Abstract
In migrating cells, the cytoskeleton coordinates signal transduction and re-distributions of transmembrane proteins, including integrins and growth factor receptors. Supervillin is an F-actin- and myosin II-binding protein that tightly associates with signaling proteins in cholesterol-rich, “lipid raft” membrane microdomains. We show here that supervillin also can localize with markers for early and sorting endosomes (EE/SE) and with overexpressed components of the Arf6 recycling pathway in the cell periphery. Supervillin tagged with the photoswitchable fluorescent protein, tdEos, moves both into and away from dynamic structures resembling podosomes at the basal cell surface. Rapid integrin recycling from EE/SE is inhibited in supervillin-knockdown cells, but the rates of integrin endocytosis and recycling from the perinuclear recycling center (PNRC) are unchanged. A lack of synergy between supervillin knockdown and the actin filament barbed-end inhibitor, cytochalasin D, suggests that both treatments affect actin-dependent rapid recycling. Supervillin also enhances signaling from the epidermal growth factor receptor (EGFR) to extracellular signal-regulated kinases 1 and 2 (ERK) and increases the velocity of cell translocation. These results suggest that supervillin, F-actin, and associated proteins may coordinate a rapid, basolateral membrane recycling pathway that contributes to ERK signaling and actin-based cell motility.
Keywords: supervillin, integrin, recycling, motility, actin, myosin, membranes
Membrane trafficking coordinates with the actin cytoskeleton during cell motility and invasion (1-4), but the molecular mechanisms are still being elucidated. The canonical events in the actin-based motility cycle are: (a) lamellipodial protrusion at the front of the cell, (b) cell-substrate attachment through integrins at focal adhesions or similar specialized sites, (c) myosin II-mediated contraction of the cell body, and (d) detachment of substrate adhesion sites at the cell rear (5-7). These cytoskeletal processes occur in conjunction with the endocytosis, scaffolding, and re-externalization of integrins, growth factor receptors, and other signaling proteins within locomoting cells (8, 9).
Membrane protein internalization and recycling takes place through multiple intracellular pathways, which are especially well-characterized for integrins (8-10). In fibroblasts, integrin-mediated adhesion regulates EGF and ERK signaling by controlling the surface organization of the EGFR and internalization of cholesterol-rich membrane microdomains called “lipid rafts” (11, 12). Slow, “long-loop” recycling of β1-integrin can be coordinated with EGFR recycling by transit through Rab4- and Rab5-associated EE/SE in the cell periphery to a Rab11-associated PNRC; recycling from the PNRC to the plasma membrane is regulated by Rab11 and Arf6 (13, 14). A rapid, “short-loop” recycling pathway involves Rab4-, Rab11-, and/or Rab-22-associated EE/SE (15). Rapid recycling also is mediated by Arf6 and its interactors and can be spatially restricted (16-20). Both slow and fast recycling pathways regulate cell motility, with β3-integrin contributing to migrational persistence, and β1-integrin promoting rapid cell migration and 3D invasion (13, 20-22).
Mechanisms of cross-regulation between trafficking membranes and the cytoskeleton are beginning to emerge. Microtubules and vesicle-associated motors control long-distance transport to and from the PNRC (13). Actin assembly promotes endocytosis, exocytosis, and membrane trafficking (11, 23), and actin tracks support myosin V-mediated rapid recycling (24, 25). Myosin II regulates membrane trafficking and exocytosis, in addition to all steps in the actin motility cycle (26-29). Yet, the intermediaries between these cytoskeletal proteins and the trafficking membranes are largely unknown.
We have been studying the membrane skeleton protein, supervillin, which regulates each step of the motility cycle. Supervillin slows the rate of cell spreading and increases myosin II contractility by scaffolding the myosin II heavy chain with the activating kinase, long (L)-myosin light chain kinase (L-MLCK) (30). Supervillin also reduces the strength of cell-substrate adhesion and decreases the formation or stability of large, mature focal adhesions through binding to the focal adhesion protein, thyroid receptor-interacting protein 6 (TRIP6)/zyxin-related protein 1 (31). In addition, supervillin increases extracellular matrix degradation, promotes the formation of invadopodia at the basal cell surface, and reorganizes the actin assembly-promoting protein, cortactin, into these structures (32). Supervillin co-isolates with signaling proteins in cholesterol-rich “lipid raft” membranes and binds tightly to F-actin, making it a candidate bridge between the cytoskeleton and membranes involved in motile processes (30, 33-35).
We report here that supervillin promotes the rapid recycling of β1- and β3-integrins through a peripheral, actin-associated endosomal compartment, without significantly affecting integrin endocytosis or recycling from the PNRC. Consistent with these results, EGFP-supervillin associates with dynamic vesicle clusters and co-localizes with markers for EE/SE and Arf6-associated membranes. Time-lapse microscopy of supervillin tagged with the photoswitchable protein, tdEos, shows both a relatively stable association with dynamic structures in the cell periphery and movements as “punctae” and “tubules” at the basal cell surface. Supervillin knockdown inhibits rapid recycling to about the same extent as does cytochalasin D, and the effects of cytochalasin D and reduced supervillin levels are not additive, suggesting that both treatments affect an actin-dependent step. Supervillin knockdown also decreases ERK phosphorylation in response to EGFR activation, and reduces nondirected cell velocity. These results suggest a role for supervillin in the cross-talk between the actin-based cytoskeleton and rapidly recycling membranes during cell movement.
Results
Supervillin associates with dynamic internal membranes (Fig. 1; Movies 1, 2)
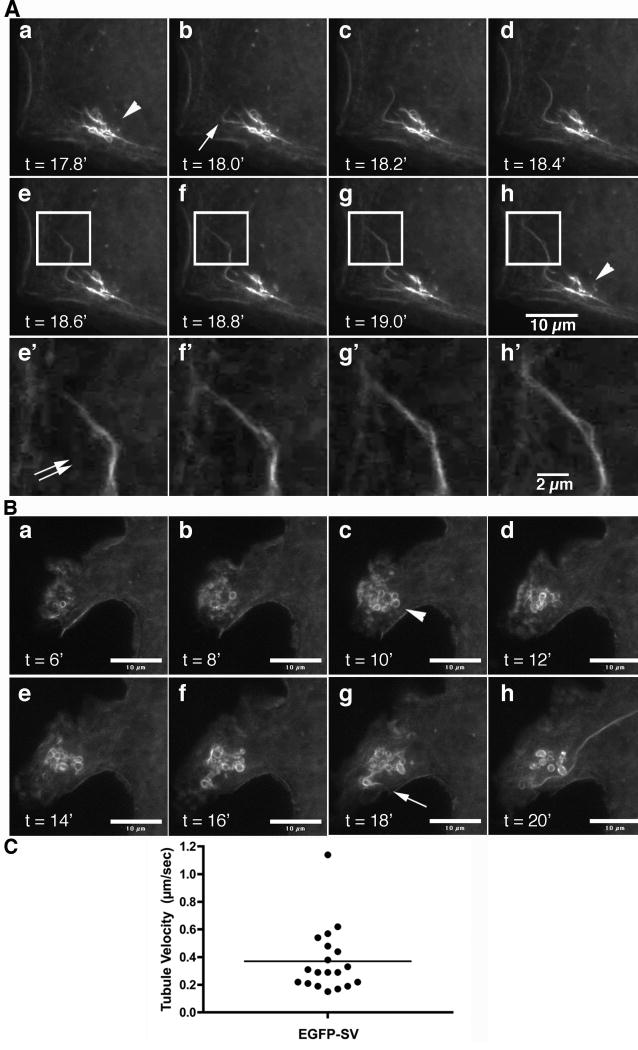
Figure 1. EGFP-supervillin associates with dynamic tubulovesicles.
EGFP-tagged supervillin in COS7-2 cells can associate with rapidly elongating tubules (arrows) that usually emerge from areas of clustered vesicles (arrowheads). As the tubules elongate, signal associated with the clusters diminishes. Locations of vesicle clusters are stable for 15-20 minutes. (A) Images from Movie 1 captured every 0.2 min. Bar, 10 μm. Enlargements (e′-h′) of the boxed regions in e-h show splaying of the elongating tubule (double arrows). Bar, 2 μm. (B) Stills from Movie 2 show large, relatively stable vesicles preceding tubule elongation, times as noted. Bars, 10 μm. (C) Instantaneous rates of tubule extension, bar indicates mean.
To visualize supervillin dynamics in vivo, we transfected COS7-2 cells with a plasmid encoding EGFP-supervillin (36). Wide-field fluorescence images were collected every 0.2 min with the focus at the ventral membrane surface (Fig. 1; Movies 1, 2). As documented previously, EGFP-supervillin localizes to peripheral bundles of F-actin and myosin II, to stress fibers, and to “punctae” with podosome/invadopodial scaffold proteins at the basolateral cell surface (30-32, 36). In addition to the rapid dynamics of the supervillin punctae (32), we found that 30-50% of COS7-2 cells expressing EGFP-supervillin contained brightly stained clusters of vesicles (Fig. 1A-B, arrowheads). In cells expressing ∼5 times endogenous levels of supervillin (31), long tubules (Fig. 1A-B, arrows) sporadically emerged from these clusters, which were usually a few micrometers behind ruffling areas of plasma membrane. Although the intensities of EGFP-supervillin at individual vesicles and the diameters of these structures were variable, their locations were usually stable for the duration of filming (15-20 min). An occasional tubule would travel towards the cell nucleus, but most trajectories ended within 10 μm of the cell margin. Instantaneous rates of tubule extension averaged 0.37 ± 0.23 μm/sec (mean ± s.d., n = 19), with a range from 0.15 to 1.14 μm/sec (Fig. 1C), consistent with a number of motile mechanisms. Changes in tubule directionality were sometimes accompanied by an apparent splaying of tubule membranes (Fig. 1A, e-h, e′-h′, double arrow), which distinguishes these structures from actin ‘comets’ (37, 38).
