Abstract
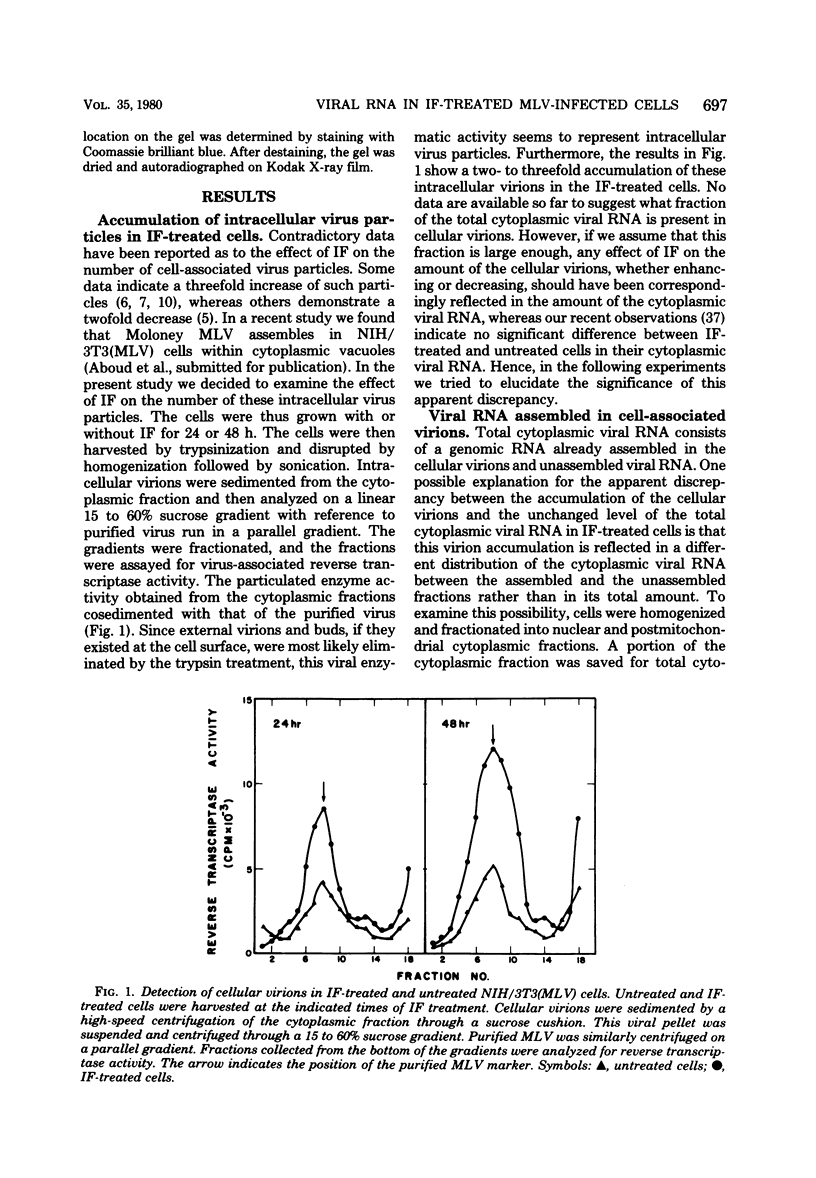
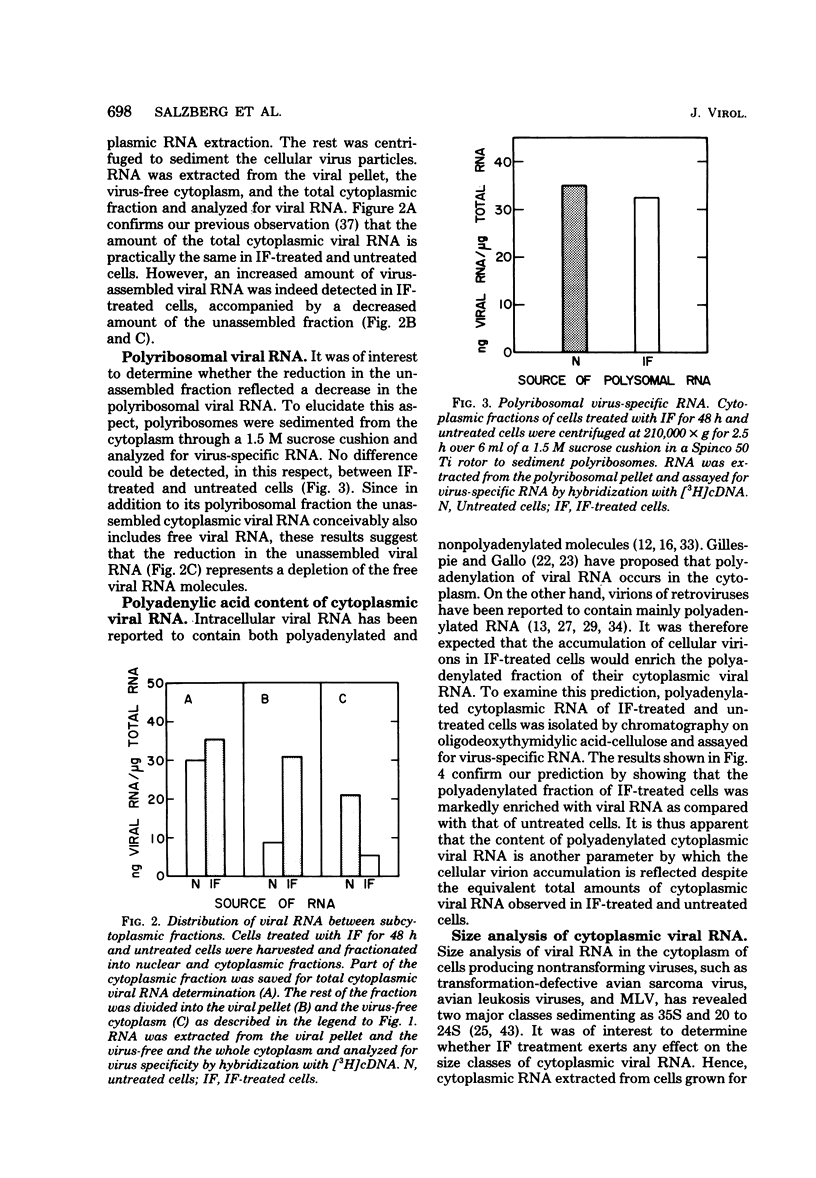
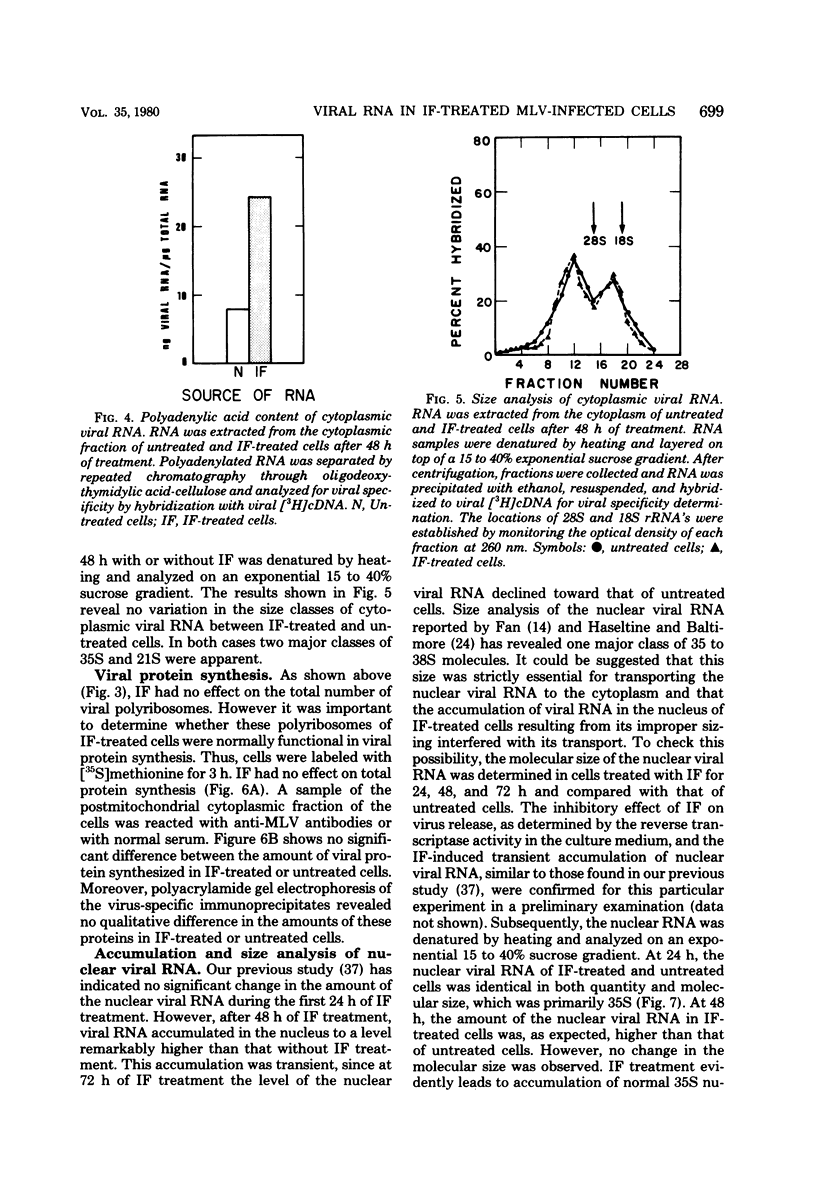
We have recently found that Moloney murine leukemia virus assembles within cytoplasmic vacuoles of chronically infected NIH/3T3 cells rather than at their surface (submitted for publication). In the present study we found that if these cells were treated with interferon (IF) for 24 to 48 h the intracellular virus particles accumulated at a two- to threefold-higher level than that observed in untreated cells. Nevertheless, despite this accumulation, no difference between IF-treated and untreated cells was observed in the amount of the total cytoplasmic viral RNA or in its 35S or 21S species. When cellular virions were sedimented from the cytoplasmic fraction, a markedly higher amount of viral RNA was detected in the viral pellet of IF-treated cells than was detected in untreated cells, whereas the amount of viral RNA left in the virus-free cytoplasm of IF-treated cells was much lower than that in the untreated cells. Furthermore, the amount of the cytoplasmic polyriboadenylic acid-containing viral RNA was also remarkably higher in the IF-treated cells. Viral polyribosomes appeared to be fully functional in IF-treated cells, since no effect of IF on viral protein synthesis could be detected. Analysis of the nuclear viral RNA showed no difference between IF-treated and untreated cells after 24 h of IF treatment. Both contained a comparable amount of 35S viral RNA. However, at 48 h a significant accumulation of viral RNA was observed in the nucleus of the IF-treated cells as compared with the untreated cells, although in both cases only 35S species were evident. This accumulation appeared to activate a degradation process which destroyed nuclear viral RNA, since a dramatic shift toward smaller-sized molecules of viral RNA and a remarkable reduction in its amount were observed after 72 h of IF treatment.
