Abstract
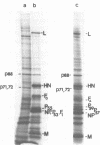
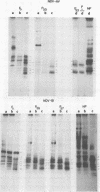
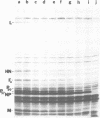
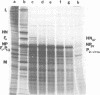
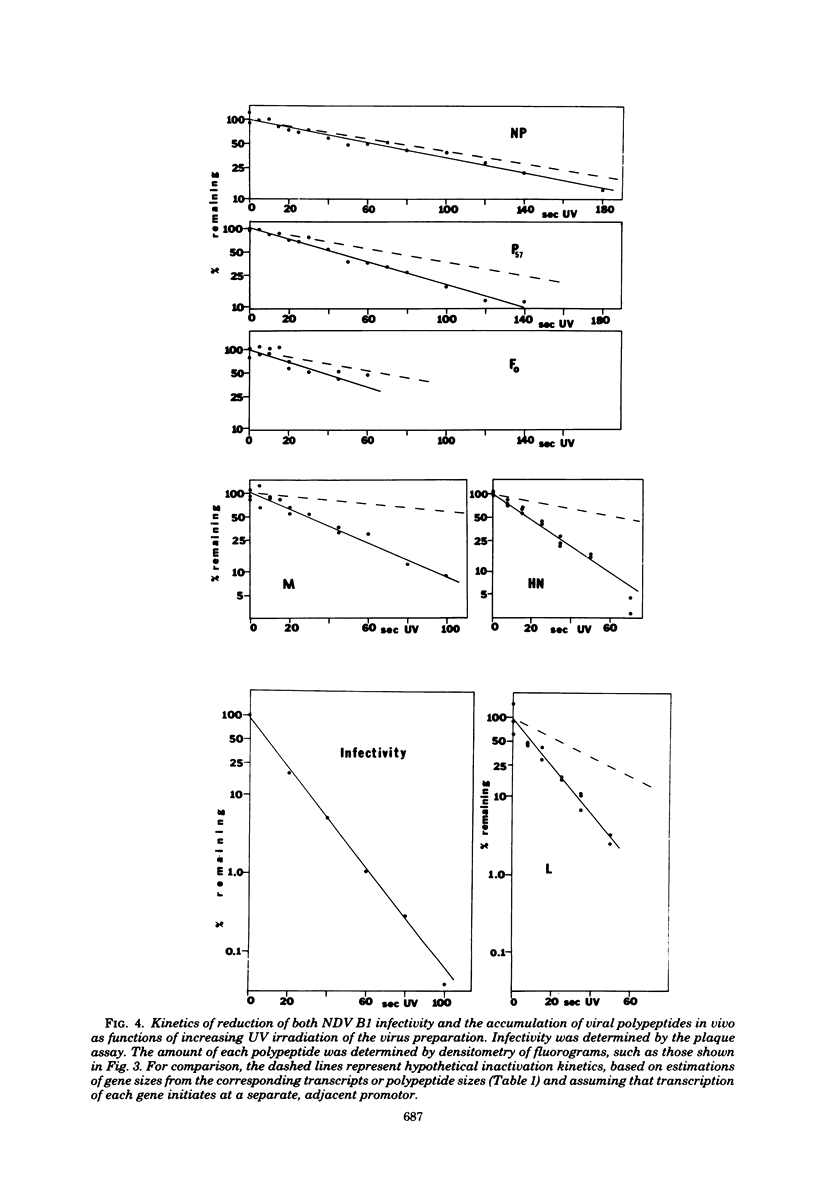
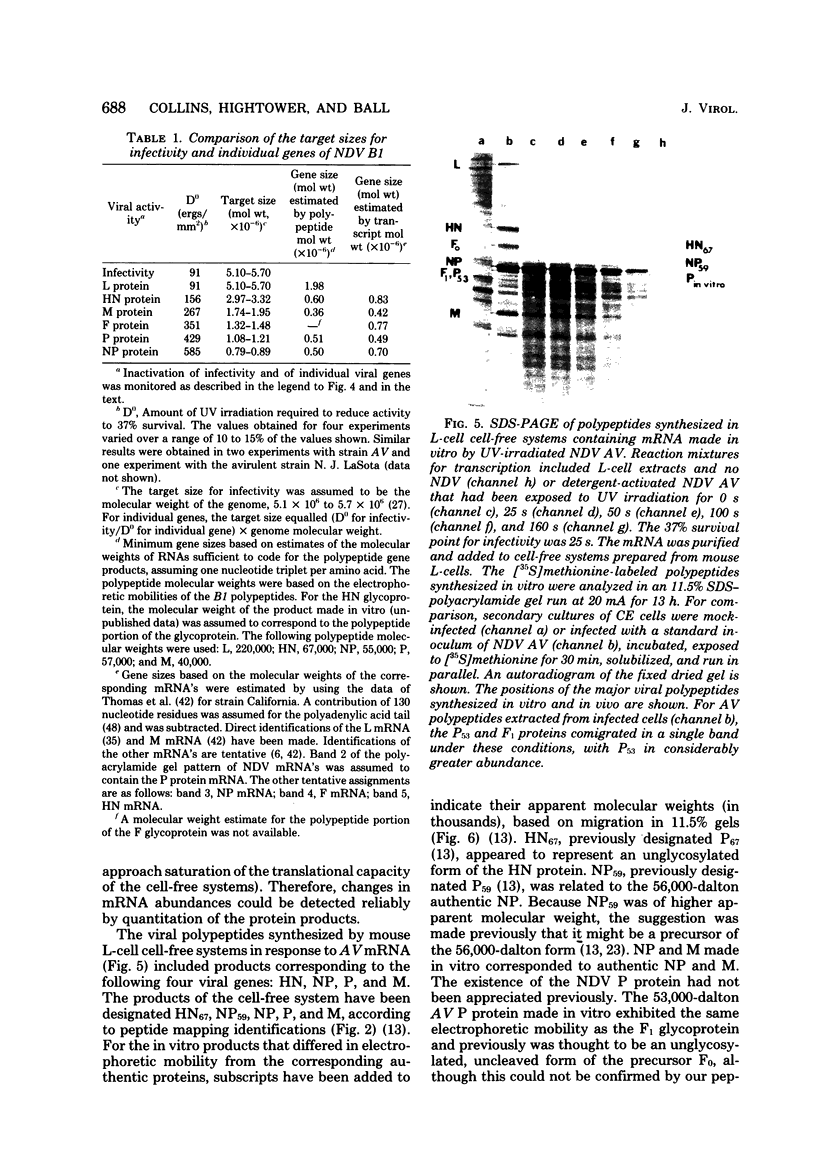
A transcriptional map of Newcastle disease virus was determined by measuring the kinetics of UV inactivation of the transcription of individual genes and of viral infectivity. The inactivation of single genes was monitored by measuring the reduction in the accumulation of viral gene products in vivo and in vitro. In vivo, the accumulation of viral polypeptides in infected cells was measured after reversal of a cycloheximide treatment designed to inhibit secondary transcription. Actinomycin D and a hypertonic medium were used to decrease selectively the synthesis of host cell polypeptides in infected cells. In vitro, mRNA's synthesized by irradiated viruses were analyzed by translation in cell-free systems under conditions in which the amount of each polypeptide synthesized reflected the relative abundance of the corresponding mRNA. UV target sizes were obtained for the genes coding for the HN, F0, NP, M, L, and P polypeptides; the 47,000-dalton protein was not detected. A comparison of the UV target sizes with the corresponding gene sizes suggested that transcription of these genes initiated at a single promotor and proceeded in the order NP, P, (F0, M), HN, L. These experiments were performed with Newcastle disease virus strains Australia-Victoria and B1-Hitchner; for both strains, two forms of the P polypeptide which differed in electrophoretic mobility were detected. Proof that the P protein is virus specific was obtained. In addition, infection of chicken embryo cells with avirulent strain B1-Hitchner enhanced the accumulation of at least four polypeptides that appeared to be specified by the host cell rather than by the infecting virus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. Transcriptional mapping of vesicular stomatitis virus in vivo. J Virol. 