Supervillin moves both outward from and inward to dynamic vesicle clusters (Fig. 2; Movie 3)
Figure 2. Supervillin moves both into and out of vesicle clusters in association with dynamic tubules and punctae.
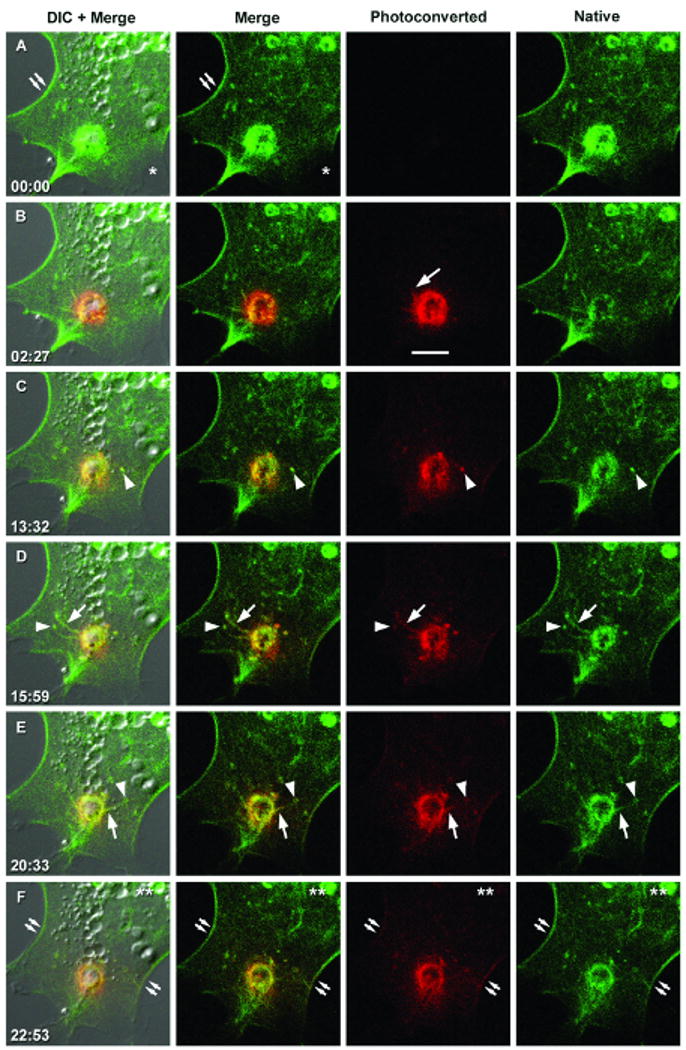
Laser scanning confocal imaging of the dynamics of tdEos-supervillin at the basolateral surface of a transfected COS7-2 cell (A) before and (B-F) at the designated times (minutes:seconds) after photoconversion of the native green tdEos fluorescence to a red fluorescent species. Images from Movie 3. Bar, 10 μm. Labels denote the positions of lateral plasma membrane filament bundles (double arrows), ruffling membrane (asterisk), presumptive membrane tubules (arrows), supervillin-associated “punctae” (arrowheads), and the direction of the cell nucleus (double asterisk).
To better examine movement directionalities, we labeled supervillin with tdEos, a green-fluorescent protein that is photoconverted to a red-fluorescent species by a pulse of 405-nm light (39). As with EGFP-supervillin (Fig. 1) (30, 32, 35), tdEos-supervillin is relatively depleted from membrane ruffles (Fig. 2A, asterisk) and concentrates with actin and myosin II filaments at plasma membrane bundles (Fig. 2A, double arrows) and with basolateral punctae, tubules, and vesicle clusters (Fig. 2B-F). Using DIC and green and red fluorescence channels, we monitored the basal ∼0.7 micron of COS7-2 cells expressing tdEos-supervillin before (Fig. 2A) and after (Fig. 2B-F) photoconversion at a dynamic vesicle cluster (Fig. 2B vs. Fig. 2A). The photoconverted red fluorescent signals were relatively stable but began to move outwards by 2.5 min, either as linear structures presumed to be membrane tubules (Fig. 2B, 2D, 2E, arrows) or as large fluorescent punctae (Fig. 2C, arrowheads). The outward-moving tubules sometimes converged in the vicinity of basolateral punctae that appeared fixed relative to the substrate but exhibited temporal fluctuations in fluorescence intensity (Fig. 2D, 2E, arrowheads). Simultaneously, green fluorescence from native tdEos-supervillin that was initially outside the illuminated spot reappeared at the vesicle clusters (Fig. 2C-2F vs. 2B). Over time, red fluorescence increased in supervillin punctae distal to the illuminated site and then appeared at plasma membrane filament bundles (Fig. 2F, double arrows). By contrast, little red fluorescence moved towards the cell nucleus over this 23-minute time course (Fig. 2F, double asterisk). These results suggest that supervillin moves both away from and into dynamic clusters of vesicles in the peripheral cytoplasm.
Supervillin localizations overlap with markers for early endosomes, but not with markers for the perinuclear recycling endosome, multivesicular bodies, or lysosomes (Fig. 3, S1, S2)
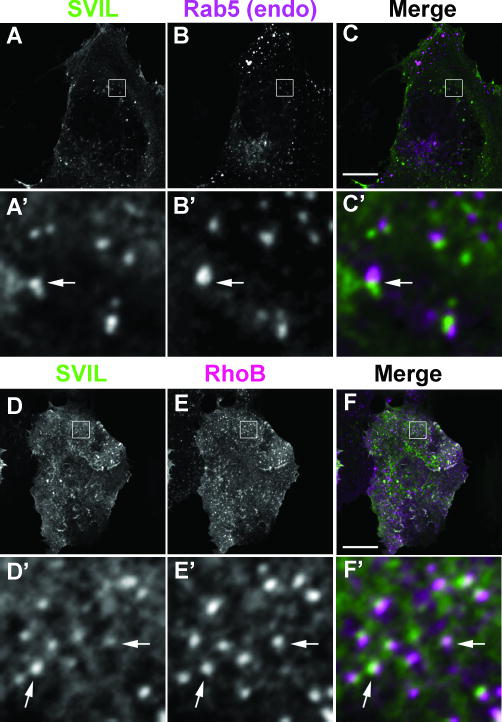
Figure 3. Supervillin localizes in proximity to Rab5 and RhoB in early endosomes.
Immunofluorescence localizations of Flag-tagged supervillin near and at early endosomes visualized with anti-Rab5 monoclonal antibody (A-C) or HA-tagged RhoB (D-F). COS7-2 cells transiently expressing very low levels of tagged protein(s) were fixed, immunostained for endogenous Rab5 and protein tags, and imaged by confocal microscopy. Pseudo-colored images show supervillin (left column, green in merge), Rab5 or RhoB (middle column, magenta in merge), and merged images (right column). Bars, 10 μm. A′ - F′ show 10× enlargements of the boxed regions in A - F. Areas of overlap (arrows) appear white.
As a first step towards identifying the vesicles associated with EGFP-supervillin, we co-expressed trace levels of tagged supervillin with endogenous or low levels of tagged proteins that mark and expand various intracellular compartments (40-44). In confocal micrographs, low levels of Flag-tagged supervillin localized in proximity to early endosomes containing endogenous Rab5 (Fig. 3A-C′) or low levels of HA-tagged RhoB (Fig. 3D-F′), both of which concentrate in early endosomes. Although we saw no significant association with the very early endosome marker, EEA1 (Fig. S1, A-C), supervillin was proximal to co-expressed Rab5 family members, Rab21, and Rab22, as well as to Rab5c (Fig. S1, D-O). Overexpression of the EE/SE marker, EGFP-Rab4, caused cell rounding and increased the numbers of supervillin punctae, many of which overlapped with Rab4 foci (Fig. S2, A-F, arrows). We further interrogated a potential interaction with Rab4 by co-expressing supervillin with mutant Rab4 proteins (45). Rab4 with a strong dominant-negative mutation in the GTP-binding site mislocalizes supervillin without significant signal overlaps (Fig. S2, G-I), suggesting that these proteins cross-talk without necessarily functioning in the same compartment. Except for strong overlaps with highly overexpressed Rab5c (Fig. S1, G-I), supervillin localizations with the EE/SE markers were proximate, rather than directly overlapping, suggesting an association with an endosome subdomain or attached cytoskeletal structure. By contrast, supervillin did not significantly co-localize with the majority of overexpressed Rab11 (Fig. S2, J-L, arrowhead) or Rab25 (not shown) at the PNRC although some proximate staining was observed in the peripheral cytoplasm (Fig. S2, L′). Neither did supervillin localize with Rab8-associated tubules (Fig. S2, M-O), structures that traffic newly synthesized proteins to the plasma membrane (46, 47), or with the predominant Rab7 signal in juxtanuclear late endosomes (Fig. S2, P-R; arrowhead). Finally, supervillin is absent from multivesicular bodies labeled with CD63 (Fig. S2, S-U, arrowhead) and from LAMP1-associated lysosomes (Fig. S2, V-X, arrowhead). Taken together, these results predicted cross-communication between supervillin and early/sorting endosome compartments.
