Abstract
The mechanisms through which estrogens modulate neuronal physiology, brain morphology, and behavior in recent years have proven to be far more complex than previously thought. For example, a second nuclear estrogen receptor has been identified, a new family of coregulatory proteins regulating steroid-dependent gene transcriptions was discovered and, finally, it has become clear that estrogens have surprisingly rapid effects based on their actions on cell membranes, which in turn results in the modulation of intracellular signaling cascades. This paper presents a selective review of new findings in this area related to work in our laboratories, focusing on the role of estrogens in the activation of male sexual behavior. Two separate topics are considered. We first discuss functions of the steroid receptor coactivator-1 (SRC-1) that has emerged as a key limiting factor for behavioral effects of estradiol. Knocking-down its expression by antisense oligonucleotides drastically inhibits male-typical sexual behaviors. Secondly, we describe rapid regulations of brain estradiol production by calcium-dependent phosphorylations of the aromatase enzyme, themselves under the control of neurotransmitter activity. These rapid changes in estrogen bioavailability have clear behavioral consequences. Increases or decreases in estradiol concentrations respectively obtained by an acute injection of estradiol itself or of an aromatase inhibitor lead within 15-30 min to parallel changes in sexual behavior frequencies. These new controls of estrogens action offer a vast array of possibilities for discrete local controls of estrogen action. They also represent a formidable challenge for neuroendocrinologists trying to obtain an integrated view of brain function in relation to behavior.
Keywords: aromatase, non genomic action of steroids, steroid receptor coregulators, preoptic area, male sexual behavior, estrogen receptors
1. Introduction
Since the origins of the field of behavioral neuroendocrinology, the way we understand how sex steroids influence behavior has undergone a tremendous evolution. For example, it was originally thought that steroid hormones relevant to the control of reproductive behaviors are secreted mostly if not exclusively by the gonads and act through a single mode of action involving the binding to intracellular receptors and modulation of gene transcription. During the last few years, this simplistic view has progressed markedly and it is now clear that steroids are produced by a variety of tissue types, including the brain itself, and that they act in the brain through multiple pathways resulting in the modulation of a wide array of physiological and behavioral processes including reproduction, metabolism, development and cognition [1]. One important cornerstone in the development of our current understanding of steroid action in the brain was the discovery that circulating steroids often need to be metabolized into more active molecules to exert their effects [2-4]. One well established example is that many effects of androgens in the brain are mediated via their local conversion into estrogens by the enzyme aromatase [5-7]. For instance, testosterone activates male sexual behavior in many vertebrate species through its active metabolites including 17β-estradiol [8, 9]. This conclusion is supported by experiments demonstrating that behavioral effects of testosterone are mimicked in castrates by treatments with estrogens or aromatisable androgens, but not by non-aromatisable androgens, and that they are blocked by aromatase inhibitors and anti-estrogens but not, or less so, by anti-androgens [7].
Another important development in the study of the mechanisms of steroid action arises from the identification of two types of fundamentally different modes of steroid action that result in the modulation of an enormous diversity of intracellular signaling pathways. The classic genomic mode of action involves the binding of the hormone-receptor complex to specific sequences of the DNA known as “hormone-responsive elements” resulting in the regulation of the transcription of genes encoding a wide array of proteins [10, 11]. These actions occur relatively slowly and develop with latencies ranging from one hour to several days. Besides this genomic mode of action, steroids also exert rapid and presumably short-lived effects through their interaction with the cell membrane (non-genomic effects). For instance, estradiol can within a few seconds or minutes affect the firing rate of neurons or activate a variety of intracellular signaling pathways resulting in the modulation of intracellular calcium concentrations or of protein phosphorylations (for review, see [12-15]). The present review will focus on the new advances on these genomic and non-genomic modes of action of testosterone and its estrogenic metabolite, 17β-estradiol in the brain and their relevance to the control of male sexual behavior. We shall specially highlight work performed in our laboratories on the Japanese quail (Coturnix japonica), a species that has proved to be a very suitable model for the study of steroid action in the brain in relation with the control of male reproductive behavior.
2. The genomic action of steroids: role of the coactivators
Many effects of steroid hormones are mediated via the activation of specific receptors that are part of the nuclear receptors superfamily. According to the standard model of nuclear receptor action [16, 17], the unbound steroid receptor is present in the cytoplasm and interacts with several chaperone proteins such as heat shock proteins (HSP: [18, 19]). The function of these proteins is to prevent anarchic folding by stabilizing the unbound receptor and avoiding the aggregation, presence in the wrong subcellular compartment and non-specific binding of the receptor to DNA (see for example [20]). The specific binding of the steroid ligand to its receptor induces the release of HSPs, a conformational change and the hyperphosphorylation of the receptor [21]. These changes generally lead to the homodimerization of the receptor and its binding to a specific DNA sequence known as the Hormone Response Element (HRE: [22]), usually located upstream of the target gene sequence (see however [23]). In some cases, activated steroids receptors dimerize with other steroid receptors (e.g., the heterodimers between the two estrogen receptors (ER) of the alpha and beta subtypes; ERα-ERβ) or even bind to other transcription factors such as AP-1 or sp1 [24]. The receptor will then recruit several general transcription factors such as the general transcription factors IIB, TFIIB [25] to induce the transcription of specific target genes that will modulate specific brain responses, ultimately leading to changes in behavior. This basic mechanism of steroid action involving a “ligand-receptor-promoter” pathway is, however, an oversimplified vision of steroid action on physiology. Research from a few laboratories suggests that a large family of proteins called steroid receptors coregulators play a key role in the control of transcription initiated by steroid receptors. In this section we shall selectively review results from our and other laboratories on recent progress made in the understanding of steroid action mediated by the slow “classical” genomic pathway.
2.1 Steroid receptor coactivators
The key initial observation that led to the discovery of steroid receptor co-factors was made by Meyer and colleagues [26] who observed that the induction of a reporter gene by the progesterone receptor is inhibited in HeLa cells co-transfected with ER or, to a lesser extent, with glucocorticoid receptor. They hypothesized that steroid receptors, and nuclear receptors in general, require common cofactors that would constitute a limiting factor when competition between different receptors (squelching) takes place. Subsequent advances in molecular biology have brought forward the importance of coregulatory molecules that modulate the transcriptional activity of steroid receptors. O’Malley and his group cloned the first receptor coactivator and confirmed that squelching, resulting in the repression of the transcriptional activity of one steroid receptor by another, is reversed by the addition of coactivators [27], therefore demonstrating that these coactivators are truly limiting factors. In vitro studies using antibodies against nuclear receptor coactivators confirmed that recruitment of coactivators is rate-limiting in steroid receptor-mediated gene transcription [28]. It should be noted that coactivators do not act as single regulatory proteins but act in a synergistic manner as a multiprotein complex [29-31]. Each member of these complexes will influence transcription through a variety of mechanisms, including acetylation, methylation, phosphorylation, chromatin remodeling and mRNA splicing [32]. We know now that nuclear receptor activity can be enhanced or decreased by coactivators and corepressors, respectively and approximately 300 of these proteins have currently been identified [33]. However, there is still much to be learned about the role and importance of coregulatory proteins in the modulation of specific responses following steroid receptor activation.
2.2. Functional significance of coactivators
Although coactivators have now been studied for the past 15 years, relatively little is known about their in vivo function, especially in the brain. The physiological importance of coactivators has been studied through the targeted gene disruption technique (often referred to as the knock-out method) in mice. Although the complete suppression of the expression of some coactivators was lethal [34, 35], other knockout mice did not present any significant adverse effects [36]. For example, females in which expression of the steroid receptor coactivator-1 has been eliminated (SRC-1-/-) are fully fertile (functional pituitary-ovarian axis) and exhibit normal proceptive and receptive sexual behavior [36] despite the fact that, in vitro, SRC-1 is known to be critical for the transcriptional activity of ER. SRC-1-/- knock out males and females are fertile and only show weak resistance to steroid and thyroid hormones [36-38]. It should be noted that the absence of major deficit is due to a compensatory adaptation resulting from the over-expression of another related coactivator, SRC-2 [39]. To define whether a reduction of coactivator expression can affect cell phenotype and behavior, our laboratory and others used the antisense oligonucleotide technique to knock-down gene expression as an alternative method that would not be susceptible to compensatory adaptation during ontogeny.
