Abstract
Survival after Hodgkin lymphoma (HL) is generally favorable, but may vary by patient demographic characteristics. The authors examined HL survival according to race/ethnicity and neighborhood socioeconomic status (SES), determined from residential census block group at diagnosis. For 12,492 classical HL patients ≥15 years diagnosed in California during 1988-2006 and followed through 2007, we determined risk of overall and HL-specific death using Cox proportional hazards regression; analyses were stratified by age and Ann Arbor stage. Irrespective of disease stage, patients with lower neighborhood SES had worse overall and HL-specific survival than patients with higher SES. Patients with the lowest quintile of neighborhood SES had a 64% (patients aged 15-44 years) and 36% (≥45 years) increased risk of HL-death compared to patients with the highest quintile of SES; SES results were similar for overall survival. Even after adjustment for neighborhood SES, blacks and Hispanics had increased risks of HL-death 74% and 43% (15-44 years) and 40% and 17% (≥45 years), respectively, higher than white patients. The racial/ethnic differences in survival were evident for all stages of disease. These data provide evidence for substantial, and probably remediable, racial/ethnic and neighborhood SES disparities in HL outcomes.
Keywords: Hodgkin disease, survival, mortality, social class, census
INTRODUCTION
Over half of Hodgkin lymphomas (HL) diagnosed in the United States (US) occur in persons under 35 years of age, making HL one of the most commonly diagnosed cancers in young adults and ranking it third among all cancers in average years of life lost to a malignancy (1). Treatment successes have led to substantial reductions in HL mortality, such that five-year relative survival for US HL patients now exceeds 80% (1) and cure rates exceed 75% (2). Although these statistics place HL among the most favorable of all cancers in terms of survival, long-term HL survivors face substantial ongoing threats to their health and quality of life from both HL recurrence and treatment-related sequelae, including second primary malignancies and cardiovascular disease (2-4).
Some of these risks can be minimized through state-of-the-art initial treatment and close post-treatment medical surveillance. Access to such medical care is known to be influenced by patient social standing, including education, income or other measures of socioeconomic status (SES) (5-7), and HL outcomes generally have been reported to be poorer for persons of lower SES (8-10) or non-white race/ethnicity (11, 12). However, prior studies to determine how SES impacts survival after HL have been limited by being conducted in clinical series (8, 10, 13), involving small sample sizes (8, 10, 13), combining HL with a variety of other cancers (8), or not controlling for particular prognostic factors (9). In addition, although SES and race/ethnicity are strongly correlated in the US (14), no studies to date have examined the joint effects of these factors on survival after HL.
Better understanding of social disparities in HL outcome is important to identifying modifiable barriers leading to the disparities, which in turn should facilitate steps to improve overall outcomes after HL for all patients. Therefore, we assessed the impact of neighborhood SES and race/ethnicity on overall, disease-specific and relative survival after HL in a large population-based case series with a median follow-up of 79 months and substantial heterogeneity in race/ethnicity and SES. Analyses of all-cause death as an endpoint allow us to consider whether the well-documented late complications of HL treatment and disease (2), in addition to the direct health consequences of HL itself, vary by SES and race/ethnicity. Utilizing cancer registry data from patients diagnosed with classical HL from 1988 through 2006 in California, we tested the hypothesis that neighborhood SES and non-Hispanic white race/ethnicity were inversely associated with hazard of death after controlling for other prognostic factors.
MATERIALS AND METHODS
Patients
Cases eligible for the study were all California residents newly diagnosed with classical HL (International Classification of Diseases—Oncology, 3rd edition (15) morphology codes 9650-9667, excluding codes 9659 (nodular lymphocyte predominance), 9661 (Hodgkin granuloma) and 9662 (Hodgkin sarcoma)) during the period January 1, 1988 through December 31, 2006 and reported by state mandate to the California Cancer Registry (CCR). From the CCR, we obtained information routinely recorded at diagnosis for each patient on age, sex, race/ethnicity (non-Hispanic white (hereafter called “white”), Hispanic, black, and Asian/Pacific Islander), summary stage [localized (Ann Arbor stage I), regional (stage II), advanced (stage III/IV)], extent of disease, tumor histologic subtype (nodular sclerosis, mixed cellularity, lymphocyte depletion, lymphocyte rich, or not otherwise specified), and census-block group of residence. With information on extent of disease, we were able to classify patients by the presence of B symptoms (weight loss, night sweats, and fever) and human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS). In addition, we obtained registry information on treatment modality (radiation (yes, no), chemotherapy (yes, no/unknown) or combined modality within four months of diagnosis); vital status (routinely determined by the CCR through hospital follow-up and linkages to vital status and other databases) as of December 2007; and, for the deceased, the underlying cause of death as routinely coded by state vital statistics personnel.
