + 0.022 %Ca) directly in the AAS auto-sampler cup. The AS-60 autosampler was programmed to deposit 10 μL of sample, equivalent to 10 μg of bone, along with 2 μg of Ca + 20 μg of NH4H2PO4 on the platform. The method detection limit (3SD) is 0.6 μg/g dry weight, with typical between-run precision < 5%. The method accuracy was previously established as better than 1%, through the use of NIST Standard Reference Material (SRM) 1486 Bone Meal and SRM 1400 Bone Ash (Zong, 1996). However, during the present study, additional samples of these SRMs were analyzed along with an internal QC material derived from a Pb-dosed caprine.
3. Results and discussion
First, analytical data were obtained from two digestion methods, (a) microwave-assisted heating and (b) room-temperature digestion for 48 h, to investigate whether the two methods were equally effective for digestion of bone samples for Pb determination. Three different materials, two NIST SRMs and one internal QC material were analyzed, and results are given in Table 1. It is clear that room-temperature digestion provides accurate and precise values that are equivalent to those obtained by microwave digestion, and t-tests (significance threshold, 0.05) on the data indicate no significant difference between the two digestion procedures. Indeed, both techniques produced results that were within the uncertainty limits given for the NIST SRMs. Therefore, these two procedures were judged equally suitable for digestion of bone for Pb determination. The only disadvantage of room temperature digestion is that it is more time consuming; however, this is offset by the advantage that room temperature digestion does not require use of pressurized digestion vessels, which must be thoroughly acid-washed between successive uses (Zong, 1996). Accordingly, bone Pb data reported hereafter were obtained with the room-temperature digestion.
Table 1.
Comparison between microwave-assisted and room-temperature digestion procedures for determination of Pb in bone.
| Bone Material | * Bone weight (μg) | Pb μg/g (SEM) dry weight | ||
|---|---|---|---|---|
| Microwave- assisted digestion N = 5 |
Room-temperature digestion N = 5 |
Certified value† N = 5 |
||
| SRM 1400 Bone Ash | 10 | 9.1 (0.1) | 9.0 (0.1) | 9.07 ±1.12 |
| 20 | 9.0 (0.2) | 8.9 (0.2) | ||
| SRM 1489 Bone Meal | 50 | 1.3 (0.1) | 1.2 (0.1) | 1.335 ±0.014 |
| 100 | 1.2 (0.1) | 1.2 (0.1) | ||
| NYS Goat 2–7 Bone Powder (Internal QC) | 10 | 44.5 (0.4) | 44.0 (0.3) | Not certified |
equivalent bone mass deposited on the platform via a 10-μL injection of acid digest.
NIST-calculated uncertainty expressed as the half-width of a 95% confidence interval.
Relationship between tibia Pb concentration and cumulative Pb dose
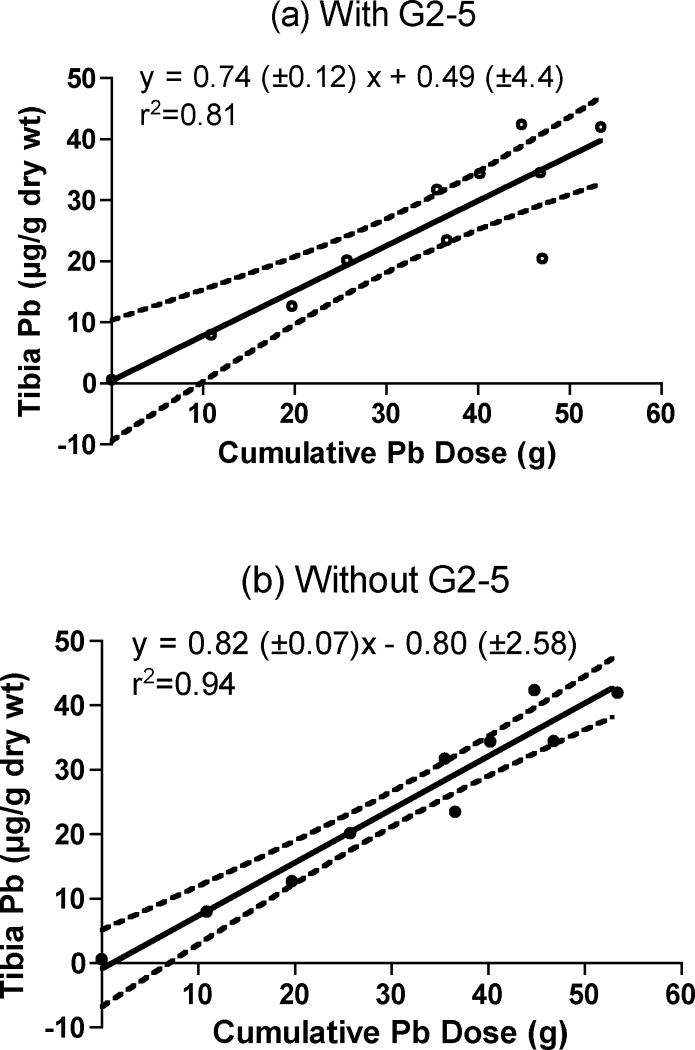