We focused our attention on defining the role of SRC-1 in the physiological responses to testosterone in the context of the activation of sexual behavior. Studies were centered on steroid action in the preoptic area (POA). This region is well known to play a critical role in the control of male sexual behavior [7]. In quail, in particular, testosterone action in the medial preoptic nucleus (POM) is necessary and sufficient for the activation of male sexual behavior by testosterone [40, 41]. To test whether this coactivator was required in the control of steroid-dependent male sexual behavior and its associated neuroplasticity, antisense oligonucleotides were used to specifically decrease the expression of SRC-1.
In a first set of experiments [42], testosterone-treated castrated male Japanese quail were implanted with an injection cannula to allow the infusion of the antisense directly into the third ventricle, at the level of the POA. Two or three days after the implantation of testosterone-filled capsules, male-typical behaviors including neck grab, cloacal contact movement and pre- and post-copulatory displays were observed in the control groups injected with saline or scrambled oligonucleotides. The birds treated with antisense showed a weaker response to testosterone, as attested by significantly decreased frequencies of neck grab, cloacal contact movement and strut (Fig. 1A-B).
Figure 1.
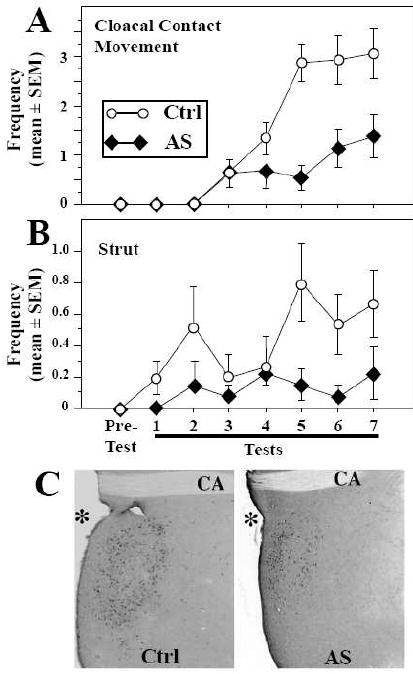
SRC-1 knock-down by antisense injections (AS) significantly inhibits testosterone–induced sexual behavior (A-B) and aromatase induction (C) in castrated male quail in comparison with control birds injected with scrambled antisense (Ctrl). Sexual behaviors are illustrated here by the estrogen-dependent cloacal contact movements and by the strictly androgen–dependent display, strutting (B). The SRC-1 knock-down also blocked the induction by testosterone of aromatase expression resulting in a smaller number of aromatase-immunoreactive cells delimiting a smaller surface of the medial preoptic area (C). See [42] for original data and their statistical analysis.
In quail, behaviors of the copulatory sequence sensu stricto such as neck grabs and cloacal contact movements are activated by testosterone only if the steroid can be aromatized into an estrogen. Pre- and post-copulatory displays such as crowing and strutting do not require testosterone aromatization and are therefore strictly androgen-dependent [7, 8]. Interestingly, while previous studies suggested that SRC-1 interacts principally with ERα in vitro and does not seem to modulate androgen receptors [43, 44], our data showed that SRC-1 is important not only for aspects of male sexual behavior influenced by estrogens (copulatory behavior per se) but also for a strictly androgen-dependent behavior (strut).
After the final behavioral test, brains were collected for histological analysis and protein quantification. Five birds from the antisense (AS) treated group were maintained in the experiment to test whether their behavior would recover after the injections of the antisense (AS) ceased. AS injections were then replaced by control scrambled oligonucleotides (SC) injections for two additional days (AS-SC group). Males in the AS-SC group showed an important increase of the behavioral response to testosterone two days only after the interruption of the AS treatment, so that their sexual behaviors were now expressed with frequencies exceeding those of control animals whose performance had apparently reached a plateau for three consecutive days.
Western blot analysis of SRC-1 expression in the preoptic area-hypothalamus (POA-HYP) confirmed the down-regulation of this protein in AS subjects as compared to controls. These analyses also showed that the AS-SC birds had a stronger expression of SRC-1 compared to control animals at the time of brain collection, probably as a result of compensatory mechanisms activated during the antisense treatment (e.g., accumulation of untranslated mRNA that would produce an excess of protein after the cessation of the antisense infusions). This SRC-1 over-expression could explain the enhanced behavioral response to testosterone observed in these AS-SC subjects.
The reduction of sexual behavior induced by the down-regulation of SRC-1 expression was associated with significant neuroanatomical and neurochemical changes in the preoptic region. In quail, testosterone regulates multiple male-biased sex differences in the POA such as the total volume of the POM, the number of cells expressing aromatase in this nucleus and the density of fibers immunoreactive for the peptide vasotocin innervating the area via its estrogenic metabolites [45, 46]. In agreement with the idea that SRC-1 is involved in the modulation of steroid activity in the brain, the volume of the POM as defined by Nissl staining was markedly decreased in birds treated with SRC-1 antisense. In parallel, the volume of the POM defined by the cluster of aromatase-immunoreactive neurons was also smaller in the AS group as compared to controls (Fig. 1C) and the density of the expression of vasotocin in the POA was significantly reduced. Finally, the integrated density of aromatase immunoreactivity in the POM, an index reflecting aromatase content in this nucleus, was decreased in the AS as compared to the control group. It is also interesting to note that the cessation of the AS injections (group AS-SC) was associated not only with a major rebound of copulatory behavior but also with a quite rapid increase of the POM volume defined by Nissl staining and by aromatase immunoreactivity. The aromatase index was also significantly higher in the AS-SC group compared to the control group [42]. Together, these data support the idea that SRC-1 is a key limiting factor for the in vivo production of behavioral and physiological responses to testosterone. A down-regulation of the coactivator decreased both estrogen- and androgen-dependent aspects of male sexual behavior and the associated neuroplasticity, while the over-expression of this protein in AS-SC subjects was associated with an increase of these steroid-dependent responses.
In a subsequent set of experiments, we analyzed in more detail the time-course of the effects of SRC-1 inhibition on the steroid-dependent activation of male sexual behavior and of aromatase expression in the Japanese quail brain. It was previously demonstrated that the induction of aromatase activity is a prerequisite for the activation of male sexual behavior in this species, with aromatase activity reaching its maximal level 48 hours after the beginning of a treatment with testosterone while copulatory behavior occurrence frequencies are maximal 96 hours after steroid implantation [47]. Somewhat surprisingly, the decrease of SRC-1 expression significantly blocked steroid-dependent male sexual behavior and decreased the density of the aromatase immunoreactivity in the POA, but aromatase activity in the POA was not affected by the repeated injection of SRC-1 antisense. This absence of an effect on the enzymatic activity paralleled by a significant decrease in the apparent concentration of the enzyme as assessed semi-quantitatively by immunohistochemistry is potentially explained by a compensatory increase in enzymatic activity of the remaining enzymatic molecules (see [48] for additional discussion).
We also performed an additional experiment to analyze in more detail the rapid increase of neurochemical attributes of the POA during the behavioral recovery period following interruption of an SRC-1 antisense treatment (AS-SC condition). Birds were first treated for 6 days with the antisense targeting SRC-1 expression and then received for three additional days scrambled injections (AS-SC group) while control subjects were injected for the entire treatment duration with either AS or SC. As expected, subjects of the AS-SC group increased their behavioral response to testosterone within a day of the cessation of AS injections, as attested by the doubling of the cloacal contact movement frequencies, which confirmed results of our previous study [42]. However, the histological analysis of these brains at the end of the experiment failed to identify significant differences in POM volume and in various measures of aromatase expression in the POA between the AS-SC, SC and more surprisingly the AS group [48]. Unlike what we had observed in previous experiments [42], the POM volume defined by Nissl staining or by aromatase immunoreactivity as well as the optical density of the aromatase signal and the aromatase index reflecting the enzyme content in the POM were similar in the three groups.
