Abstract
Prevalence of microalbuminuria is increased in patients with HIV. Microalbuminuria is associated with increased mortality in other populations, including diabetics, for whom microalbuminuria testing is standard of care. We investigated whether microalbuminuria is associated with mortality in HIV-infected women not receiving antiretroviral therapy.
Methods
Urinalysis for proteinuria and semi-quantitative testing for microalbuminuria were performed in specimens from two consecutive visits in 1,547 HIV-infected women enrolled in the Women’s Interagency HIV Study in 1994–1995. Time to death was modeled using proportional hazards analysis.
Results
Compared to women without albuminuria, the hazard ratio (HR) for all-cause mortality was increased in women with one (HR 3.4; 95% CI 2.2–5.2) or two specimens positive for either proteinuria or microalbuminuria (HR 3.9; 95% CI 2.1–7.0). The highest risk was observed in women with both specimens positive for proteinuria (HR 5.8; 95% CI 3.4–9.8). The association between albuminuria and all-cause mortality risk remained significant after adjustment for demographics, HIV disease severity, and related comorbidities. Similar results were obtained for AIDS death.
Conclusions
We identified a graded relationship between albuminuria and the risk of all-cause and AIDS mortality.
Keywords: HIV, microalbuminuria, proteinuria, mortality
Introduction
Chronic kidney disease and overt proteinuria have been associated with adverse outcomes in HIV-infected patients.1 2 Lower levels of urinary albumin excretion, or microalbuminuria, have also been associated with increased mortality in other patient populations, and routine microalbuminuria testing is now considered standard of care in diabetics. 3 Several studies have demonstrated an increased prevalence of microalbuminuria in HIV-infected individuals both before 4 and after the introduction of highly active antiretroviral therapy (HAART); 5 6 however, the prognostic significance of microalbuminuria in HIV-infected patients is not known.
The Women’s Interagency HIV Study (WIHS) has collected information on HIV disease characteristics, comorbidities, and outcomes in a prospective cohort of women enrolled in the pre-HAART era. 7 An earlier analysis in WIHS demonstrated an association between overt proteinuria and increased mortality risk. 1 We sought to determine whether microalbuminuria testing also provides prognostic information.
Methods
The WIHS design has been described previously. 7 Briefly, WIHS is an ongoing prospective cohort study that enrolled HIV-infected and at-risk seronegative women in five metropolitan areas in the United States. Only HIV-infected women enrolled between October 1994 and November 1995 were included in the current analysis. Demographics and social, medical, and detailed HIV disease history were collected by standardized interview. Prior AIDS-defining illness (ADI) was defined based on the 1993 Centers for Disease Control case definition for AIDS, excluding CD4 <200 cells/mm3. Laboratory data including CD4, HIV-RNA, hepatitis serologies, hemoglobin, serum albumin, and routine urinalysis were collected at enrollment and semi-annually. Serum creatinine testing was performed at enrollment and annually. Glomerular filtration rate (eGFR) was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation. Because currently available GFR estimates have not been validated in HIV-infected populations, we selected the abbreviated MDRD equation because of the established relationship between decreased MDRD eGFR and increased mortality in other patient populations. 8, 9 The WIHS was approved by the institutional review boards of all sites, and participants provided written informed consent. The current analysis was also approved by the institutional review board of the Mount Sinai School of Medicine.
Urine specimens were collected at study entry and every 6 months. To minimize artifact from cystitis, specimens were excluded from this analysis if the corresponding urinalysis demonstrated pyuria (leukocyte esterase 1+ with a positive or missing urine culture, or leukocyte esterase >1+). By WIHS protocol, urine specimens were not collected during menses. Women with two eligible urine specimens were included in this analysis. The index visit was defined as the first visit at which an eligible specimen was obtained. Specimens with protein <1+ were tested for microalbuminuria. Semi-quantitative microalbuminuria testing was performed in banked urine specimens under standardized conditions using Clinitek Microalbumin Reagent Strips and the Clinitek 50 Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). This point-of-care dipstick assay has a sensitivity of 86% compared to clinical laboratory testing, and was selected because of its applicability to clinical practice. Frozen specimens (−70C) from the Division of AIDS central repository were thawed completely before testing. Quality assurance testing was performed with standardized controls (Bio-Rad Laboratories, Hercules, CA). Microalbuminuria was defined as an albumin: creatinine ratio > 30 mg/g.
The study exposure was hierarchically defined as follows; 1) Women with urinalysis protein ≥1+ at both the index visit and the next consecutive eligible visit were considered to have “confirmed proteinuria.” 2) Women with microalbuminuria at both visits or with microalbuminuria at one visit and proteinuria at the other were considered to have “confirmed microalbuminuria.” 3) Women with proteinuria or microalbuminuria at only one visit were considered to have “unconfirmed albuminuria.” 4) Women without proteinuria or microalbuminuria on either visit were considered to have no albuminuria.