Characteristics of the 11 goats that were examined as part of this study are summarized in Table 2. Initially, we collected only a single cross-section at the mid-shaft the tibia (along with single samples from other bone sites) as this is the most common sampling site used for XRF measurements in humans. Each single tibia cross-section was analyzed for lead content and these data were then compared to administered dose. The bone Pb data reported in Table 2 represent the analysis of a single 1-cm cross-section of the tibia, removed from the mid-shaft region of the bone. The bone lead result for each sample represents the mean of 5 replicate injections into the graphite furnace. Figure 1 shows a plot of tibia Pb concentration as a function of the cumulative dose received by each of these animals. Ordinary least squares analysis of the data (Fig 1a) indicates a strong linear correlation between tibia Pb and cumulative Pb dose (r2= 0.81). However, one goat (G2–5) appeared to have an suspiciously low tibia lead concentration given a cumulative dose of 47 g Pb. It is possible that this point represents “biological” or perhaps locational variability. It is also equally possible that for unknown reasons, this particular goat did not absorb ingested lead as efficiently as did the others. Other (unlikely) possibilities include analytical blunder or that the goat was not dosed as indicated in the records. Ordinary least squares analysis was repeated (Fig 1b) excluding the data from G2–5, indicating a stronger fit (r2= 0.94) although the difference in slopes may not be statistically significant given the uncertainty. The bone-Pb content of an undosed animal (G0–11) was less than 1 μg/g (Table 2), and close to the method detection limit; it represented exposure to background Pb levels in the environment.
Table 2.
Details of lead dosed goats and tibia Pb levels.
| ID# | Gender | Age (y) | # of years dosed with Pb | Cumulative Pb dose (g) | Tibia-Pb (± SEM)a (μg/g dry wt.) |
|---|---|---|---|---|---|
| G0–11 | F | 14 | 0 | 0 | 0.7 ± 0.1 |
| G2–15 | F | 9 | 1.2 | 10.9 | 8.0 ± 0.2 |
| G2–14 | M | 8 | 3.3 | 19.7 | 12.7 ± 0.2 |
| G106 | F | 13 | 4.3 | 25.7 | 20.2 ± 0.3 |
| G2–13 | M | 9 | 4.6 | 35.5 | 31.8 ± 0.3 |
| G2–11 | M | 12 | 6.8 | 36.6 | 23.5 ± 0.3 |
| G2–8 | M | 12 | 7.8 | 40.2 | 34.4 ± 0.4 |
| G1–9 | F | 10 | 7.7 | 44.8 | 42.4 ± 0.5 |
| G2–7 | F | 12 | 8.0 | 46.8 | 34.5 ± 0.4 |
| G2–5 | M | 12 | 8.0 | 47.0 | 20.5 ± 0.3 |
| G1–5 | M | 14 | 8.5 | 53.4 | 42.0 ± 0.5 |
Standard Error of the Mean (SEM) represents analytical uncertainty (n=5), rather than biological variation within the tibia.
Fig. 1.
Plot of tibia Pb concentration as a function of cumulative Pb dose. Ordinary least squares analysis of the data was conducted (a) for all goats (open circles, n=11), and (b) excluding one goat, G2–5 (closed circles, n=10). The slope and intercepts are shown (±SE), along with the 95% confidence intervals.
