Abstract
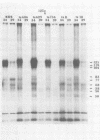
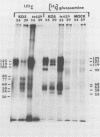
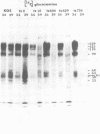
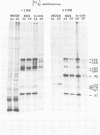
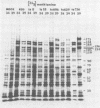
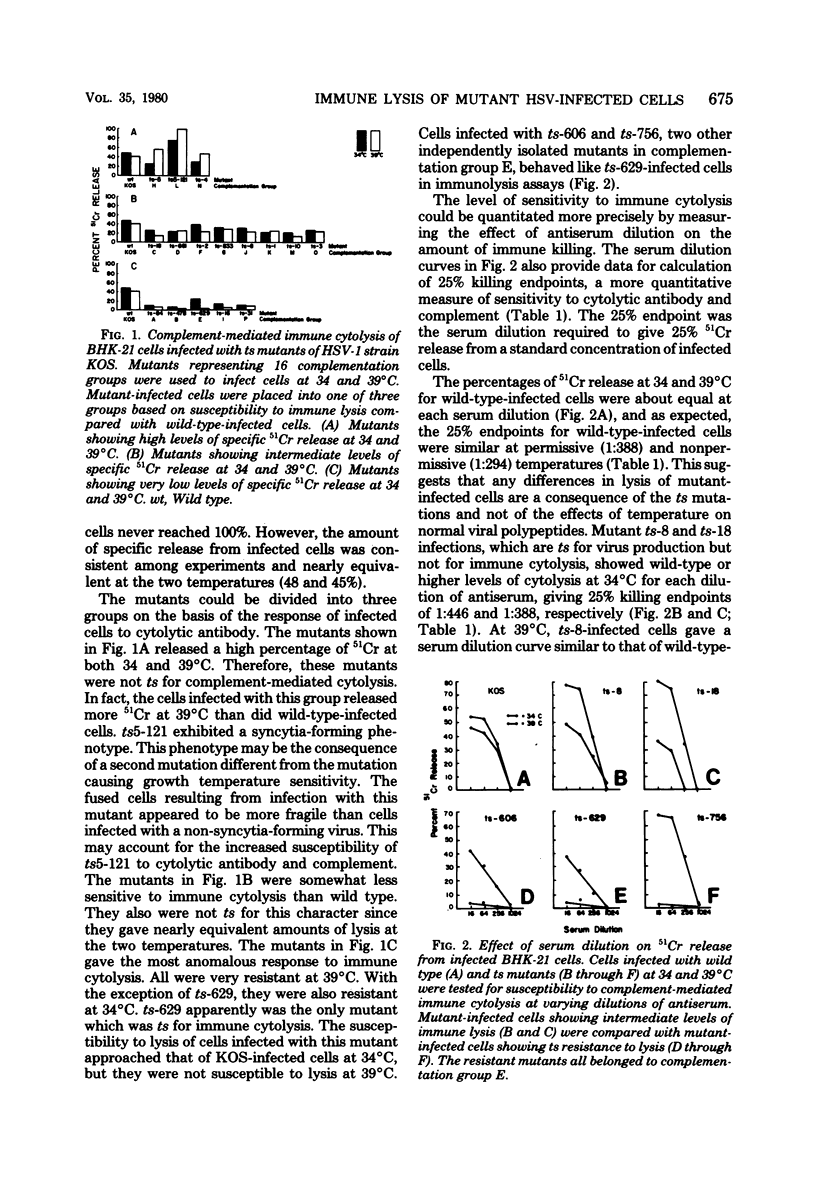
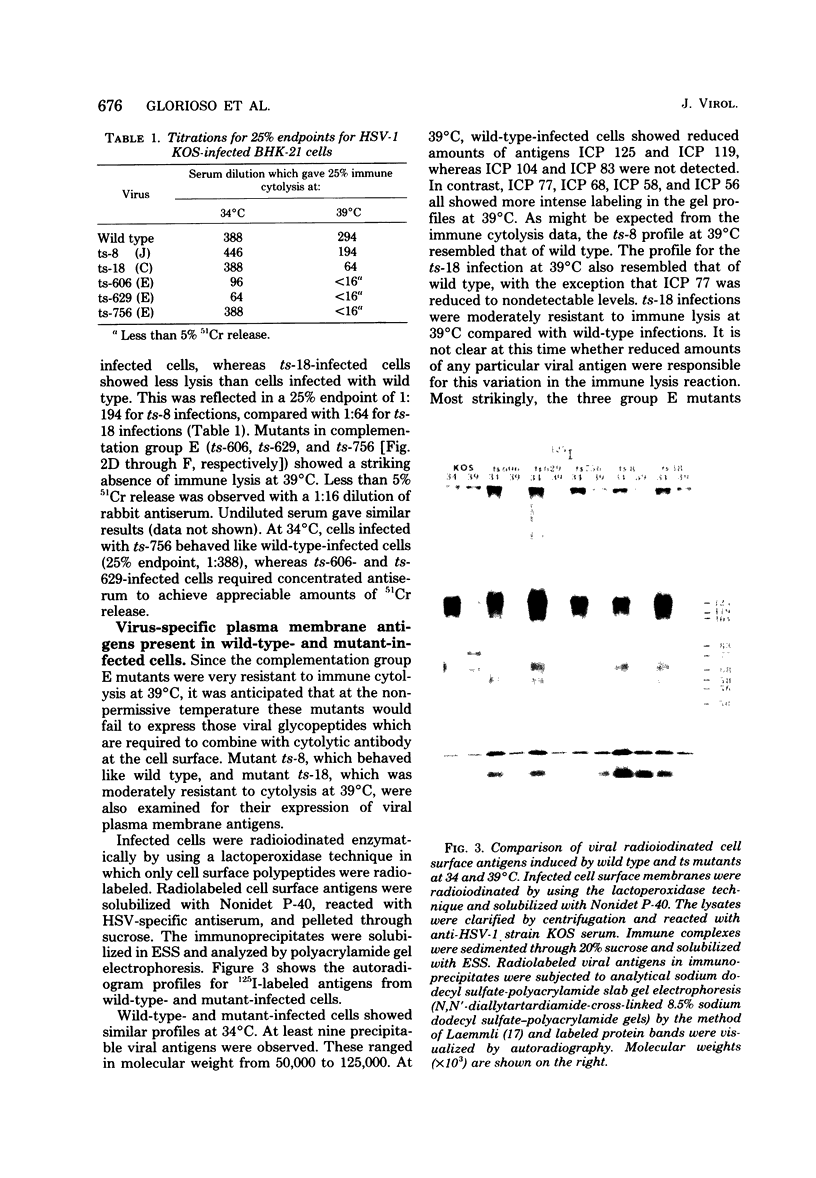
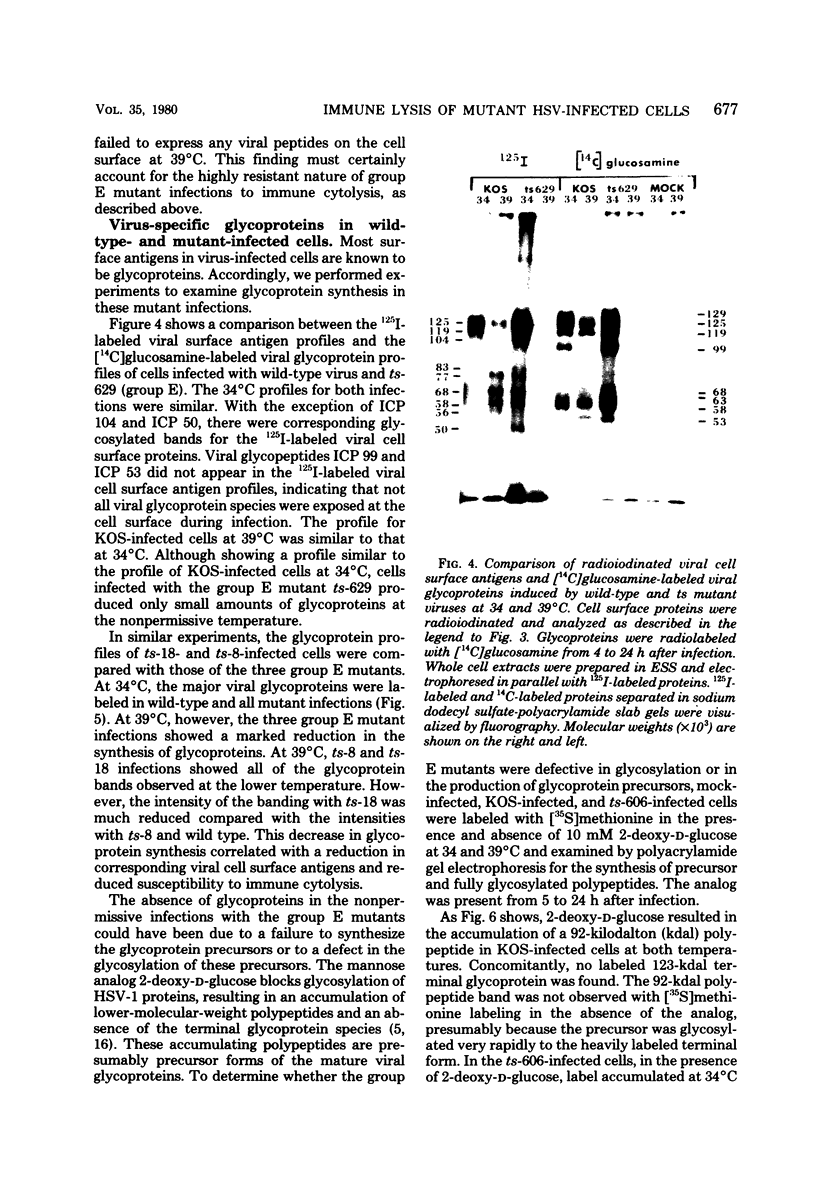
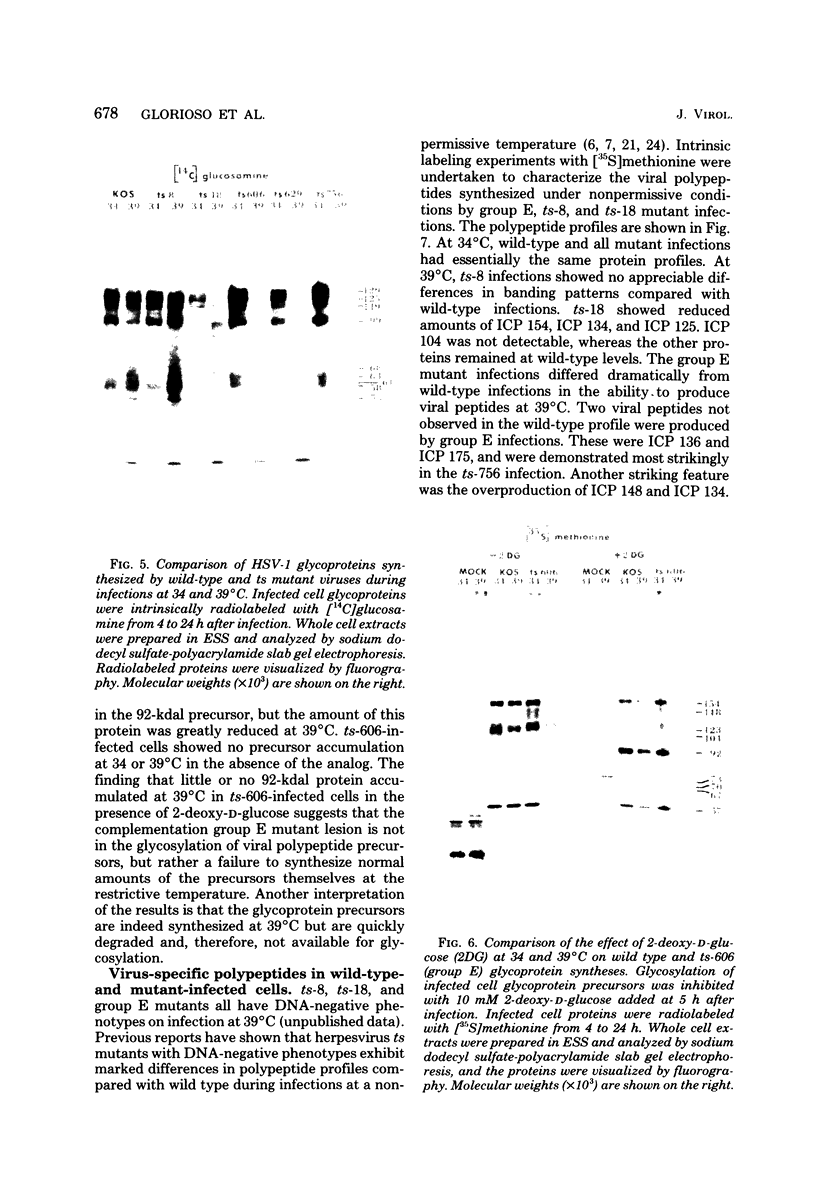
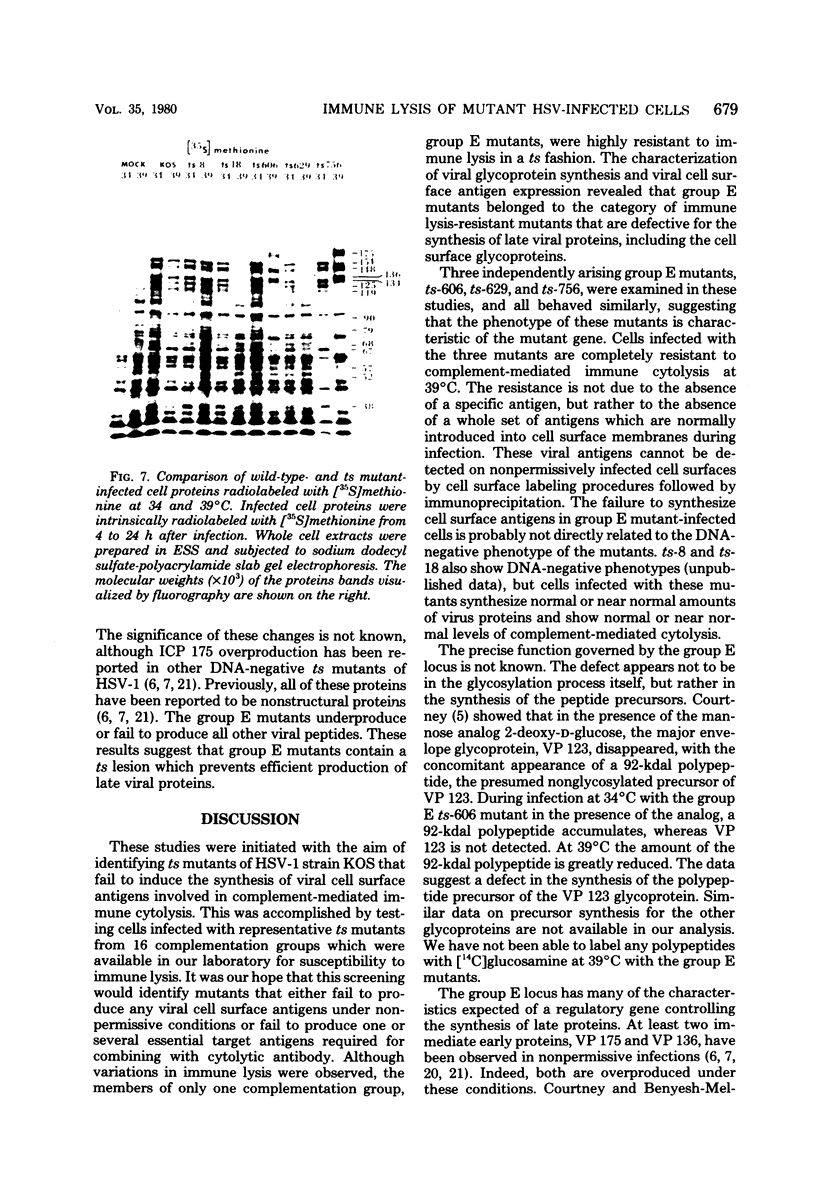
BHK-21 cells infected with temperature-sensitive mutants of herpes simplex virus type 1 strain KOS representing 16 complementation groups were tested for susceptibility to complement-mediated immune cytolysis at permissive (34 degrees C) and nonpermissive (39 degrees C) temperatures. Only cells infected by mutants in complementation group E were resistant to immune cytolysis in a temperature-sensitive manner compared with wild-type infections. The expression of group E mutant cell surface antigens during infections at 34 and 39 degrees C was characterized by a