Abstract
Background and Aims
Studies evaluating the effect of legume consumption on cholesterol have focused on soybeans, however non-soy legumes, such as a variety of beans, peas, and some seeds, are commonly consumed in Western countries. We conducted a meta-analysis of randomized controlled trials evaluating the effects of non-soy legume consumption on blood lipids.
Methods and Results
Studies were retrieved by searching MEDLINE (from January 1966 through July 2009), EMBASE (from January 1980 to July 2009), and the Cochrane Collaboration's Central Register of Controlled Clinical Trials using the following terms as medical subject headings and keywords: fabaceae not soybeans not isoflavones and diet or dietary fiber and cholesterol or hypercholesterolemia or triglycerides or cardiovascular diseases. Bibliographies of all retrieved articles were also searched. From 140 relevant reports, 10 randomized clinical trials were selected which compared a non-soy legume diet to control, had a minimum duration of 3 weeks, and reported blood lipid changes during intervention and control. Data on sample size, participant characteristics, study design, intervention methods, duration, and treatment results were independently abstracted by 2 investigators using a standardized protocol. Data from 10 trials representing 268 participants were examined using a random-effects model. Pooled mean net change in total cholesterol for those treated with a legume diet compared to control was −11.8 mg/dL (95% confidence interval [CI], −16.1 to −7.5); mean net change in low density lipoprotein cholesterol was −8.0 mg/dL (95% CI, −11.4 to −4.6).
Conclusion
These results indicate that a diet rich in legumes other than soy decreases total and LDL cholesterol.
Keywords: legumes, fabaceae, meta-analysis, randomized controlled trial, cholesterol, cardiovascular diseases
INTRODUCTION
Worldwide, cardiovascular diseases (CVD) are estimated to be the leading cause of death and loss of healthy life years resulting from disability (1). In the United States, CVD causes 1 of every 3 deaths (2). Recent data show that 71.3 million people in the United States have two or more risk factors for heart disease (2). Studies have consistently shown that risk factor modification can decrease the prevalence of cardiovascular diseases, such as coronary heart disease and strokes (3-6). Diet is an important modifiable risk factor for many types of heart disease (7, 8).
Observational epidemiologic studies have strongly indicated an inverse relationship between fruit and vegetable consumption and the incidence of cardiovascular events (7, 9). So much so that not consuming fruits and vegetables daily may be responsible for up to 13.7% of acute myocardial infarcts in one estimate(10). Several studies have also shown that persons who consume diets high in whole grains and fiber have lower blood pressure and total cholesterol levels (11, 12). The Dietary Guidelines for Americans suggest consuming 3 cups of legumes, which are rich in soluble dietary fiber and vegetable protein, per week; however less than a third of the population meets this guideline (13, 14). Legume consumption has been associated with lower risks of coronary heart disease in observational epidemiologic studies (15, 16) and has been shown to decrease total cholesterol and low-density lipoprotein cholesterol in clinical trials (17, 18). However, the majority of studies that have evaluated the hypocholesterolemic effects of legume consumption examined soybeans specifically rather than the many non-soy legumes, which are more commonly consumed in the Western hemisphere (19). Non-soy legumes include a variety of beans such as navy, pinto, kidney, garbanzo and lima beans and peas such as split green peas or lentils. Randomized controlled trials that have examined the potential hypocholesterolemic effects of a diet rich in non-soy legumes have differed in their findings (20-31), with some finding no effect (22, 24, 31), while other identified a significant cholesterol lowering effect (20, 21, 27). We conducted a meta-analysis of randomized controlled trials to quantify the direction and magnitude of the potential effect that consumption of non-soy legumes may have on serum cholesterol concentrations.
METHODS
Study Selection
We searched the online databases MEDLINE (from January 1966 through July 2009) using the following terms as medical subject headings and keywords: fabaceae not soybeans not isoflavones and diet or dietary fiber and cholesterol or hypercholesterolemia or triglycerides or cardiovascular diseases. An EMBASE database search (from January 1980 to July 2009) was also performed using the database-specific medical subject headings and keywords: legume or bean not soybean not isoflavone and dietary fiber or high fiber diet and cholesterol or hypercholesterolemia or triacylglycerol or cardiovascular disease. Both searches were limited to human subjects but not limited in language. The authors also performed a search of the Cochrane Central Register of Controlled Trials with the same search criteria and limits. A manual examination of the references in the published studies and in suitable review articles was also performed. Experts in the field were contacted to determine if any other studies were near completion.
The titles and abstracts of 140 studies were identified through the literature search and were reviewed independently by two investigators (M.T.T., C.H.N.) in duplicate to determine whether they met eligibility criteria for inclusion. Where discrepancies between investigators occurred for inclusion or exclusion, a third investigator (L.A.B.) was involved to conduct additional evaluation of the study, and discrepancies were resolved by consensus. Studies were eligible for inclusion if they met the following criteria: (1) the study design was a randomized control trial; (2) the study had similar total energy and macronutrient values in the control and legume diets; (3) the intervention and control groups reported total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and/or triglycerides; (4) the intervention consisted of non-soy legume consumption; (5) the duration of intervention was at least 3 weeks; and (6) study participants were adults.