Locational distribution of Pb along the diaphyses of caprine long bones
Long bones, including the femur, tibia, humerus and radius were removed from four goats and were further analyzed for Pb content in a more in-depth study. The fours goats were selected based on total lead dose received, such that the moderate to high dose range was covered. Each long bone was separated into consecutive cross sections of ~1 cm in width. Each 1-cm cross section was further sub-divided into three for analysis, and the three determinations were averaged to produce a mean value (±SEM) for bone Pb. Each sub-sample was digested and analyzed separately for Pb content by ETAAS thus providing information on the local biological variability in Pb concentration. Therefore, in spite of the limitations of triplicate analysis (n=3), the calculated SEM (or standard uncertainty) included not only the analytical component of uncertainty but also biological variability. The data confirm that the latter is by far the dominant component of the uncertainty in bone lead measurements by ETAAS.
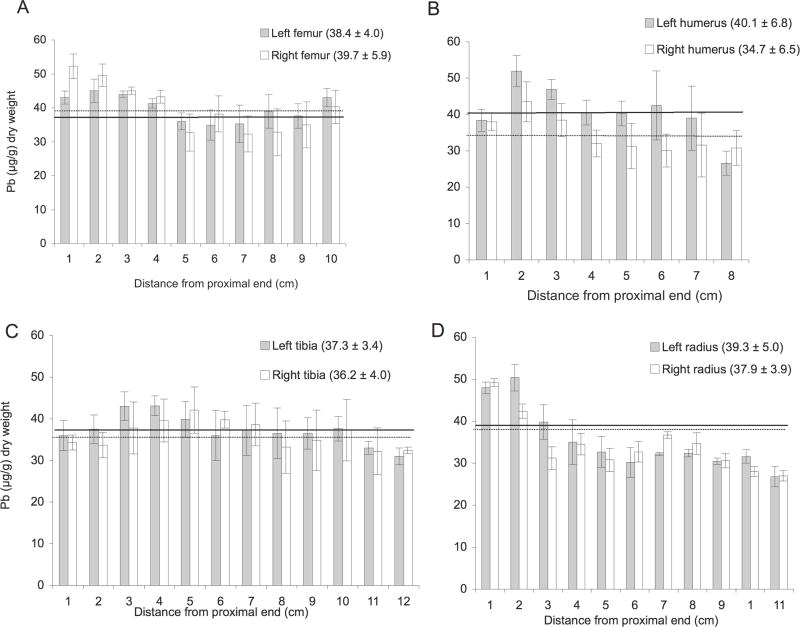
In Figure 2, the locational distribution of Pb is shown in the femur, tibia, humerus, and radius removed post mortem from a single goat with a lifetime cumulative Pb dose of 46.8 g. This specific goat (G2–7) was selected from a sub-group of three having similar cumulative Pb doses, and that preserved our options for later experimental studies. It is clear that considerable variation in bone Pb concentration exists even within a single animal. This intra-individual variability in bone lead concentrations reflects the lack of understanding as to the mechanism(s) in which Pb is taken up, transported to and accumulated in different long bones, and even between triplicate sub-samples of the same bone or, of a bone pair. It is interesting to note that no significant difference was found between the mean concentrations of Pb found in the left and right femur, or in between the mean concentrations of Pb found in the left and right tibia (two-tailed t-test, P>0.005). For those left/right bone pairs (humerus and radius), where the underlying data were non-normal, the median Pb concentration differences were either not quite or not significant respectively (Many-Whitney test, p=0.065, and p= 0.921). With respect to the former, range of Pb concentrations found in the humerus of G2–7 varied from 27 to 52 μg/g, a factor of almost 2. An example of the proximal-distal variation in Pb concentration is shown in the radius of G-27. In contrast, there is greater Pb enrichment at the epiphyses in the radius than in the diaphyses. Overall, the locational distribution pattern among the four major long bones from a single dosed goat is not consistent, and thus, generalizations across long bone types should not be made.
Fig. 2.
Locational distribution of Pb in the left (black bars) and right (white bars) hind long bones from dosed goat G2–7. The error bars denote the SEM of Pb concentration values found for the three sub-segments analyzed. The bars in each graph are sorted from left to right to show location, i.e., with increasing distance from the proximal end where the diaphyses were separated from the epiphyses. The overall mean Pb concentration, i.e., the mean of all the Pb data points obtained for each specific bone, is represented as a horizontal line in each panel, with the mean value (μg/g) ±SEM given in parenthesis.