Full text
PDF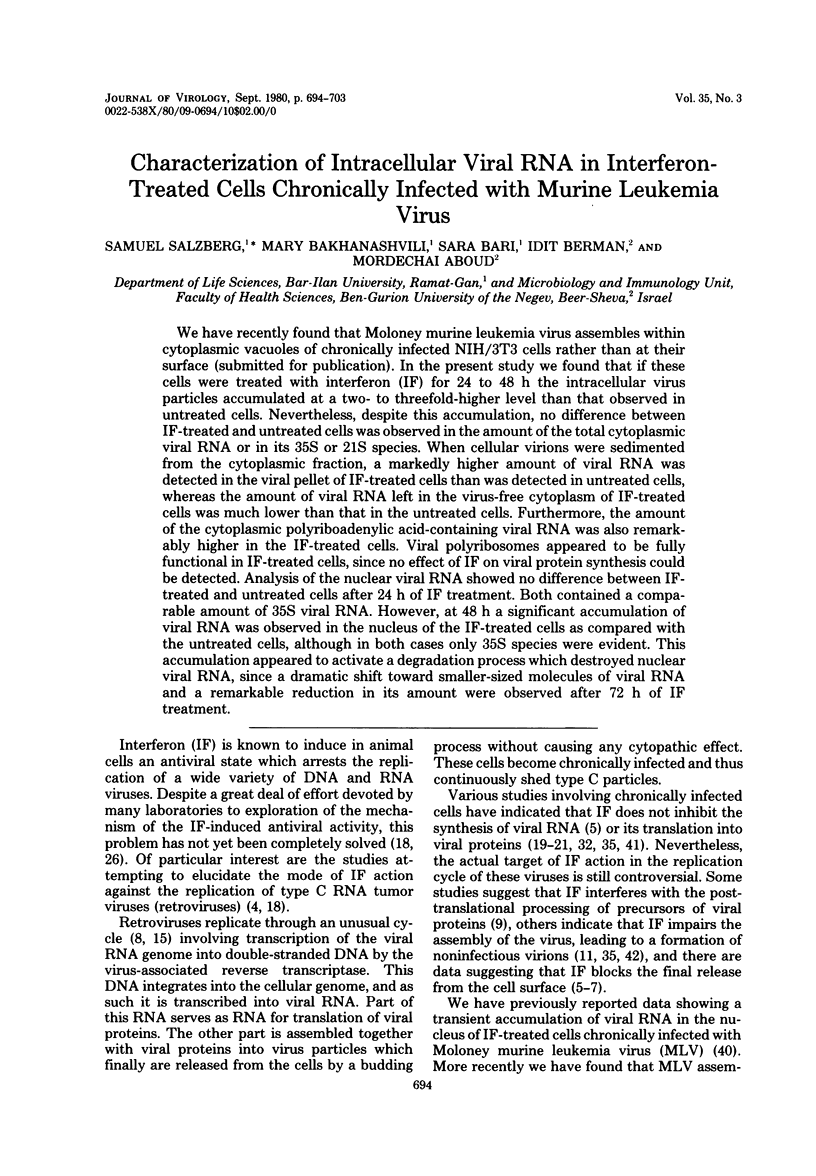





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Shoor R., Salzberg S. Adsorption, penetration, and uncoating of murine leukemia virus studied by using its reverse transcriptase. J Virol. 1979 Apr;30(1):32–37. doi: 10.1128/jvi.30.1.32-37.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud M., Weiss O., Salzberg S. Rapid quantitation of interferon with chronically oncornavirus-producing cells. Infect Immun. 1976 Jun;13(6):1626–1632. doi: 10.1128/iai.13.6.1626-1632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Sobis H., de Somer P. Influence of interferon on virus-particle synthesis in oncornavirus-carrier lines. II. Evidence for a direct effect on particle release. Int J Cancer. 1974 Sep 15;14(3):335–340. doi: 10.1002/ijc.2910140306. [DOI] [PubMed] [Google Scholar]
- Billiau A. Effect of interferon on RNA tumor viruses. Tex Rep Biol Med. 1977;35:406–419. [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Maeyer-Guignard J., De Somer P. Trapping of oncornavirus particles at the surface of interferon-treated cells. Virology. 1976 Sep;73(2):537–542. doi: 10.1016/0042-6822(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Somer P. Influence of interferon on the synthesis of virus particles in oncornavirus carrier cell lines. IV. Relevance to the potential application of interferon in natural infectious diseases. J Infect Dis. 1976 Jun;133 (Suppl):A51–A55. doi: 10.1093/infdis/133.supplement_2.a51. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Friedman R. M. A large glycoprotein of Moloney leukemia virus derived from interferon-treated cells. Biochem Biophys Res Commun. 1977 Jul 11;77(1):392–398. doi: 10.1016/s0006-291x(77)80210-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Myers M. W., Wong P. K., Friedman R. M. The inhibitory effect of interferon on a temperature-sensitive mutant of Moloney murine leukemia virus. Virology. 1977 Apr;77(2):625–636. doi: 10.1016/0042-6822(77)90487-1. [DOI] [PubMed] [Google Scholar]
- East J. L., Chan J. C., Bartlett R. J., Knesek J. E. Quantitative measurement of intracellular RNA genomes of Rauscher murine leukemia virus by competition hybridization in DNA excess. J Virol. 1979 Feb;29(2):818–824. doi: 10.1128/jvi.29.2.818-824.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H. Expression of RNA tumor viruses at translation and transcription lebels. Curr Top Microbiol Immunol. 1978;79:1–41. doi: 10.1007/978-3-642-66853-1_1. [DOI] [PubMed] [Google Scholar]
- Fan H., MacIsaac P. Virus-specific RNA synthesis in interferon-treated mouse cells productively infected with Moloney murine leukemia virus. J Virol. 1978 Aug;27(2):449–452. doi: 10.1128/jvi.27.2.449-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. RNA metabolism of murine leukemia virus: size analysis of nuclear pulse-labeled virus-specific RNA. Cell. 1977 Jun;11(2):297–305. doi: 10.1016/0092-8674(77)90046-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Chang E. H., Ramseur J. M., Myers M. W. Interferon-directed inhibition of chronic murine leukemia virus production in cell cultures: lack of effect on intracellular viral markers. J Virol. 