These data indicate a clear dissociation between effects of testosterone on male copulatory behavior and on the anatomical and neurochemical plasticity in the POM of Japanese quail. The specific mechanisms mediating the inhibition of male sexual behavior during the present experiment remain, however, unclear. As previously mentioned, aromatase expression in the POA is steroid-sensitive and plays a critical role in the activation of male sexual behavior. Our first antisense experiment suggested that the inhibition of male sexual behavior following depletion of SRC-1 expression was mediated, at least in part, by the decrease of preoptic aromatase content. However, we now observed in two independent experiments that sexual behavior was inhibited by injections of SRC-1 antisense in the absence of significant changes in measures of brain aromatase activity or in the volume of the POM defined by aromatase staining. It is likely that the antisense treatment additionally affected the control by steroids of other neurochemical systems of the POA besides aromatase (e.g., various neuropeptides or neurotransmitters) and that these changes played a major role in the suppression of behavior following SRC-1 depletion.
This set of experiments suggests that different steroid-sensitive neurochemical systems underlying the activation of sexual behavior such as aromatase, vasotocin and other neuropeptides or transmitters that were not considered in these studies are differentially affected by the reduction of SRC-1. While the reduction of SRC-1 expression significantly inhibited testosterone-dependent male sexual behavior in all these experiments, the associated neurochemical changes were more variable. This raises a large number of questions concerning the exact role of SRC-1 in biochemical and physiological cascades leading to the activation of behavior and regarding the importance of SRC-1 for individual target genes. It would thus be necessary to define which steroid-sensitive enzymatic and peptidergic systems besides aromatase are involved in the activation by testosterone of male sexual behavior and require SRC-1 expression. In addition, it is important to note here that in the studies performed so far we focused on SRC-1 but several other steroid coactivators are likely to be involved in the control of the physiological and behavioral responses to testosterone [39, 49].
Similar experiments were performed to define the role of coactivators in the sexual differentiation, during ontogeny, and activation, in adulthood, of hormone-dependent female sexual behavior in rats. It was found that females treated neonatally with testosterone who received hypothalamic SRC-1 antisense infusions during development had a smaller sexually dimorphic nucleus of the medial preoptic area (SDN) compared to testosterone-treated control females, suggesting that interfering with SRC-1 disrupts the masculinization of the SDN [50]. Males and testosterone-treated females infused with SRC-1 antisense during the neonatal period also exhibited high levels of adult female sexual behavior that were comparable to control females, suggesting that SRC-1 is critical in mediating the defeminizing actions of testosterone on adult female sexual behavior. Interestingly the knock-down of CBP (CREB-Binding Protein) expression during ontogeny by the same antisense strategy also interfered with testosterone’s ability to defeminize sexual behavior [51].
Steroid receptor coregulators also affect the expression of adult female sexual behavior. Adult female rats treated with antisense to both SRC-1 and CBP into the ventromedial nucleus of the hypothalamus (VMN) display reduced levels of hormone-dependent female sexual receptivity as compared to scrambled-treated controls [49]. Another study supported these findings with SRC-1 alone and extended them to include a role for SRC-2 in steroid-dependent behavior [39]. These two studies thus demonstrated that the activation by estrogens of female sexual behavior is inhibited by a knock-down of coactivators, including CBP, SRC-1 and SCR-2. In addition, they showed that the estrogen-dependent progesterone receptor (PR) induction in the VMN is also significantly decreased by these treatments. Interestingly, combining the knock-down of several coactivators, such as SRC-1/CBP [49] or SRC-1 or -2/SRA [52], has more prominent inhibitory effects as compared to single knock-downs, thus confirming the importance of coactivator complexes in the regulation of nuclear receptor activity. The effects of these nuclear receptor coactivators on both ER- and PR-dependent aspects of female sexual behavior were also specifically investigated and it was confirmed that SRC-1 enhances activity of both ER and PR.
These experiments demonstrate that SRC-1 expression is critical for the hormone-mediated sexual differentiation and activation of neural gene expression and of male and female sexual behavior. This set of data also demonstrates that SRC-1 is required for androgen, estrogen and progesterone action in vivo and suggests that spontaneous variations of the expression of these coactivators in physiological conditions could modify in a significant manner the responses to steroids of brain and behavior.
This conclusion obviously raises the question of whether these coactivators are constitutively expressed in a fairly constant manner or if their expression is acutely regulated by the environment. Very few studies have investigated the potential regulation of the expression of coactivators in the brain and they mostly concern SRC-1 and other members of the SRC family. Overall our work in Japanese quail as well as studies performed in other laboratories, mainly on rats, clearly indicate that SRC-1 expression in the brain is not constitutive but, rather, is regulated by multiple endogenous and exogenous factors, including the neuroanatomical site considered, the sex of the subject, its endocrine condition (sex steroid hormones circulating concentrations), and potentially the time of the day [53, 54].
In mammals, 17β-estradiol treatment reduces the levels of SRC-1 in rat pituitary cells both in vivo and in vitro [55], while the same treatment reverses the down-regulation of SRC-1 expression in the rat ventromedial hypothalamus caused by ovariectomy [56]. SRC-1 levels in female rat hypothalamus vary during the estrous cycle; they are lowest during diestrus, and highest at proestrus and estrus [57]. In male Siberian hamsters, short days reduce SRC-1 expression in the posteromedial bed nucleus of the stria terminalis and posterodorsal medial amygdala independently of testosterone treatment [58]. Interestingly, both testosterone treatment and day length significantly altered SRC-1 expression in the anterior amygdala [58].
Regulation of coactivator concentrations is clearly achieved at the transcriptional level, but in vitro experiments have additionally demonstrated the important role of protein degradation. Post-translational modifications such as phosphorylation and methylation lead to the ubiquitination of the coactivators and direct them to the ubiquitin-proteasome pathway [59, 60]. Although the importance of proteasome has been demonstrated in progesterone receptor-dependent behavior [61], no information is currently available on the function at the organismal level of this proteasome-induced coactivator degradation.
Much remains to be done to understand the exact mechanisms involved in the control of the expression of SRC-1. Moreover, relatively little is known about potential regulation of other coactivators. Coactivators exist as preformed complexes that seem to be dynamically regulated [62]. To date, no study has simultaneously investigated the control of the expression of the different coregulators that are part of a specific transcriptional regulatory complex within a particular cell type or brain region. Together, cellular differences in coactivator concentrations and ratios as well as their unique post-translational modifications are likely to be involved in cell-specific responses to hormones [63, 64]. Understanding the recruitment of the different coactivators that are part of a same transcription complex will be critical for the understanding of how hormones function in the brain to regulate integrated responses such as sexual behavior [33, 65].
3. Non-genomic effects of estrogens and rapid changes in aromatase activity
Besides their genomic mode of action, steroids, including the intensively studied 17β-estradiol, also exert effects that are too rapid (seconds to minutes) to be mediated through the modulation of DNA transcription and protein synthesis [66-68]. Similar rapid effects of other steroids such as testosterone have also been described (e.g., [69, 70]) but these are not the focus of the present special issue.
Although purely cytoplasmic effects have been described [71], non-genomic effects generally appear to be initiated at the plasma membrane by an estrogen receptor located at the membrane such as GPR30, STX-binding protein, or ER-X that shows features resembling those of G protein coupled receptors (GPCR). Additionally, the nuclear receptors ERα and ERβ seem able to associate to the neuronal membrane and interact with G proteins (for review see [15]). These effects result in the activation of a wide variety of intracellular signaling pathways including the modulation of intracellular calcium concentrations [72] and the phosphorylation of a variety of proteins such as the mitogen activated protein kinase (MAPK) and cAMP response element binding protein (CREB) [73-77]. The activated intracellular pathways lead to modulations of electrical activity [12, 73] and to neuronal activation [74, 78-81] in various brain regions which can ultimately result in the modulation of gene transcription through mechanisms independent of classical estrogen responsive element (indirect genomic effects; for review see [14, 68, 82].