Data abstraction
All data were independently abstracted in duplicate by 2 investigators (M.T.T., C.H.N.) using a standardized data collection form. Discrepancies were resolved by discussion with a third investigator (L.A.B.) and by referencing the original articles. If necessary, we contacted authors for missing data. Publication characteristics were recorded as follows: primary author's name, publication source and year, country of origin, study design (parallel, factorial, or crossover trial), blinding (open, single, or double), whether there was a washout period, if the treatment allocation was concealed, if intention-to-treat analysis was used, study duration, sample size, percentage of male participants, mean age and range, baseline mean levels of lipids, inclusion and exclusion criteria, and a description of legume preparation. Dietary components for both treatment and control groups were recorded as follows: caloric amount (kcal/d), protein (g/d), carbohydrates (g/d), total fat (g/d), saturated fat (g/d), mono-unsaturated fat (g/d), poly-unsaturated fat (g/d), dietary cholesterol (mg/d), total dietary fiber (g/d) and soluble dietary fiber (g/d). Mean study endpoints for the legume and control diets were recorded for total cholesterol, HDL cholesterol, LDL cholesterol, VLDL cholesterol, and triglycerides.
Statistical analysis
Some studies reported lipid levels in mg/dL, which required conversion to mmol/L prior to computations. For conversion, 1 mg/dL=0.0259 mmol/L was used for cholesterol and 1 mg/dL=0.0113 mmol/L was used for triglycerides.
The mean total cholesterol, HDL, LDL, VLDL, and triglycerides at baseline and at the end of intervention period were used to calculate mean net changes for each study. For parallel trials, mean net changes for the outcomes listed above were calculated as the difference (legume diet minus control diet) of the changes (baseline minus endpoint) in mean values. For crossover trials, mean net changes for the outcomes were calculated as the difference (legume diet minus control diet) in values at the end of the intervention and control phases. In studies where two amounts of the same legume were tested (23), we used the average values from the two intervention arms for the change in cholesterol levels. One trial used a 3 phase crossover design to examine the effects of pinto beans and black-eyed peas separately with only one control group (31). In order to avoid double use of the control group, we used only the data from pinto beans to reduce possible between-study heterogeneity due to the variety of legumes included in the meta-analysis.
Variance of the mean net changes of the outcomes (total cholesterol, HDL, LDL, VLDL, and triglycerides) for each trial was calculated using standard deviations or standard errors. To calculate pooled mean net changes, each study was assigned a weight, calculated as the reciprocal of the variance for the mean net changes.
The homogeneity of the effect size among studies was tested using a χ2 test. Our tests indicated homogeneity across the studies included in the meta-analysis for total cholesterol (p=0.26) and LDL cholesterol(p=0.65), and heterogeneity across the studies included for HDL (p=0.005), VLDL (p<0.0001), and triglycerides (p= <0.0001). Due to the heterogeneity identified between studies, we used DerSimonian and Laird random-effects models, which take into account between-study variations, to calculate the pooled mean net change of lipids comparing the legume diets with control diets (32). A meta-regression was also performed to examine sources of heterogeneity and determine the influences of study characteristics including age of participants, proportion of male participants, study design, country of origin, study size, and the duration of intervention, on effect-size estimates.
To assess for potential publication bias, we constructed funnel plots for each outcome in which the mean net change was plotted against the study size (33). In addition, Begg's rank correlation test was used to examine the association between mean net change and its variance, and Egger's linear regression test, which regresses z statistics on the reciprocal of the standard error (SE) for each study, was also used to detect publication bias (34, 35). We conducted an influence analysis where each trial was excluded in turn to evaluate the influence of that trial on the pooled estimate and determine if that study was an outlier. All analyses were conducted in STATA version 9.2 (StataCorp, College Station, TX). We conformed to QUOROM (Quality of Reporting of Meta-analyses) guidelines in the report of this meta-analysis of randomized controlled trials (36).
RESULTS
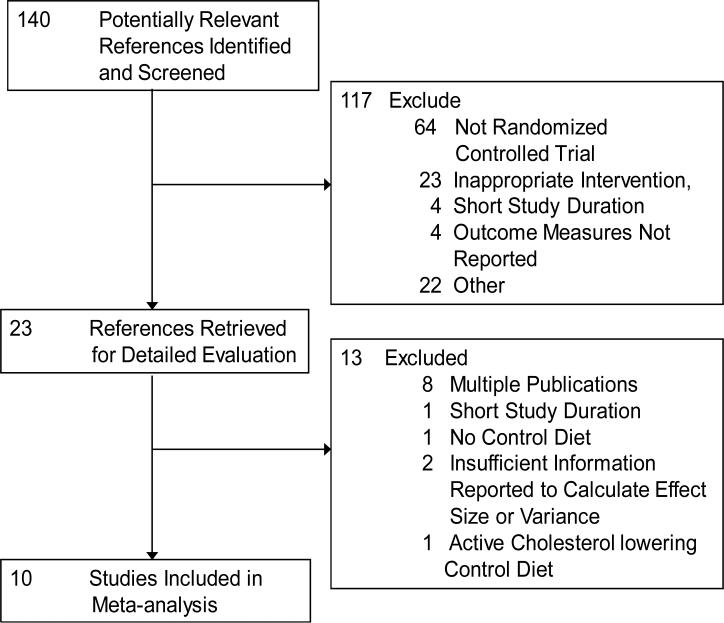
Figure 1 depicts the flow of study selection for the analysis. Of the 140 potentially relevant references identified, 117 were excluded following review of abstract and title. A total of 23 full-text articles were retrieved and reviewed for inclusion. We further excluded 8 articles due to multiple publications from an individual trial, 1 study was shorter than 3 weeks in duration, 1 was excluded due to lack of control diet, 2 articles were excluded because they reported insufficient information to calculate an effect size and/or its variance and 1 was eliminated because the control diet was an active cholesterol lowering intervention. A total of 10 randomized controlled trials were included in this meta-analysis, representing data collected from 268 participants.(21-26, 28-31)
Figure 1.