Supervillin accumulates with β1-integrin at intracellular vesicles in the presence of a trafficking inhibitor
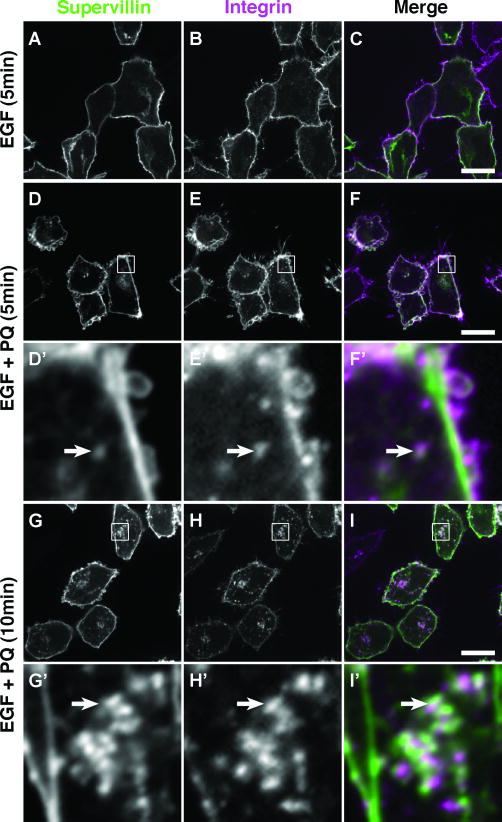
Because Rab4 and Rab5 family proteins are involved in integrin trafficking (48-53), we looked for a potential association of supervillin with endosomes containing recently internalized antibody against β1-integrin (Fig. 4). In serum-starved HeLa S3 cells stimulated for 5 minutes with EGF, EGFP-supervillin and β1-integrin antibody co-localized primarily at the plasma membrane (Fig. 4A-C). In the presence of primaquine to inhibit recycling by slowing vesicle fusion events (15, 54), EGF stimulation caused the co-accumulation of supervillin and internalized anti-β1-integrin at vesicle clusters in the peripheral cytoplasm (Fig. 4D-I), not unlike those observed with EGFP- and tdEos-supervillin (Fig. 1, 2; Movies 1-3). These results suggest associations with endosomes involved in rapid integrin recycling (55). This suggestion is supported by the overlaps of Rab5 and, to a lesser extent, Arf6 with the β1-integrin-associated endosome clusters in primaquine-treated cells (Fig. S3) and by the overlap of supervillin at and near the plasma membrane with co-expressed markers for the rapid integrin recycling pathway (Fig. S4). These partially overlapping markers include wild-type and constitutively active forms of the Arf6 GTPase (11, 16, 49, 56) (Fig. S4, A-F′), wild-type and dominant-negative phospholipase D2 (PLD2) (57, 58) (Fig. S4, G-L′), syntaxin 4 (18, 59) (Fig. S4, M-O′), and protein kinase D1 (PKD1) (55) (Fig. S4, P-R′). In the cell interior, supervillin localized in punctae proximal to endosomes enlarged by dominant-negative Arf6-T27N (Fig. S4, S-U′) and, to a much lesser extent, to punctae proximal to syntaxin 3 (Fig. S4, V-X′). By contrast, supervillin did not significantly co-localize with EHD1, or EHD3 (Fig. S4, Y-D″), which mark other intracellular trafficking pathways (59-62).
Figure 4. Supervillin localizes with β1-integrin at the plasma membrane and at intracellular vesicles after inhibition of membrane recycling.
HeLa S3 cells stably expressing EGFP-supervillin were serum-starved, incubated at 4°C with anti-β1-integrin antibody for 1 hour, washed, and stimulated with warm 0.1 μg/ml EGF in DMEM alone (A-C) or in the presence of the membrane-trafficking inhibitor, primaquine (PQ, 120 μM), for 5 min (D-F) or for 10 min (G-I). Cells were fixed, stained with secondary antibody, and visualized by confocal microscopy. Pseudo-colored images show EGFP-supervillin (left column, green in merge), β1-integrin (middle column, magenta in merge), and merged images (right column). Bars, 10 μm. D′ – F′ and G′ – I′ show 10× enlargements of the boxed regions in D – F and G – I, respectively. Areas of overlap (arrows) appear white.
Endogenous supervillin reduces the net uptake of integrins (Fig. 5)
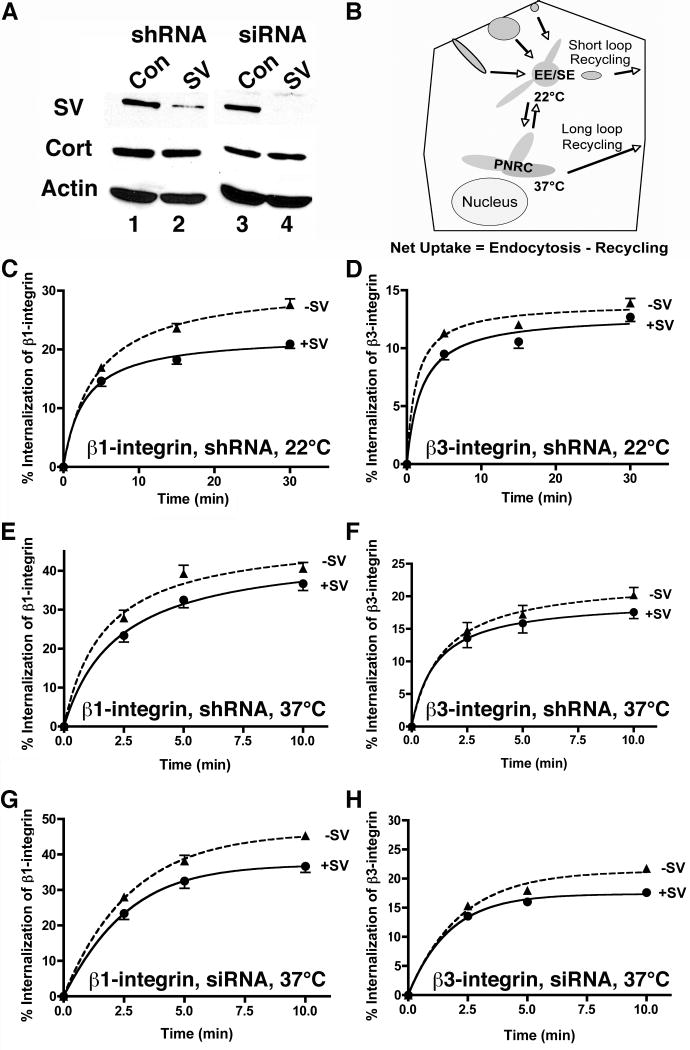
Figure 5. Supervillin knockdown increases the net uptake of both β1-integrin and β3-integrin.
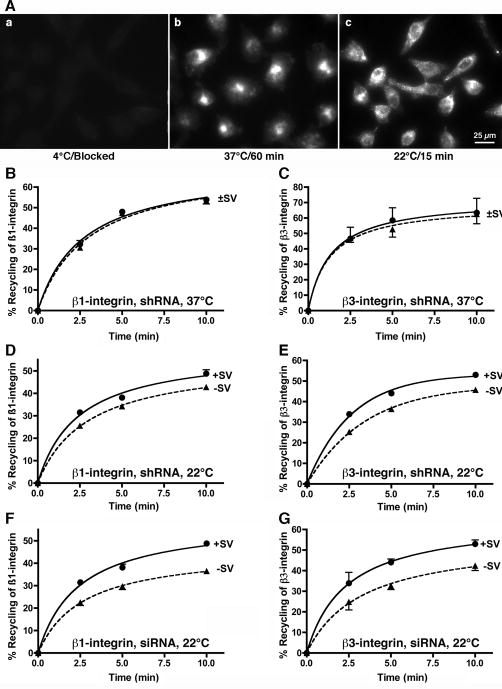
(A) HeLa S3 cells were stably (lanes 1,2) or transiently (lanes 3,4) transfected with a control shRNA plasmid (Con, lane 1), shRNA targeting supervillin nucleotides 2657-2677 (SV, lane 2), control dsRNA (Con, lane 3), or dsRNA targeting supervillin nucleotides 1680-1704 (SV, lane 4). Residual supervillin was quantified relative to cortactin (Cort) and β-actin (Actin). (B) Diagram showing integrin trafficking pathways. Integrins preferentially collect in early/sorting endosomes (EE/SE) after a 15-min incubation at 22°C and at the perinuclear recycling center (PNRC) after a 1-h pre-incubation at 37°C. Net uptake occurs when the rate of endocytosis exceeds the combined rates of fast recycling from EE/SE and slow recycling from the PNRC. (C-H) Serum-starved HeLa S3 cells stably expressing control (+SV) or supervillin-specific (-SV) shRNA (panels C-F) or transfected with dsRNA (panels G, H) were assayed for net uptake of β1-integrin (panels C, E, G) and β3-integrin (panels D, F, H) at both 22°C and 37°C in the presence of EGF (0.1 μg/ml). Means ± s.d. (n = 3 experiments, 3 replicates/experiment); the sizes of the symbols (➂, control; ➉, supervillin-specific) eclipse some error bars. Experiments were performed on (C, D) 3 or (E-H) 2 different days. All differences are statistically significant, p < 0.05.
To directly examine the role of supervillin in integrin trafficking, we analyzed HeLa cell lines that either stably expressed a control or supervillin-specific shRNA (SV, Fig. 5A, lanes 1, 2) or had been transfected twice over 4 days with control or supervillin-specific double-stranded RNAi (SV, siRNA) against a different supervillin sequence (Fig. 5A, lanes 3, 4). Based on densitometric comparisons with cortactin and β-actin loading controls, only ∼20% and ∼10% of endogenous supervillin remained in cells treated with shRNA or siRNA, respectively (Fig. 5A, lanes 2, 4). We followed the net uptake of surface-biotinylated β1-integrin and β3-integrin over time with capture-ELISA assays (15). Cell surface proteins were biotinylated at 4°C with a cleavable reagent (sulfo-NHS-SS-biotin), and the cells were washed to remove excess reagent and warmed to 22°C or 37°C in the presence of EGF to initiate endocytosis and recycling. We removed biotin remaining at the cell surface with the cell-impermeable reducing agent, MesNa, and measured the relative amounts of cell-associated, biotinylated integrins by capture with specific antibodies in ELISA wells. Levels of associated biotin were quantified with horseradish peroxidase-coupled streptavidin (14, 15, 63). Net uptake, defined as the difference between the rates of endocytosis and recycling (Fig. 5B), was assayed at both 22°C (Fig. 5C-D) and 37°C (Fig. 5E-H). At both temperatures and for both β1- and β3-integrins, we observed a significant increase of ∼15% in net uptake in cells in which supervillin levels were stably or transiently reduced (Fig. 5C-H; -SV vs. +SV). These results suggest that endogenous supervillin either inhibits an endocytic pathway or promotes exocytic recycling of integrins from an internal cell compartment.
