Abstract
4-Monochlorobiphenyl (PCB3) is readily converted by xenobiotic-metabolizing enzymes to dihydroxy-metabolites and quinones. The PCB3 hydroquinone (PCB3-HQ; 2-(4’-chlorophenyl)-1,4-hydroquinone) induces chromosome loss in Chinese Hamster V79 cells, whereas the para-quinone (PCB3-pQ; 2-(4’-chlorophenyl)-1,4-benzoquinone) very efficiently induces gene mutations and chromosome breaks. Apparently, each of these two metabolites, which are a redox pair, has a different spectrum of genotoxic effects due to different, metabolite-specific mechanisms. We hypothesized that the HQ requires enzymatic activation by peroxidases with the formation of reactive oxygen species (ROS) as the ultimate genotoxin, whereas the pQ reacts directly with nucleophilic sites in DNA and/or proteins. To examine this hypothesis, we employed two cell lines with different myeloperoxidase (MPO) activities, MPO-rich HL-60 and MPO-deficient Jurkat cells, and measured cytotoxicity, DNA damage (COMET assay), MPO activity, intracellular levels of reactive oxygen species (ROS) and intracellular free –SH groups (monochlorobimane assay, MCB) and free GSH contents (enzyme recycling method) after treatment with PCB3-HQ and PCB3-pQ. We also examined the modulation of these effects by normal/low temperature, pre-treatment with an MPO inhibitor (succinylacetone, SA), or GSH depletion. PCB3-p-Q increased intracellular ROS levels and induced DNA damage in both HL-60 and Jurkat cells at 37 °C and 6 °C, indicating a direct, MPO-independent mode of activity. It also strongly reduced intracellular free –SH groups and GSH levels in normal and GSH-depleted cells. Thus the ROS increase could be caused by reduced protection by GSH or non-enzymatic autoxidation of the resulting PCB3-HQ-GSH adduct. PCB3-HQ did not produce a significant reduction of intracellular GSH in HL-60 cells and reduced intracellular free –SH groups only at the highest concentration tested in GSH depleted cells. Moreover, PCB3-HQ induced DNA damage and ROS production only at 37 °C in HL-60 cells, not at 6 °C or in Jurkat cells at either temperature; no significant DNA damage and ROS production was observed in HL-60 cells at 37 °C if MPO activity was inhibited by SA. These studies show that the effects of PCB3-HQ are enzyme dependent, i.e. PCB3-HQ is oxidized by MPO in HL-60 cells with the generation of ROS and induction of DNA damage. However, this is not the case with the PCB3-pQ, which may produce DNA damage by the reactivity of the quinone with the DNA or nuclear proteins, or possibly by indirectly increasing intracellular ROS levels by GSH depletion. These different modes of action explain not only the different types of genotoxicity observed previously, but also suggest different organ specificity of these genotoxins.
Keywords: PCB3 hydroquinone, PCB3 para-quinone, Reactive Oxygen Species, DNA damage, Comet assay, Myeloperoxidase
INTRODUCTION
Polychlorinated biphenyls (PCBs) were produced between 1929 and the 1980s and are now an ubiquitous class of persistent environmental pollutants. They were used for a large number of technical applications, such as coolants and lubricants, as dielectric fluids in transformers and capacitors, as additives in paints, plastics, adhesives, and in sealants (ATSDR 2000). It is estimated that world-wide two million tons of commercial PCB mixtures were produced, with roughly 0.2 million tons remaining in various environmental reservoirs (WHO 2003). In addition, a significant amount of PCB mixtures are still in use in older electrical equipment and may be introduced into the environment through careless maintenance. Therefore, PCBs still pose a high risk to human health.
Commercial PCBs are mixtures of the 209 possible congeners, which differ in the number and position of chlorine atoms attached to the biphenyl core. Higher chlorinated congeners (6 or more chlorine atoms) are only slowly metabolized and bioaccumulate in the body and biomagnify in the food chain. Airborne PCBs are lower chlorinated (fewer than 6 chlorine atoms) and are subject to metabolic attack. Lower chlorinated biphenyls are constituents in commercial PCB mixtures and have recently been detected in indoor and outdoor air (Imsilp et al. 2005; Uraki et al. 2004). One study showed that the congeners measured in indoor air in houses built in an Aroclor-1260 contaminated district were mostly 4-monochlorobiphenyl (PCB3) and 2-monochlorobiphenyl (PCB1) (Davis 2002). Human exposure to PCBs occurs through both diet and inhalation (ATSDR 2000; Safe 1998). However, for children, inhalation exposure can be the major source for daily PCB uptake (Wilson et al. 2001). The health consequences of this exposure are not clear and epidemiological studies concerning cancer induction by PCBs in humans, including the risk of leukemia and lymphomas, are contradictory (Cocco et al. 2008; Cogliano 1998; Colt et al. 2005; Engel et al. 2007a; Engel et al. 2007b; McGlynn et al. 2009; Prince et al. 2006; Rubin et al. 2007; Spinelli et al. 2007). Therefore a better understanding of the mechanisms of action (MOA) would help to better judge potential risks from exposure to these compounds.
PCBs are complete carcinogens in rodents (Mayes et al. 1998; Silberhorn et al. 1990). Commercial PCB mixtures and individual congeners which induce cytochrome P-450 dependent monooxygenases (CYPs) in the liver, have been shown to be promoters in two stage hepatocarcinogenesis assays (Dean et al. 2002; Haag-Gronlund et al. 2000). Evidence for tumor initiating activity of lower chlorinated PCBs which can be metabolically activated (PCB3, PCB15, PCB52 and PCB77) was provided using a modified Solt-Farber protocol in male Fischer 344 rats (Espandiari et al. 2003; Espandiari et al. 2004). Also, recently the induction of gene mutations, a typical characteristic of initiators, by PCB3 in the liver and lung of transgenic male Fischer 344 (BigBlue) rats was reported (Lehmann et al. 2007; Maddox et al. 2008). Thus PCB mixtures could be complete carcinogens due to the initiating activity of their lower chlorinated congeners and the promoting activity of the higher chlorinated congeners (Ludewig et al. 2008). However, the mechanism(s) of their cancer initiating potential remains unclear and disputed.
PCB3 can be metabolized by microsomal enzymes to mono- and dihydroxy-metabolites. Dihydroxy-metabolites, which have two hydroxyl group in ortho (catechols, cat) or para position to each other (hydroquinones, HQ), can be further metabolized by peroxidases and/or prostaglandin synthase to semiquinones and ortho- or para-quinones, respectively (McLean et al. 1996a; Srinivasan et al. 2001; Wangpradit et al. 2009). This process is accompanied in vitro by a production of reactive oxygen species (ROS) and DNA damage (Oakley et al. 1996a; Srinivasan et al. 2001). PCB quinones can bind to various amino acids, particularly cysteine, and to nucleic acids and may undergo redox-cycling, with the production of even more ROS (Amaro et al. 1996; McLean et al. 1996b; McLean et al. 2000; Oakley et al. 1996b; Srinivasan et al. 2002). In cells in culture different PCB3 metabolites produce ROS, DNA strand breaks, micronuclei, gene mutations, and a marked decrease in telomere length (Jacobus et al. 2008; Srinivasan et al. 2001; Zettner et al. 2007).
Our goal was to investigate the mechanism(s) by which the hydroquinone (PCB3-HQ) and para-quinone of PCB3 (PCB3-pQ) produce their different genotoxic effects in blood cells. We hypothesized that an enzyme dependent metabolism pathway based on myeloperoxidase (MPO) and/or production of reactive oxygen species should play a crucial role for PCB3-HQ and PCB3-pQ induction of genotoxicity. We therefore investigated the reactivity of these PCB3 metabolites with respect to DNA damage and ROS induction and effects on cellular –SH groups and MPO activity in MPO rich HL-60 cells and MPO-poor Jurkat cells. We cultured cells at 37 °C and 6 °C (to inhibit enzyme activities) and employed the normal Comet assay and modified fragment length analysis using repair enzymes (FLARE) COMET assay, as well as determinations of intracellular ROS, lipid peroxidation, MPO activity, and GSH and free thiol levels in normal and GSH-depleted cells and HL-60 cells with depleted MPO activity.
MATERIALS AND METHODS
Chemicals
2-(4’-chlorophenyl)-1,4-hydroquinone (PCB3-HQ) and 2-(4’-chlorophenyl)-1,4-benzoquinone (PCB3-pQ) were synthesized and characterized as previously described (Amaro et al. 1996; McLean et al. 1996a). Cell culture media and serum were obtained from GIBCO (Grand Island, NY). All other reagents were from Fisher Scientific (Pittsburg, PA), unless otherwise stated.