1975 Sep;16(3):569–574. doi: 10.1128/jvi.16.3.569-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Costa J. C., Ramseur J. M., Meyers M. W., Jay F. T., Chang E. H. Persistence of the viral genome in interferon-treated cells infected with oncogneic or nononcogenic viruses. J Infect Dis. 1976 Jun;133 (Suppl):A43–A50. doi: 10.1093/infdis/133.supplement_2.a43. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Gallo R. C. Concepts on the interference of replication and expression of RNA tumor viruses. Ann N Y Acad Sci. 1977 Mar 4;284:576–584. doi: 10.1111/j.1749-6632.1977.tb21989.x. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Gallo R. C. RNA processing and RNA tumor virus origin and evolution. Science. 1975 May 23;188(4190):802–811. doi: 10.1126/science.47650. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Baltimore D. Size of murine RNA tumor virus-specific nuclear RNA molecules. J Virol. 1976 Aug;19(2):331–337. doi: 10.1128/jvi.19.2.331-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. The mechanism of action of interferon. Ann N Y Acad Sci. 1977 Mar 4;284:711–716. doi: 10.1111/j.1749-6632.1977.tb22007.x. [DOI] [PubMed] [Google Scholar]
- Keith J., Gleason M., Fraenkel-Conrat H. Characterization of the end groups of RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4371–4375. doi: 10.1073/pnas.71.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- King A. M., Wells R. D. All intact subunit RNAs from Rous sarcoma virus contain poly (A). J Biol Chem. 1976 Jan 10;251(1):150–152. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D., Voloch Z., Aviv H., Nudel U., Revel M. Effects of interferon on hemoglobin synthesis and leukemia virus production in Friend cells. Mol Biol Rep. 1974 Dec;1(8):447–451. doi: 10.1007/BF00360670. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Lewis P., Dierks P. Purification of virus-specific RNA from chicken cells infected with avian sarcoma virus: identification of genome-length and subgenome-leghth viral RNAs. J Virol. 1978 Jul;27(1):227–238. doi: 10.1128/jvi.27.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Park J. J., Hollis V. W., Jr Polyriboadenylate sequences at the 3'-termini of ribonucleic acid obtained from mammalian leukemia and sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4366–4370. doi: 10.1073/pnas.71.11.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S., Bakhanashvili M., Aboud M. Effect of interferon on mouse cells chronically infected with murine leukaemia virus: kinetic studies on virus production and virus RNA synthesis. J Gen Virol. 1978 Jul;40(1):121–130. doi: 10.1099/0022-1317-40-1-121. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Levi Z., Aboud M., Goldberger A. Isolation and characterization of DNA-DNA and DNA-RNA. Biochemistry. 1977 Jan 11;16(1):25–29. doi: 10.1021/bi00620a004. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. A possible requirement for protein synthesis early in the infectious cycle of the murine sarcoma-leukemia virus. Virology. 1977 Jan;76(1):341–351. doi: 10.1016/0042-6822(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. Appearance of virus-specific RNA, virus particles, and cell surface changes in cells rapidly transformed by the murine sarcoma virus. Virology. 1973 May;53(1):186–195. doi: 10.1016/0042-6822(73)90477-7. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., Billiau A. Synthesis and cleavage processing of oncornavirus proteins during interferon inhibition of virus particle release. Infect Immun. 1977 Jun;16(3):742–747. doi: 10.1128/iai.16.3.742-747.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