Supervillin has no effect on integrin endocytosis (Fig. 6)
Figure 6. Integrin endocytosis in the absence of membrane recycling is independent of supervillin.
In the presence of 30 μM primaquine to inhibit recycling, serum-starved HeLa S3 cells stably transfected with control (➂, solid lines) or supervillin-specific shRNA (➉, dashed lines) exhibited identical rates of endocytosis of β1-integrin (A, C) and β3-integrin (panels B, D) at both 22°C (panels A, B) and 37°C (C, D). Means ± s.d. (n = 3 experiments, 3 replicates/experiment); some error bars are eclipsed by the size of the symbols. Experiments were performed on (A, B) 2 or (C, D) 3 different days. No differences are statistically significant.
To look for effects of supervillin knockdown on endocytosis alone, we measured the net uptake of biotinylated integrins in the presence of primaquine, which halts recycling pathways (54, 55). Primaquine treatment increased the net uptake of both β1- and β3-integrins at both 22°C (Fig. 6A-B) and 37°C (Fig. 6C-D), as compared with net uptakes in its absence (Fig. 5C-D and Fig. 5E-F, respectively). However, no difference between control and supervillin-knockdown cells was observed for integrin uptake in the absence of recycling (Fig. 6A-D; ±SV), indicating that supervillin has no effect on integrin endocytosis.
Supervillin enhances integrin recycling from peripheral endosomes, but not from the PNRC
The two major recycling compartments within cells are the PNRC, into which most integrins become sequestered after 1 h of internalization at 37°C (Fig. 5B; Fig. 7A, panel b) (14), and the more peripheral EE/SE, which are preferentially populated after 15 min of uptake at 22°C (Fig. 5B; Fig. 7A, panel c) (15). The steady-state levels of total and surface integrin (Fig. S5), i.e. during serum starvation, and the intracellular localizations of Rab5 and β1-integrin (not shown) are not significantly different in the absence of endogenous supervillin, suggesting no gross effects of supervillin depletion on integrin levels or endosome populations. After pre-loading the PNRC or EE/SE with surface-biotinylated integrins, we initiated recycling by adding media containing EGF at 37°C. Biotinylated integrins that returned to the cell surface were removed by MesNa cleavage. We quantified recycling as the percentage of the initially internalized integrin-associated biotin that became cleavable after EGF addition. Depletion of endogenous supervillin had no effect on the recycling of either β1- or β3-integrin internalized at 37°C (Fig. 7B-C, ±SV), indicating no effect of supervillin on the slow, “long-loop” recycling from the PNRC (48, 64, 65). By contrast, recycling of both β1- and β3-integrin from the 22°C compartment was significantly inhibited by supervillin knockdown (Fig. 7D-G, -SV). HeLa S3 cells with ∼20% of endogenous supervillin exhibited maximal differences of 19-25% at 2.5 min after onset of recycling (Fig. 7D-E). Similar reductions of 23-27% were observed with cells containing only ∼10% of endogenous supervillin (Fig. 7F-G). These results suggest that supervillin plays a role in fast integrin recycling from a population of EE/SE (49, 66, 67).
Figure 7. Supervillin promotes recycling from early/sorting endosomes (EE/SE), but not from the perinuclear recycling center (PNRC).
(A) Visualization of the cellular compartments involved in integrin recycling. Serum-starved HeLa S3 cells were incubated at 4°C for 1 h with monoclonal β1-integrin IgG, washed, and incubated at the specified temperatures to (a) retain primary antibody at the cell surface (4°C, 1 h) or to internalize the β1-integrin and bound IgG into (b) the PNRC (37°C, 60 min) or (c) peripheral EE/SE (22°C, 15 min). Surface-bound IgG was blocked with unlabeled goat anti-mouse secondary IgG, the cells were fixed and permeabilized, and the internalized anti-β1-integrin IgG was visualized with fluorescently labeled goat anti-mouse IgG. Recycling assays were performed after similar internalizations of surface-biotinylated proteins in serum-starved HeLa S3 cells that (B-E) stably expressed control (➂, solid lines) or supervillin-specific (➉, dashed lines) shRNAs or that (F-G) had been pre-treated with dsRNAs (siRNA) against a different supervillin sequence. Biotinylated proteins were internalized into either (B, C) the PNRC or (D-G) EE/SE. Recycling was initiated by adding media with 0.1 μg/ml EGF at 37°C, and the amounts of internalized β1- and β3-integrins were quantified by capture ELISAs after the indicated times, as described in Materials and Methods. Means ± s.d. (n = 3 experiments, 3 replicates/experiment); some error bars are eclipsed by the size of the symbols. Experiments were performed on (B-E) 3 or (F, G) 2 different days. (B, C) no differences are statistically significant; (D, E, F) p < 0.001; (G) p ≤ 0.05.
Supervillin promotes cytochalasin D-sensitive integrin recycling
The actin-polymerization inhibitor, cytochalasin D (68), inhibits a fraction of the recycling of β1-integrin, the EGFR, and β-adrenergic receptors (14, 24, 69). Because supervillin binds directly to F-actin and regulates actin organization (33), we investigated the relationship between decreased recycling mediated by cytochalasin D and by supervillin knockdown. As reported previously for the β-adrenergic receptor (24), we found no difference in net uptake of β1-integrin in the presence or absence of cytochalasin D (not shown). We also found that a ∼80% decrease in supervillin levels is ∼78% as effective at reducing the rate of β1-integrin recycling from EE/SE at 2.5 minutes after EGF addition as is treatment with 1 μM cytochalasin D (Fig. 8, -SV). Furthermore, the rate of recycling from EE/SE is independent of the presence of supervillin in cytochalasin D-treated cells at all times examined (Fig. 8, ±SV + CD). The lack of synergy between cytochalasin D and supervillin knockdown strongly suggests that both treatments affect the same recycling pathway.
Figure 8. Supervillin knockdown and cytochalasin D apparently inhibit the same recycling pathway.
Serum-starved HeLa S3 cells pre-treated with control (circles, +SV) or SV-specific siRNA (triangles, -SV) were surface biotinylated, incubated at 22°C for 20 min with or without 1 μM cytochalasin D (open symbols, ±SV + CD) to preferentially internalize membranes into EE/SE, cleared of surface biotin, and assayed for EGF-induced recycling of β1-integrin. Means ± s.e.m. (n = 3 experiments, 3 replicates/experiment). Some error bars are eclipsed by the size of symbols. All differences are significant (p < 0.001), except for those between the −SV data and the CD-treated samples at 2.5 min and for the CD-treated samples +/- SV at all time points (p > 0.05).
Supervillin increases ERK signaling downstream of EGFR activation
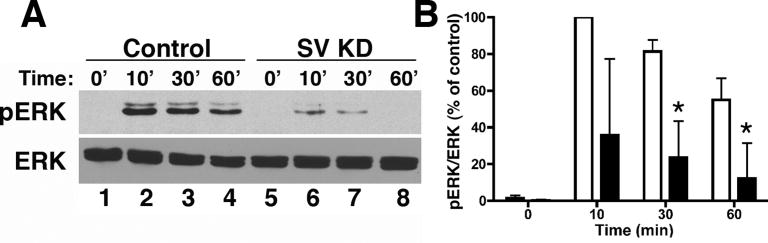
ERK activation is synergistically promoted by cross-talk between integrins and receptor protein tyrosine kinases, including the EGFR (70, 71). Mechanisms include the coordinated recycling of β1-integrin and EGFR and the scaffolding of the EGFR with integrin or F-actin at the cell surface or within endosomes (8, 72, 73). Because the larger, smooth muscle isoform of supervillin has been proposed as a scaffold for stimulus-mediated ERK signaling (74), we investigated EGF-mediated ERK activation in cells with reduced levels of supervillin (Fig. 9A-B). We found that supervillin knockdown enhanced EGFR uptake at 37°C by 17% to 23% (not shown), comparable to results obtained for β1- and β3-integrins (Fig. 5). By contrast, ERK signaling (pERK/ERK ratio) in response to EGF addition was inhibited by at least 48-73% in supervillin-knockdown cells; differences were especially apparent 30-60 min after EGF addition (Fig. 9). These results are comparable to those observed after reduction of the larger supervillin isoform in aorta cells (75), suggesting that both nonmuscle and smooth muscle isoforms of supervillin promote ERK signaling.
Figure 9. Supervillin increases EGF-stimulated ERK activation.
(A) Immunoblot of cell lysates from HeLa S3 cells stably expressing control (lanes 1-4) or supervillin (SV)-specific shRNA (lanes 5-8). Cells were serum-starved and stimulated with EGF at 37°C. Lysates were harvested at 0 min (lanes 1,5), 10 min (lanes 2,6), 30 min (lanes 3,7), or 60 min (lanes 4,8), resolved by SDS-PAGE, and immunoblotted for phosphorylated ERK (pERK) and ERK. (B) ERK and pERK were quantified, and pERK/ERK ratios were normalized to the maximal control pERK/ERK ratio at 10 min. Differences at 30 min and 60 min are statistically significant by two-tailed unpaired t tests; means +/- s.d., p < 0.05 (n = 3).