Cell Culture Conditions and Treatment
The human promyelocytic leukemia cell line HL-60 and the human T-cell leukemia cell line Jurkat (Clone E6-1) were obtained from the American Type Culture Collection (ATCC; Rockville, MD). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin at 37 °C and humidified air with 5% CO2. For experiments, unless otherwise stated, cells were collected by centrifugation, washed once with phosphate buffered saline (PBS), and resuspended in Minimum Essential Medium (MEM) with Hanks salts with antibiotics, but without FBS, to which PCB3-HQ (5, 10 and 20 μM), PCB3-pQ (2.5, 5 and 7.5 μM), or solvent alone (0.5% DMSO final concentration in medium) was added. Exposure to test compounds occurred for 1 or 3 h in an incubator without CO2 at 37 °C or a refrigerator at 6 °C as indicated for the specific assay. Cells cultured at 6 °C did not show any changes in viability and cell proliferation compared to cells at 37 °C (data not shown). This is in agreement with results obtained after maintaining cells for days and even weeks at 6 °C (Hunt et al. 2005).
Evaluation of the Cytotoxic Potential of PCB Hydroquinone and Quinone
To identify the non-toxic concentration range of the test compounds, cell viability after exposure to various concentrations was determined using the trypan blue exclusion assay and resazurin reduction assay (G. Ciapetti 2000; Sandoval et al. 1997). Cell viability at all concentrations and exposure times chosen for study was above 80% for both PCB3-HQ and PCB3-pQ.
Detection of Oxidative Products with DCFH-DA
This method is based on a fluorometric assay described by Rosenkranz and coworkers (Rosenkranz et al. 1992). Briefly, 1 × 106/mL HL-60 or Jurkat cells were incubated in MEM with 5 μM DCFH-DA (Molecular Probes, Eugene, OR) for 30 min, washed twice with PBS, resuspended in MEM, distributed in 200 μl aliquots into 96-well plates, and treated with the different concentrations of the test compounds or solvent alone. After 1 and 3 h of exposure reactive oxygen species formation was determined by measuring fluorescence of DCF per well using a Tecan GeniosPro microplate reader with an excitation wavelength of 485 nm and emission wavelength of 530 nm. All experiments were carried out in triplicate and repeated at least three times.
Comet Assay – Standard Procedure
The Comet assay was performed following the instructions provided in the Trevigen Comet Assay kit. Briefly, 1 × 106 HL-60 or Jurkat cells in 1 mL MEM medium without FBS were seeded into the wells of 24-well plates. Test compounds were added to the wells and cells were incubated for 1 h at both 37 °C and 6 °C. After treatment, cells were collected and washed twice with cold PBS, then resuspended in cold PBS and the cell density adjusted to 1 × 105/mL. Cell aliquots were combined with 1% low-melting agarose at a ratio of 1:10 and pipetted onto Trevigen CometSlides. After the cell/agar mixture had solidified, the cells were lysed at 4 °C in lysis buffer (100 mM Na2EDTA, 2.5 M NaCl, 10 mM Tris (pH 10.0), 1% Triton X-100, and 1% sodium lauroyl sarcosinate) for 45 min. The slides were then immersed in freshly prepared alkali solution (200 mM EDTA adjusted with 1 M NaOH to pH >13) for 30 min, followed by alkaline electrophoresis for 30 min at 1 V/cm. The samples were washed, then fixed with 70% ethanol for 5 min, air dried in the dark overnight, stained with SYBR green for 5 min, and air dried in the dark again. Comets were measured using an Axio A1 Imager epifluorescence microscopy with 10-fold objective, an Axio Cam MRm camera (Zeiss, Jena, Germany), and the free Comet Score™ 1.5 software (http://www.autocomet.com/products_cometscore.php). Data from at least 100 randomly chosen cells per data point were used to calculate the Olive tail moment, which is the Tail length minus Head length multiplied with the %DNA in the Tail divided by 100 [(mean Tail – mean Head) × Tail % DNA/100]. Each experiment was repeated at least three times.
Comet Assay for Oxidative Damage
To identify DNA strand breaks caused by oxidative damage, the Trevigen hOGG1 FLARE kit was used. Briefly, cells were treated with test compounds, washed and resuspended (1 × 105 cells/mL) in cold PBS, combined with 1% low-melting agarose, fixed onto Trevigen CometSlides and lyzed at 4 °C in lysis buffer for 30 min as described above. The samples were then equilibrated with FLARE buffer (10 mM HEPES/KOH (pH 7.4) and 0.1 M KCl) for 15 min. One slide per data point was incubated for 60 min at 37 °C with the hOGG1 enzyme as described in the manual, a second slide was treated with buffer-only as control. Following equilibration with alkali solution (500 mM EDTA adjusted with 5 M NaOH to pH 12.5), the slides underwent electrophoresis for 30 min at 1 V/cm. The samples were then fixed with 70% ethanol, stained with SYBR green, and scored as described above. The difference between the score of the hOGG1-treated samples and buffer controls indicates the amount of oxidative damage to the DNA bases.
Thiobarbituric Acid Reactive Substances (TBARS)
TBARS were measured with a modified method (Wagner et al. 1992). After treatment with test compounds 2.5 × 106 HL-60 or Jurkat cells were centrifuged at 300 × g for 10 min, washed once with PBS and 0.9% NaCl, resuspended in 2 mL 0.9% NaCl, and then immediately frozen at -20 °C until use. Samples were thawed, vortexed vigorously, and 230 μL of trichloroacetic acid-saturated solution (250 g of trichloroacetic acid per 100 mL of water) was added. Samples were then vortexed and centrifuged at 3000 × g (10 min) to precipitate the protein. The supernatant (1.6 mL) was placed in glass test tubes and 200 μL of 14.4 mg/ml 2-thiobarbituric acid in 0.1 N NaOH solution was added. The samples were incubated for 30 min at 75 °C. Standards were prepared from the hydrolysis of 1,1,2,2-ethoxypropane in a 40% trichloroacetic acid solution. After cooling, the absorbance of the samples at 535 nm was measured and TBARS values were calculated as nanomoles/2.5 × 106 cells.
Measurement of MPO Enzyme Activity
Cells treated with PCB3-HQ and PCB3-pQ for 1 and 3 h, were washed once with PBS before MPO was assayed by monitoring the conversion of guaiacol to tetraguaiacol (Wiemels et al. 1999). Briefly, 5×105 cells in 0.08 mL phosphate buffer were incubated with 0.02 mL of 0.02% acetyltrimethylammonium bromide for 5 min at room temperature. To each sample 0.9 mL of assay buffer (10 mM sodium phosphate (pH 7.0), 0.003% H2O2, and 14 mM guaiacol) was added, and the optical density at 470 nm was monitored for 30 s with a Perkin-Elmer Lambda 3B spectrophotometer. Four moles of H2O2 is required to produce 1 mol of tetraguaiacol product which has an extinction coefficient of 26.6 mM-1cm-1. MPO units of activity were calculated as follows:
Where Vt is the total volume (in mL), Vs is the sample volume (in mL), OD is the density change, and t is the time of measurement (minutes).
Glutathione Determination
HL-60 cells, with and without pretreatment with 1 mM diethylmaleate (DEM) for 6 h to deplete intracellular glutathione, were incubated for 1 h with the test compounds and then used to determine the levels of intracellular GSH using the following two methods. GSH-depleted PCB exposed cells were also used to measure DNA damage and intracellular levels of ROS as described above.
(A) Monochlorobimane Assay
Monochlorobimane (MCB) was used as a sensitive and specific probe to analyze GSH in intact cells (Fernandez-Checa and Kaplowitz 1990). Briefly, 3×106 HL-60 cells per sample were washed once with PBS, resuspended in 1 mL PBS containing 100 μmol/L MCB, and maintained at 37 °C in the dark for 30 min before analysis. The formation of the fluorescent adduct (GS-MCB) was monitored with a Tecan GeniosPro microplate reader using excitation and emission wavelengths of 395 and 482 nm, respectively. The GSH content was calculated as nanomoles per 106 cells based on a GSH standard curve.
(B) Enzyme recycling Method
Determination of glutathione was carried out according to a modified method (Baker et al. 1990; Slim et al. 2000). After treatment, cellular protein of HL-60 cells (1×106/mL in MEM in 24 well plate) was precipitated by adding 200 μL of ice-cold 0.09% sulfosalicylic acid (SSA) to each well and incubating at 40°C for 15 min, after which the cell lysates were collected by centrifugation at 10,000 × g for 5 min. Glutathione levels were determined spectrophotometrically using the glutathione-linked 5,5’-dithiobis(2-nitrobenzoic acid, DTNB) recycling assay. Sample cuvettes contained 50 μL of supernatant and 100 μL of 125 mM phosphate buffer containing 0.225 mM DTNB, 0.302 mM NADPH, and glutathione reductase at a concentration of 1.25 U/mL. The blank contained 50 μL of 0.09% 5-SSA instead of supernatant, and the control reaction contained a glutathione standard in place of the supernatant. The mixtures were equilibrated at room temperature for 3 min, and the reaction was started by the addition of 100μL of the reaction buffer. The absorbance was measured at 405 nm in a Tecan Genios pro microplate reader. The intracellular GSH content was calculated as nanomoles/106 cells.