combination of cell surface radioiodination, specific immunoprecipitation, and gel electrophoretic analysis of immunoprecipitates. Resistance to immune lysis at 39 degrees C correlated with the absence of viral antigens exposed at the cell surface. Intrinsic radiolabeling of group E mutant infections with [14C]glucosamine revealed that normal glycoproteins were produced at 34 degrees C but none were synthesized at 39 degrees C. The effect of 2-deoxy-D-glucose on glycosylation of group E mutants at 39 degrees C suggested that the viral glycoprotein precursors were not synthesized. The complementation group E mutants failed to complement herpes simplex virus type 1 mutants isolated by other workers. These included the group B mutants of strain KOS, the temperature-sensitive group D mutants of strain 17, and the LB2 mutant of strain HFEM. These mutants should be considered members of herpes simplex virus type 1 complementation group 1.2, in keeping with the new herpes simplex virus type 1 nomenclature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Courtney R. J. Herpes simplex virus protein synthesis in the presence of 2-deoxy-D-glucose. Virology. 1976 Aug;73(1):286–294. doi: 10.1016/0042-6822(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Schaffer P. A., Powell K. L. Synthesis of virus-specific polypaptides by temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1976 Dec;75(2):306–318. doi: 10.1016/0042-6822(76)90030-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Randall R. E., Killington R. A., Watson D. H. Some properties of recombinants between type 1 and type 2 herpes simplex viruses. J Gen Virol. 1977 Sep;36(3):471–484. doi: 10.1099/0022-1317-36-3-471. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman M. R. Herpes simplex vaccines. Cancer Res. 1976 Feb;36(2 Pt 2):857–858. [PubMed] [Google Scholar]