Supervillin contributes to rapid cell motility
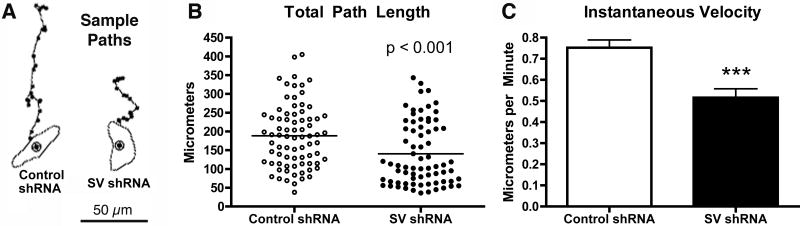
The rapid recycling pathway and ERK activation are both linked to increased rates of cell translocation (20, 76-78). In previous work, we showed that supervillin regulates cell surface protrusion, substrate adhesion, and myosin II contractility (30, 31). To determine the effects of supervillin knockdown on overall cell translocation, we analyzed the random motility of HeLa S3 cells containing control or supervillin-specific shRNA (Fig. 10; Movies 4, 5). We traced the outlines of individual cells every 10 minutes for ∼4 hours and plotted the paths of their centroids over time (Fig. 10A). These paths were analyzed for differences in motile parameters using Dynamic Image Analysis Software (79). Supervillin knockdown cells traveled only ∼74% of the distances covered by control cells (Fig. 10B), with total path lengths of 140 μm ± 10 μm (n = 72) vs. 188 μm ± 10 μm (n = 77) for controls (means ± s.e.m.; p < 0.001). Also, the mean instantaneous velocity of 0.52 μm/min ± 0.04 μm/min of the supervillin-depleted cells was 32% lower than the mean velocity of 0.75 μm/min ± 0.04 μm/min (p < 0.001) exhibited by control cells (Fig. 10C). The median path length and median instantaneous velocity of supervillin-knockdown cells (106.6 μm, 0.40 μm/min) were even lower than the corresponding mean values, whereas the median path length and velocity for control cells (186.8 μm, 0.73 μm/min) were nearly the same as the means. These results suggest that variable amounts of residual supervillin in the knockdown cells may have partially masked differences as large as 57%. Statistically significant, but minor, differences were observed for net path length, cross-sectional area, and magnitude of directional change, with supervillin-depleted cells exhibiting 24% decreases (p < 0.05), 16% decreases (p < 0.01), and 14% increases (p < 0.05), respectively, in these parameters. The persistence of migration in a single direction, i.e. speed divided by direction change (79), was not significantly affected. These results indicate that supervillin affects the rate at which cells translocate but not their migrational persistence.
Figure 10. Supervillin-depleted cells are less motile.
(A) Sample paths of the centroids of representative HeLa S3 cells containing control or supervillin (SV)-specific shRNA. These cells exhibited total path lengths of 196 μm (control) and 107 μm (SV-specific) and instantaneous velocities of 0.73 μm/min (control) and 0.40 μm/min (SV-specific), values near the medians for each group. Initial cell outlines with centroids are at the bottom; subsequent centroid locations are shown as dots. Bar, 50 μm. Means ± s.e.m. for (B) total path lengths and (C) instantaneous velocities. ***, p < 0.001; n = 77 cells (control shRNA), 72 cells (SV shRNA) in 12 fields from 2 experiments.
Discussion
We report here that the lipid raft-associated protein, supervillin, increases the rate of rapid integrin recycling (Fig. 5, 6, 7), a result that agrees with the observed co-localizations of supervillin with and near markers for early and sorting endosomes (Fig. 3, 4, S1, S2, S3). The movements of supervillin-associated tubules away from vesicle clusters and towards basolateral punctae and the cell periphery also are consistent with this conclusion (Fig. 1, 2; Movies 1-3). Similar outward movements have been seen by immunostaining of endogenous levels of the smooth muscle isoform of supervillin after agonist stimulation of aorta cells (75). Although this previous study did not address whether the motile structures were membrane- or actin-based, our current results suggest that supervillin-associated movements may involve both intracellular membranes and associated cytoskeletal proteins.
As has been reported for other overexpressed endosome-associated proteins (18, 44, 80, 81), the average ∼5-fold increased expression of fluorescent supervillin needed for time-lapse microscopy (31) may affect endosome morphology by increasing tubulation. Signal overlaps with endogenous Rab5 in COS7 cells and with internalized integrin in primaquine-treated HeLa S3 cells appear mostly with vesicular, not linear, supervillin structures (Fig. 3, 4), many of which are probably stress fibers or peripheral actomyosin filament bundles (30, 31, 36). Conversely, supervillin appearance is altered after co-expression with some endosomal proteins. The numbers of intracellular supervillin punctae proximal to vesicles increase after co-expression of RhoB, Rab5, Rab21, Rab22, Rab4, or dominant-negative Arf6-T27N (Fig. 3, S1, S2, S4). Similarly, the preferential association of supervillin at the cell edge with constitutively activated Arf6, PLD2, syntaxin 4, or PKD1 (Fig. S4) may be related to the changes in membrane trafficking induced by these proteins (55, 82-85). Thus, supervillin both redistributes within the cell in response to alterations in EE/SE trafficking and potentially affects membrane equilibria by inhibiting endosome fission or by promoting tubulation of recycling vesicles.
Supervillin appears to selectively regulate actin-dependent rapid recycling because cytochalasin D and supervillin knockdown have comparable effects at early times after EGF stimulation and because supervillin knockdown exerts no additional effect on recycling beyond that of cytochalasin D alone (Fig. 8). Both supervillin knockdown and cytochalasin D treatment maximally inhibit only 25-30% of the recycling from peripheral endosomes. Partial inhibition of recycling is expected because multiple trafficking pathways exist, and inhibition of one pathway can cause preferential shifts into alternative routes (8, 17, 86-88). Even without drug treatment, most supervillin associated with vesicle clusters is not rapidly diffusible (Fig. 2, Movie 3), as is expected for a protein with multiple attachments to the actin cytoskeleton (30, 31, 33). These results suggest that supervillin is part of an actin-associated cytoskeleton that regulates peripheral membrane recycling in a pathway that communicates with Rab4 and Rab5 endosomes, but is distinguishable from them.
In other major findings reported here, we show that supervillin enhances ERK phosphorylation downstream of EGF receptor activation (Fig. 9) and increases the rate of cell translocation (Fig. 10). These observations are likely to be functionally related because ERK activation increases the rate of cell migration (76, 89, 90). One possibility is that nonmuscle supervillin is an essential component of an ERK-activating scaffold, as has been proposed for the smooth muscle splice-form of this protein (74). Multiple ERK scaffolds, including many at endosomes, control ERK interactions with substrates (78). An association of supervillin with ERK scaffolds could facilitate ERK regulation of L-MLCK and myosin II (76, 89, 90), both of which bind directly to supervillin (30, 33). Myosin II activation, in turn, increases the rates of cell motility, vesicle trafficking, and exocytosis (26, 29, 91).
The supervillin-associated cytoskeleton also might increase the rate of cell motility in response to growth factors by accelerating the recycling of activated integrins and signaling proteins at the cell anterior (20). Rapid recycling of the EGFR, which can be coordinated with β1-integrin trafficking, prolongs the duration of ERK signaling and promotes increased rates of random cell motility (13, 92, 93). ERK activation, in turn, promotes rapid recycling through an actin- and Arf6-associated pathway that promotes localized retention of integrins and active GTPases in lamellipodia (19, 40, 94). This hypothesis is consistent with the effect of supervillin on net EGFR uptake and with the observed proximities of supervillin to Arf6, Rab5, Rab22, and other proteins in the lamellipodial recycling pathway (Fig. 3, S1-S4) (8, 9).
However, supervillin probably does not facilitate integrin recycling directly to lamellipodia. First, ruffling membranes contain comparatively little supervillin (Fig. 1, 2; Movies 1-3) in the absence of co-overexpression with an activator of the Arf6 pathway (Fig. S4). Second, any supervillin-mediated increases in surface integrins are transient because steady-state levels of surface integrins are unaffected by supervillin knockdown (Fig. S5). Finally, the formation of lamellipodia is inversely related to the level of supervillin, which binds directly to the lamellipodial protein, cortactin, and reorganizes cortactin into punctae and apparent precursors of invadopodia near and at the basal cell membrane (32).
The basolateral cell surface is a much more feasible destination for the supervillin-mediated recycling pathway. Much outward trafficking of supervillin from vesicle clusters occurs near the basal cell surface, and tubules often appear to terminate at or near stationary basal punctae (Fig. 2, Movie 3), which are biochemically and morphologically similar to podosomes and invadopodial precursors (32, 95). Both β1- and β3-integrins associate with basally localized invadopodia (96, 97). Arf6 and cortactin, both of which conditionally co-localize with supervillin (Fig. S4) (32), also promote invadopodia formation (4, 98, 99) as well as endosome trafficking (56, 69). Co-overexpression experiments also show extensive overlaps between supervillin and the basolateral targeting protein, syntaxin-4, but not with apically targeted syntaxin-3 (Fig. S4) (100). Furthermore, supervillin enhances the formation of invadopodia, the degradation of extracellular matrix, and matrix invasion (32). All of these observations suggest preferential functioning at the basal cell surface.
The mechanism for supervillin facilitation of rapid recycling may involve interactions with actin nucleators at endosomes or the plasma membrane. Actin dynamics have been implicated in receptor endocytosis, endosome trafficking, vesicle fission, intracellular sorting, and the stabilization of sites of exocytosis at the plasma membrane (23, 101-104). Cortactin-mediated actin assembly is specifically implicated in EGFR recycling, growth factor signaling, and invadopodia formation (69, 95, 105, 106). Because ERK phosphorylation induces cortactin-mediated actin polymerization and the formation of invadopodia (107, 108), supervillin-mediated activation of ERK could lead to the targeting of cortactin-associated endosomes to invadopodia, as well as to enhanced myosin II contractility.
Most likely, the roles of supervillin in promoting rapid integrin recycling, ERK activation, cell translocation, myosin contractility, and matrix degradation are intertwined during the invasive spread of tumor cells. Supervillin may coordinate myosin II activation with vesicle trafficking or exocytosis (27, 28, 109), facilitate the translocation of both integrins and cortactin to the basal cell surface (32), and help anchor both myosin II and actin filaments in proximity to membrane-based signals (34). In this context, it is notable that myosin II and MLCK also are implicated in matrix degradation by invadopodia (110). We conclude that the lipid raft-associated protein, supervillin, is uniquely positioned to coordinate membrane trafficking with actin- and myosin II-based cytoskeletal functions during cell motility and invasion.