Suppression of MPO
HL-60 cells were incubated for 72 h with 200 μM succinylacetone (SA) dissolved in RPMI 1640 medium. Cell viability, determined microscopically by Trypan blue dye exclusion, was 98%. MPO activity was determined as described above and the MPO protein level was assessed by immunoblotting (Nauseef et al. 1983). Briefly, 1.5 ×105 cells were sonicated on ice and protein from cells was separated by electrophoresis on 12.5% polyacrylamide gels. Separated proteins were transferred onto nitrocellulose membranes and blocked with 5% dry milk in 0.01 M Tris/0.15 M NaCl buffer, pH 8.0, and 0.1% Tween 20. Blots were rinsed three times and incubated with monospecific rabbit polyclonal antiserum against MPO (which was a generous gift from Dr. William M. Nauseef, University of Iowa, diluted 1:500 for use) for 1 h at room temperature. After further washing, the blot was incubated in goat anti-rabbit IgG conjugated with horseradish peroxidase (Santa Cruz Biotechnology Inc, Santa Cruz, CA) at a 1:10,000 dilution for 1 h at room temperature. Blots were washed again, and bands were visualized using chemiluminescence.
MPO-depleted HL-60 cells were used following the same regime of exposure to PCB3-HQ and PCB3-pQ and methods to measured DNA damage and intracellular levels of ROS as described above.
Statistical Analysis
All data are expressed as the mean ± standard deviations computed from at least three independent experiments. ANOVA was used for comparison among multiple groups. The post hoc Tukey's HSD test was used to determine the differences between two groups. A p-value less than 0.05 was considered to be significant.
Results
Evaluation of the Cytotoxic Potential of PCBs hydroquinone and quinone
To establish appropriate concentrations of the PCB3 metabolites for the assays, it was necessary to measure cytotoxicity at various concentrations, time points and cultured conditions. We chose very short 1 h and 3 h time points to determine in parallel genotoxicity, ROS levels, lipid peroxidation, and MPO enzyme activity, and a 1 h time point to correlate with DNA damage and GSH determination. Using V79 cells we had previously observed that 2.5 μM was the lowest concentration that did not reduce the number of cells compared to controls after 24 h continuous exposure to either compound (Zettner et al. 2007). Since this cytotoxicity assay is not applicable for short exposure times we used the trypan blue exclusion assay and the resazurin reduction assay to determine cytotoxicity under our exposure conditions. Concentration of up to 20 μM PCB3-HQ and 7.5 μM PCB3-pQ resulted in >87% viability in both of these assays immediately after the 1 h or 3 h exposure (data not shown). To avoid confounding effects from cytotoxicity these concentrations were selected as upper limit for all of assays described below.
DNA Damage Induction (COMETS) by PCB3 metabolites
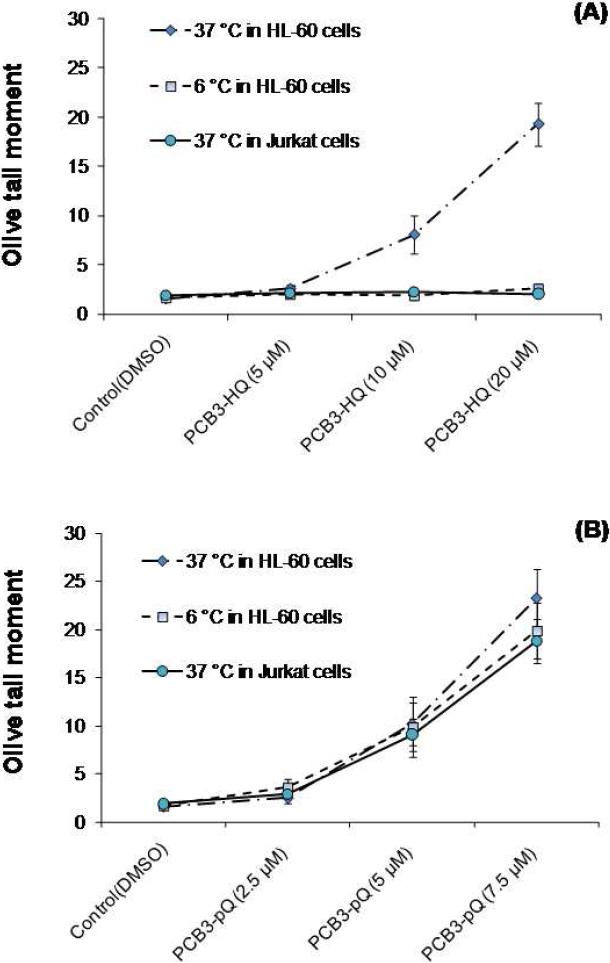
HL-60 and Jurkat cells were exposed to PCB3-HQ and PCB3-pQ for 1 h after which DNA damage was determined with the alkaline COMET assay, which measured single strand breaks and alkaline-labile DNA sites in individual cells. The Olive Tail moment was calculated and used to quantify the damage. As can be seen in Fig. 1A, PCB-HQ induced a dose-dependent increase in DNA damage if treatment occurred at 37 °C. No effect on DNA integrity was observed if cells were exposed at 6 °C. No damage was visible in Jurkat cells. PCB3-pQ was a more efficacious and potent inducer of DNA damage in HL-60 cells at 37 °C than PCB3-HQ (Fig 1B). In addition, PCB-pQ was similarly genotoxic at 6 °C in HL-60 cells and at 37 °C in Jurkat cells compared to the effects in HL-60 cells at 37 °C.
Figure 1.
DNA damage induced by PCB3-HQ (A) and PCB3-pQ (B) in HL-60 and Jurkat cells measured by the standard comet assay. The Olive tail moment is used as the metric for DNA damage. Treatments were conducted for 1 h at 37 and 6 °C.
Generation of Intracellular ROS in HL-60 and Jurkat cells
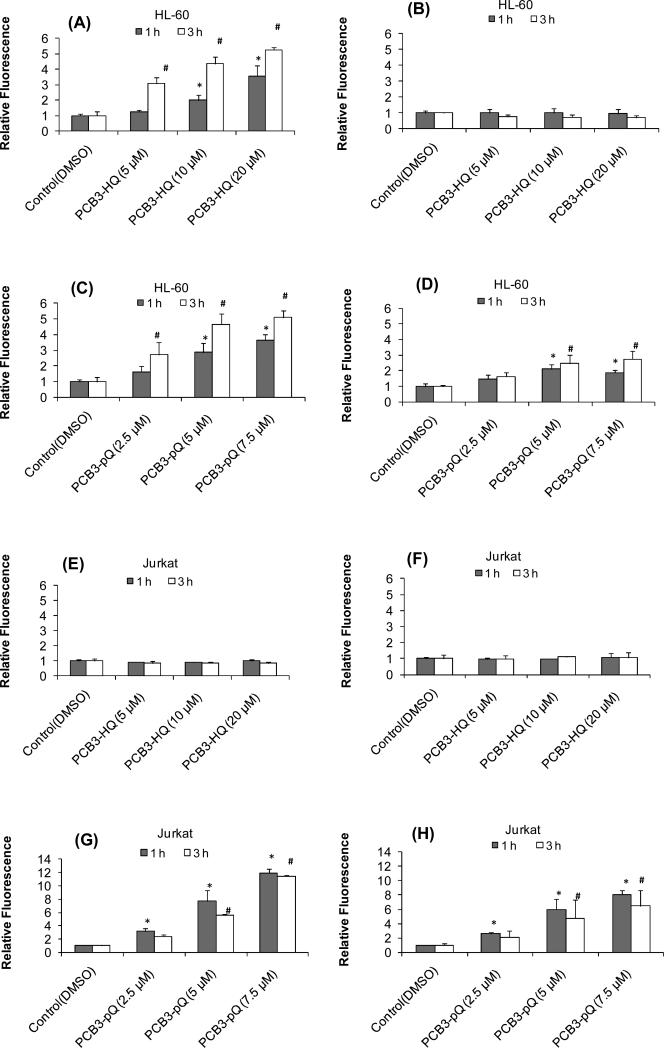
The oxidant sensitive dye DCFH-DA was employed to assess intracellular ROS levels in both HL-60 (MPO rich) and Jurkat (MPO deficient) cells after treatment with test compounds at 37 and 6 °C. Treatment with PCB3-HQ affected intracellular ROS only in HL-60 cells and only at 37 °C. The relative fluorescence showed a dose-dependent increase to 3.5 and 5 times that of the solvent-treated control after 1 and 3 h, respectively (Fig. 2A). No changes in ROS levels were observed in HL-60 cells treated at 6 °C (Fig 2B), or in Jurkat cells treated at 37° and 6 °C for 1 and 3 h (Fig. 2E, F). PCB3-pQ induced an increase in intracellular fluorescence within 1 and 3 h in a dose-dependent manner in both HL-60 (Fig 2C, D) and Jurkat (Fig 2G, H) cells at 37 and 6 °C. HL-60 cells treated with 7.5 μM PCB3-pQ at 37 °C exhibited a relative fluorescence that was 4 and 5.5 times that of the solvent-treated control after 1 and 3 h exposure, respectively; with Jurkat cells the relative fluorescence was 12 and 11.5 times that of solvent-treated controls. After treatment at 6 °C the relative fluorescence was 2.5 and 3 times that of solvent-treated controls in HL-60 cells, and 8 and 6.5 times that of the solvent-treated control in Jurkat cells after 1 and 3 h, respectively.
Figure 2.