1977 Jan;21(1):411–414. doi: 10.1128/jvi.21.1.411-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinkscales C. W., Bratt M. A., Morrison T. G. Synthesis of Newcastle disease virus polypeptides in a wheat germ cell-free system. J Virol. 1977 Apr;22(1):97–101. doi: 10.1128/jvi.22.1.97-101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. S., Bratt M. A. Separation of the messenger RNAs of Newcastle disease virus by gel electrophoresis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2544–2548. doi: 10.1073/pnas.70.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
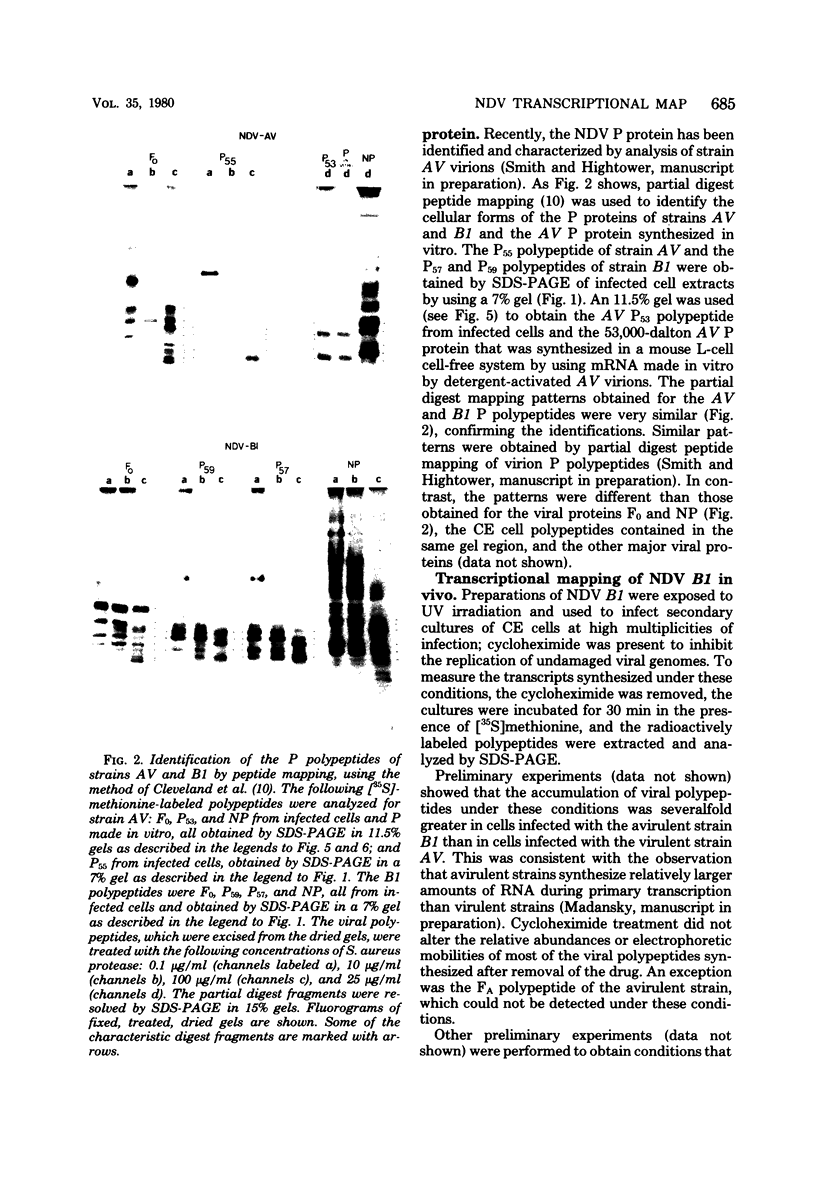
- Collins P. L., Hightower L. E., Ball L. A. Transcription and translation of Newcastle disease virus mRNA's in vitro. J Virol. 1978 Oct;28(1):324–336. doi: 10.1128/jvi.28.1.324-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F. Transcriptional mapping of rabies virus in vivo. J Virol. 1978 Nov;28(2):518–523. doi: 10.1128/jvi.28.2.518-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Adler S., Lazzarini R. A., Colonno R. J., Banerjee A. K., Westphal H. Intervening polyadenylate sequences in RNA transcripts of vesicular stomatitis virus. Cell. 1978 Oct;15(2):587–596. doi: 10.1016/0092-8674(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Morrison T. G., Bratt M. A. Relationships among the polypeptides of Newcastle disease virus. J Virol. 1975 Dec;16(6):1599–1607. doi: 10.1128/jvi.16.6.1599-1607.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma M., Nagai Y., Maeno K., Yoshida T., Matsumoto T. Studies on the assembly of Newcastle disease virus: incorporation of structural proteins into virus particles. J Gen Virol. 