As the CCR does not collect individual-level patient SES, we determined neighborhood SES using an index incorporating census block-group averages of education, income, occupation, and cost of living, as described previously (16, 17). This SES index was available for the 95% of patients whose residential census block group at diagnosis could be geocoded; patients with a missing block group were randomly assigned to a block group within their county of residence at diagnosis. Each HL patient was assigned an SES quintile based on the distribution in the study population; SES quintiles were then collapsed into two groups (higher- and lower-SES), as described below.
The final study population included 12,492 HL patients ≥ 15 years of age at diagnosis after exclusion, in a hierarchical manner, of those with: 1) unknown race/ethnicity (n=167); 2) registry or death certificate evidence of HIV or AIDS (n=598), because of the substantially poorer outcome of HIV-associated HL during the study period (18, 19); 3) HL diagnosis at autopsy, by death certificate only, or with zero/invalid survival time (n=121); and 4) American Indian/Alaskan native race/ethnicity (n=10), as SES-specific life tables were not available for this group. All study protocols were approved by the Institutional Review Board of the Northern California Cancer Center.
Statistical analyses
Outcomes of interest included overall survival, which considers death from all causes, and HL-specific survival, which considers death from HL. For deceased patients, survival time was measured in months from the date of diagnosis to the date of death of any cause for overall survival, and to the date of death from HL for HL-specific survival. Patients who died from other causes were censored at the time of death in analyses of HL-specific survival. Patients alive at the study end date (12/31/2007) were censored at this time or at the date of last known contact. Ninety-four percent of censored patients had a follow-up date within two years of the study end date; neighborhood SES did not differ between patients with and without recent follow-up information. However, the percentage of patients with follow-up within two years was slightly higher for whites (96%), Blacks (96%), and APIs (93%) than for Hispanics (90%).
To evaluate associations with survival controlling for known prognostic factors, we used Cox proportional hazards regression to estimate hazard ratios (HR) and associated 95% confidence intervals (CI). Multivariate regression models included variables significant at p<0.15 in univariate models or with a priori reasons for inclusion (e.g., age, race/ethnicity, and gender). All variables were included in the multivariate analyses with the exception of initial treatment, which was correlated with stage at diagnosis (p < 0.01), the primary factor influencing treatment selection (20). For stratified analyses (Tables 1, 4 and 5), neighborhood SES quintiles were collapsed into two groups (quintiles 1, 2, and 3 (lower SES), quintiles 4 and 5 (higher SES)) due to similarities across quintiles in survival patterns. Because of previously established HL survival differences by age (11), we present analyses separately by age group (< 45, ≥45 years). Effect modification was assessed between SES and race/ethnicity, stage at diagnosis, histologic subtype, and gender, and between race/ethnicity and stage at diagnosis, by including interaction terms in the multivariable models. No interaction terms were statistically significant. In all models, the proportional hazards assumption was assessed numerically based on cumulative sums of Martingale residuals (21) and visually based on inspection of the survival curves (log (−log) of the survival distribution function by log (months)). There was evidence of a violation of this assumption with stage; therefore, Cox models were stratified by stage. Regression analyses were conducted using SAS version 9.1 software (SAS institute Inc., Cary, NC, USA).
Table 1.
Characteristics of Hodgkin lymphoma (HL) patients (N = 12,492) by age at diagnosis and neighborhood socioeconomic status (SES) group, California, 1988-2006.