Flow diagram of articles identified and evaluated during the study selection process.
The baseline characteristics of the study participants and designs of the randomized controlled trials are presented in Table 1. Out of the 10 trials, 4 were conducted in the United States, 2 in Australia, 2 in Spain, and 1 each in Chile, and New Zealand. A total of 268 participants were included in the analysis. There were 188 men in the trials, representing 70.1% of all participants and 5 trials had exclusively male participants. Study participants ranged in age from 18 to 78. Participants with high, borderline high, and normal cholesterol levels were included and no studies included participants who were taking cholesterol-lowering drugs. Total cholesterol at baseline was reported for 8 studies and study means ranged from 199 to 294.6 mg/dL(22-26, 29-31). Mean baseline LDL- and HDL-cholesterol were reported in 6 studies and mean LDL cholesterol ranged from 138 to 200.4 mg/dL while mean HDL cholesterol ranged from 39.0 to 58.0 mg/dL.(22-26, 30, 31) Trials primarily employed the crossover design, however 2 were parallel and 1 was factorial in design. Most trials matched macronutrient and energy content between the legume diet and control diet groups, including amounts of saturated and total fat in the diets (Table 2). Various non-soy legumes were represented; intervention diets included the addition of mixed legume dishes, whole chickpeas, field beans ground into flour, whole pinto beans, canned baked beans, whole peas, and whole navy beans, among others (Table 1). Comparison groups consisted of calorie and macronutrient-matched control diets, often with a wheat-based or canned vegetable substitution. Intervention durations ranged from 3 to 8 weeks. Most of the studies were conducted in free-living adults, though 1 trial was conducted in a metabolic lab (26) and in one trial all meals were eaten at the study kitchen.(21)
Table 1.
Baseline characteristics of participants and design characteristics of randomized controlled trials of non-soy legume consumption.
| Source | N | Mean Age, y | Men, % | Total Cholesterol, mg/dL | LDL, mg/dL | HDL, mg/dL | TG, mg/dL | Type of Legume | Design* | Legume, g/d | Length of Intervention (Phase), d | Type of Control Diet† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nervi et al, 198921 | 20 | 18-22§ | 100 | -- | -- | -- | -- | Peas, lentils | C | 120 | 30 | matched |
| Cobiac et al, 199022 | 20 | 29-65§ | 100 | 246.3 | 181.1 | 48.3 | 99.1 | Baked beans | C | 440 | 28 | spaghett |
| Anderson et al 199023 | 24 | 58.0 | 100 | 294.6 | 200.4 | 41.7 | 255.8 | Baked beans | C | 120;162 | 21 | matched |
| Mackay et al, 199224 | 39 | 28-66§ | 56.4 | 266.8 | 161.8 | 44.4 | 134.5 | Pinto, haricot, kidney | F | 80 | 42 | matched |
| Fruhbeck et al, 199725 | 40 | 18-21§ | 100 | 223.9 | 155.4 | 39.0 | 148.9 | Field bean flour | P | 90 | 30 | matched |
| Duane WC, 199726 | 9 | 58.0 | 100 | 204 | -- | -- | -- | Red, navy, lima, peas, lentils | C | 120 | 42-49 | matched |
| Pittaway et al, 200628 | 47 | 53.0 | 40.4 | -- | -- | -- | -- | Chickpeas | C | 140 | 35 | wheat |
| Crujeiras et al, 200729 | 30 | 36.0 | 57 | 199 | -- | -- | -- | Lentils, chickpeas, peas, fava | P | -- | 56 | matched |
| Winham et al, 200730 | 23 | 45.9 | 43.48 | 218 | 138 | 58 | 133.0 | Baked beans (Navy beans) | C | 130 | 56 | carrots |
| Winham et al, 200731 | 16 | 43.0 | 43.75 | 217 | 138 | 54 | 150.0 | Pinto OR black-eyed peas | C | 130 | 56 | carrots |
P denotes parallel; C denotes crossover; F denotes factorial.
The age range of participants were provided in lieu of a mean age.
matched denotes macronutrient and total energy contents of intervention and control diets are the same.
Table 2.