3.1. Functional significance of the non-genomic effects of estrogens for behavior control
Evidence is accumulating that these acute effects of estrogens that have been described in some detail at the cellular level influence a variety of physiological and behavioral processes in different species. Non-genomic effects of estrogens on sexual behavior have been described in both sexes. In females, acute administration of 17β-estradiol facilitates lordosis behavior partly through non-genomic actions that potentially involve the interaction of activated ERα with metabotropic glutamate receptors [15, 83, 84]. In males, Cross and Roselli first showed that, in castrated rats, the acute injection of a high dose of 17β-estradiol stimulates mounts and anogenital investigations within 35 min [85]. In quail and mice, a single injection of a bolus of 17β-estradiol facilitates the expression of most aspects of male sexual behavior within 10-15 min and this effect vanishes after 30 min [86-88]. These effects are best observed when subjects are pre-treated with a sub-optimal dose of steroids (testosterone or 17β-estradiol) suggesting that these non-genomic effects require some steroid priming to occur. In male plain midshipman fishes, an intramuscular injection of 17β-estradiol increases within 5 min the duration of fictive vocalizations stimulated by electrical stimuli in an in vitro preparation [89]. This effect persists for 15 to 30 min. Interestingly, 17α-estradiol or testosterone do not produce similar effects. Hayden-Hixson and colleagues also showed that a microinjection of 17β-estradiol in the anterior hypothalamus stimulates agonistic behavior (flank marking) within 15 min in male hamsters [90].
A few generalizations about the non-genomic effects of estrogens on behavior can be drawn from these and other data:
Fast effects of estrogens, and of other steroids, have been identified in different vertebrate species suggesting that the non-genomic mechanisms of action of steroids are conserved across species (For review, see [91]).
These behavioral effects occur with latencies ranging from 5 min to 1 hour, most of them being observed around 15-30 min. This time course of action is a lot faster than the several days usually observed for transcription-dependent activation of behavior [14].
Most of these rapid effects on behavior occur only during estrogen exposure and rapidly disappear when estrogens are eliminated. These actions appear transient when compared to the long lasting effects mediated by transcriptional actions.
These non-genomic effects of estrogens have been described in both males and females. Interestingly, males do not produce high circulating levels of estrogens.
Although some rapid effects of estrogens are elicited in vitro at high picomolar to low nanomolar concentrations [68, 72, 81, 92, 93], many acute cellular and behavioral effects of estrogens appear to require concentrations higher (i.e., micro to millimolar) than normal circulating levels. This conclusion is based on several sets of observations: a) many in vitro non-genomic effects of estrogens are only elicited at concentrations superior to physiological levels [74, 78, 94, 95], and b) the doses of 17β-estradiol acutely administered in vivo are estimated to reach circulating levels that are 2 to 3 orders of magnitude above the endogenous plasma levels. However, estrogen concentrations exceeding circulating levels have been measured in brain regions such as the hypothalamus, the preoptic area and the hippocampus [96, 97] and therefore the very high doses of steroids required to trigger these non-genomic effects could reflect the physiological concentrations that are present locally in the brain (for more detail and a discussion of the physiological relevance of these concentrations, see [14]).
3.2. What is the source of estrogens?
One question arising from these observations concerns the nature of the mechanism(s) that are able to produce and rapidly modulate the high local concentrations of estrogens on a timescale compatible with their acute effects [14, 66]. An example of transient increase of estrogens is the preovulatory surge of 17β-estradiol. This surge could potentially explain rapid effects of estrogens seen in females but, as we have seen, rapid effects of estrogens also exist in males. In addition, it is unlikely that this surge provides a concentration of the steroid that is high enough to explain all non-genomic effects. Finally, the time course of the preovulatory estradiol surge is relatively slow as compared to the latencies of non-genomic effects. Even if non-genomic effects were triggered only when 17β-estradiol reaches its maximal concentration, the use of this peak to trigger rapid non-genomic effects would still probably result in a waste of the temporal resolution offered by these rapid effects. In the following section we develop a suite of arguments supporting the involvement of aromatase in the rapid control of local brain estrogen concentrations and we analyze the functional significance of rapid changes in this enzymatic activity for the control of behavioral responses such as male sexual behavior.
Rapid modulations of estrogens concentrations in the brain could result from rapid changes in brain aromatase activity or from rapid changes in the concentration of substrate available to the enzyme. Plasma concentrations of testosterone do not seem to vary rapidly enough and with sufficient amplitude to provide the desired changes in aromatase substrate concentration. However, the expression of all enzymes involved in the steroidogenic pathway, including StAR, P450ssc, 3β-HSD and 17β-HSD, was demonstrated in the brain of many species including the Japanese quail [98-100]. Rapid changes in brain steroid production upstream of aromatase could thus also influence the availability of androgenic substrate. However, very few data exists indicating a rapid regulation of these brain enzymes (see however [101, 102]). Based on castration experiments, it also seems that most of, if not all, androgens that support male sexual behavior in quail originate from the testes (castration completely abolishes behavior; [103, 104]). This does not exclude that part of the androgenic substrate used by brain aromatase to activate rapid changes in behavior could not be produced locally but there is no actual data supporting this notion at the present time. The potential implication of locally produced androgens in the determination of brain estradiol concentration will therefore not be discussed further in this review.
As previously mentioned, preoptic aromatase activity is critical for the activation of male sexual behavior. Chronic inhibition of aromatase activity by specific inhibitors delivered to the POA results in the complete suppression of copulatory behavior within a few days [105]. In castrates, the behavioral sequence can be restored by the local administration of testosterone as well as estrogens [106]. These results indicate that the expression of male sexual behavior is tied to changes in aromatase concentration and activity in the POA [47].
Previous studies demonstrated that changes in preoptic aromatase activity are paralleled by changes in enzyme concentration that are largely mediated by the synergistic transcriptional action of estrogenic and androgenic metabolites of testosterone [7, 107]. However, beside this genomic control of aromatase activity, it has also been established that this enzymatic activity can be modulated much more rapidly in a fashion that does not depend on changes in the concentration of the enzyme. In vitro studies conducted in our laboratory showed that aromatase activity measured in preoptic-hypothalamic homogenates is markedly inhibited within 10-15 min after homogenates have been exposed to elevated but physiological concentrations of ATP, Mg2+ and Ca2+. This inhibition is prevented by compounds that chelate divalent ions or by kinase inhibitors thus indicating that it is caused by calcium-dependent phosphorylation processes [108, 109]. The presence of several consensus sites of phosphorylation in the sequence of aromatase fits in well with this interpretation [108, 110].
Interestingly, rapid inhibitions of aromatase activity were detected in quail preoptic/hypothalamic explants, in which the cellular integrity of the neurons and a large part of their connectivity is maintained. In such explants, aromatase activity is rapidly (within 5 min) and reversibly inhibited by conditions that increase the intracellular Ca2+ concentration such as a K+-induced depolarization (Fig. 3A) or the exposure to thapsigargin, a drug that mobilizes intracellular pools of Ca2+ [109]. These data indicated that the rapid modulations of aromatase activity can also be observed in morphologically intact neurons.
Figure 3.