Materials and Methods
Antibodies and Plasmids
Antibodies were purchased as follows: anti-ß1-integrin (clone TS2/16) from Biolegend (San Diego, CA); anti-ß1-integrin (3H1192) from Santa Cruz Biotechnology, Inc.; anti-ß3-integrin (2C9.G2), anti-Arp3 (Clone 4), anti-Rab5 (Clone 1/Rab5) and anti-EEA1 (clone 14) from BD Biosciences (San Jose, CA); anti-cortactin (4F11), anti-ERK1/2, and anti-actin (C4) antibodies from Millipore Corporation (Bedford, MA); anti-CD63 (H5C6) from the Developmental Studies Hybridoma Bank (Iowa City, IA); anti-Flag M2 (F7425) and streptavidin-peroxidase polymer (S2438) from Sigma (Saint Louis, MI); anti-HA-Tag (262K), anti-EGFR, anti-phospho-EGFR (Tyr1045), and anti-phospho-ERK1/2(Thr202/Tyr204) from Cell Signaling (Danvers, MA, E10). Affinity-purified rabbit polyclonal anti-supervillin IgG (H340) has been described (34, 111), as has cloning of the full-length supervillin cDNA into pEGFP-C1 (Clontech) (36). Plasmids encoding tdEos- and mRFP-tagged supervillin were constructed by excising the EGFP coding sequence between the NheI and KpnI restriction sites in pEGFP-supervillin and substituting cassettes encoding tdEos (112) or mRFP after PCR amplification from a cassette generously provided by Dr. Roger Tsien (Howard Hughes Medical Institute, UCSD) (113). Flag-tagged supervillin plasmid was constructed in two steps by sequentially excising the coding sequence at the EcoRI / XbaI and EcoRI sites and cloning into the EcoRI / XbaI and EcoRI sites of p3XFLAG-myc-CMV24 (Sigma, E6401). EGFP fusions with human Rab4a-WT, murine Rab5c-WT, murine Rab7-WT, human Rab21-WT, and murine Rab22a-WT (114) were generated by PCR amplification with Vent polymerase (New England Biolabs), restricted with BamH1 and SalI, and subcloned in frame into the BglII/SalI restriction sites in pEGFP-C vectors (Clontech). Site-specific mutations were performed using the QuickChange kit (Stratagene). Plasmids encoding the short-loop trafficking markers, GFP-Rab4-S22N, YFP-Rab4-N121I, HA-Rab25, and GFP-PKD1, were the kind gift of Dr. Jim Norman (Beatson Institute for Cancer Research) (15, 40, 55, 115). Other plasmids used as markers were generously supplied as follows: mCherry-Rab8a-WT from Dr. James Goldenring (Vanderbilt-Ingram Cancer Center) (116); Rab11-WT (Addgene plasmid 12674) from Dr. Richard Pagano (Mayo Clinic) (117); LAMP1-RFP (Addgene plasmid 1817) from Dr. Walther Mothes (Yale U. Sch. Med.) (118); EGFP-mPLD2 and EGFP-mPLD2-K758R from Dr. Michael A. Frohman (SUNY, Stonybrook) (119), myc-EHD1 and myc-EHD3 from Dr. Steven Caplan (U. Nebraska Medical Center) (116), myc-tagged syntaxin 3 (Addgene plasmid 12372) and syntaxin 4 (Addgene plasmid 12377) from Dr. Thomas Weimbs (UC, Santa Barbara) (59, 120); 3xHA-tagged RhoB from UMR cDNA Resource Center; and HA-tagged wild type (WT) Arf6 (Addgene plasmid 10834), constitutively active HA-Arf6-Q61L (Addgene plasmid 10835) and dominant-negative HA-Arf6-T27N (Addgene plasmid 10831) from Dr. Thomas Roberts (Florida State) (121).
Cells and Tissue Culture
COS7-2 cells were grown and transfected in 6-well dishes for 24 hours with 0.4-1.0 μg/well EGFP- or mRFP-tagged supervillin and/or with 0.1-0.3 μg/well marker plasmids (31). HeLa S3 Tet-Off cells (Clontech) were maintained in DMEM (Gibco), 10% fetal calf serum. For RNAi, HeLa S3 cells were transfected with Lipofectamine 2000 (Invitrogen) and a Stealth dsRNA (Invitrogen) targeting human supervillin coding nucleotides 1680-1704 (hSV1); the Stealth duplex sequence, 5′-CAGAAUAAAGGAUCUAUAAUCCGCU-3′, was used as a control (30). Supervillin depletion required two dsRNA treatments over 4 days: ∼50% confluent cells were transfected with 10 nM dsRNA for 2 days, split 1:3, re-transfected with 10 nM dsRNA, split again 8-10 hours later, and used in experiments after another 40-48 hours of growth. Stable HeLa S3 cell lines (Clontech) expressing control or supervillin-specific shRNA or tet-regulated EGFP-supervillin were created by 24-hr transfections of 3 × 105 cells with Effectene (Qiagen) and 0.5 μg of pSM2C/control or pSM2C/SH2533-H1 (supervillin coding nt 2657-2677, Open-Biosystems), or pTRE2pur/EGFP-supervillin plasmid DNA. Cells were selected for 2 weeks in 10-cm plates with 3 μg/ml puromycin, 10% FBS, DMEM. Colonies were isolated and identified for depletion of supervillin by immunoblotting with H340 antibody. Individual colonies were expanded and used for ≤10 passages.
Live Cell Imaging and Motility Assays
COS7-2 cells transfected with EGFP-supervillin were imaged in DMEM, 10% FCS, 10 mM HEPES, without phenol red, with a 63× HCX PL Apo (1.32 NA) oil-immersion objective lens on a DMIRE 2 inverted microscope with a mechanical stage (Leica), a Retiga Exi cooled CCD camera (QImaging) and Openlab 3.5.2 software (Improvision). After temperature equilibration for ≥2 hr in a Plexiglass environmental chamber heated to 37°C, 100 images were acquired for EGFP every 12 s with the initial focus on the ventral cell surface. Images were processed with Openlab to enhance contrast and to generate movie files with intervals of 0.2 s (Movie 1, 60× real time) or 0.5 s (Movie 2, 24× real time). Stills were exported to Adobe Photoshop, where regions of interest were sized and additionally contrast enhanced.
Translocation of stably transfected HeLa S3 cells was followed similarly. Cells (∼1 × 104/ml) were plated on coverslips pre-coated with 10 μg/ml fibronectin (Sigma). After temperature equilibration, fields of cells were selected and imaged by DIC every 10 min for ∼4 h with a 10× objective lens (NA 0.30) and 1.5× tube magnification. Movies were generated using QuickTime (Apple Inc). Cell outlines were traced, and migration parameters were determined using DIAS software (79, 122). Cells that contacted another cell or left the field of view during filming were excluded from analysis.
In photoconversion experiments, COS7-2 cells expressing low levels of tdEos-supervillin were imaged at 37°C with a 40× UPlanapo (1.0 NA) oil-immersion objective lens on an Olympus FluoView FV1000 confocal laser scanning microscope using a prism-based spectral detector. The green emission channel (native tdEos) was recorded over a bandwidth of 500-530 nm whereas the red emission channel (photoconverted tdEos) was recorded over a bandwidth of 555-655 nm. A 3.5-second laser pulse at a power of 45 μW from the 405-nm SIM (photoactivation) scanner in tornado mode was used to photoconvert tdEos from its native state of green fluorescence to a red-fluorescent species (112). DIC and fluorescence images were simultaneously collected every 7 seconds for 23 min, and assembled using Adobe Photoshop, Premier, and After Effects software.
Antibody Internalization and Cell Imaging
Endocytosis of ß1-integrin was followed by light or confocal microscopy, as described (14). HeLa S3 cells, with or without EGFP-supervillin expression, were grown on glass coverslips for 48 hours and serum-starved overnight in DMEM with 0.01% BSA (DMEM/BSA). Antibody against ß1-integrin was diluted in DMEM/BSA to 10 μg/ml and allowed to bind to surface integrin for 1 h at 4°C. Excess antibody was removed with 2 rapid washes of cold DMEM/BSA. Cells were incubated in pre-warmed DMEM/BSA for 1 hour at 37°C or for 15 min at 22°C and fixed. For experiments with primaquine, cells were incubated at 37°C for 5 or 10 min in pre-warmed DMEM/BSA containing 0.1 μg/ml EGF with or without primaquine (120 μM), then rapidly washed by DPBS and fixed with 4% paraformaldehyde, as indicated.
HeLa and COS7-2 cells were fixed for 15 minutes with 4% paraformaldehyde in PBS, and permeabilized for 5 min with 0.1% Triton X-100 in PBS before blocking for 30 min with 1% BSA, 0.5% Tween-20 in PBS and immunostaining. For wide-field fluorescence, slides were analyzed with a 40× (N.A. 0.90) or 100× (NA 1.3) Plan-NeoFluar oil immersion objective on a Zeiss Axioskop fluorescence microscope with a RETIGA 1300 CCD camera (QImaging) and OpenLab 3.5.2 software. Confocal images were obtained with a 60× Plan Apo objective lens (N.A. 1.4) on a Nikon TE-2000E2 inverted microscope (Nikon Instruments, Melville, NY) with a Yokogawa CSU10 Spinning Disk Confocal Scan Head (Solamere Technology Group, Salt Lake City, UT), Rolera-MGi Plus camera (QImaging), and MetaMorph 7.6 software. Images were adjusted uniformly for contrast and brightness, and merged images were assembled using Adobe Photoshop or ImageJ 1.42l (rsb.info.nih.gov/ij). Co-localization was quantified using ImageJ with the Intensity Correlation Analysis plug-in (www.macbiophotonics.ca/imagej/). Co-localizations were accepted as representing significant overlap if the mean Pearson's correlation coefficient from 4-5 cells was 0.6 or higher, on a scale of -1 to 1 (123), and if rotation of one image by 90° significantly reduced the degree of overlap (p < 0.05, n = 5); the images shown are representative. Enlarged images were re-adjusted uniformly for contrast and brightness.