Dietary composition of non-soy legume diets as compared to control diets.
|
Nutrients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Diet | Total Energy (kcal/d) | Protein (g/d) | Carbohydrate (g/d) | Dietary Fiber (g/d) | Fat (g/d) | SFA (g/d) | MUFA (g/d) | PUFA (g/d) | Dietary Cholesterol (mg/d) |
| Nervi et al, 198921 | Legume | 3219 | 116 | 438 | 12.4 | 118 | -- | -- | -- | 300 |
| |
Control |
3219 |
118 |
430 |
12.5 |
120 |
-- |
-- |
-- |
302 |
| Cobiac et al, 199022 | Legume | 2318 | 99 | 279 | 22.5 | 85 | 35 | 28.9 | 13 | -- |
| |
Control |
2287 |
95 |
283 |
11 |
82 |
32.7 |
26.8 |
12.4 |
-- |
| Anderson et al 199023 | Legume | 1855 | 89 | 204 | 22.6 | 78 | 25.3 | 32.3 | 10.3 | 415 |
| |
Control |
1812 |
89 |
203 |
12.9 |
77 |
26.3 |
32.3 |
9.5 |
412 |
| Mackay et al, 199224 | Legume | 1710 | 71 | 227 | -- | 51.8 | 16.6 | -- | -- | 161 |
| |
Control |
1664 |
67 |
221 |
-- |
53.6 |
18.5 |
-- |
-- |
188 |
| Fruhbeck et al, 199725 | Legume | 3302 | 75 | 272 | 20 | 39 | 13 | 16 | 5 | 221 |
| |
Control |
3298 |
74 |
271 |
19 |
38 |
12 |
16 |
5 |
218 |
| Duane WC, 199726 | Legume | -- | -- | -- | 24.1 | -- | -- | -- | -- | 350 |
| |
Control |
-- |
-- |
-- |
19.1 |
-- |
-- |
-- |
-- |
350 |
| Pittaway et al, 200628 | Legume | 1959 | 89 | 224 | 30.5 | 69.7 | 26.5 | 25.6 | 11.9 | -- |
| |
Control |
2005 |
95 |
221 |
27.9 |
73.8 |
29.0 |
30.0 |
11.7 |
-- |
| Crujeiras et al, 200729 | Legume | 1462 | 69 | 184 | 25 | 50.9 | -- | -- | -- | 81 |
| |
Control |
1462 |
69 |
184 |
18 |
54.3 |
|
-- |
-- |
93 |
| Winham et al, 200730 | Legume | 2046 | 77 | 262 | 23 | 73 | 23 | 16 | 8 | 211 |
| |
Control |
2214 |
91 |
275 |
21 |
80 |
27 |
19 |
8 |
227 |
| Winham et al, 200731 | Legume | 2086 | 88 | 263 | 25.5 | 73 | 24 | 18 | 7.8 | 280 |
| Control | 2122 | 88 | 265 | 20.7 | 79 | 27 | 17 | 7.6 | 276 | |
*SFA denotes saturated fatty acids; MUFA denotes monounsaturated fatty acids; PUFA denotes polyunsaturated fatty acids.
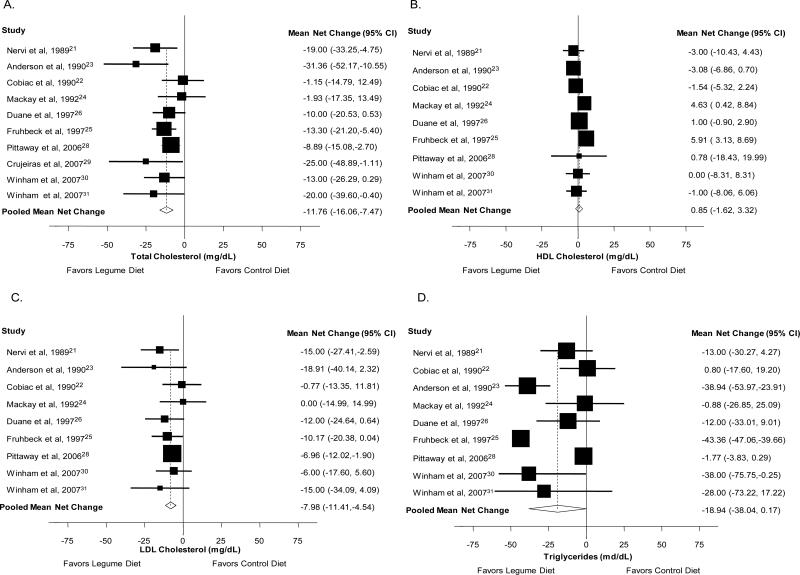
Mean net changes and corresponding 95% CIs for total serum cholesterol were reported in 10 studies (21-26, 28-31), and HDL, LDL cholesterol and triglycerides were reported in 9 studies (21-26, 28, 30, 31). For total cholesterol, the mean net changes in each study ranged from 1.2 to −31.4 mg/dL. The pooled mean net change from a legume diet was −11.76 mg/dL (95%CI: −16.06, −7.47, p<0.001; Χ2 for heterogeneity p=0.26), Figure 2, Panel A. The mean net changes for HDL cholesterol ranged from −4.03 to 5.91 mg/dL, and the pooled mean net change was 0.85 mg/dL (95%CI: −1.62, 3.32, p=0.05; Χ2 for heterogeneity p=0.005), Figure 2, Panel B. For serum LDL cholesterol, mean net changes ranged from −18.91 to 0.0 mg/dL and pooled mean net change was −7.98 mg/dL (95%CI: −11.41, −4.54, p<0.001; Χ2 for heterogeneity p=0.65), Figure 2, Panel C. For triglycerides, mean net changes ranged from −43.36 to 0.80 mg/dL. Pooled mean net change for serum triglycerides was −18.94 mg/dL (95%CI: −38.04, 0.17, p=0.05; Χ2 for heterogeneity p<0.001), Figure 2, Panel D. Only 3 studies reported information on VLDL as an outcome measure (not shown), and mean net changes ranged from −8.24 mg/dL to 1.00 mg/dL and the pooled mean net change was −3.34 mg/dL (95%CI: −9.13, 2.45, p=0.26; Χ2 for heterogeneity p<0.001).