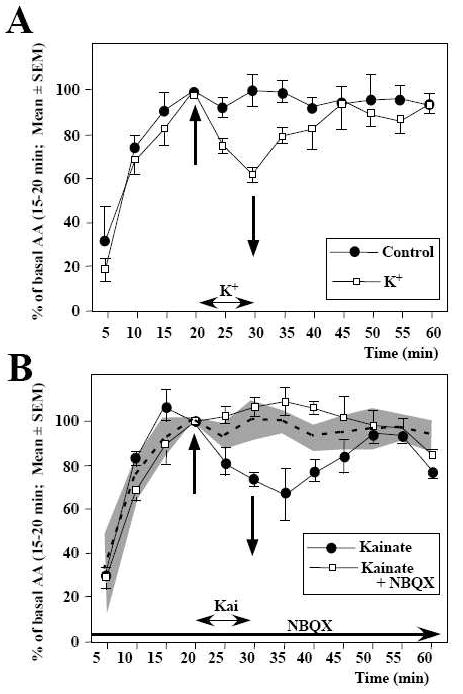
Rapid changes in aromatase activity (AA) are observed in paired quail preoptic-hypothalamic explants maintained in vitro. A. One explant was exposed for 10 min [between 20 min (upward arrow) and 30 min (downward arrow)] to a K+-induced depolarization. Data are expressed as percentages of basal release, defined as the activity during the period preceding the experimental manipulation (15–20 min). B. Both explants were exposed for 10 min (between 20 and 30 min) to the glutamate agonist kainate (100 mM) but one explant had been pre-incubated with the non-NMDA glutamate antagonist NBQX. The gray area represents the control values, observed in the absence of any manipulation, derived from the experiment described in A. See [109] and [111] for detail of original data and their statistical analysis
Similarly, the application on these preoptic/hypothalamic explants of glutamatergic agonists (AMPA and kainate and to a lesser extent NMDA), but not of GABA, also resulted in a rapid inhibition of aromatase activity. These effects were reversible and could be blocked by specific antagonists (Fig. 3B) [111]. They are likely mediated by a direct action of glutamatergic agonist on aromatase-expressing neurons. Indeed, previous electrophysiological studies had demonstrated that the POA of quail and rats displays a nearly continuous neuronal firing and that this tonic electrical activity is suppressed by blockers of glutamatergic and GABAergic transmission [112-114]. Intracellular recordings combined with the biocytin labeling of the recorded neurons also indicated that the electrical activity of aromatase-positive neurons is directly sensitive to glutamate, dopamine and norepinephrine [112, 113]. It is therefore likely that direct glutamatergic inputs to aromatase cells regulate in an acute manner (within min) the activity of the enzyme. This mechanism could explain why aromatase activity in the POA decreases within a few min following expression of copulatory behavior in quail ([115] and see below for additional description of these data). It is indeed known that there is a major release of glutamate in the rat POA following copulation [116]. Whether a similar release takes place in quail has not been determined.
Similar interactions between neurotransmitter action and aromatase activity have also been reported recently in another avian species, the zebra finch. In this species, retrodialysis of glutamatergic agonists in the caudo-medial nidopallium, a brain region expressing elevated concentrations of aromatase, induced a local decrease in estrogen synthesis, thus confirming the inhibitory role of glutamate on estrogen synthesis in vivo [117]. Recent data indicate that the acute delivery of estradiol in this auditory brain region results in a rapid modulation of electrical responses to species-specific sounds [118]. Collectively, these data indicate that rapid changes in aromatase activity mediated by conformational changes of the enzyme are able to rapidly regulate brain estrogen concentrations. It is likely that this mechanism, conserved across species and common to both males and females, plays a pivotal role in the control of acute effects of estrogens making it possible to a) to reach the high local concentrations required to trigger non-genomic actions of estrogens and b) regulate endogenous estrogen concentrations on a timescale compatible with their acute effects.
This conclusion is also supported by the presence of aromatase in brain regions where rapid effects of estrogens have been described. Indeed, in birds and mammals, a dense expression of aromatase is found in the preoptic area, the bed nucleus of the stria terminalis, the mediobasal hypothalamus, the amygdala and in the spinal cord [119-123]. A reproducible although lower expression of the enzyme is also found in the hippocampus and the dorsal root ganglion (DRG) [122-124]. Songbirds such as zebra finches also exhibit abundant aromatase expression throughout most of the telencephalon in auditory areas and in regions surrounding nuclei that are part of a specialized network involved in the control of song production [123]. Interestingly, non-genomic actions of estrogens have been described in most of these brain regions [74, 81, 84, 94, 95, 118, 125-129]. Finally, although males generally display a higher aromatase activity than females, both male and female brains express aromatase [130-133]. This observation fits in well with the fact that cellular and behavioral non-genomic effects of estrogens occur in both sexes (see above).
3.3. Aromatase and rapid controls of male sexual behavior
Recent work from our laboratory investigated the implications of the rapid modulations of local estrogen brain production in the control of male-typical behaviors using two complementary strategies. In a first set of experiments, knowing that acute injections of estradiol facilitate the expression of both appetitive and consummatory aspects of male sexual behavior (see above and Fig. 4A), we reasoned that the pharmacological blockade of estrogen synthesis by aromatase inhibitors should rapidly inhibit the expression of these behaviors. Accordingly, systemic injections of a large dose of vorozole™, a non-steroidal aromatase inhibitor, rapidly reduced most aspects of male sexual behavior in sexually active male quail (gonadally intact males or castrates implanted with 40 mm testosterone-filled capsules; Fig. 4B). The same effect was observed following injections of androstatrienedione (ATD), another type of aromatase inhibitor. In both cases, this behavioral inhibition reached a maximum after 30 min and started to diminish 15 to 30 minutes later [86]. Enzymatic assays performed on homogenized preoptic-hypothalamic blocks collected 30 min after the injection of the aromatase inhibitor demonstrated that within 30 min both vorozole™ and ATD had reached the preoptic area and completely inhibited aromatase activity. When applied to the extracellular milieu of preoptic-hypothalamic explants maintained in vitro, Vorozole™ actually blocked aromatase activity within 5 min. These enzyme assays thus confirmed that the behavioral effects of aromatase inhibitors are correlated with the complete blockade of the enzymatic activity [86].
Figure 4.
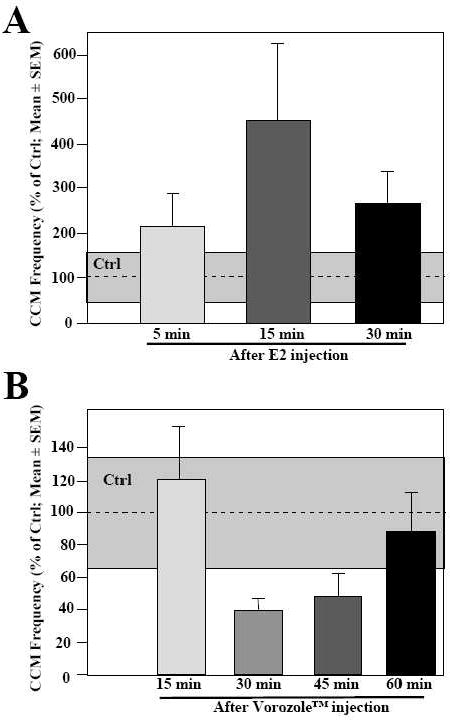
Behavioral effects of rapid changes in estrogen availability following an acute injection of estradiol (A) or of the aromatase inhibitor Vorozole™ (B). A. A single injection of estradiol (E2) performed 5, 15 or 30 min before the beginning of the behavioral test significantly increases the expression of sexual behavior measured here by the frequency of cloacal contact movements (CCM). B. Significant inhibition by a single injection of the aromatase inhibitor Vorozole™ performed 15, 30, 45 or 60 min before the beginning of the behavioral test of the expression of sexual behavior measured by the frequency of CCM. Control values for birds injected with the control vehicle solution are represented by the gray areas. Redrawn from data in [88] and [86]
Similarly, in mice the systemic acute injection of vorozole™, ATD or its metabolite 17-OH-ATD also completely suppressed mount and intromission behaviors within 10-20 minutes [87]. Such inhibitions were not observed in mice with targeted disruption of the aromatase gene (i.e. aromatase knock-out mice) in which sexual behavior has been activated by a chronic treatment with exogenous estrogens. This lack of effect of aromatase inhibitors in mice that do not express the enzyme but display sexual behavior activated by exogenous estrogens provides additional evidence for the specificity of the aromatase involvement in the acute modulation of sexual behavior.