1971 Sep;12(3):239–247. doi: 10.1099/0022-1317-12-3-239. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Barrett T., Brown C. M., Almond J. W. The smallest genome RNA segment of influenza virus contains two genes that may overlap. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3790–3794. doi: 10.1073/pnas.76.8.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverin N. V., Varich N. L. Newcastle disease virus-specific RNA: polyacrylamide gel analysis of single-stranded RNA and hybrid duplexes. J Virol. 1974 Feb;13(2):253–260. doi: 10.1128/jvi.13.2.253-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W., Rochovansky O. M. Ultraviolet inactivation of influenza virus RNA in vitro and vivo. Virology. 1979 Aug;97(1):183–189. doi: 10.1016/0042-6822(79)90385-4. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varich N. L., Lukashevich I. S., Kaverin N. V. Newcastle disease virus-specific RNA: an analysis of 24 S and 35 S RNA transcripts. Acta Virol. 1979 Jul;23(4):273–283. [PubMed] [Google Scholar]
- Varich N. L., Lukashevich I. S., Kaverin N. V. Newcastle disease virus-specific RNA: hybridization-competition of the non-dissociable 35 S RNA with individual 18 S RNA species. Acta Virol. 1979 Jul;23(4):341–343. [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]
- Waterson A. P., Pennington T. H., Allan W. H. Virulence in Newcastle disease virus. A preliminary study. Br Med Bull. 1967 May;23(2):138–143. doi: 10.1093/oxfordjournals.bmb.a070534. [DOI] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Comparative electrophoresis of the 18-22S RNAs of Newcastle disease virus. J Virol. 1976 Apr;18(1):316–323. doi: 10.1128/jvi.18.1.316-323.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]