- Hughes R. G., Jr, Munyon W. H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975 Aug;16(2):275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Ruyechan W. T., Roizman B., Halliburton I. W. Molecular genetics of herpes simplex virus: demonstration of regions of obligatory and nonobligatory identity within diploid regions of the genome by sequence replacement and insertion. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3896–3900. doi: 10.1073/pnas.75.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Abnormal properties of an immediate early polypeptide in cells infected with the herpes simplex virus type 1 mutant tsK. J Virol. 1979 Nov;32(2):357–369. doi: 10.1128/jvi.32.2.357-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Courtney R. J., McCombs R. M., Benyesh-Melnick M. A temperature-sensitive mutant of herpes simplex virus defective in glycoprotein synthesis. Virology. 1971 Nov;46(2):356–368. doi: 10.1016/0042-6822(71)90037-7. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Norrild B. Early damage of herpes-infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specified cell-surface antigens and input virus. J Immunol. 1979 Nov;123(5):2239–2244. [PubMed] [Google Scholar]
- Smith J. W., Glorioso J. C. Effect of cross-immunization on monotypic antibody responses to herpes simplex virus types 1 and 2. J Immunol. 1976 Apr;116(4):898–903. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Subak-Sharpe J. H., Wilkie N. M. Physical mapping of herpes simplex virus type 1 mutations by marker rescue. J Virol. 1978 Oct;28(1):182–192. doi: 10.1128/jvi.28.1.182-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 by intertypic marker rescue. Virology. 1978 Oct 1;90(1):1–11. doi: 10.1016/0042-6822(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Honess R. W. Polypeptides and antigens of herpes simplex virus; their nature and relevance in chemotherapy and epidemiology of herpes infections. J Antimicrob Chemother. 1977 Mar;3 (Suppl A):33–46. doi: 10.1093/jac/3.suppl_a.33. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]