Intracellular level of ROS measured by DCF fluorescence after exposure to PCB3-HQ for 1h and 3 h in HL-60 cells at (A - D) and Jurkat cells (E – H) at 37°C (graphs in left column) and 6°C (graphs in right column). *, significantly different compared to the 1 h control, P<0.05; # significantly different compared to the 3 h control, p<0.05.
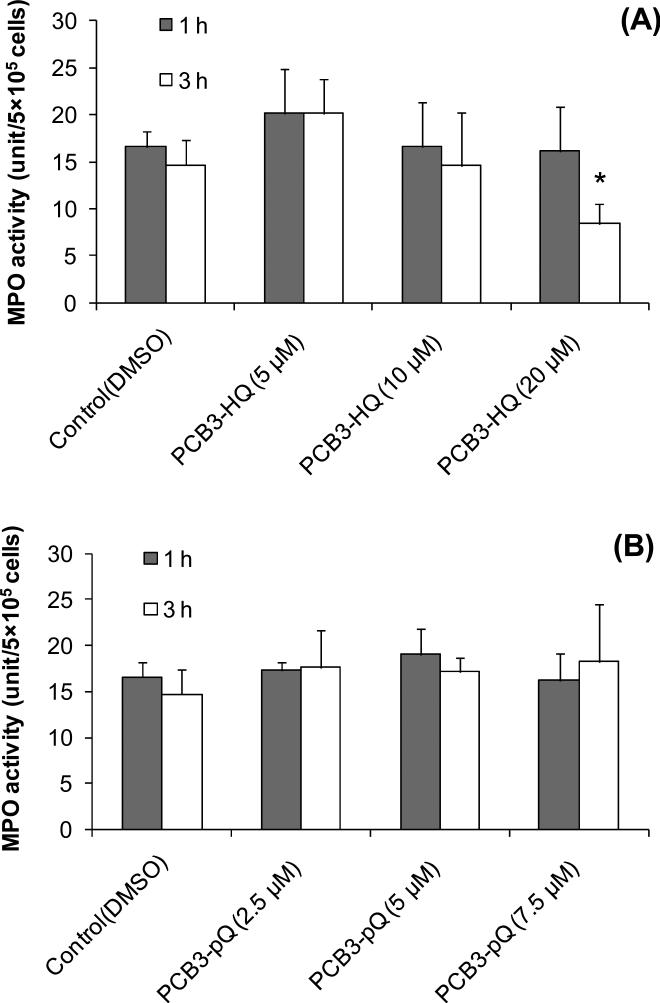
MPO Activity
HL-60 cells contain appreciable MPO activity, whereas Jurkat cells contain only trace amounts of activity (Fig. 3). MPO activity in HL-60 cells (MPO rich) and Jurkat cells (MPO deficient) were 22.0±3.8 unit/5×105 cells and 1.3±0.5 unit/5×105 cells, respectively. After cultivation of HL-60 cells for 72 h in the presence of 200 uM succinylacetate the MPO activity was reduced to 2.2±0.7 unit/5×105 cells.
Figure 3.
MPO activity and protein levels in HL-60 cells, HL-60 cells after 72h incubation with the MPO heme synthesis inhibitor succinylacetone (SA), and in Jurkat cells determined by the guaiacol oxidation assay and western blot analysis (insert), respectively. *, significantly different from HL-60 cells without SA treatment, p<0.05.
The assay used to determine MPO activity measures the activity of heme-peroxidase, and although optimized for MPO and eosinophil peroxidase, it is not specific for any one form of the enzyme (Bozeman et al. 1990). Therefore, we performed Western analysis using an antibody to MPO (Nauseef et al. 1983). Figure 3 shows that the heavy subunit of MPO and precursor MPO are present in the HL-60 cells. No MPO protein was detected in Jurkat cells. Exposure of HL-60 cells to SA strongly reduced MPO protein levels in these cells.
Exposure of HL-60 cells for 1 and 3 h to PCB3-HQ produced a biphasic effect: 5 μM PCB3-HQ produced an increase in MPO activity by 25% and 33% after 1 and 3 h treatment, respectively, compared to solvent treated control (Fig. 4A). No change in MPO activity was observed with 10 μM at both time points and with 20 μM PCB3-HQ after 1 h incubation. Longer exposure of HL-60 cells to PCB3-HQ resulted in an about 50% decrease in MPO activity compared to the control. Treatment of HL-60 cells with PCB3-pQ for 1 and 3 h exposure had no effect on MPO activity at any concentration tested (Fig. 4B).
Figure 4.
MPO activity in HL-60 cells treated for 1 and 3h with PCB3-HQ (A) or PCB3-pQ (B). *, significantly different from the corresponding control, p<0.05.
ROS generation in MPO-depleted HL-60 cells
Reduction of intracellular MPO protein levels and activity strongly reduced ROS generation by PCB3-HQ in HL-60 cells. Exposure to PCB3-HQ for 1 h and 3 h at 37 °C in MPO-depleted HL-60 cells did not cause a change in intracellular ROS levels, whereas a concentration- and time-dependent increase in ROS was seen in HL-60 with normal MPO levels (Fig 5A, B).
Figure 5.
Intracellular level of ROS in HL-60 cells with and without exposure to 200 μM succinylacetone for 72 h to inhibit MPO followed by exposure to PCB3-HQ for 1h (A) and 3h (B) or PCB3-pQ for 1 (C) and 3h (D). a, significantly different compared to normal control cells, p<0.05 ; b, significantly different compared to SA-treated controls, p<0.05 ; c, significant difference between corresponding normal and SA-pretreated cells, p<0.05.
Compared with the normal HL-60 cells, PCB3-pQ induced a similar dose- and time-dependent increase of intracellular ROS production in MPO-depleted HL-60 cells at 37 °C (Fig. 5C, 5D). The increase in fluorescence was even higher in HL-60 cells with very low MPO levels compared to normal HL-60 cells, which was particularly strong after 1 h exposure time and was 5.5 and 6.2 times greater than the solvent-treated control for 1 h and 3 h exposure to 7.5 μM PCB3-pQ, respectively.
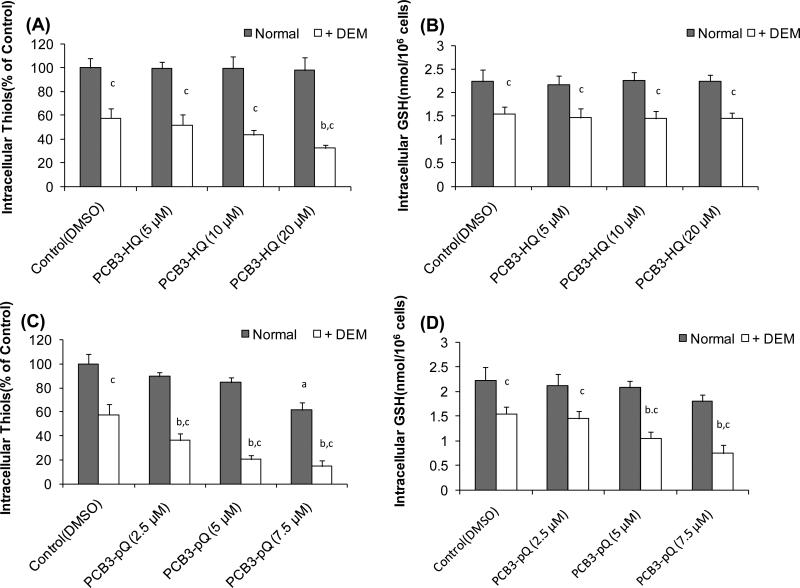
GSH and Free Thiol Depletion by PCB Metabolites in HL-60 Cells in Culture
Monochlorobimane (MCB) was used to measure the quantity of cellular free thiol groups. MCB can diffuse into living cells and forms fluorescent adducts after binding to free -SH groups (Fernandez-Checa and Kaplowitz 1990). Since GSH is the major thiol present in cells, most, but not all, of the fluorescence is due to the binding to intracellular reduced GSH. Exposure of HL-60 cells for 1 h at 37° C to PCB3-HQ did not reduce intracellular free thiol levels (Fig. 6A). In contrast, a dose-dependent decline in intracellular free thiols was found with PCB3-pQ which reached significance at 7.5 μM PCB3-pQ (Fig. 5B). Pretreatment of cells for 6 h with DEM, a GSH depleting compound, reduced intracellular free thiol groups to about 60% of control levels (Fig 6 A, B). In these partially GSH-depleted cells treatment with PCB3-HQ slightly further reduced the free thiol groups in a dose-dependent manner reaching significance at the highest concentration tested (Fig 6A). PCB3-pQ, on the other hand, significantly further depleted the intracellular free thiol groups in a dose-dependent manner in these DEM-treated cells. The effect was more than additive at all 3 concentrations. The maximum depletion in intracellular free thiol groups by DEM plus PCB3-pQ was 80% compared to the normal solvent-treated control at 7.5 μM (Fig. 6B).
Figure 6.
Intracellular thiol (A,C) and GSH (B,D) status in HL-60 cells and HL-60 cells pretreated with the GSH-depleting DEM after exposure to PCB3-HQ (A,B) or PCB-pQ (C,D). a, significantly different compared to normal control cells, p<0.05 ; b, significantly different compared to DEM-pretreated controls, p<0.05 ; c, significant difference between corresponding normal and DEM-pretreated cells, p<0.05.