Based on these studies, we anticipated that the preoptic aromatase activity should change significantly during or after the expression of sexual behavior in order to regulate the short-term variations in behavioral frequencies. This hypothesis was experimentally tested during a second group of studies conducted in quail. Sexually experienced male quail were given visual access to a receptive female or copulated with a receptive female for 1, 5 or 15 minutes. Control subjects were simply handled and returned to their home cage for the duration of the test. Immediately after the behavioral interaction brains were collected from all subjects. The block of brain tissue containing the preoptic-hypothalamic region was dissected out and aromatase activity was assayed in these samples. These enzymatic assays identified a decrease of aromatase activity in samples collected after 1 min of visual access to or copulatory interaction with the female. The reduction of activity peaked (-20%) after 5 min of interaction and was almost back to control activities after 15 min (Fig. 5) [115]. Ongoing experiments indicate that this 20% reduction of enzymatic activity following the expression of copulatory behavior results primarily from a decrease in enzymatic activity in the POM and the mediobasal hypothalamus (Cornil and Balthazart, unpublished data). Although the functional significance of such a reduction in brain aromatase activity still remains to be determined, these data demonstrate that aromatase activity is rapidly modulated in vivo in behaviorally relevant situations and in the relevant brain areas.
Figure 5.
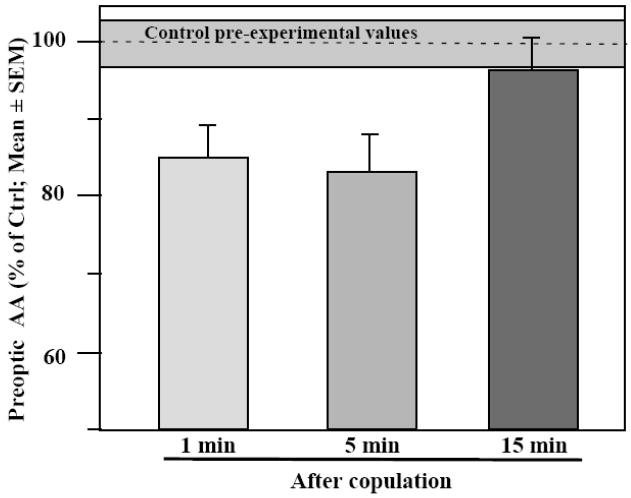
Rapid changes in aromatase activity (AA) are observed in quail following copulation with a sexually mature female for 1, 5 or 15 min. A significant decrease is observed at the 5 min time point. Control birds were simply handled and returned to their home cage (Values in gray area). Brains were collected immediately after the end of the behavioral test and AA was quantified in the preoptic area-hypothalamus. Redrawn from data in [115] where detail of statistical analyses can be found.
3.4. Rapid inactivation of brain estrogens
The rapid inhibition of sexual behavior following acute inhibition of brain aromatase prompted us to consider what terminates non-genomic estrogen actions when estrogen synthesis is reduced or interrupted. Although a single injection of an aromatase inhibitor almost immediately blocks estrogen synthesis (within 5 min or less, see above), one must indeed consider that high concentrations of estrogens should be present when this inhibition is installed and they should continue to activate for quite some time the neural mechanisms that mediate the activation of behavior. One would expect this estrogen-dependent neural activation to be blocked itself by some currently unidentified mechanism.
One possibility is that estrogens that are potentially present in very high concentrations at their site of synthesis (e.g., in presynaptic boutons, see [133-135]) simply diffuse away from their site of synthesis and action. Since the loci where this synthesis occurs produce high but very local concentrations that are presumably anatomically discrete, passive diffusion could fairly rapidly reduce these concentrations below the minimal threshold that is required to activate the non-genomic effects. Because these effects apparently require the presence of estrogen concentrations that are markedly higher than the circulating peripheral concentrations (see above), diffusion away from the localized synthesis sites should rapidly bring concentrations below the active threshold.
A non-exclusive alternative involves the rapid catabolism of the newly synthesized estrogens into less active and water-soluble steroids that can be eliminated in feces and/or urine. Estrogens are metabolized through oxidations, hydroxylations, and conjugation processes such as glucuronidation, sulfonation and/or O-methylation [136]. Although these metabolic pathways have been mainly described in the liver, detectable levels of metabolic activity have also been reported in the brain.
One of the best-characterized enzymes mediating this catabolism is the 2-hydroxylase which transforms estrogens into 2-hydroxy- or catechol-estrogens. Interestingly, it has been proposed that, depending on the available substrate, aromatase not only has the capacity to synthesize estrogens from androgens, but also displays an estrogen 2-hydroxylase activity. This conclusion is based on various independent observations including the fact that when cell lines that do not express aromatase nor 2-hydroxylase activities are transfected with the aromatase gene, they begin to express both enzymatic activities. Both reactions should thus be catalyzed by the same enzymatic protein [137]. This conclusion is also consistent with the fact that the distributions of these two enzymatic activities are nearly identical in the quail brain [138, 139]. Because catechol-estrogens are less active than their parent estrogens, the co-existence of these two enzymatic activities could provide a mechanism through which estrogens would be produced in high concentration, but also catabolized and partly inactivated in the exact same brain loci.
Regardless of the mechanism underlying estrogen inactivation, indirect evidence derived mainly from behavioral studies suggests that brain-synthesized estrogens are rapidly eliminated [13]. Together with the rapid modulation of estrogen synthesis through conformational changes of aromatase, these rapid enzymatic changes provide a mechanism to rapidly switch on and off the non-genomic actions of estrogens.
4. General conclusion
The last two decades have witnessed the identification of an unsuspected diversity of mechanisms through which steroids are produced and affect brain activity. Sex steroids are not only produced by the gonads and adrenals but the brain itself is able to synthesize these hormones directly from cholesterol [99] even if the functional significance of this synthesis still remains somewhat unclear. Two fundamentally different modes of steroid action in the brain are also clearly distinguishable now. A large part of their effects result from the modulation of the transcription of a vast array of specific genes but, additionally, more rapid effects are mediated by changes in intracellular signaling usually elicited by interactions of the steroids with the plasma membrane. We have presented here a selective review of a few recent developments in this field focusing specifically on the actions of estrogens in the brain as they relate to the activation of male sexual behavior.
The genomic effects of estrogens are known to be mediated by two different types of intracellular receptors (ERα and ERβ) that interact with a broad variety of coregulators to modulate gene expression. Work in our and other laboratories has shown that two of these coregulators, SRC-1 and SRC-2, play a key role in the activation by estrogens of male sexual behavior but a huge amount of work remains to determine how other regulators and transcription factors interfere with and control the estrogen-dependent genomic regulation.
On a shorter time scale, a variety of estrogen actions on neuronal physiology have been identified and they seem to be triggered by the interaction of estrogens with new membrane receptors (GPR30, STX-binding protein, ER-X,…) as well as with the classical nuclear receptors (ERα and ERβ) that become associated to the neuronal membrane. It is now firmly established in a variety of animal species that these rapid non-genomic actions of estrogens at the neuronal level play a significant role in the control of short-term variations in reproductive behavior. In quail, we demonstrated the existence of two independent mechanisms that regulate the local synthesis of estrogens in the brain: (1) a transcriptional control of the concentration of the enzyme aromatase and (2) conformational changes (phosphorylations) leading to rapid changes in the enzymatic kinetics independent of changes in enzyme concentration. These two processes seem to nicely match the time scale of genomic and non-genomic actions of estrogens on behavior. Changes in aromatase activity mediated by phosphorylations of the enzyme indeed provide a transient source of high local concentrations of estrogens that are necessary to trigger most of the non-genomic effects described in vitro and in vivo. In this case also, many questions remain, however, unanswered concerning namely the nature of the membrane receptors implicated in the rapid control of behavior by estrogens and the mechanisms that are able to rapidly inactivate local estradiol action when its synthesis is blocked either pharmacologically or due to physiological changes in neurotransmitter activity. One might have thought at the end of the 1980s that the mechanisms of steroid action had been (almost) completely identified and research could turn to other important questions. Recent developments that are briefly and selectively summarized here clearly demonstrate that this is far from being the case and there are still many significant questions that require additional experimental investigations.