| Characteristic | Age 15 – 44 years | Age ≥ 45 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 8228 |
n = 4264 |
|||||||||
| Lower-SES (Quintiles 1-3) |
Higher-SES (Quintiles 4,5) |
Lower-SES (Quintiles 1-3) |
Higher-SES (Quintiles 4,5) |
|||||||
| n=4408 | % | n=3820 | % | P-value | n=2325 | % | n=1939 | % | P-value | |
| Sex | ||||||||||
| Male | 2322 | 52.7 | 1961 | 51.3 | 1326 | 57.0 | 1104 | 56.9 | ||
| Female | 2086 | 47.3 | 1859 | 48.7 | 0.22 | 999 | 43.0 | 835 | 43.1 | 0.95 |
| Year of diagnosis | ||||||||||
| 1988-1992 | 1190 | 27.0 | 1113 | 29.1 | 526 | 22.6 | 471 | 24.3 | ||
| 1993-1997 | 1131 | 25.7 | 969 | 25.4 | 581 | 25.0 | 476 | 24.6 | ||
| 1998-2002 | 1119 | 25.4 | 937 | 24.5 | 630 | 27.1 | 553 | 28.5 | ||
| 2003-2006 | 968 | 22.0 | 801 | 21.0 | 0.18 | 588 | 25.3 | 439 | 22.6 | 0.16 |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 2551 | 57.9 | 3033 | 79.4 | 1426 | 61.3 | 1578 | 81.4 | ||
| Black | 407 | 9.2 | 128 | 3.4 | 172 | 7.4 | 53 | 2.7 | ||
| Hispanic | 1284 | 29.1 | 407 | 10.7 | 624 | 26.8 | 198 | 10.2 | ||
| Asian/Pacific | 166 | 3.8 | 252 | 6.6 | <0.01 | 103 | 4.4 | 11 | 5.7 | <0.01 |
| Stage | ||||||||||
| I | 606 | 13.8 | 568 | 14.9 | 435 | 18.7 | 397 | 20.5 | ||
| II | 1982 | 45.0 | 1880 | 49.2 | 581 | 30.5 | 592 | 30.5 | ||
| III/IV | 1543 | 35.0 | 1155 | 30.2 | 1102 | 47.4 | 818 | 42.2 | ||
| Missing/unknown | 277 | 6.3 | 217 | 5.7 | <0.01 | 207 | 8.9 | 132 | 6.8 | <0.01 |
| B Symptoms | ||||||||||
| No | 1425 | 32.3 | 1503 | 39.4 | 679 | 29.2 | 682 | 35.2 | ||
| Yes | 1525 | 34.6 | 1222 | 32.0 | 817 | 35.1 | 654 | 33.7 | ||
| Missing/unknown | 1458 | 33.1 | 1095 | 28.7 | <0.01 | 829 | 35.7 | 603 | 31.1 | <0.01 |
| Histologic subtype | ||||||||||
| Nodular sclerosis | 3243 | 73.6 | 3027 | 79.2 | 990 | 42.6 | 939 | 48.4 | ||
| Mixed cellularity | 531 | 12.1 | 376 | 9.8 | 666 | 28.7 | 491 | 25.3 | ||
| Lymphocyte depletion | 61 | 1.4 | 27 | 0.7 | 111 | 4.8 | 59 | 3.0 | ||
| Lymphocyte rich | 123 | 2.8 | 81 | 2.1 | 113 | 4.9 | 84 | 4.3 | ||
| Not otherwise specified | 450 | 10.2 | 309 | 8.1 | <0.01 | 445 | 19.1 | 366 | 18.9 | <0.01 |
| First course of treatment | ||||||||||
| Combined modality | 1409 | 32.0 | 1535 | 40.2 | 299 | 12.9 | 399 | 20.6 | ||
| Radiation only | 514 | 11.7 | 570 | 14.9 | 261 | 11.2 | 269 | 13.9 | ||
| Chemotherapy only | 2010 | 45.6 | 1367 | 35.8 | 1262 | 54.3 | 957 | 49.4 | ||
| None | 475 | 10.8 | 348 | 9.1 | <0.01 | 503 | 21.6 | 314 | 16.2 | <0.01 |
| Vital status | ||||||||||
| Alive | 3681 | 83.5 | 3398 | 89.0 | 958 | 41.2 | 958 | 49.4 | ||
| Death from HL | 402 | 9.1 | 226 | 5.9 | 600 | 25.8 | 410 | 21.1 | ||
| Death from Non-Hodgkin lymphoma or leukemia |
66 | 1.5 | 24 | 0.6 | 172 | 7.4 | 129 | 6.7 | ||
| Death from other cancer | 34 | 0.8 | 27 | 0.7 | 110 | 4.7 | 91 | 4.7 | ||
| Death from circulatory disease |
42 | 1.0 | 24 | 0.6 | 186 | 8.0 | 107 | 5.5 | ||
| Death from other cause | 104 | 2.4 | 82 | 2.2 | 217 | 9.3 | 179 | 9.2 | ||
| Death from unknown cause |
79 | 1.8 | 39 | 1.0 | <0.01 | 82 | 3.5 | 65 | 3.4 | <0.01 |
Table 4.
Multivariate adjusted* hazard ratios and 95% confidence interval (95% CI) estimates for death from all causes (overall survival) and death from Hodgkin lymphoma (HL-specific survival) in HL patients diagnosed between 1988 and 2006 in the state of California by age group, stage of disease at diagnosis, race/ethnicity and neighborhood socioeconomic status (SES).