Biochemical Uptake and Recycling Assays
Following established protocols (14, 15, 63, 124), HeLa S3 cells starved for 6 hours in serum-free DMEM were plated onto plates pre-coated with 10 μg/ml fibronectin. Cells were washed twice with cold PBS, and their surfaces were labeled for 30 min at 4°C in PBS with 0.2 mg/ml sulfo-NHS-SS-biotin (Pierce). Labeled cells were washed rapidly with cold PBS, and internalization of surface biotin was followed after transfer to DMEM with or without 0.1 μg/ml EGF (Sigma) in the absence or presence of 300 μM primaquine to inhibit membrane recycling (54). At indicated times, cells were washed twice with ice-cold PBS. Biotin remaining on cell surfaces was removed by 2 treatments for 10 min at 4°C with 20 mM 2-mercaptoethanesulfonic acid (MesNa, Sigma) in 100 mM NaCl, 50 mM Tris, pH 8.6. Unreacted MesNa was quenched for 10 min at 4°C with 20 mM iodoacetamide (Sigma) in 150 mM NaCl, 50 mM Tris, pH 8.0. Cells were lysed by RIPA lysis buffer containing 1% NP-40, 1% sodium deoxycholate, 150 mM NaCl, 50 mM Tris, pH 7.4, 1.5 mM Na3VO4, 5 mM EGTA, 50 μg/ml leupeptin, 50 μg/ml aprotinin, 5 μM pepstatin, 5 μM E64, 10 μM calpain inhibitor III, 1 mM phenylmethanesulphonylfluoride. Total protein was quantified by BCA (Pierce), and biotinylated integrins were analyzed by capture-ELISAs with specific antibodies.
In recycling assays, biotin-labeled cells were pre-incubated in serum-free DMEM for 60 min at 37°C or for 15 min at 22°C to allow integrin internalization into either the PNRC or early endosomes, respectively. Cells were washed twice with ice-cold PBS, and surface biotin was removed with MesNa, as above. Recycling of internalized biotin to the plasma membrane was followed after cell stimulation with 0.1 μg/ml EGF in warmed DMEM. Recycling experiments with cytochalasin D were performed similarly, except that cells were pre-incubated with 1 μM cytochalasin D or 0.1% DMSO at 22°C for 20 min before the initiation of recycling. At indicated times, cells were washed with ice-cold PBS and cell surface biotin was removed by a second MesNa incubation. Biotinylated integrins were quantified by capture-ELISA. The percentage of recycled integrin was calculated from control cells maintained on ice. Lysates were stored at -80°C until assay by capture-ELISA.
Capture-ELISAs were performed as described (15, 125). In brief, 96-well plates (Apogent) were coated overnight at 4°C with 5 μg/ml anti-integrin antibodies in coating buffer (0.1 M NaHCO3, 0.03 M Na2CO3, pH 9.5) and blocked for 2 h at room temperature with 3% BSA in PBS containing 0.05% Tween-20 (PBS-T). Biotinylated proteins in cell lysates were captured by overnight incubation at 4°C. Unbound proteins were removed by washing with PBS-T, and plates were incubated for 30 min at room temperature with streptavidin-conjugated horseradish peroxidase polymer (Sigma) in PBS-T. After eight washes with PBS-T, color was developed with freshly prepared 0.1% hydrogen peroxide, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (#A3219, Aldrich-Sigma) and read at 405 nm. Values are means ± s.d. of 9 replicates from 3 independent experiments that were performed on 2 or 3 separate days, as noted in legends.
Immunoprecipitation and Immunoblotting
To measure the relative levels of ERK phosphorylation, cells were lysed with RIPA + protease inhibitors at specified times after EGF addition. Blots were probed for phosphorylated ERK1/2 with monoclonal antibody E10 (1:1000) and re-probed with polyclonal antibody against ERK1/2 (1:1000) for detection of total ERK1/2 using a Kodak Image Station and molecular imaging software (Rochester, NY).
The steady-state levels of total and surface β1-integrin were determined for HeLa S3 cells pre-treated once with 20 nM control or supervillin-specific siRNA. After 3 days in growth media, cells were washed with cold-DPBS and labeled with 0.2 mg/ml sulfo-NHS-SS-biotin (Pierce) for 30 min at 4°C. Excess sulfo-NHS-SS-biotin was removed by rapid washing with cold DPBS. Cells were lysed in RIPA buffer plus protease inhibitors. Cell lysates were incubated with Protein A/G beads (Pierce) and anti-ß1-integrin antibody (Santa Cruz Biotechnology, Inc.) for 4 h at 4°C with constant rotation. Beads were washed with lysis buffer and boiled in 2× Laemmli sample buffer (126). Cell lysates and immunoprecipitated pellets were run on 6-15% polyacrylamide SDS-gels; and transferred to nitrocellulose (31, 32). Total supervillin, ß1-integrin, and β1-actin (loading control) were visualized on immunoblots of cell lysates; cell surface integrin in the immunoprecipitated pellets was visualized with peroxidase-conjugated streptavidin. Primary antibodies and dilutions were: anti-supervillin (H340), 0.5 μg/ml; anti-cortactin, 1:2000; and anti-ß-actin, 1:5000. HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Jackson Laboratories, West Grove, PA) were diluted 1:10,000 and detected by chemiluminescence with SuperSignal WestPico (Pierce).
Flow Cytometry
Steady-state surface β1- and β3-integrin levels were measured by flow cytometry (127). Cells were washed with DPBS and detached using pre-warmed Enzyme-free Dissociation Solution (Aldrich-Sigma) for 5 min at 37°C, and fixed in 4% paraformaldehyde-PBS. Cells were incubated with anti-ß1 or anti-ß3-integrin antibodies for 2 h at room temperature and then stained with a goat anti-mouse secondary antibody. Surface integrin was quantified by FACS and analyzed by FlowJo (Version 8.2, Tree Star, Inc.).
Supplementary Material
Acknowledgments
We thank Dr. Paul Furcinitti (University of Massachusetts Medical School Digital Light Microscopy Core Facility) for assistance in acquiring confocal images. We also thank Drs Steven Caplan, Michael Frohman, James Goldenring, Walther Mothes, Jim Norman, Richard Pagano, Thomas Roberts, Roger Tsien, and Thomas Weimbs for plasmids. This project was supported by NIH grants GM33048 (to E.J.L.) and GM5634 (to D.G.L.) and a Charles King Postdoctoral Fellowship (to A.D.). This research also benefited from NIH Biomedical Instrumentation Grant #1S1ORR015775 awarded to Dr. Greenfield Sluder for the shared high-resolution multimode microscope used in live cell imaging.
References
- 1.Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989;8:1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark ES, Weaver AM. A new role for cortactin in invadopodia: Regulation of protease secretion. Eur J Cell Biol. 2008;87:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher SJ, Rappoport JZ. Moving forward: polarised trafficking in cell migration. Trends Cell Biol. 2010;20:71–78. doi: 10.1016/j.tcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Sabe H, Hashimoto S, Morishige M, Ogawa E, Hashimoto A, Nam JM, Miura K, Yano H, Onodera Y. The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic. 2009;10:982–993. doi: 10.1111/j.1600-0854.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 6.Murray J, Vawter-Hugart H, Voss E, Soll DR. Three-dimensional motility cycle in leukocytes. Cell Motil Cytoskeleton. 1992;22:211–223. doi: 10.1002/cm.970220308. [DOI] [PubMed] [Google Scholar]
- 7.Wehrle-Haller B, Imhof B. The inner lives of focal adhesions. Trends Cell Biol. 2002;12:382–389. doi: 10.1016/s0962-8924(02)02321-8. [DOI] [PubMed] [Google Scholar]
- 8.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 9.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007;9:1381–1391. doi: 10.1038/ncb1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 13.Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 16.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 18.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 20.White DP, Caswell PT, Norman JC. αvβ3 and α5β1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–526. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–673. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Millman EE, Zhang H, Zhang H, Godines V, Bean AJ, Knoll BJ, Moore RH. Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic. 2008;9:1958–1971. doi: 10.1111/j.1600-0854.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Q, Sun W, Kujala P, Lotfi Y, Vida TA, Bean AJ. CART: an Hrs/actinin-4/BERP/myosin V protein complex required for efficient receptor recycling. Mol Biol Cell. 2005;16:2470–2482. doi: 10.1091/mbc.E04-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loubéry S, Coudrier E. Myosins in the secretory pathway: tethers or transporters? Cell Mol Life Sci. 2008;65:2790–2800. doi: 10.1007/s00018-008-8350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neco P, Giner D, Viniegra S, Borges R, Villarroel A, Gutierrez LM. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 28.Vascotto F, Lankar D, Faure-Andre G, Vargas P, Diaz J, Le Roux D, Yuseff MI, Sibarita JB, Boes M, Raposo G, Mougneau E, Glaichenhaus N, Bonnerot C, Manoury B, Lennon-Dumenil AM. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol. 2007;176:1007–1019. doi: 10.1083/jcb.200611147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takizawa N, Ikebe R, Ikebe M, Luna EJ. Supervillin slows cell spreading by facilitating myosin II activation at the cell periphery. J Cell Sci. 2007;120:3792–3803. doi: 10.1242/jcs.008219. [DOI] [PubMed] [Google Scholar]
- 31.Takizawa N, Smith TC, Nebl T, Crowley JL, Palmieri SJ, Lifshitz LM, Ehrhardt AG, Hoffman LM, Beckerle MC, Luna EJ. Supervillin modulation of focal adhesions involving TRIP6/ZRP-1. J Cell Biol. 2006;174:447–458. doi: 10.1083/jcb.200512051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowley JL, Smith TC, Fang Z, Takizawa N, Luna EJ. Supervillin reorganizes the actin cytoskeleton and increases invadopodial efficiency. Mol Biol Cell. 2009;20:948–962. doi: 10.1091/mbc.E08-08-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Takizawa N, Crowley JL, Oh SW, Gatto CL, Kambara T, Sato O, Li X, Ikebe M, Luna EJ. F-actin and myosin II binding domains in supervillin. J Biol Chem. 2003;278:46094–46106. doi: 10.1074/jbc.M305311200. [DOI] [PubMed] [Google Scholar]