Figure 2.
Mean net change in total (Panel A), HDL (Panel B), and LDL cholesterol (Panel C) and triglycerides (Panel D) and corresponding 95% confidence intervals by trial and pooled.
We examined the potential for publication bias by plotting sample sizes versus mean net change for total cholesterol, HDL, LDL, VLDL, and triglycerides among the trials included in this meta-analysis using Begg's rank correlation test (p=0.09, p=0.75, p=0.30, p=0.12 and p=0.99 for total, HDL, LDL and VLDL cholesterol, and triglycerides, respectively) and Egger's linear regression tests (p=0.19, p=0.53, p=0.41, p=0.01 and p=0.55 for total, HDL, LDL and VLDL cholesterol, and triglycerides, respectively).
We also examined heterogeneity between studies. Heterogeneity among the effect sizes of individual trials for total, LDL, HDL and VLDL cholesterol and triglycerides had I-square values of 19.7%, 63.8%, 0%, 89.0% and 97.9% respectively. Significant heterogeneity remained between studies for HDL and VLDL cholesterol and triglycerides, therefore, we performed a meta-regression analysis to examine characteristics of the trials and/or their study populations which may affect the heterogeneity in mean net change for lipid parameters. Significant predictors (p<0.05) of the mean net change in total cholesterol among studies included the number of male participants and the length of the intervention phase. For LDL cholesterol, significant predictors included mean age, number of male participants, study design, number of participants and duration of the study. For triglycerides only mean age was a significant predictor and for HDL cholesterol none of the study characteristics were significant predictors.
We then performed sensitivity analyses on net change of lipid concentration using gender distribution (trials with 100% vs. <100% male participants), median duration of the intervention (< 5 vs. ≥5 weeks), study design (crossover vs. parallel or factorial design), and type of control diet (matched vs. other), shown in Table 3. In the influence analysis, exclusion of any single study did not change the significance of the pooled estimates for total cholesterol, LDL, HDL, VLDL, or triglycerides.
Table 3.
Sensitivity analysis of mean net change in serum lipid concentrations using different exclusion criteria.
| Total Cholesterol |
LDL Cholesterol |
HDL Cholesterol |
Triglycerides |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
N |
Net Change (95% CI) |
I2 |
N |
Net Change (95% CI) |
I2 |
N |
Net Change (95% CI) |
I2 |
N |
Net Change (95% CI) |
I2 |
| Gender Distribution | ||||||||||||
| 100% male | 5 | −13.0 (−20.3, −5.8) | 41.7% | 5 | −10.3 (−16.0, −4.6) | 0.0% | 5 | 0.33 (−2.98, 3.64) | 79.2% | 5 | −22.5 (−41.0, −4.0) | 89.5% |
| <100% male |
5 |
−10.2 (−15.1, −5.2) |
0.0% |
4 |
−6.7 (−11.0, −2.3) |
0.0% |
4 |
2.59 (−0.68, 5,86) |
0.0% |
4 |
−8.0 ( −22.4, 6.3) |
37.5% |
| Study Duration | ||||||||||||
| <5 weeks | 4 | −14.4 (−24.2, −4.6) | 54.2% | 4 | −9.9 (−16.7, −3.0) | 10.9% | 4 | −0.11 (−5.2, 5.0) | 84.3% | 4 | −24.8 (−45.2, −4.5) | 90.4% |
| ≥5 weeks |
6 |
−10.1 (−14.6, −5.6) |
0.0% |
5 |
−7.2 (−11.3, −3.1) |
0.0% |
5 |
1.4 (−0.2, 3.0) |
0.0% |
5 |
−7.0 (−17.3, 3.2) |
29.7% |
| Study Design | ||||||||||||
| Crossover | 7 | −12.0 (−17.5, −6.5) | 27.4% | 5 | −8.2 (−11.9, −4.4) | 0.0% | 7 | −0.25 (−1.71, 1.21) | 0.0% | 7 | −15.8 (−29.6, −2.0) | 79.9% |
| P* or F* |
3 |
−11.8 (−21.3, −2.0) |
30.5% |
4 |
−6.6 (−16.1, −2.9) |
17.2% |
2 |
5.52 ( 3.20, 7.84) |
0.0% |
2 |
−24.1 (−65.6, 17.3) |
90.1% |
| Type of Control Diet | ||||||||||||
| Matched | 6 | −14.1 (−20.6, −7.7) | 27.3% | 5 | −10.8 (−16.6, −4.9) | 0.0% | 5 | 1.54 (−1.81, 4.88) | 78.7% | 5 | −23.9 (−40.9, −6.9) | 85.8% |
| Other | 4 | −9.2 (−14.2, −4.1) | 0.0% | 4 | −6.5 (−10.8, −2.3) | 0.0% | 4 | −1.17 (−4.23, 1.88) | 0.0% | 4 | −5.9 (−17.8, 6.0) | 38.7% |
N=Number of Studies included in each sensitivity analysis; *P= Parallel; *F = Factorial;
DISCUSSION
CVD remains the leading cause of death in the US and other Western countries despite advances in care (1). Therefore, modification of risk factors is an essential part of any strategy to decrease the number of CVD events and deaths. Our results indicate that non-soy legume consumption has a significant beneficial effect on serum cholesterol levels, one of the most important risk factors for CVD. Both total and LDL cholesterol decreased, while HDL cholesterol did not change significantly, when non-soy legumes were supplemented. On average, men, who composed the majority of participants in these study populations, achieved lower cholesterol levels (total and LDL) than women while consuming legume-supplemented diets.