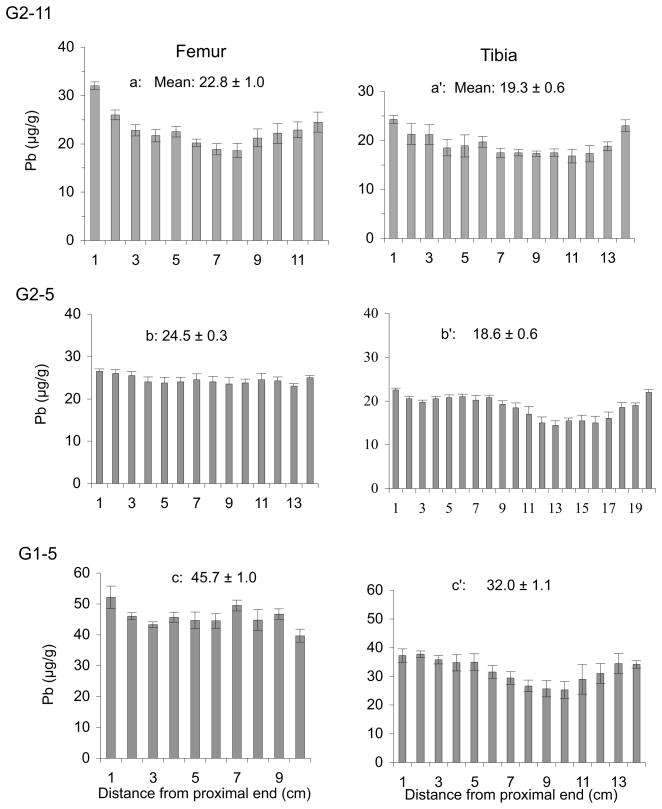
Further analyses for Pb content were carried out on long bones from three other dosed goats. Figure 3 compares the Pb concentration found in the femur and tibia from three dosed goats, G2–11, G2–5 and G1–5, that had received respective lifetime Pb doses of 36.6 g, 47.0 g, and 53.4 g. Pb concentrations were generally greater at each end of the femur and the tibia for G2–11, and also at each end of the tibiae from G2–5 and G1–5. However, this proximal-distal variation did not hold true for the femurs from G2–5 and G1–5, the two goats that received the highest cumulative dosages. Interestingly, the grand mean Pb concentration found for each femur was significantly greater than that found in the corresponding tibia for each of the three animals (two-tailed t-test, P=0.006, G2–11; P<0.0001, G2–5 and G1–5). Results also indicate that Pb concentration along the diaphysis (shaft) of bones from dosed animals is highly heterogeneous, varying by as much as a factor of 2 (within a 1-cm × 3-cm cross section). In some cases, Pb appears to accumulate more toward the ends (epiphyses) of the bone than in the mid-shaft.
Fig. 3.
Distribution of Pb in femur and tibia from dosed goats G2–11, G2–5, and G1–5. The overall mean Pb concentration (± SEM) in each long bone is given along with locational data ranked left to right, proximal to distal.
Characterization of the intra-individual variation in Pb concentration in sub-samples of bone taken at the mid-point of the tibia is interesting, since that site is the sampling target frequently analyzed by practitioners of in vivo XRF techniques. It is also useful in the present case since the particular goat tibiae analyzed here were previously suggested as good candidates for an interlaboratory round robin for in vivo XRF facilities (Parsons et al., 1995), and have indeed been used for this purpose (Parsons et al., 2008). Just recently, we reported preliminary data on the microdistribution of Pb in a cross-section of the tibia from a lead-dosed goat (Bellis et al., 2009). Those data were obtained using a prototype instrument based on MμXRF coupled with a low power source and DCC optics, and indicate that Pb appears to accumulate in thin band near the periosteal surface (Bellis et al., 2009).