Data on vital status were collected from medical records, providers, and personal contacts of WIHS participants and from the National Death Index. In cases where the death certificate was not available, the cause of death was ascertained from medical records, providers, or personal contacts, in that order. The cause of death was categorized as AIDS, non-AIDS, or indeterminate, as previously described. 10
Clinical characteristics at the index visit were summarized for each albuminuria group. When data were missing at the index visit, the value was obtained from the most recent prior visit. Continuous and categorical variables were compared using Kruskall-Wallis and chi-square tests, respectively. All p-values are 2-sided. Kaplan-Meier and proportional hazards models evaluated associations of albuminuria with time to death. Proportional hazards models included baseline characteristics that have previously been associated with short-term mortality in WIHS:1 11 demographics, prior ADI, hepatitis C co-infection, log transformed HIV-RNA, CD4, hemoglobin, and serum albumin. Diabetes, blood pressure, and eGFR were also considered for inclusion because of their known association with albuminuria. Survival analyses were left-truncated at the second eligible study visit, and were censored at 31 October 1997, based on a prior analysis of WIHS demonstrating minimal HAART use and little effect of HAART exposure on the relationship between other covariates and death prior to this date. 11 In addition, a sensitivity analysis including HAART use as a time dependent variable in the multivariate models was performed. All analyses were performed using SAS (version 9.1.3; SAS).
Results
Among 2,059 HIV-infected women enrolled in WIHS during 1994–1995, 1,547 (75%) had at least two eligible urine specimens available. Women with inadequate specimens were similar to included participants with respect to age and race-ethnicity (data not shown). One-third of women included in this analysis reported a history of ADI at or prior to the index visit, and only 1% reported HAART use at the time of the index visit (Table 1). Fifty-seven women (3.7%) had confirmed proteinuria, 64 (4.1%) had confirmed microalbuminuria, and 165 (10.7%) had unconfirmed albuminuria. Compared to participants with no albuminuria, women with any level of albuminuria had more advanced HIV disease and were more likely to classify their race as black and to have an eGFR below 60ml/min/1.73m2.
Table 1.
Characteristics of WIHS participants at the index visit.
| No Albuminuria n=1261 (81.5%) |
Unconfirmed Albuminuria n=165 (10.7%) |
Confirmed Microalbuminuria n=64 (4.1%) |
Confirmed Proteinuria n=57 (3.7%) |
|
|---|---|---|---|---|
| Age, years*** | 36.5 (7.7) | 38.2 (8.0) | 40.2 (7.9) | 36.2 (8.5) |
| Race*** Black (including Hispanic) White (including Hispanic) Other |
659 (52.3%) 277 (22.0%) 325 (25.8%) |
114 (69.1%) 29 (17.6%) 22 (13.3%) |
51 (79.7%) 6 (9.4%) 7 (10.9%) |
45 (79.0%) 10 (17.5%) 2 (3.5%) |
| CD4 cell count, cells/mm3*** | 408.1 (279.0) | 350.7 (276.7) | 280.8 (282.7) | 304.4 (255.2) |
| Log10 HIV-RNA*** | 4.2 (0.9) | 4.4 (0.9) | 4.6 (1.1) | 4.8 (0.9) |
| Prior AIDS-defining illness** | 400 (31.7%) | 62 (37.6%) | 28 (43.8%) | 28 (49.1%) |
| Hepatitis C co-infection** | 420/1225 (34.4%) | 74/160 (46.3%) | 30/63 (47.6%) | 20/56 (35.7%) |
| Hepatitis B co-infection | 30 (2.4%) | 7 (4.2%) | 2 (3.1%) | 3 (5.3%) |
| Diabetes mellitus | 31 (2.5%) | 3 (1.8%) | 4 (6.3%) | 2 (3.5%) |
| Systolic blood pressure, mmHg** | 115.6 (14.9) | 118.4 (17.0) | 123.7 (21.6) | 120.5 (18.8) |
| Diastolic blood pressure, mmHg*** | 74.4 (10.3) | 77.2 (13.3) | 80.7 (14.4) | 78.8 (12.6) |
| Smoking, current or former | 924/1260 (73.3%) | 124/164 (75.6%) | 45/64 (70.3%) | 44/57 (77.2%) |
| eGFR < 60mL/min/1.73m2*** | 80/1244 (6.4%) | 14/162 (8.6%) | 12/62 (19.4%) | 14/55 (25.5%) |
| Hemoglobin, g/dL*** | 12.5 (1.3) | 12.1 (1.7) | 11.8 (1.4) | 11.3 (1.6) |
| Serum albumin, g/dL*** | 4.2 (0.4) | 4.2 (0.4) | 3.9 (0.6) | 3.7 (0.5) |
| All-cause death*** | 74 (5.9%) | 31 (18.8%) | 13 (20.3%) | 17 (29.8%) |
| AIDS death*** | 52 (4.1%) | 23 (13.9%) | 11 (17.2%) | 11 (19.3%) |
| New AIDS-defining illness** | 156 (12.4%) | 28 (17.0%) | 15 (23.4%) | 15 (26.3%) |
p-value: * < 0.05,
<0.01 and
< 0.001.