A method that determines total GSH in cells, i.e. oxidized and reduced, is the enzyme recycling method of (Tietze 1969). The intracellular total GSH content of normal HL-60 cells was approximately 2.2 nmol/106 cells, which is in agreement with that previously reported (Srinivasan et al. 2002). One h exposure to 5-20 μM PCB3-HQ had no influence on the level of intracellular GSH (Fig. 6C). Treatment with PCB3-pQ showed a slight dose-dependent decrease in GSH content but it did not reach significance (Fig. 6D). Pretreatment of cells for 6 h with DEM followed by 1 h exposure to solvent alone reduced intracellular GSH levels by 32% to 1.5 nmol/106 cells. PCB3-HQ treatment of DEM-pretreated cells did not result in any additional reduction in intracellular GSH (Fig. 6C). However, PCB3-pQ caused a further dose-dependent decrease in the intracellular GSH content (Fig 6D). This effect was additive (with 2.5 μM) or more than additive (with 5 and 7.5 μM) and the effect was significant with 5 and 7.5 μM PCB3-pQ, where a reduction to 1.1 nmol/106 and 0.8 nmol/106 cells, equivalent to a reduction of 50% and 64%, respectively, was seen (Fig. 6D).
ROS generation in GSH depleted HL-60 cells
Pretreatment of HL-60 cells for 6 h with the GSH synthesis inhibitor DEM did not have an effect on intracellular ROS as determined by DCF-fluorescence. DEM-pretreatment also did not have a significant effect on the previously described increase in intracellular ROS level due to treatment with PCB3-pQ for 1 or 3 h (Fig 7C, 7D) or PCB3-HQ for 1 h (Fig 7A). However, the PCB3-HQ-induced increase in fluorescence was significantly lower in partially GSH-depleted cells if exposure to the HQ was extended to 3h (Fig 7B).
Figure 7.
Intracellular ROS level in HL-60 cells with and without pretreatment with 1mM DEM for 6 h to deplete cellular GSH followed by exposure to PCB3-HQ for 1h (A) or 3h (B) or PCB3-pQ for 1h (C) or 3h (D). a, significantly different compared to normal control cells, p<0.05 ; b, significantly different compared to DEM-pretreated controls, p<0.05 ; c, significant difference between corresponding normal and DEM-pretreated cells, p<0.05.
Quantification of ROS-produced DNA Damage and Modulation of DNA Damage by Changes in Intracellular MPO and GSH Levels in HL-60 Cells
Intracellular ROS generation induces lipid, protein, and DNA oxidation and transcription factor(s) activation (Nishigori et al. 2004). DNA strand breaks and formation of 8-oxodeoxyguanosine have been extensively used as markers for DNA oxidation (Floyd 1990; Nishigori et al. 2004). An enzymatic modification of the standard comet assay, the hOGG1 FLARE comet assay, allows for the qualitative determination of oxidized bases (Singh et al. 1988). Total and oxidized DNA damage was determined in normal and SA- and DEM-pretreated cells after 1h exposure to PCB3-HQ and PCB3-pQ at 37C.
Baseline DNA damage in solvent controls was low and about a third of it was due to oxidized bases (Fig 8). Reduction of intracellular MPO activity with SA did not have a significant effect on total DNA damage, but slightly increased the baseline levels of oxidized bases to ~40% of total damage. Reduction of intracellular GSH with DEM slightly increased the baseline level of DNA damage which was primarily due to roughly a doubling of the normal level of oxidized bases to >50% of total damage.
Figure 8.
DNA damage induced by PCB3-HQ (A) and PCB3-pQ (B) in HL-60 cells determined by the standard comet assay and hOGG1 FLARE comet assay. The Olive tail moment is used as the metric for DNA damage. Treatments were conducted for 1 h at 37°C with and without pre-treatment with succinylacetone (SA) to reduce MPO activity or diethylmaleate (DEM) to lower intracellular GSH levels. * and #, significantly different compared to the control in Comet and Flare assay, respectively, P<0.05.
The dose-dependent increase in total DNA damage caused by PCB3-HQ was reduced by 60-90% in cells with low MPO activity (Fig 8A). The DNA damage due to oxidized bases was similarly affected. In contrast, no effect of MPO reduction was seen in cells treated with PCB3-pQ in either total or oxidized DNA damage (Fig 8B). DEM-pretreatment slightly increased total DNA damage in PCB3-HQ treated cells, an effect that was primarily due to a relatively strong increase in oxidized DNA damage (Fig 8A). No significant effect of partial GSH-depletion on total and oxidized DNA damage was seen in cells treated with PCB3-pQ (Fig 8B).
Thiobarbituric Acid Reactive Substances
The levels of TBARS from HL-60 cells treated with different concentrations of PCB3-HQ and PCB3-pQ were evaluated for the 1 and 3 h exposure time points. Only trace amounts of TBARS production was observed in solvent-treated controls and no significant changes were seen after exposure to PCB3-HQ and PCB3-pQ at all concentrations tested (data not shown). This suggests that lipid peroxidation is not being induced to any great extent by the PCB3-HQ and PCB3-pQ.
Discussion
It is well established that lower chlorinated PCBs are subject to metabolic activation and that their metabolism may lead to the formation of electrophiles and ROS (Ludewig et al. 2008; Ludewig et al. 2000; Safe 1989). More specifically, PCB3 can be metabolized by cytochrome P450s to form initially mono- and then di-hydroxylated derivatives (McLean et al. 1996a). The dihydroxy-metabolites, which include the catechols and hydroquinones, can be further oxidized by peroxidases and/or prostaglandin synthase (PGS) to the semiquinones and quinones which is accompanied by the generation of ROS (Oakley et al. 1996a; Wangpradit et al. 2009). Furthermore, PCB3-pQ can be non-enzymatically reduced via Michael addition of GSH, and the resulting PCB3-HQ-GSH adduct can re-enter the same enzymatic or non-enzymatic redox cycling with more generation of ROS (Amaro et al. 1996; Srinivasan et al. 2001). Among these compounds, PCB3 itself does not induce genotoxic damage in V79 Chinese hamster cells in culture, but several of the monohydroxy-, dihydroxy, and quinone derivatives induced various types of genotoxic damage (Zettner et al. 2007). Reactive electrophilic intermediates like the quinones and ROS can bind/modify cellular macromolecules, including DNA, and induce DNA damage in vitro (Oakley et al. 1996a; Srinivasan et al. 2001). PCB3 was mutagenic in the livers of rats in vivo, presumably after metabolic activation to one of these reactive derivatives (Lehmann et al. 2007). In any case the underlying mechanism(s) of these genotoxic effects and crucial components, such as which enzyme-dependent or non-enzymatic pathway(s) and which reactive electrophilic forms play the crucial role of the genotoxicity of PCB3 are still unknown. In the present study we analyzed the markers of oxidative stress, DNA damage and certain oxidant enzymes in order to better understand the genotoxic potentials and its probable pathway(s) for the redox pair PCB3-HQ and PCB3-pQ.
To study the importance of certain enzymes we employed two different human cell lines, HL-60 (promyelocytic leukemia) and Jurkat (T-cell leukemia). PCB3-HQ induced DNA damage only in HL-60 cells and only at 37°C, indicating that enzyme activity, present in the promyelotic but not the T-cell leukemia line, was required for activation of the HQ to a genotoxic agent. PCB3-pQ, on the other hand, produced DNA damage at low concentrations in both cell lines and at all temperatures, indicating that no enzymatic activation was required.
Since both metabolites may undergo redox reactions, we hypothesized that generation of reactive oxygen species (ROS) may be involved in the genotoxic activity of either or both compounds. Measurement of intracellular ROS with the DCFH-DA assay showed that PCB3-HQ increased ROS in a dose-dependent way, but only in HL-60 cells and only at 37°C. This is exactly the same pattern as observed for DNA damage induction, indicating that metabolic generation of ROS may be the cause of the genotoxicity of this compound. At 6°C the metabolizing enzymes lose most of their catalyzing activity, which means slower speed of metabolism and lower product generation. Although this is a general principle for all cellular enzymes and is not limited to certain enzymes, like MPO, this finding indicates that metabolic activity is a requirement for ROS generation and genotoxicity of PCB3-HQ and that Jurkat cells miss the crucial enzyme(s) that could perform this biotransformation.