Figure 2.
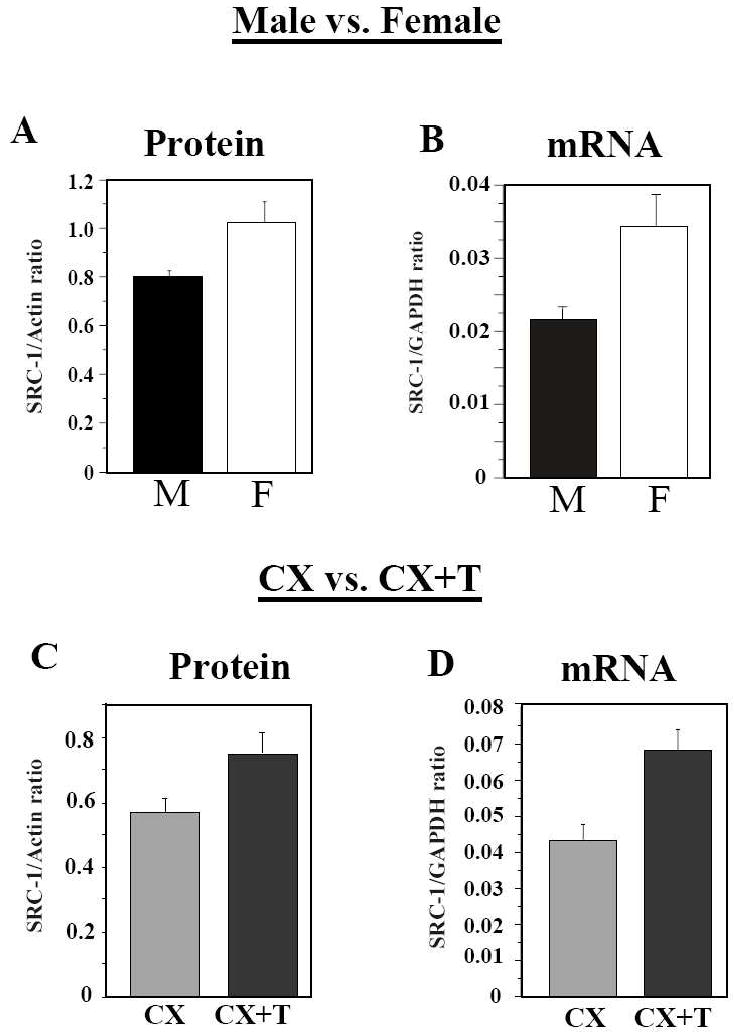
Expression of SRC-1 in the preoptic area-hypothalamus is significantly affected by the sex and endocrine condition of the subjects. The concentration of the SRC-1 protein (A, C) and of the corresponding mRNA (B, D) were measured by Western blot and quantitative real-time PCR respectively in sexually mature males (M) and females (F) (panels A, B) and in castrated males that had been treated with testosterone (CX+T) or not (CX) (panels C, D). SCR-1 is expressed at higher concentrations (both mRNA and protein) in males than in females and in testosterone-treated castrates than in castrates. Both the sex difference and the effect of testosterone were however not replicated in one other experiment (not shown here) for reasons that are not completely identified (see [54] for discussion). Redrawn from data in [54] where detail of statistical analyses can also be found.
Acknowledgments
The experimental work described in this review was supported by grants from the NIH (R01 MH 50388) to GFB and JB and from the Belgian Fonds de la Recherche Fondamentale collective (FRFC 2.4537.09) to JB. CAC is a F.R.S.-FNRS Research associate. TDC is a F.R.S.-FNRS Postdoctoral Researcher.
Footnotes
Disclaimers: the authors have nothing to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Celotti F, Massa R, Martini L. Metabolism of sex steroids in the central nervous system. In: DeGroot LJ, editor. Endocrinology. Grune & Stratton; New York: 1979. pp. 41–53. [Google Scholar]
- 3.Martini L, Celotti F, Lechuga MJ, Melcangi RC, Motta M, Negri-Cesi P, Poletti A, Zoppi S. Androgen metabolism in different target tissues. Ann N Y Acad Sci. 1990;595:184–198. doi: 10.1111/j.1749-6632.1990.tb34292.x. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. Vol. 3. Springer Verlag; Berlin: 1989. pp. 105–159. [Google Scholar]
- 5.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 6.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 9.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 10.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 12.Kelly MJ, Ronnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Brain and Behavior. Vol. 3. 2002. pp. 361–380. [Google Scholar]
- 13.Cornil CA. Rapid regulation of brain oestrogen synthesis: the behavioural roles of oestrogens and their fates. J Neuroendocrinol. 2009;21:217–226. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS. Neural gonadal steroid actions. Science. 1981;211:1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 18.Joab I, Radanyi C, Renoir M, Buchou T, Catelli MG, Binart N, Mester J, Baulieu E-E. Common non-hormone binding component in non transformed chick oviduct receptors of four steroid hormones. Nature. 1984;308:850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- 19.Pratt WB, Toft DO. Regulation fo signaling protein function and trafficking by hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 20.Jensen EV. Steroid hormones, receptors, and antagonists. Ann N Y Acad Sci. 1996;784:1–17. doi: 10.1111/j.1749-6632.1996.tb16223.x. [DOI] [PubMed] [Google Scholar]
- 21.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple functions. Mol Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 22.Payvar F, Franco D, Firestone GL, Edgar B, Wrange O, Okret S, Gustafsson J-A, Yamamoto K. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983;35:381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- 23.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acid Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Molec Endocrinol. 2008;41:263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ing NH, Beekman JM, Tsai SY, Tsai M-J, O’Malley BW. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II) J Biol Chem. 1992;267:17617–17623. [PubMed] [Google Scholar]
- 26.Meyer ME, Gronemeyer H, Turcotte B, Bocquel M-T, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- 27.Oñate SA, Tsai SY, Tsai M-J, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 28.Torchia J, Rose DW, Inostrazo J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Koh SS, Zhang X, Cheng X, Stallcup MR. Synergy among nuclear receptor coactivators: selective requirement for protein methyltransferase and acetyltransferase activities. Mol Cell Biol. 2002;22:3621–3632. doi: 10.1128/MCB.22.11.3621-3632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Huang SM, Stallcup MR. Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J Biol Chem. 2000;275:40810–40816. doi: 10.1074/jbc.M005459200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Benveniste EN. Transcriptional activation of human matrix metalloproteinase-9 gene expression by multiple co-activators. J Mol Biol. 2008;383:945–956. doi: 10.1016/j.jmb.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonard DM, O’Malley BW. Expanding functional diversity of the coactivators. TIBS. 2005;30:126–132. doi: 10.1016/j.tibs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–342. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Naruse I, Hongo T, Xu M-J, Nakahata T, Maekawa T, Ishii S. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech Dev. 2000;95:135–145. doi: 10.1016/s0925-4773(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 35.Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai M-J, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 37.Weiss RE, Gehin M, Xu J, Sadow PM, O’Malley BW, Chambon P, Refetoff S. Thyroid function in mice with heterozygous and homozygous disruptions of SRC-1 and TIF-2 coactivators: evidence for haploinsufficiency. Endocrinology. 2002;143:1554–1557. doi: 10.1210/endo.143.4.8828. [DOI] [PubMed] [Google Scholar]
- 38.Weiss RE, Xu J, Ning G, Pohlenz J, O’Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO. 1999;18:1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apostolakis EM, Ramamurphy M, Zhou D, Onate SA, O’Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 40.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on the male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 41.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sexual behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monks DA, Xu J, O’Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen -mediated sexual differentiation of spinal motoneurons. Neuroendocrinology. 2003;78:45–51. doi: 10.1159/000071705. [DOI] [PubMed] [Google Scholar]
- 44.Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC-1. Molec Cell Biol. 1999;19:8383–8392. doi: 10.1128/mcb.19.12.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 46.Viglietti-Panzica C, Aste N, Balthazart J, Panzica GC. Vasotocinergic innervation of sexually dimorphic medial preoptic nucleus of the male Japanese quail: Influence of testosterone. Brain Res. 1994;657:171–184. doi: 10.1016/0006-8993(94)90965-2. [DOI] [PubMed] [Google Scholar]
- 47.Balthazart J, Foidart A, Hendrick C. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 48.Charlier TD, Harada N, Ball GF, Balthazart J. Targeting steroid receptor coactivator-1 expression with locked nucleic acid antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res. 2006;172:333–343. doi: 10.1016/j.bbr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 50.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Nat Acad Sci USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavaretta IT, Mukopadhyay R, Lonard DM, Cowsert LM, Bennet CF, O’Malley BW. Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERalpha transcriptional activity and MCF-7 proliferation. Molec Endocrinol. 2002;16:253–270. doi: 10.1210/mend.16.2.0770. [DOI] [PubMed] [Google Scholar]
- 53.Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;76:297–315. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- 54.Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator1 in the japanese quail brain: Effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;140:1381–1394. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Misiti S, Shomburg L, Yen PM, Chin WW. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- 56.Mitev YA, Wolf SS, Almeida OFX, Patchev VK. Developmental expression profiles and distinct regional estrogen responsiveness suggest novel role for the steroid receptor coactivator SRC-1 as a discriminative amplifier of estrogen signaling in the rat brain. FASEB J. 2003;17:518–519. doi: 10.1096/fj.02-0513fje. [DOI] [PubMed] [Google Scholar]