Uptake and deposition of Pb in the bones of the caprine hind limb
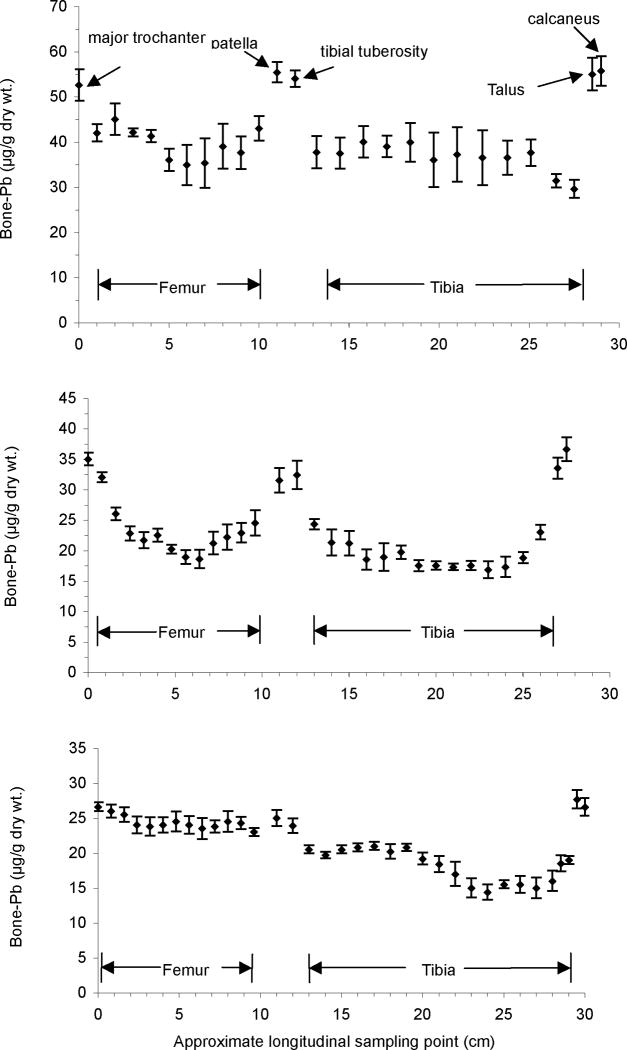
The accumulation of Pb in the various bones of the caprine hind limb was investigated. A complete set of hind limb bones that included the epiphyses was obtained from three dosed animals. In addition to the principal long bones, i.e., the femur and tibia, the patella, talus and calcaneus were collected and analyzed for Pb content. We removed three 1cm × 1cm sub-samples in increments of 1cm from each bone, as described previously. The variation in bone Pb concentration along the caprine hind limbs from these animals is shown in Figure 4. Results for goats G2–7 (Panel A) and G2–11 (Panel B) indicate that Pb accumulated preferentially in the epiphyses, at the major trochanter (femur), in the patella, at the tibial tuberosity, in the talus, and in the calcaneus, compared to the diaphyses. For goat G2–5 (Panel C), Pb was highly enriched in the talus and in the calcaneus, and Pb concentrations in the femur and patella were greater than those found in the tibia.
Fig. 4.
Distribution of Pb along the left hind limb of three lead-dosed goats, G2–7, G2–11, and G2–5. Each data point represents a mean Pb concentration (± SEM) of 3 sub-samples taken at that location. The longitudinal sampling points are estimated to be within ±2 mm.
Based on the bone Pb measurements in the hind limbs from two of the three dosed animals, Pb concentrations were clearly higher in spongy (trabecular) bones, such as the patella and calcaneus, than in compact (cortical) bones, such as the mid-shaft of the tibia and femur.
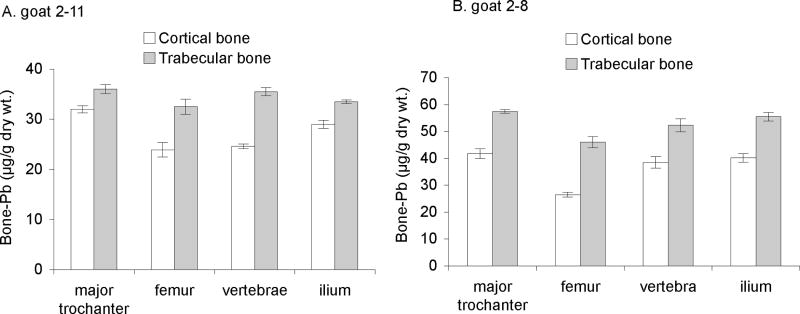
Accumulation of Pb in cortical and trabecular bone
Analytical data based on replicate measurements indicated that Pb was not uniformly distributed in these bones. In fact, Pb was clearly enriched in spongy bone relative to compact bone. We carried out additional analyses of long bone samples by physically separating the spongy from compact bone material, and determining the Pb content in each. The precise procedure was modified from that reported by other workers (Inskip et al., 1992). Samples of the major trochanter, femur, vertebrae, and ilium, were collected from two goats (G2–11 and G2–8) in the mid-dose range, and the spongy and compact bone materials were separated and analyzed for Pb content. Results shown in Figure 5 indicate clearly, at least for these two animals, that Pb accumulates preferentially in blood-rich trabecular (spongy) bone.
Fig. 5.