Chi-square test for categorical variables and Kruskal-Wallis for continuous variables. Hepatitis C co-infection, hepatitis C antibody positive and RNA positive or unknown; hepatitis B co-infection, hepatitis B surface antigen positive; eGFR, estimated glomerular filtration rate.
During a median follow-up of 2.1 years, there were 135 deaths, including 97 from AIDS. Kaplan-Meier curves demonstrated a graded relationship between the severity of albuminuria and time to all-cause and AIDS death (Figure 1). Compared to women with no albuminuria, women with unconfirmed albuminuria (HR 3.4; 95% CI 2.2–5.2) and confirmed microalbuminuria (HR 3.9; 95% CI 2.1–7.0) had a higher risk of all-cause mortality, while women with confirmed proteinuria had the highest risk (HR 5.8; 95% CI 3.4–9.8). A similar relationship was observed for AIDS death.
Figure 1.
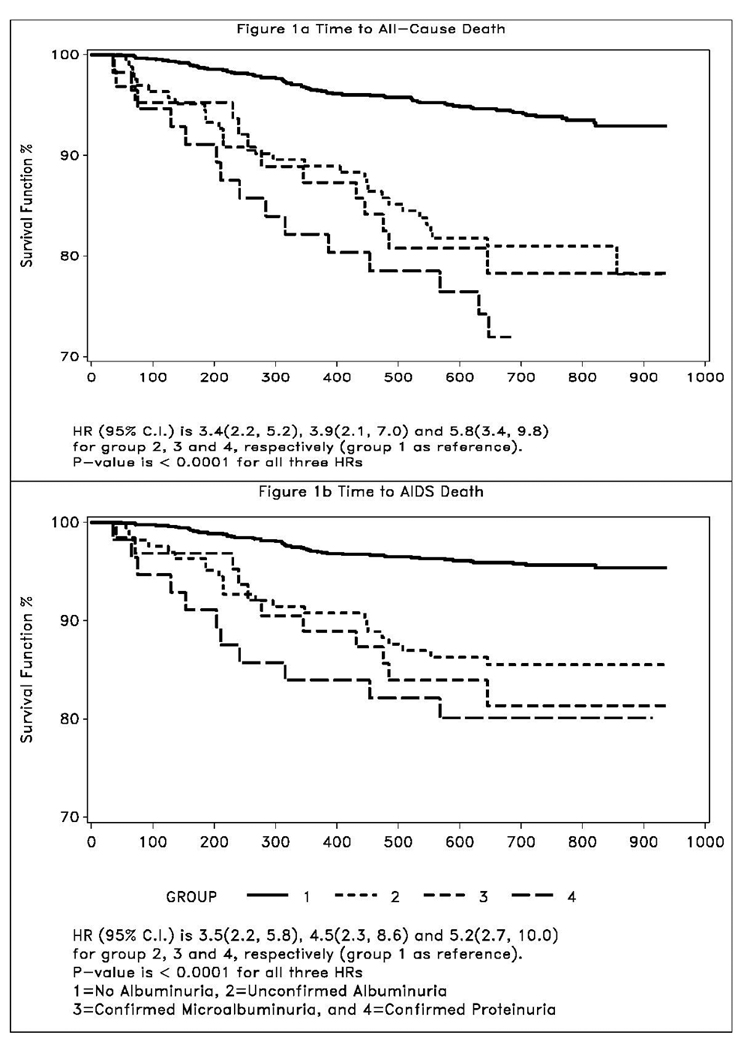
Kaplan-Meier estimates of time to all-cause death and time to AIDS death.
After adjustment, the association of albuminuria with all-cause mortality was partially mitigated but remained significant (Supplemental Digital Content, Table 2). Compared to women with no albuminuria, the adjusted mortality risk was 2-fold higher in women with unconfirmed albuminuria (adjusted HR 2.0; 95% CI 1.2–3.2) or confirmed microalbuminuria (adjusted HR 1.9; 95% CI 1.0–3.8), and nearly 3-fold higher in women with confirmed proteinuria (adjusted HR 2.8; 95% CI 1.5–5.2). Other variables that were independently associated with mortality included CD4, HIV-RNA, prior ADI, and hemoglobin. Diabetes was excluded from the final model because there were no deaths among the small subgroup with diabetes at the index visit (0/40 versus 135/1372 with no diabetes, p=0.04), which prevented the proportional hazards model from converging. Systolic and diastolic blood pressures were highly correlated; only systolic blood pressure was included in the final model. Similar results were obtained in multivariate analysis when the outcome was AIDS death, and in sensitivity analyses including HAART use as a time-dependent covariate (data not shown).