PCB3-pQ, on the other hand, increased ROS in both cell lines and at both temperatures, again the same pattern as for genotoxicity induction, which indicates that the mechanisms of ROS production are different for these two compounds. It seems that PCB3-pQ produces oxidative stress and genotoxicity by a non-enzymatic mechanism in these cell lines. The fact that ROS production was higher at 37° than at 6°C in both cell lines may be due to changes in membrane fluidity or transport processes at the lower temperature, which may reduce uptake of the compound into the cells, or a partial involvement of enzymes in the ROS generation and DNA damage. ROS production by PCB3-pQ was twice as high in Jurkat compared to HL-60 cells. This suggests that the two cell lines differ not only in the activating enzyme activity, but possibly also in antioxidant enzyme levels, such as in SOD or glutathione peroxidase. Despite the much higher level of ROS in Jurkat cells, DNA damage was slightly lower in Jurkat than in HL-60 cells. This suggests that ROS is not the only or the main cause for the DNA damage, but that binding of the PCB3-pQ to nucleophilic sites in DNA, topoisomerase II, and other proteins could be a major factor in the generation of the observed DNA damage. Also, prolonged exposure (3 instead of 1 h) resulted in increased ROS levels in HL-60 cells but not in Jurkat cells. It could be speculated that in HL-60 cells a second, enzymatic pathway, may be responsible for the ongoing ROS generation. Since non-enzymatic binding of GSH to PCB3-pQ leads to the generation of a GSH-PCB3-HQ adduct, this secondary mechanisms could be due to enzymatic oxidation of this adduct to the corresponding quinone with ROS generation.
HL-60 cells are rich in MPO activity and MPO was shown to catalyze benzene bioactivation by converting the hydroquinone to the quinone accompanied by the production of ROS and increased levels of oxidative DNA damage (Subrahmanyam et al. 1991; Wiemels et al. 1999). We hypothesized that in HL-60 cells the PCB3-HQ requires enzymatic activation by MPO with the formation of reactive derivatives (ROS or PCB3-derived) as the ultimate genotoxic species. Measurements of MPO activity and protein content confirmed Jurkat cells are basically deficient of MPO, whereas HL-60s express a high level of MPO. This supports but does not prove our hypothesis about MPO-involvement in PCB3-HQ bioactivation. The test compounds did not affect MPO activity with the exception of the highest concentration (20 μM) of PCB3-HQ, which significantly decreased the MPO activity after 3 h of treatment. Thus enzyme inactivation by the PCB3-pQ or induction by PCB3-HQ was not involved in the different activity patterns of these compounds. We then used succinylacetone to inhibit MPO synthesis in HL-60 cells to produce a sister cell that differed only in this one enzyme activity. Succinylacetone (SA) efficiently reduces MPO by blocking the heme synthesis (Fan et al. 2006; Kagan et al. 2000; Nakazato et al. 2007). During MPO maturation, heme is incorporated into apoproMPO and then the heme containing proMPO becomes enzymatically active MPO (Hansson et al. 2006). Pretreatment of HL-60 cells with succinylacetone generated apoproMPO without peroxidase activity due to the lack of heme incorporation (Fig 3). HL-60 cells without MPO activity no longer produced ROS or DNA damage during exposure to PCB3-HQ (Fig 5, 8). This supports our hypothesis that MPO and not any other enzyme is an essential requirement for the generation of ROS and DNA damage by PCB3-HQ in HL-60 cells. The correlation in ROS and DNA strand break production suggests, but does not prove, that the former is responsible for the latter. Use of a selective ROS, scavenger that does not influence PCB3-derivative levels during this process could clarify whether it is indeed an ROS and which one that causes the DNA damage. ROS and DNA damage by PCB3-pQ was not inhibited by MPO depletion in HL-60 cells; if anything, ROS production increased in the absence of MPO (Figure 5, 8). Also, at high concentrations of PCB3-pQ, ROS levels were significantly higher in SA-treated cells compared to the normal cells, but DNA damage was not similarly increased. This indicates that other factors, like formation of DNA and protein adducts, are, at least in part, probable mechanisms of the genotoxicity induced by PCB3-pQ.
We have shown previously that PCB3-pQ instantaneously and very efficiently binds to sulfhydryl groups (Amaro et al. 1996; Srinivasan et al. 2002). We therefore hypothesized that the non-enzymatic induction of DNA damage by this compound involved binding to protein or GSH sulfhydryl-groups, whereas PCB3-HQ should not bind GSH or only after being oxidized to the quinone. To study this hypothesis, we measured free sulfhydryl groups (MCB assay) and total GSH levels (enzyme recycling method) in normal cells and cells that were pretreated with the GSH-depleting agent DEM, and then exposed the cells to the test compounds. GSH is a cellular peptide which has important protective functions through conjugation with electrophilic xenobiotics and through scavenging of ROS and their reaction products (Hanna and Mason 1992; Spear and Aust 1995). Thus GSH depletion may result in increased oxidative stress due to the reduction in ROS-scavenging activity. Indeed the baseline level of DNA damage was slightly higher in DEM-pretreated cells because of an increase in oxidative DNA damage, even though the ROS was not increased, indicating that the FLARE-Comet assay is more sensitive to changes in oxidative stress than the DCF-DA assay and/or that the nucleus/nuclear DNA is particularly vulnerable to increased oxidative stress.
As expected, PCB3-HQ did not produce a significant change in free thiol or GSH content with the exception of a dose-dependent decrease in free –SH in GSH-depleted cells (Figure 6). This may be partially due to GSH oxidation by ROS to GSSG, which would only be discovered by the MCB assay, not by the enzyme cycling method measuring total GSH. However, ROS levels were slightly lower in GSH-depleted cells compared to normal cells after PCB3-HQ treatment for 3 h and MPO-activity was significantly inhibited with 20 μM PCB3-HQ. Thus binding of the oxidation product of PCB3-HQ to protein –SH at high levels of the test compound may have occurred.
PCB3-pQ decreased the free thiol and GSH levels in a dose-dependent manner, supporting our hypothesis that sulfhydryl-binding by the quinone is responsible for its genotoxic effects. GSH depletion did not influence PCB3-pQ-induced oxidative stress or DNA damage, possibly because it was never severe enough to cross a threshold level. It was reported that only severe GSH depletion will lead to an abrupt and extensive lipid peroxidation (Comporti et al. 1991) and GSH depletion by 70-80% of the total levels is needed to impair the cell's defense against oxidative damage from H2O2 and lead to cell death (Pascoe and Reed 1989). The highest level of GSH-depletion that we reached was ~40% in normal cells and ~70% in DEM-pretreated cells, not enough to produce lipid peroxidation-derived TBARS. It seems that GSH was never compromised enough to be responsible for the ROS or DNA damage. Both must be due to a more direct effect of PCB3-pQ or the GSH-adduct.
The modified COMET assay can be used to quantify two types of oxidative DNA damage, 8-oxo-deoxyguanine (8-oxo-dG) and formamidopyrimidine moieties. In this assay cells are treated with a repair enzyme for these oxidative DNA base modifications, the human 8-oxoguanine DNA glycosylase 1 (hOGG1), and the results are compared with the normal COMET assay. In HL-60 control cells about 30% of DNA damage was due to these oxidized bases, which increased to >50% in cells pretreated with DEM, indicating that partial GSH depletion had a negative effect on DNA integrity in the cell nucleus.
PCB3-HQ significantly increased in a dose-dependent manner both total and oxidative DNA damage in HL-60 cells, and the increase of oxidized DNA damage was about 10-25% above the solvent control, although no dose-dependency was observed (Figures 1, 8). This supports the hypothesis that this compound is genotoxic at least to a large part through ROS generation and oxidative DNA damage. A similar increase in DNA damage, 8-oxo-dG, and ROS was seen in HepG2 cells treated with similar concentrations of the hydroquinone of benzene and the authors came to the same conclusion, namely that ROS may be responsible for most or much of the DNA damage (Ibuki and Goto 2004; Luo et al. 2008). A similar relative increase in oxidative DNA base damage was seen with the lowest concentration of PCB3-pQ, but the percent of oxidative DNA base damage decreased with increasing concentration of the test compound. This may indicate that at low concentrations of PCB3-pQ, a relatively larger proportion of DNA damage is due to ROS, whereas at higher concentrations other mechanisms, for example, adduct formation with DNA or nuclear proteins, becomes the dominating mechanism.
MPO, a heme-protein, is a component of the oxygen-dependent antibacterial system of the cell (Babior 1978; Klebanoff 1968). It catalyzes the conversion of H2O2 into HOCl and has the capacity to oxidize Cl- to Cl+ at physiologic pH, a property that is unique to MPO (Hansson et al. 2006). It is abundant in the azurophilic granules of neutrophils, neutrophil precursors and monocyte/macrophages, and is present at the promyelocyte stage of myeloid maturation (Pember and Kinkade 1983; Subrahmanyam et al. 1991). Several environmental chemicals are reported to be activated by MPO to genotoxic metabolites (Arlt et al. 2006; Isola et al. 1993; Kagan et al. 2000; Kalf et al. 1991; Lakshmi et al. 2000; Shen et al. 1990), which could be the basis of selective target organ toxicity and carcinogenicity of certain compounds.
Our studies demonstrate that PCB3-HQ increased ROS levels and DNA damage by an enzyme dependent mechanism, that MPO playd a crucial role, and that ROS may be the major cause for the DNA damage. PCB3-pQ increased ROS levels and DNA damage by enzyme-independent mechanisms, and DNA- and/or protein adduction, which may inhibit nuclear proteins like topoisomerase II (Bender et al. 2006), seem to be significant factors in DNA damage induction. These different modes of action explain not only the different types of genotoxicity observed with these two compounds, but also suggest possible target organs. Investigations revealed that hydroxylated metabolites of PCBs are retained in human plasma and in rodent tissue (Bergman et al. 1994; Sjodin et al. 2000; Wehler et al. 1989) and the formation of quinone-derived protein adducts in the liver and brain of PCB-exposed rats has been reported (Haag-Gronlund et al. 2000; Lin et al. 2000). Thus a scenario of hydroxylated PCB metabolites reaching the bone marrow and other organs containing peroxidases and there being activated to genotoxic compounds, as shown here, cannot be excluded and certainly should be considered in the risk assessment for human health exposure to lower chlorinated PCBs.