Lead accumulation in cortical and trabecular bone obtained from two dosed goats; error bars denote the SEM of values from triplicate analyses.
Conclusion
Lead is taken up into the bone compartment of dosed goats and distributes unevenly among different bones within an individual animal. Furthermore, Pb tends to accumulate preferentially in blood-rich trabecular (spongy) bone as opposed to cortical bone. The enrichment of Pb in bone types that are predominantly trabecular, such as the patella and calcaneus, is consistent with data acquired by KXRF on occupationally-exposed human subjects. It should be noted that the KXRF-based observation of greater lead concentration in trabecular (cf. cortical) bone arises, in part because of the lower mineral content of the trabecular bones, in combination with the concentration units of KXRF (μg/g bone mineral) being different to those used here (μg/g dry weight) with acid digestion and AAS. Lead also accumulates within the epiphyses of long bones, sites at which bone growth and turnover are most rapid.
Acknowledgments
This project was supported in part by grant R01 ES12424 from the National Institute of Environmental Health Sciences (NIEHS), a division of the National Institutes of Health (NIH), USA. The contents of this paper are, however, solely the responsibility of its authors and do not represent the official views of the NIEHS or the NIH. The authors gratefully recognize the contribution of the Wadsworth Center’s Facility Veterinarians, Dr. Ronald Gruhn and Dr. Frank Blaisdell, Wadsworth Center’s Griffin Laboratory animal care staff, and members of the Trace Elements Laboratory involved in lead dosing, collection and archiving of animal materials over many years.
Institutional Animal Care and Use Committee (IACUC) approvals.
Research conducted with animals as described in this manuscript was conducted in accordance with a protocol that is approved by the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC), protocol number 07-096. The Wadsworth Center’s Animal Care and Use Program complies with the requirements of federal and state regulatory agencies under
*PHS Animal Welfare Assurance #A3183-01
*USDA Class R Research Facility Registration #21-R-0124
*NYSDOH Permit for the Use of Living Animals AW ID# A002
and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aufderheide AC, Wittmers LE., Jr Selected aspects of the spatial distribution of lead in bone. Neurotoxicology. 1992;13:809–819. [PubMed] [Google Scholar]
- Barbosa FJ, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environmental Health Perspectives. 2005;113:1669–74. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PSI. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PSI, Mossman D. Lead concentrations in human tissues. Br J Ind Med. 1970;27:339–351. doi: 10.1136/oem.27.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis DJ, Li D, Chen Z, Gibson WM, Parsons PJ. Measurement of the microdistribution of strontium and lead in bone via benchtop monochromatic microbeam X-ray fluorescence with a low power source. Journal of Analytical Atomic Spectrometry. 2009 doi: 10.1039/B820067J. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JAA, McNeill FE, Webber CE, Chettle DR. Grid search: an innovative method for the estimation of the rates of lead exchange between body compartments. Journal of Environmental Monitoring. 2005;7:241–247. doi: 10.1039/b416054a. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Preventing lead poisoning in young children [Report] U.S. Department of Health and Human Services; Atlanta, GA: 2005. [Google Scholar]
- Chettle DR. Three decades of in vivo x-ray fluorescence of lead in bone. X-Ray Spectrometry. 2005;34:446–450. [Google Scholar]
- Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskip MJ, Franklin CA, Subramanian KS, Blenkinsop J, Wandelmaier F. Sampling of cortical and trabecular bone for lead analysis: method development in a study of lead mobilization during pregnancy. Neurotoxicology. 1992;13:825–34. [PubMed] [Google Scholar]
- Jones KW, Bockman RS, Bronner F. Microdistribution of lead in bone: a new approach. Neurotoxicology. 1992;13:835–41. [PubMed] [Google Scholar]