This meta-analysis is one of the first to assess the effects of non-soy legume supplemented diets on measures of CVD risk such as total cholesterol, LDL, HDL, VLDL, body weight, or BMI. We identified only one previous analysis, conducted in 2002, which examined the cholesterol lowering effects of non-soy legumes (17). However, in the latter study, investigators did not describe a specific search strategy, present inclusion or exclusion criteria, forest plots, sensitivity analyses, assessments for heterogeneity among studies, or assessments for publication bias. In addition, several new, larger randomized controlled trials of legume supplementation have been conducted in recent years (28-31).
The majority of previous meta-analyses have focused primarily on soy-based interventions. For example, in a recent meta-analysis of 41 randomized controlled trials examining the effect of isolated soy protein supplementation on cholesterol levels, Reynolds et al. identified a significant reduction in total cholesterol (−5.26 mg/dL, 95%CI: −7.14, −3.38), LDL cholesterol (−4.25 mg/dL, 95%CI: −6.00, −2.50), and triglycerides, and an increase in HDL cholesterol (0.77 mg/dL, 95% CI: 0.20, 1.34) when soy protein supplements were incorporated into the diet of participants (19). An earlier meta-analysis by Anderson et al. which focused on soy-based dietary interventions examined 38 controlled clinical trials, however not all studies included in the analysis used random assignment. Also, this meta-analysis included studies with adults and children and both isolated soy protein and textured soy protein supplementation. The authors reported a 9.3 percent reduction in total cholesterol (23.2 mg/dL, 95% CI: 13.5, 32.9) and 12.9 percent reduction in LDL cholesterol (21.7 mg/dL, 95% CI: 11.2, 31.7), as well as a modest, but non-significant 2.4 percent (1.2 mg/dL, 95% CI: −3.1, 5.4) increase in HDL cholesterol (37). While soy-based supplementation appears to be beneficial, soybean consumption is not a traditional part of Western dietary habits whereas the consumption of other legumes and seeds is traditional. Thus, systematically examining the potential benefits of non-soy legume consumption has important clinical implications in Western populations. The results of our meta-analysis shows that a non-soy legume diet have similar effects as diet employing soy-based supplementation with significant reductions in total and LDL cholesterol.
Several components of legumes are likely to contribute to their cholesterol-lowering effects. Soluble fiber, in particular, is thought to bind to bile acids in the intestines and prevent re-absorption into the body. Consequently, an increase in the production of bile acids decreases the liver pool of cholesterol and increases uptake of serum cholesterol by the liver thereby decreasing circulating cholesterol in the blood (38). In addition, prospective epidemiologic studies have identified an association between higher intakes of dietary total and soluble fiber and a lower incidence of coronary heart disease events (12, 39, 40). Currently, the Dietary Guidelines for Americans recommends 14 g of dietary fiber for each 1,000 kcal of energy consumed per day (13). Legumes are a particularly good source of dietary fiber. Among the non-soy legumes included in this meta-analysis, one-half cup of cooked beans or peas can provide a range of dietary fiber from 4.6 g in fava beans up to 9.6 g fiber in navy beans, with a half cup of chick peas (garbanzo beans) providing 6.2 g of total fiber, and 1.3 grams soluble dietary fiber (see Appendix 1in supplemental materials for the nutrient content of beans included in the meta-analysis). Specific phytochemicals may also contribute to the hypocholesterolemic effects of legumes. For instance, phytosterols, a component of plant cell membranes, have been shown to reduce blood cholesterol levels and are present in small to moderate amounts in many types of legumes, such as chickpeas (41).
This meta-analysis has several important strengths which lend confidence to our findings. Given that our meta-analysis draws on the results of randomized controlled trials, findings are less likely to be subject to confounding and bias than those from observational studies. We did not find strong evidence of publication bias on testing; however it should be noted that since the studies included in the meta-analysis had small sample sizes, the random error is likely to be more widely scattered around the mean effect. Also small studies with large effect sizes are more likely to be published therefore, the possibly of publication bias cannot be completely excluded. In addition, our sensitivity analysis showed minimal influence on the combined results for any single trial. Finally, while a diversity of non-soy legumes were included in the intervention diets, they were similar in nutrient content; the nutrient content of the control diets were also similar to legume diets in total energy and macronutrients.