Acknowledgements
The authors would like to thank Dr. William M. Nauseef for generously providing the antiserum against MPO and Dr. Hans-Joachim Lehmler for synthesizing and characterizing the study chemicals. This research was supported by grant number P42 ES 013661 from the National Institute of Environmental Health Sciences (NIEHS), NIH, DAMD17-02-1-0241 from the Department of Defense (DOD) and the University of Iowa Center for the Health Effect of Environmental Contaminants (CHEEC). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic Activation of PCBs to Quinones: Reactivity toward Nitrogen and Sulfur Nucleophiles and Influence of Superoxide Dismutase. Chem. Res. Toxicol. 1996;9(3):623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Henderson CJ, Wolf CR, Schmeiser HH, Phillips DH, Stiborova M. Bioactivation of 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone: evidence for DNA adduct formation mediated by cytochrome P450 enzymes and peroxidases. Cancer Lett. 2006;234(2):220–31. doi: 10.1016/j.canlet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- ATSDR Toxicological Profile for Polychlorinated Biphenyls. 2000. [PubMed]
- Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978;298(13):721–5. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Analytical Biochemistry. 1990;190(2):360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIalpha: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45(33):10140–52. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994;102(5):464–9. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeman PM, Learn DB, Thomas EL. Assay of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Journal of Immunological Methods. 1990;126(1):125–133. doi: 10.1016/0022-1759(90)90020-v. [DOI] [PubMed] [Google Scholar]
- Cocco P, Brennan P, Ibba A, de Sanjose Llongueras S, Maynadie M, Nieters A, Becker N, Ennas MG, Tocco MG, Boffetta P. Plasma polychlorobiphenyl and organochlorine pesticide level and risk of major lymphoma subtypes. Occup Environ Med. 2008;65(2):132–40. doi: 10.1136/oem.2007.033548. [DOI] [PubMed] [Google Scholar]
- Cogliano VJ. Assessing the cancer risk from environmental PCBs. Environ Health Perspect. 1998;106(6):317–23. doi: 10.1289/ehp.98106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colt JS, Severson RK, Lubin J, Rothman N, Camann D, Davis S, Cerhan JR, Cozen W, Hartge P. Organochlorines in carpet dust and non-Hodgkin lymphoma. Epidemiology. 2005;16(4):516–25. doi: 10.1097/01.ede.0000164811.25760.f1. [DOI] [PubMed] [Google Scholar]
- Comporti M, Maellaro E, Del Bello B, Casini AF. Glutathione depletion: its effects on other antioxidant systems and hepatocellular damage. Xenobiotica. 1991;21(8):1067–76. doi: 10.3109/00498259109039546. [DOI] [PubMed] [Google Scholar]
- Davis B, Beach J, Wade M, Klein AK, Hoch K. Risk assessment of polychlorinated biphenyls (PCBs) in indoor air. The Toxicologist, Supplement to Toxicological Sciences. 2002;66:516. [Google Scholar]
- Dean CE, Jr., Benjamin SA, Chubb LS, Tessari JD, Keefe TJ. Nonadditive Hepatic Tumor Promoting Effects by a Mixture of Two Structurally Different Polychlorinated Biphenyls in Female Rat Livers. Toxicol. Sci. 2002;66(1):54–61. doi: 10.1093/toxsci/66.1.54. [DOI] [PubMed] [Google Scholar]
- Engel LS, Laden F, Andersen A, Strickland PT, Blair A, Needham LL, Barr DB, Wolff MS, Helzlsouer K, Hunter DJ. Polychlorinated biphenyl levels in peripheral blood and non-Hodgkin's lymphoma: a report from three cohorts. Cancer Res. 2007a;67(11):5545–52. doi: 10.1158/0008-5472.CAN-06-3906. others. [DOI] [PubMed] [Google Scholar]
- Engel LS, Lan Q, Rothman N. Polychlorinated biphenyls and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007b;16(3):373–6. doi: 10.1158/1055-9965.EPI-07-0055. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186(1):55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol Sci. 2004;79(1):41–6. doi: 10.1093/toxsci/kfh097. [DOI] [PubMed] [Google Scholar]
- Fan Y, Schreiber EM, Giorgianni A, Yalowich JC, Day BW. Myeloperoxidase-Catalyzed Metabolism of Etoposide to Its Quinone and Glutathione Adduct Forms in HL60 Cells. Chem. Res. Toxicol. 2006;19(7):937–943. doi: 10.1021/tx0600595. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190(2):212–9. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- G. Ciapetti DGECLSDCAP Cytotoxic effect of bone cements in HL-60 cells: Distinction between apoptosis and necrosis. Journal of Biomedical Materials Research. 2000;52(2):338–345. doi: 10.1002/1097-4636(200011)52:2<338::aid-jbm13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Haag-Gronlund M, Conolly R, Scheu G, Warngard L, Fransson-Steen R. Analysis of Rat Liver Foci Growth with a Quantitative Two-Cell Model after Treatment with 2,4,5,3′,4′-Pentachlorobiphenyl. Toxicol. Sci. 2000;57(1):32–42. doi: 10.1093/toxsci/57.1.32. [DOI] [PubMed] [Google Scholar]
- Hanna PM, Mason RP. Direct evidence for inhibition of free radical formation from Cu(I) and hydrogen peroxide by glutathione and other potential ligands using the EPR spin-trapping technique. Arch Biochem Biophys. 1992;295(1):205–13. doi: 10.1016/0003-9861(92)90507-s. [DOI] [PubMed] [Google Scholar]
- Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Archives of Biochemistry and Biophysics. 2006;445(2):214–224. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hunt L, Hacker DL, Grosjean F, De Jesus M, Uebersax L, Jordan M, Wurm FM. Low-temperature pausing of cultivated mammalian cells. Biotechnol Bioeng. 2005;89(2):157–63. doi: 10.1002/bit.20320. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, Goto R. Dysregulation of apoptosis by benzene metabolites and their relationships with carcinogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2004;1690(1):11–21. doi: 10.1016/j.bbadis.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Imsilp K, Wiedenmann L, Bordson GO, Morrow CK, Cope R, Hansen LG. Time- and tissue-dependent polychlorinated biphenyl residues in hairless mice after exposure to polychlorinated biphenyl-contaminated soil. Arch Environ Contam Toxicol. 2005;49(1):105–18. doi: 10.1007/s00244-004-0116-y. [DOI] [PubMed] [Google Scholar]
- Isola VJ, Hartman TC, Trumble SJ, Ruzek MC, Gentile JM. Metabolism of 2-aminofluorene by human polymorphonuclear leukocytes: more evidence for the association between inflammation and cancer. Environ Health Perspect. 1993;101(Suppl 3):27–31. doi: 10.1289/ehp.93101s327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus JA, Flor S, Klingelhutz A, Robertson LW, Ludewig G. 2-(4′-Chlorophenyl)-1,4-Benzoquinone Increases the Frequency of Micronuclei and Shortens Telomeres. Environ Toxicol Pharmacol. 2008;25(2):267–272. doi: 10.1016/j.etap.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Kuzmenko AI, Shvedova AA, Kisin ER, Tyurina YY, Yalowich JC. Myeloperoxidase-Catalyzed Phenoxyl Radicals of Vitamin E Homologue, 2,2,5,7,8-Pentamethyl-6-hydroxychromane, Do Not Induce Oxidative Stress in Live HL-60 Cells. Biochemical and Biophysical Research Communications. 2000;270(3):1086–1092. doi: 10.1006/bbrc.2000.2564. [DOI] [PubMed] [Google Scholar]
- Kalf G, Shurina R, Renz J, Schlosser M. The role of hepatic metabolites of benzene in bone marrow peroxidase-mediated myelo- and genotoxicity. Adv Exp Med Biol. 1991;283:443–55. doi: 10.1007/978-1-4684-5877-0_60. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95(6):2131–8. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi VM, Hsu FF, Davis BB, Zenser TV. N-Acetylbenzidine-DNA adduct formation by phorbol 12-myristate-stimulated human polymorphonuclear neutrophils. Chem Res Toxicol. 2000;13(8):785–92. doi: 10.1021/tx0000320. [DOI] [PubMed] [Google Scholar]
- Lehmann L, L.Esch H, A.Kirby P, W.Robertson L, Ludewig G. 4-Monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28(2):471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A, Swenberg JA. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2′,5, 5′-tetrachlorobiphenyl. Chem Res Toxicol. 2000;13(8):710–8. doi: 10.1021/tx000030f. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ Toxicol Pharmacol. 2008;25(2):241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig G, Srinivasan A, Robertson LW. Mechanisms of toxicity of PCB metabolites: generation of reactive oxygen species and glutathione depletion. Cent Eur J Public Health. 2000;8(Suppl):15–7. [PubMed] [Google Scholar]