- Jones KW, Schidlovsky G, Burger DE, Milder FL, Hu H. Distribution of lead in human bone: III. Synchrotron x-ray microscope measurements. Basic Life Sciences. 1990;55:281–286. doi: 10.1007/978-1-4613-1473-8_38. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environmental Health Perspectives. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Kim D, Galeano MA, Paul CJ, Hull AP, Morgan SP. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environ Health Perspect. 2007;115:1242–7. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons PJ, Bellis DJ, Hetter KM, Geraghty C, Berglind NA, Ginde NR, Mata P, Todd AC. An interlaboratory comparison of bone lead measurements via K-shell x-ray fluorescence. X-Ray Spectrometry. 2008;37:76–83. [Google Scholar]
- Parsons PJ, Hetter KM, Zong YY, Bellis D, Blaisdell FS, Ginde NR, Todd AC. In: Alpoim MC, Morais PV, Santos MA, Cristóvão AJ, Centeno JA, Collery P, editors. Measuring lead in bone by non-invasive K-shell X-ray fluorescence and atomic absorption spectrometry: lead distribution, homogeneity, and validation issues; Metal Ions in Biology and Medicine: Proceedings of the 9th International Symposium on Metal Ions in Biology and Medicine; May 21–24, 2006; Portugal, Europe: John Libbey, Eurotext, Lisboa; 2006. pp. 326–330. [Google Scholar]
- Parsons PJ, Reilly AA, Esernio-Jenssen D, Werk LN, Mofenson HC, Stanton NV, Matte TD. Evaluation of blood lead proficiency testing: Comparison of open and blind paradigms. Clinical Chemistry. 2001;47:322–330. [PubMed] [Google Scholar]
- Parsons PJ, Zong YY, Matthews MR. In: Predecki PK, Bowen DK, Gilfrich JV, Goldsmith CC, Huang TC, Jenkins R, Noyan IC, Smith DK, editors. Development of Bone-Lead Reference Materials for Validating in vivo XRF Measurements; Advances in X-Ray Analysis (Proceedings of the Forty-third Annual Conference on Applications of X-Ray Analysis; August 1–5, 1994; SteamBoat Springs, Colorado. New York: Plenum Press; 1995. pp. 625–632. [Google Scholar]
- Preiss IL, Washington W. Skin thickness effects on in vivo LXRF. Advances in X-ray Analysis. 1995;38:607–613. [Google Scholar]
- Rosen JF, Slatkin DN. A commentary on in vivo lead X-ray fluorescence with reference to the 1992 workshop. Neurotoxicology. 1993;14:537–540. [PubMed] [Google Scholar]
- Schidlovsky G, Jones KW, Burger DE, Milder FL, Hu H. Distribution of lead in human bone: II. Proton microprobe measurements. Basic Life Sciences. 1990;55:275–280. doi: 10.1007/978-1-4613-1473-8_37. [DOI] [PubMed] [Google Scholar]
- Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115:483–92. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AC, Carroll S, Geraghty C, Khan FA, Moshier EL, Tang S, Parsons PJ. L-shell X-ray fluorescence measurements of lead in bone: accuracy and precision. Physics in Medicine and Biology. 2002a;47:1399–1419. doi: 10.1088/0031-9155/47/8/312. [DOI] [PubMed] [Google Scholar]
- Todd AC, Chettle DR. In vivo x-ray fluorescence of lead in bone: review and current issues. Environmental Health Perspectives. 1994;102:172–177. doi: 10.1289/ehp.94102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AC, Landrigan PJ, Bloch P. Workshop on the X-ray fluorescence of lead in bone: conclusions, recommendations and summary. Neurotoxicology. 1993;14:145–154. [PubMed] [Google Scholar]
- Todd AC, Parsons PJ, Carroll S, Geraghty C, Khan FA, Tang S, Moshier EL. Measurements of lead in human tibiae. A comparison between K-shell x-ray fluorescence and electrothermal atomic absorption spectrometry. Physics in Medicine and Biology. 2002b;47:673–687. doi: 10.1088/0031-9155/47/4/309. [DOI] [PubMed] [Google Scholar]
- Todd AC, Parsons PJ, Tang SD, Moshier EL. Individual variability in human tibia lead concentration. Environmental Health Perspectives. 2001;109:1139–1143. doi: 10.1289/ehp.011091139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmers LE, Jr, Aufderheide AC, Wallgren J, Rapp G, Jr, Alich A. Lead in bone. IV. Distribution of lead in the human skeleton. Arch Environ Health. 1988;43:381–91. doi: 10.1080/00039896.1988.9935855. [DOI] [PubMed] [Google Scholar]
- Zong YY. Ph.D. Thesis. The University at Albany; Albany, New York: 1996. Atomization, determination and distribution of lead in bone by electrothermal atomic absorption spectrometry; pp. 1–184. [Google Scholar]
- Zong YY, Parsons PJ, Slavin W. Accurate and precise measurements of lead in bone using electrothermal atomic absorption spectrometry with Zeeman-effect background correction. Journal of Analytical Atomic Spectrometry. 1996;11:25–30. [Google Scholar]