Discussion
In this well-characterized cohort of HIV-infected women enrolled prior to the widespread introduction of HAART, we identified a graded relationship between the degree of albuminuria and the risk of mortality. While overt proteinuria has been associated with adverse outcomes in previous studies, 1 2 the prognostic significance of microalbuminuria has not been described in HIV-infected individuals. In the current analysis, the detection of proteinuria or microalbuminuria on a single urine specimen was associated with an increased risk of mortality, even after adjustment for markers of HIV disease severity. These data suggest that microalbuminuria testing may provide useful prognostic information in HIV-infected individuals.
While this is the first study to investigate the prognostic significance of microalbuminuria in patients with HIV, a number of limitations must be acknowledged. First, this analysis included only women enrolled prior to the widespread use of HAART, and results may not be generalizable to men or HAART-treated individuals. Although more than 50% of participants had a CD4 > 350 cells/mm3 at the index visit, the majority of deaths occurred in women with lower CD4. The relationship between albuminuria and all-cause death was consistent when stratified at a CD4 of 350 cells/mm3, although we had limited power to detect differences in the strength of this relationship between CD4 strata (data not shown). Because more than 70% of deaths were attributed to AIDS, we had limited power to analyze the relationship between albuminuria and non-AIDS death. Second, a small number of participants initiated HAART before the end of the study period. In a previous WIHS analysis using the same study period, the percentage of person-time on HAART was very low, and HAART use had no significant influence on mortality.11 Results of the current analysis were similar when HAART use was included as a time-dependent variable. Finally, it is possible that misclassification occurred due to the use of angiotensin converting enzyme (ACE) inhibitors and other agents known to reduce albuminuria, limited sensitivity of dipstick assays for protein or microalbumin, or decreased sensitivity of microalbuminuria testing in banked urine specimens. 12 This potential misclassification would be expected to bias our results towards the null.
The current study suggests that microalbuminuria testing may identify HIV-infected patients at increased risk for mortality. Although the mechanism of the observed association is not known, microalbuminuria has been hypothesized to reflect generalized endothelial dysfunction. 3 In patients with HIV, microalbuminuria is associated with more advanced HIV disease 5 and longer duration of infection. 6 Consistent with these reports, the incremental risk of mortality associated with microalbuminuria was reduced, but not eliminated, after adjusting for markers of HIV disease severity. Nonetheless, microalbuminuria could serve as a non-invasive and accessible prognostic marker, particularly in resource-limited settings where extensive laboratory testing is not feasible. Future studies should investigate whether microalbuminuria is associated with mortality in other HIV-infected populations, and whether microalbuminuria testing is a cost-effective approach to identify patients who may benefit from earlier intervention.
Supplementary Material
Acknowledgements
Data in this manuscript were collected by the WIHS Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (NIAID) (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). CMW is supported by the National Institute of Diabetes and Digestive and Kidney Disease (K23-DK-077568). PCT is supported by NIAID (K23-AI-66943). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. All authors contributed to the design of this analysis, interpretation of data, and critical revision of the manuscript. We are grateful to Johanna Goderre and Henry Sacks for their assistance.
Funding: Support for this work was provided by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199–1206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 2.Gardner LI, Holmberg SD, Williamson JM, et al. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 4.Kimmel PL, Umana WO, Bosch JP. Abnormal urinary protein excretion in HIV-infected patients. Clin Nephrol. 1993;39:17–21. [PubMed] [Google Scholar]
- 5.Szczech LA, Grunfeld C, Scherzer R, et al. Microalbuminuria in HIV infection. AIDS. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baekken M, Os I, Sandvik L, Oektedalen O. Microalbuminuria associated with indicators of inflammatory activity in an HIV-positive population. Nephrol Dial Transplant. 2008;23:3130–3137. doi: 10.1093/ndt/gfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MH, French AL, Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anastos K, Kalish LA, Hessol N, et al. The relative value of CD4 cell count and quantitative HIV-1 RNA in predicting survival in HIV-1-infected women: results of the women's interagency HIV study. AIDS. 1999;13:1717–1726. doi: 10.1097/00002030-199909100-00016. [DOI] [PubMed] [Google Scholar]
- 12.Brinkman JW, de Zeeuw D, Lambers Heerspink HJ, et al. Apparent loss of urinary albumin during long-term frozen storage: HPLC vs immunonephelometry. Clin Chem. 2007;53:1520–1526. doi: 10.1373/clinchem.2007.088823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