- 57.Camacho-Arroyo I, Neri-Gomez T, Gonzales-Arenas A, Guerra-Araiza C. Changes in the content of steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid hormone receptors in rat brain during estrous cycle. J Steroid Bioch Mol Biol. 2005;94:267–272. doi: 10.1016/j.jsbmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Tetel MJ, Ungar TC, Hassan B, Bittman EL. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in siberian hamster brain. Mol Brain Res. 2004;131:79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan F, Gao X, Lonard DM, Nawaz Z. Specific ubiquitin-conjugating enzymes promote degradation of specific nuclear receptor coactivators. Mol Endocrinol. 2003;17:1315–1331. doi: 10.1210/me.2002-0209. [DOI] [PubMed] [Google Scholar]
- 60.Lonard DM, O’Malley BW. SRC-3 transcription-coupled activation, degradation, and the ubiquitin clock: is there enough coactivator to go around in cells? Sci Signal. 2008;1:pe16. doi: 10.1126/stke.113pe16. [DOI] [PubMed] [Google Scholar]
- 61.Gonzales-Flores O, Guerra-Araiza C, Cerbón M, Camacho-Arroyo I, Etgen AM. The 26S proteasome participates in the sequential inhibition of estrous behavior induced by progesterone in rats. Endocrinology. 2004;145:2328–2336. doi: 10.1210/en.2003-1162. [DOI] [PubMed] [Google Scholar]
- 62.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 63.Auboeuf D, Dowhan DH, Kang YK, Larkin K, Lee JW, Berget SM, O’Malley BW. Differential recruitment of nuclear receptor coactivators may determine alternative RNA splice site choice in target genes. Proc Natl Acad Sci USA. 2004;101:2270–2274. doi: 10.1073/pnas.0308133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Yi X, Sun X, Yin N, Shi B, Wu H, Wang D, Wu G, Shang Y. Differential gene regulation by the SRC family of coactivators. Genes and Development. 2004;18:1753–1765. doi: 10.1101/gad.1194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charlier TD. Importance of steroid receptor coactivators in the modulation of steroid action on brain and behavior. Psychoneuroendocrinol. 2009 doi: 10.1016/j.psyneuen.2009.05.004. In press. [DOI] [PubMed] [Google Scholar]
- 66.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 67.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system : Mechanisms and nonreproductive functions. Ann Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 68.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 69.James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus) Physiol Behav. 2002;75:287–294. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- 70.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: When it occurs and why. Front Neuroendocrinol. 2009;30:460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endo Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moss RL, Gu Q, Wong M. Estrogen, nontranscriptional signaling pathway. Rec Prog Horm Res. 1997;52:33–69. [PubMed] [Google Scholar]
- 74.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5’-monophosphatase response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 75.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)a and ERb exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 76.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells : effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 77.Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD. Estradiol (E2) elicits Src phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–5148. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- 78.Abraham IM, Han S-K, Todman MG, Korach KS, Herbison AE. Estrogen receptor Beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 81.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 83.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Nat Acad Sci USA. 2004;101:12354–11235. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 86.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 89.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiol Behav. 1991;50:793–799. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- 91.Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006;1126:27–23. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 92.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 93.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Nat Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 95.Kow L-M, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rats hypothalamic ventromedial nucleus. Brain Res. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 96.Bixo M, Backstrom T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Biol. 1995;55:297–303. doi: 10.1016/0960-0760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 97.Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Nat Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsutsui K, Ukena K, Takase M, Kohchi C, Lea RW. Neurosteroid biosynthesis in vertebrate brains. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:121–129. doi: 10.1016/s0742-8413(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 99.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Aste N, Watanabe Y, Shimada K, Saito N. Sex- and age-related variation in neurosteroidogenic enzyme mRNA levels during quail embryonic development. Brain Res. 2008;1201:15–22. doi: 10.1016/j.brainres.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 101.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 102.Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104:244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beach FA, Inman NG. Effects of castration and androgen replacement on mating in male quail. Proc Nat Acad Sci USA. 1965:1426–1431. doi: 10.1073/pnas.54.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adkins EK, Pniewski EE. Control of reproductive behavior by sex steroids in male quail. J Comp Physiol Psychol. 1978;92:1169–1178. [Google Scholar]
- 105.Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal inhibitor R76713 on testosterone-induced sexual behavior in the japanese quail (Coturnix coturnix japonica) Horm Behav. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- 106.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 107.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 108.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 109.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 110.Miller TW, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL. Aromatase is phosphorylated in situ at serine-118. J Steroid Biochem Mol Biol. 2008;112:95–101. doi: 10.1016/j.jsbmb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 112.Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Res. 2004;1029:224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 114.Hoffman NW, Kim YI, Gorski RA, Dudek FE. Homogeneity of intracellular eletrophysiological properties in different neuronal subtypes in medial preoptic slices containing the sexually dimorphic nucleus of rat. J Comp Neurol. 1994;345:396–408. doi: 10.1002/cne.903450306. [DOI] [PubMed] [Google Scholar]
- 115.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 120.Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. J Comp Neurol. 2000:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 121.Naftolin F, Horvath TL, Balthazart J. Estrogen synthetase (aromatase) immunohistochemistry reveals concordance between avian and rodent limbic systems and hypothalami. Proc Soc Exp Biol Med. 2001;226:717–725. doi: 10.1177/153537020222600802. [DOI] [PubMed] [Google Scholar]
- 122.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 123.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 124.Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia. 2009 doi: 10.1002/glia.20910. [DOI] [PubMed] [Google Scholar]
- 125.Minami T, Oomura Y, Nabekura J, Fukuda A. 17b-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 126.Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 127.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptor. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 129.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:9225–9229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61:365–374. [PubMed] [Google Scholar]
- 131.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 132.Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007 doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 133.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 135.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain - An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 136.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells : review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 137.Osawa Y, Higashiyama T, Shimizu Y, Yarborough C. Multiple functions of aromatase and the active site structure; Aromatase is the placental estrogen 2-hydroxylase. J Steroid Biochem Mol Biol. 1993;44:469–480. doi: 10.1016/0960-0760(93)90252-r. [DOI] [PubMed] [Google Scholar]
- 138.Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- 139.Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res Bull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]