An additional strength of the present meta-analysis is that there was no evidence of heterogeneity in effect size for total or LDL cholesterol and these two lipid levels are the focus of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III guidelines for reducing cardiovascular risk (3). One limitation of our study may be the sample populations included in the trials. The majority of participants were middle-aged men and many of those participating were hypercholesterolemic. While we would expect the underlying mechanisms to operate similarly in persons with other characteristics, for instance women and/or those with a normal cholesterol level, we cannot be sure of this based on the results of our meta-analysis. Further studies should be conducted which specifically enroll women, participants from racial and ethnic minority groups, pre- and post-menopausal women, and obese as well as normal weight participants.
Additionally, weight- loss can independently affect cholesterol levels. A recent study demonstrated that relatively small amounts of weight loss, ranging from 5.2 to 8.9% of body weight (approximately 5 to 8.5 kg ) produced a 2.4-7.6% (approximately 5-15 mg/dL) decrease in total cholesterol in obese participants after 6 months of lifestyle modification (42). Another study demonstrated that as little as 2.5%loss in body weight was associated with a 2.2% decrease in total cholesterol over three years of lifestyle intervention (43). In this meta-analysis, 6 of the 10 trials reported change in weight as an outcome and mean net changes in body weight ranged from −2.6 kg to 1.3 kg (about −2 to +1% body weight)(21, 23-25, 28, 29). This amount of weight loss is not likely to produce the changes in cholesterol that were demonstrated in the meta-analysis (mean net change −11.8, 95% CI −16.1, −7.5) particularly due to the relatively short duration of these trials. Similarly, exercise and physical activity could have confounded the results; however no trials reported physical activity measures. For the trials that mentioned physical activity (n=4), all stated that participants were asked to maintain their normal level of activity (22, 28, 30, 31).
In summary, this meta-analysis of randomized controlled trials provides the strongest evidence to date that non-soy legume consumption lowers serum total and LDL cholesterol, and therefore may lower the risk CVD. Existing dietary guidelines call for the consumption of 3 cups of dried beans or peas per week, however current consumption is less than half that, while consumption of starchy vegetables, primarily white potatoes, is far above current recommendations (14). Replacing white potatoes with legumes at some meals could result in improved cholesterol levels. Dietary modification strategies that target the reduction of risk factors for CVD should include an increase in legume consumption in addition to other strategies which have been of proven benefit.
Supplementary Material
ACKNOWLEDGEMENTS
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Bazzano had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Tees and Dr. Nguyen assisted in the abstraction of data and writing of the manuscript. Ms. Thompson contributed to the analysis of the data and editorial revision of the manuscript. Dr. Winham contributed to the writing and editorial revision of the manuscript and to identification of studies in the field.
Sources of Support: Dr. Bazzano was partially supported by Grant No. 2P20RR017659-06 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, Bethesda, MD via the Tulane University Hypertension and Renal Center of Excellence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Murray CJ, Lopez AD, editors. The global burden of disease: a comprehensive assessment of mortality and disability from disease, injuries, and risk factors in 1990 adn projected to 2020. Harvard School of Public Health; Boston: 1996. [Google Scholar]
- 2.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart Disease and Stroke Statistics--2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of Baseline Serum Cholesterol Levels in 3 Large Cohorts of Younger Men to Long-term Coronary, Cardiovascular, and All-Cause Mortality and to Longevity. JAMA. 2000;284:311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 5.Schnohr PL, Petera b, Scharling Henrika, Jensen Skov, Jana c. Long-term physical activity in leisure time and mortality from coronary heart disease, stroke, respiratory diseases, and cancer. The Copenhagen City Heart Study. Eur J Cardiov Prev R. 2006;13:173–179. doi: 10.1097/01.hjr.0000198923.80555.b7. [DOI] [PubMed] [Google Scholar]
- 6.Giampaoli S, Palmieri L, Panico S, Vanuzzo D, Ferrario M, Chiodini P, et al. Favorable Cardiovascular Risk Profile (Low Risk) and 10-Year Stroke Incidence in Women and Men: Findings from 12 Italian Population Samples. Am J Epidemiol. 2006;163:893–902. doi: 10.1093/aje/kwj110. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement From the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri LD, Chiara, Giampaoli Simona, Trojani Michela, Panico Salvatore, Vanuzzo Diego, Pilotto Lorenza, Cesana Giancarlo, Ferrario Marco, Chiodini Paolo, Sega Roberto, Stamler Jeremiah. Favorable cardiovascular risk profile and 10-year coronary heart disease incidence in women and men: results from the Progetto CUORE. Eur J Cardiov Prev R. 2006;13:562–570. doi: 10.1097/01.hjr.0000221866.27039.4b. [DOI] [PubMed] [Google Scholar]
- 9.Bazzano LA, Serdula M, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 11.Flight I, Clifton P. European Journal of Clinical Nutrition. Nature Publishing Group; 2006. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. pp. 1145–1159. [DOI] [PubMed] [Google Scholar]