- 34.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 35.Pestonjamasp KN, Pope RK, Wulfkuhle JD, Luna EJ. Supervillin (p205): A novel membrane-associated, F-actin-binding protein in the villin/gelsolin superfamily. J Cell Biol. 1997;139:1255–1269. doi: 10.1083/jcb.139.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wulfkuhle JD, Donina IE, Stark NH, Pope RK, Pestonjamasp KN, Niswonger ML, Luna EJ. Domain analysis of supervillin, an F-actin bundling plasma membrane protein with functional nuclear localization signals. J Cell Sci. 1999;112:2125–2136. doi: 10.1242/jcs.112.13.2125. [DOI] [PubMed] [Google Scholar]
- 37.Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277:37771–37776. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASP-dependent actin assembly. J Cell Sci. 2006;119:2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- 39.Nienhaus GU, Nienhaus K, Hölzle A, Ivanchenko S, Renzi F, Oswald F, Wolff M, Schmitt F, Röcker C, Vallone B, Weidemann W, Heilker R, Nar H, Wiedenmann J. Photoconvertible fluorescent protein EosFP: Biophysical properties and cell biology applications. Photochem Photobiol. 2006;82:351–358. doi: 10.1562/2005-05-19-RA-533. [DOI] [PubMed] [Google Scholar]
- 40.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 44.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 45.Cormont M, Bortoluzzi MN, Gautier N, Mari M, van Obberghen E, Le Marchand-Brustel Y. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol Cell Biol. 1996;16:6879–6886. doi: 10.1128/mcb.16.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 49.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 51.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiebsch RR, Raub TJ, Wattenberg BW. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J Biol Chem. 1991;266:20323–20328. [PubMed] [Google Scholar]
- 55.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell. 2004;15:1024–1030. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Padron D, Tall RD, Roth MG. Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors. Mol Biol Cell. 2006;17:598–606. doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T. Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell. 2006;17:977–989. doi: 10.1091/mbc.E05-05-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George M, Ying G, Rainey MA, Solomon A, Parikh PT, Gao Q, Band V, Band H. Shared as well as distinct roles of EHD proteins revealed by biochemical and functional comparisons in mammalian cells and C. elegans. BMC Cell Biol. 2007;8:3. doi: 10.1186/1471-2121-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 63.Bretscher MS, Lutter R. A new method for detecting endocytosed proteins. EMBO J. 1988;7:4087–4092. doi: 10.1002/j.1460-2075.1988.tb03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006;119:3723–3731. doi: 10.1242/jcs.03216. [DOI] [PubMed] [Google Scholar]
- 66.Chieregatti E, Meldolesi J. Regulated exocytosis: new organelles for non-secretory purposes. Nat Rev Mol Cell Biol. 2005;6:181–187. doi: 10.1038/nrm1572. [DOI] [PubMed] [Google Scholar]
- 67.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 68.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lladó A, Timpson P, Vila de Muga S, Moreto J, Pol A, Grewal T, Daly RJ, Enrich C, Tebar F. Protein kinase C{delta} and calmodulin regulate epidermal growth factor receptor recycling from early endosomes through Arp2/3 complex and cortactin. Mol Biol Cell. 2008;19:17–29. doi: 10.1091/mbc.E07-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 72.Kostenko O, Tsacoumangos A, Crooks D, Kil SJ, Carlin C. Gab1 signaling is regulated by EGF receptor sorting in early endosomes. Oncogene. 2006;25(50):6604–6617. doi: 10.1038/sj.onc.1209675. [DOI] [PubMed] [Google Scholar]
- 73.Yu X, Miyamoto S, Mekada E. Integrin alpha 2 beta 1-dependent EGF receptor activation at cell-cell contact sites. J Cell Sci. 2000;113:2139–2147. doi: 10.1242/jcs.113.12.2139. [DOI] [PubMed] [Google Scholar]
- 74.Gangopadhyay SS, Kengni E, Appel S, Gallant C, Kim HR, Leavis P, DeGnore J, Morgan KG. Smooth muscle archvillin is an ERK scaffolding protein. J Biol Chem. 2009;284:17607–17615. doi: 10.1074/jbc.M109.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gangopadhyay SS, Takizawa N, Gallant C, Barber AL, Je HD, Smith TC, Luna EJ, Morgan KG. Smooth muscle archvillin: A novel regulator of signaling and contractility in vascular smooth muscle. J Cell Sci. 2004;117:5043–5057. doi: 10.1242/jcs.01378. [DOI] [PubMed] [Google Scholar]
- 76.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal. 2007;19:1621–1632. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Soll DR. The use of computers in understanding how cells crawl. Int Rev Cytol. 1995;163:43–104. [PubMed] [Google Scholar]
- 80.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 81.Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 82.Jovanovic OA, Brown FD, Donaldson JG. An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling. Mol Biol Cell. 2006;17:327–335. doi: 10.1091/mbc.E05-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roth MG. Molecular Mechanisms of PLD Function in Membrane Traffic. Traffic. 2008;9:1233–9. doi: 10.1111/j.1600-0854.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 85.Weimbs T, Low SH, Li X, Kreitzer G. SNAREs and epithelial cells. Methods. 2003;30:191–197. doi: 10.1016/s1046-2023(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 86.Gong Q, Huntsman C, Ma D. Clathrin-independent internalization and recycling. J Cell Mol Med. 2008;12:126–144. doi: 10.1111/j.1582-4934.2007.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 88.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 89.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 90.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 91.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 92.Baldys A, Gooz M, Morinelli TA, Lee MH, Raymond JR, Jr, Luttrell LM, Raymond JR., Sr Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry. 2009;48:1462–1473. doi: 10.1021/bi801771g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 94.Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–657. doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, Gerber-Scokaert D, Planus E, Albiges-Rizo C. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Stylli SS, Kaye AH, Lock P. Invadopodia: At the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 99.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 100.Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 Activator WASH Controls the Fission of Endosomes through a Large Multiprotein Complex. Dev Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 102.Gomez TS, Billadeau DD. A FAM21-Containing WASH Complex Regulates Retromer-Dependent Sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res. 2007;313:560–571. doi: 10.1016/j.yexcr.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 105.Kaksonen M, Peng HB, Rauvala H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J Cell Sci. 2000;113:4421–4426. doi: 10.1242/jcs.113.24.4421. [DOI] [PubMed] [Google Scholar]
- 106.Lai FP, Szczodrak M, Oelkers JM, Ladwein M, Acconcia F, Benesch S, Auinger S, Faix J, Small JV, Polo S, Stradal TE, Rottner K. Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol Biol Cell. 2009;20:3209–3223. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 108.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Togo T, Steinhardt RA. Nonmuscle Myosin IIA and IIB Have Distinct Functions in the Exocytosis-dependent Process of Cell Membrane Repair. Mol Biol Cell. 2004;15:688–695. doi: 10.1091/mbc.E03-06-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oh SW, Pope RK, Smith KP, Crowley JL, Nebl T, Lawrence JB, Luna EJ. Archvillin, a muscle-specific isoform of supervillin, is an early expressed component of the costameric membrane skeleton. J Cell Sci. 2003;116:2261–2275. doi: 10.1242/jcs.00422. [DOI] [PubMed] [Google Scholar]
- 112.McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6:131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delprato A, Merithew E, Lambright DG. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell. 2004;118:607–617. doi: 10.1016/j.cell.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 115.Matthews S, Iglesias T, Cantrell D, Rozengurt E. Dynamic re-distribution of protein kinase D (PKD) as revealed by a GFP-PKD fusion protein: dissociation from PKD activation. FEBS Lett. 1999;457:515–521. doi: 10.1016/s0014-5793(99)01090-x. [DOI] [PubMed] [Google Scholar]
- 116.Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, Allen PG, Pypaert M, Cunningham JM, Mothes W. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 119.Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 120.Sharma N, Low SH, Misra S, Pallavi B, Weimbs T. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Cell Biol. 2006;173:937–948. doi: 10.1083/jcb.200603132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furman C, Short SM, Subramanian RR, Zetter BR, Roberts TM. DEF-1/ASAP1 is a GTPase-activating protein (GAP) for ARF1 that enhances cell motility through a GAP-dependent mechanism. J Biol Chem. 2002;277:7962–7969. doi: 10.1074/jbc.M109149200. [DOI] [PubMed] [Google Scholar]
- 122.Palmieri SJ, Nebl T, Pope RK, Seastone DJ, Lee E, Hinchcliffe EH, Sluder G, Knecht D, Cardelli J, Luna EJ. Mutant Rac1B expression in Dictyostelium: Effects on morphology, growth, endocytosis, development, and the actin cytoskeleton. Cell Motil Cytoskeleton. 2000;46:285–304. doi: 10.1002/1097-0169(200008)46:4<285::AID-CM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 123.Rodgers JL, Nicewander WA. Thirteen ways to look at the correlation coefficient. The American Statistician. 1988;42:59–66. [Google Scholar]
- 124.Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853–866. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- 125.Turvy DN, Blum JS. Detection of biotinylated cell surface receptors and MHC molecules in a capture ELISA: a rapid assay to measure endocytosis. J Immunol Methods. 1998;212:9–18. doi: 10.1016/s0022-1759(97)00206-8. [DOI] [PubMed] [Google Scholar]
- 126.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 127.Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, Cooper JA. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol. 2009;186:99–111. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.