- Luo L, Jiang L, Geng C, Cao J, Zhong L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chemico-Biological Interactions. 2008;173(1):1–8. doi: 10.1016/j.cbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Maddox C, Wang B, Kirby PA, Wang K, Ludewig G. Mutagenicity of 3-Methylcholanthrene, Pcb3, and 4-Oh-Pcb3 in the Lung of Transgenic Bigblue Rats. Environ Toxicol Pharmacol. 2008;25(2):260–266. doi: 10.1016/j.etap.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci. 1998;41(1):62–76. doi: 10.1093/toxsci/41.1.62. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Polychlorinated biphenyls and risk of testicular germ cell tumors. Cancer Res. 2009;69(5):1901–9. doi: 10.1158/0008-5472.CAN-08-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of Catechol and Hydroquinone Metabolites of 4-Monochlorobiphenyl. Chem. Res. Toxicol. 1996a;9(1):158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- McLean MR, Robertson LW, Gupta RC. Detection of PCB Adducts by the 32P-Postlabeling Technique. Chem. Res. Toxicol. 1996b;9(1):165–171. doi: 10.1021/tx9500843. [DOI] [PubMed] [Google Scholar]
- McLean MR, Twaroski TP, Robertson LW. Redox cycling of 2-(x'-mono, -di, -trichlorophenyl)- 1, 4-benzoquinones, oxidation products of polychlorinated biphenyls. Arch Biochem Biophys. 2000;376(2):449–55. doi: 10.1006/abbi.2000.1754. [DOI] [PubMed] [Google Scholar]
- Nakazato T, Sagawa M, Yamato K, Xian M, Yamamoto T, Suematsu M, Ikeda Y, Kizaki M. Myeloperoxidase Is a Key Regulator of Oxidative Stress Mediated Apoptosis in Myeloid Leukemic Cells. Clin Cancer Res. 2007;13(18):5436–5445. doi: 10.1158/1078-0432.CCR-07-0481. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Root RK, Malech HL. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983;71(5):1297–307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigori C, Hattori Y, Toyokuni S. Role of Reactive Oxygen Species in Skin Carcinogenesis. Antioxidants & Redox Signaling. 2004;6(3):561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Devanaboyina U, Robertson LW, Gupta RC. Oxidative DNA Damage Induced by Activation of Polychlorinated Biphenyls (PCBs): Implications for PCB-Induced Oxidative Stress in Breast Cancer. Chem. Res. Toxicol. 1996a;9(8):1285–1292. doi: 10.1021/tx960103o. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Robertson LW, Gupta RC. Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis. 1996b;17(1):109–114. doi: 10.1093/carcin/17.1.109. [DOI] [PubMed] [Google Scholar]
- Pascoe GA, Reed DJ. Cell calcium, vitamin E, and the thiol redox system in cytotoxicity. Free Radic Biol Med. 1989;6(2):209–24. doi: 10.1016/0891-5849(89)90118-4. [DOI] [PubMed] [Google Scholar]
- Pember SO, Kinkade JM., Jr Differences in myeloperoxidase activity from neutrophilic polymorphonuclear leukocytes of differing density: relationship to selective exocytosis of distinct forms of the enzyme. Blood. 1983;61(6):1116–1124. [PubMed] [Google Scholar]
- Prince MM, Ruder AM, Hein MJ, Waters MA, Whelan EA, Nilsen N, Ward EM, Schnorr TM, Laber PA, Davis-King KE. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). Environ Health Perspect. 2006;114(10):1508–14. doi: 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J Immunol Methods. 1992;156(1):39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Rubin CS, Holmes AK, Belson MG, Jones RL, Flanders WD, Kieszak SM, Osterloh J, Luber GE, Blount BC, Barr DB. Investigating childhood leukemia in Churchill County, Nevada. Environ Health Perspect. 2007;115(1):151–7. doi: 10.1289/ehp.9022. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs): mutagenicity and carcinogenicity. Mutat Res. 1989;220(1):31–47. doi: 10.1016/0165-1110(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Safe SH. Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds. J. Anim Sci. 1998;76(1):134–141. doi: 10.2527/1998.761134x. [DOI] [PubMed] [Google Scholar]
- Sandoval M, Zhang X-J, Liu X, Mannick EE, Clark DA, Miller MJS. Peroxynitrite-Induced Apoptosis in T84 and RAW 264.7 Cells: Attenuation by -Ascorbic Acid. Free Radical Biology and Medicine. 1997;22(3):489–495. doi: 10.1016/s0891-5849(96)00374-7. [DOI] [PubMed] [Google Scholar]
- Shen JH, Wegenke M, Wolff T. Capability of human blood cells to form the DNA adduct, C8-(N2-aminofluorenyl)-deoxyguanosine-3′-5′-diphosphate from 2-aminofluorene. Carcinogenesis. 1990;11(8):1441–4. doi: 10.1093/carcin/11.8.1441. [DOI] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20(6):440–96. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Hagmar L, Klasson-Wehler E, Bjork J, Bergman A. Influence of the consumption of fatty Baltic Sea fish on plasma levels of halogenated environmental contaminants in Latvian and Swedish men. Environ Health Perspect. 2000;108(11):1035–41. doi: 10.1289/ehp.108-1240159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim R, Toborek M, Robertson LW, Lehmler HJ, Hennig B. Cellular Glutathione Status Modulates Polychlorinated Biphenyl-Induced Stress Response and Apoptosis in Vascular Endothelial Cells. Toxicology and Applied Pharmacology. 2000;166(1):36–42. doi: 10.1006/taap.2000.8944. [DOI] [PubMed] [Google Scholar]
- Spear N, Aust SD. Effects of glutathione on Fenton reagent-dependent radical production and DNA oxidation. Arch Biochem Biophys. 1995;324(1):111–6. doi: 10.1006/abbi.1995.9921. [DOI] [PubMed] [Google Scholar]
- Spinelli JJ, Ng CH, Weber JP, Connors JM, Gascoyne RD, Lai AS, Brooks-Wilson AR, Le ND, Berry BR, Gallagher RP. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121(12):2767–75. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Lehmler H-J, Robertson LW, Ludewig G. Production of DNA Strand Breaks in Vitro and Reactive Oxygen Species in Vitro and in HL-60 Cells by PCB Metabolites. Toxicol. Sci. 2001;60(1):92–102. doi: 10.1093/toxsci/60.1.92. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Robertson LW, Ludewig G. Sulfhydryl binding and topoisomerase inhibition by PCB metabolites. Chem Res Toxicol. 2002;15(4):497–505. doi: 10.1021/tx010128+. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam VV, Ross D, Eastmond DA, Smith MT. Potential role of free radicals in benzene-induced myelotoxicity and leukemia. Free Radical Biology and Medicine. 1991;11(5):495–515. doi: 10.1016/0891-5849(91)90063-9. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Uraki Y, Suzuki S, Yasuhara A, Shibamoto T. Determining sources of atmospheric polychlorinated biphenyls based on their fracturing concentrations and congener compositions. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39(11-12):2755–72. [PubMed] [Google Scholar]
- Wagner BA, Buettner GR, Burns CP. Membrane peroxidative damage enhancement by the ether lipid class of antineoplastic agents. Cancer Res. 1992;52(21):6045–51. [PubMed] [Google Scholar]
- Wangpradit O, Mariappan SV, Teesch LM, Duffel MW, Norstrom K, Robertson LW, Luthe G. Oxidation of 4-chlorobiphenyl metabolites to electrophilic species by prostaglandin H synthase. Chem Res Toxicol. 2009;22(1):64–71. doi: 10.1021/tx800300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehler EK, Bergman A, Brandt I, Darnerud PO, Wachtmeister CA. 3,3′,4,4′-Tetrachlorobiphenyl. Excretion and tissue retention of hydroxylated metabolites in the mouse. Drug Metab Dispos. 1989;17(4):441–8. [PubMed] [Google Scholar]
- WHO . Polychlorinated biphenyls: Human health aspects. Geneva: 2003. World Health Organisation Concise International Chemical Assessment Document 55 [cited; Available from: http://www.inchem.org/documents/cicads/cicads/cicad55.htm ] [Google Scholar]
- Wiemels J, Wiencke JK, Varykoni A, Smith MT. Modulation of the toxicity and macromolecular binding of benzene metabolites by NAD(P)H:Quinone oxidoreductase in transfected HL-60 cells. Chem Res Toxicol. 1999;12(6):467–75. doi: 10.1021/tx9800811. [DOI] [PubMed] [Google Scholar]
- Wilson NK, Chuang JC, Lyu C. Levels of persistent organic pollutants in several child day care centers. J Expo Anal Environ Epidemiol. 2001;11(6):449–458. doi: 10.1038/sj.jea.7500190. [DOI] [PubMed] [Google Scholar]
- Zettner MA, Flor S, Ludewig G, Wagner J, Robertson LW, Lehmann L. Quinoid Metabolites of 4-Monochlorobiphenyl Induce Gene Mutations in Cultured Chinese Hamster V79 Cells. Toxicol. Sci. 2007;100(1):88–98. doi: 10.1093/toxsci/kfm204. [DOI] [PubMed] [Google Scholar]