- 12.Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK. Dietary Fiber Intake and Reduced Risk of Coronary Heart Disease in US Men and Women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 2003;163:1897–1904. doi: 10.1001/archinte.163.16.1897. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services. U.S. Department of Agriculture . Dietary Guidelines for American, 2005. 6th Edition Washington, D.C.: 2005. [Google Scholar]
- 14.Guenther PM, Dodd KW, Reedy J, Krebs-Smith SM. Most Americans Eat Much Less than Recommended Amounts of Fruits and Vegetables. Journal of the American Dietetic Association. 2006;106:1371–1379. doi: 10.1016/j.jada.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, et al. Legume Consumption and Risk of Coronary Heart Disease in US Men and Women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med. 2001;161:2573–2578. doi: 10.1001/archinte.161.21.2573. [DOI] [PubMed] [Google Scholar]
- 16.Kushi LH, Meyer KA, Jacobs DR., Jr Cereals, legumes, and chronic disease risk reduction: evidence from epidemiologic studies. Am J Clin Nutr. 1999;70:451S–458. doi: 10.1093/ajcn/70.3.451s. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. British Journal of Nutrition. 2002;88:263–271. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- 18.Duranti M. Grain legume proteins and nutraceutical properties. Fitoterapia. 2006;77:67–82. doi: 10.1016/j.fitote.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds K, Chin A, Lees KA, Nguyen A, Bujnowski D, He J. A Meta-Analysis of the Effect of Soy Protein Supplementation on Serum Lipids. 2006. pp. 633–640. [DOI] [PubMed]
- 20.Anderson J, Story L, Sieling B, Chen W, Petro M, Story J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am J Clin Nutr. 1984;40:1146–1155. doi: 10.1093/ajcn/40.6.1146. [DOI] [PubMed] [Google Scholar]
- 21.Nervi F, Covarrubias C, Bravo P, Velasco N, Ulloa N, Cruz F, et al. Influence of legume intake on biliary lipids and cholesterol saturation in young Chilean men. Identification of a dietary risk factor for cholesterol gallstone formation in a highly prevalent area. Gastroenterology. 1989;96:825–830. [PubMed] [Google Scholar]
- 22.Cobiac L, McArthur R, Nestel PJ. Can eating baked beans lower plasma cholesterol? Eur J Clin Nutr. 1990;44:819–822. [PubMed] [Google Scholar]
- 23.Anderson J, Gustafson N, Spencer D, Tietyen J, Bryant C. Serum lipid response of hypercholesterolemic men to single and divided doses of canned beans. Am J Clin Nutr. 1990;51:1013–1019. doi: 10.1093/ajcn/51.6.1013. [DOI] [PubMed] [Google Scholar]
- 24.Mackay S, Ball MJ. Do beans and oat bran add to the effectiveness of a low-fat diet? Eur J Clin Nutr. 1992;46:641–648. [PubMed] [Google Scholar]
- 25.Fruhbeck G, Monreal I, Santidrian S. Hormonal implications of the hypocholesterolemic effect of intake of field beans (Vicia faba L.) by young men with hypercholesterolemia. Am J Clin Nutr. 1997;66:1452–1460. doi: 10.1093/ajcn/66.6.1452. [DOI] [PubMed] [Google Scholar]
- 26.Duane W. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res. 1997;38:1120–1128. [PubMed] [Google Scholar]
- 27.Sowmya P, Rajyalakshmi P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Foods Hum Nutr. 1999;53:359–365. doi: 10.1023/a:1008021618733. [DOI] [PubMed] [Google Scholar]
- 28.Pittaway JK, Ahuja KDK, Cehun M, Chronopoulos A, Robertson IK, Nestel PJ, et al. Dietary Supplementation with Chickpeas for at Least 5 Weeks Results in Small but Significant Reductions in Serum Total and Low-Density Lipoprotein Cholesterols in Adult Women and Men. Ann Nutr Metab. 2006;50:512–518. doi: 10.1159/000098143. [DOI] [PubMed] [Google Scholar]
- 29.Crujeiras AB, Parra D, Abete I, MartÃ-nez JA. Free Radical Research. Taylor & Francis Ltd; 2007. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. pp. 498–506. [DOI] [PubMed] [Google Scholar]
- 30.Winham DM, Hutchins AM. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr Res. 2007;27:380–386. [Google Scholar]
- 31.Winham DM, Hutchins AM, Johnston CS. Pinto Bean Consumption Reduces Biomarkers for Heart Disease Risk. J Am Coll Nutr. 2007;26:243–249. doi: 10.1080/07315724.2007.10719607. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Begg C. Publication bias. In: Cooper H, Hedges L, editors. The Handbook of Research Synthesis. Russell Sage Foundation; New York, N.Y.: 1994. pp. 399–409. [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. 1999. pp. 1896–1900. [DOI] [PubMed]
- 37.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. N Engl J Med. 1995;333:276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 38.Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. The Journal of Nutritional Biochemistry. 2008;19:71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease Among Men. JAMA. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 40.Pietinen P, Rimm EB, Korhonen P, Hartman AM, Willett WC, Albanes D, et al. Intake of Dietary Fiber and Risk of Coronary Heart Disease in a Cohort of Finnish Men: The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Circulation. 1996;94:2720–2727. doi: 10.1161/01.cir.94.11.2720. [DOI] [PubMed] [Google Scholar]
- 41.Rochfort S, Panozzo J. Phytochemicals for Health, the Role of Pulses. Journal of Agricultural and Food Chemistry. 2007;55:7981–7994. doi: 10.1021/jf071704w. [DOI] [PubMed] [Google Scholar]
- 42.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of Methods for Delivering a Lifestyle Modification Program for Obese Patients: A Randomized Trial. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 43.Madsen EL, Rissanen A, Bruun JM, Skogstrand K, Tonstad S, Hougaard DM, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol. 2008;158:179–187. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.