| Stage of disease at diagnosis | Age 15 - 44 years | Age ≥ 45 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | HL-Specific Survival |
Overall Survival | HL-Specific Survival |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Stage I | ||||||||
| Race/ethnicity# | ||||||||
| Black | 1.52 | 0.80-2.89 | 1.96 | 0.73-5.26 | 1.23 | 0.76-2.01 | 2.02 | 0.90-4.54 |
| Hispanic | 0.82 | 0.46-1.45 | 1.70 | 0.84-3.46 | 1.08 | 0.81-1.43 | 1.44 | 0.87-2.37 |
| Asian/Pacific Islander | 1.21 | 0.48-3.03 | 1.15 | 0.27-4.91 | 0.83 | 0.53-1.29 | 0.69 | 0.25-1.93 |
| Neighborhood socioeconomic status group** | ||||||||
| Lower SES (Q+1-3) | 2.03 | 1.36-3.05 | 1.26 | 0.69-2.29 | 1.33 | 1.08-1.64 | 1.22 | 0.81-1.85 |
| Stage II | ||||||||
| Race/ethnicity# | ||||||||
| Black | 1.83 | 1.30-2.59 | 1.65 | 1.04-2.63 | 0.90 | 0.58-1.39 | 0.99 | 0.50-1.96 |
| Hispanic | 1.30 | 1.01-1.67 | 1.05 | 0.74-1.49 | 0.94 | 0.74-1.20 | 1.17 | 0.82-1.68 |
| Asian/Pacific Islander | 1.01 | 0.60-1.70 | 1.35 | 0.75-2.44 | 1.21 | 0.77-1.91 | 1.57 | 0.82-3.01 |
| Neighborhood socioeconomic status group** | ||||||||
| Lower SES (Q+1-3) | 1.56 | 1.26-1.92 | 1.96 | 1.47-2.62 | 1.35 | 1.12-1.62 | 1.39 | 1.04-1.87 |
| Stage III/IV | ||||||||
| Race/ethnicity# | ||||||||
| Black | 1.58 | 1.18-2.12 | 1.73 | 1.19-2.52 | 1.12 | 0.85-1.47 | 1.46 | 1.03-2.06 |
| Hispanic | 1.37 | 1.11-1.70 | 1.64 | 1.26-2.14 | 1.25 | 1.09-1.44 | 1.18 | 0.96-1.43 |
| Asian/Pacific Islander | 1.15 | 0.73-1.81 | 1.22 | 0.68-2.19 | 1.07 | 0.81-1.40 | 1.18 | 0.82-1.69 |
| Neighborhood socioeconomic status group** | ||||||||
| Lower SES (Q+1-3) | 1.28 | 1.06-1.54 | 1.21 | 0.95-1.54 | 1.27 | 1.13-1.43 | 1.19 | 1.01-1.40 |
| Unknown stage | ||||||||
| Race/ethnicity# | ||||||||
| Black | 2.50 | 1.19-5.26 | 4.01 | 1.41-11.38 | 1.14 | 0.56-2.31 | 2.14 | 0.79-5.81 |
| Hispanic | 2.40 | 1.40-4.14 | 2.58 | 1.09-6.11 | 0.83 | 0.53-1.29 | 0.82 | 0.37-1.82 |
| Asian/Pacific Islander | - | - | - | - | 0.57 | 0.26-1.24 | 0.41 | 0.09-1.77 |
| Neighborhood socioeconomic status group** | ||||||||
| Lower SES (Q+1-3) | 1.25 | 0.79-1.99 | 0.92 | 0.46-1.83 | 1.17 | 0.86-1.61 | 1.43 | 0.82-2.47 |
Adjusted for age at diagnosis (continuous), sex, year of diagnosis (1988-92, 1993-1997, 1998-2002, 2003-6) presence of B-symptoms (yes, no, unknown), histologic subtype (nodular sclerosis, mixed cellularity, lymphocyte depletion, lymphocyte rich, not otherwise specified).
Quintile
Reference group = Higher SES (Q 4,5).
Reference group = non-Hispanic white race/ethnicity.
Table 5.
5-, 10-, and 15-year relative survival (standard error (SE)) by patient characteristics, age group, and neighborhood socioeconomic status (SES) group for Hodgkin lymphoma patients (n = 12,492) diagnosed between 1988 and 2006 in the state of California.
| 5-Year Relative Survival (SE) |
10-Year Relative Survival (SE) |
15-Year Relative Survival (SE) |
||||
|---|---|---|---|---|---|---|
| Patient Characteristic | Age 15-44 years |
Age ≥ 45 years |
Age 15-44 years |
Age ≥ 45 years |
Age 15-44 years |
Age ≥ 45 years |
| White | ||||||
| Lower SES (Q+ 1-3) | 90.0 (0.6) | 61.6 (1.6) | 86.1 (0.8) | 53.2 (2.1) | 82.1 (1.1) | 44.8 (3.1) |
| Higher SES (Q+ 4,5) | 93.5 (0.5) | 69.8 (1.4) | 90.7 (0.6) | 59.8 (1.9) | 88.1 (0.8) | 53.5 (2.7) |
| Black | ||||||
| Lower SES (Q+ 1-3) | 84.1 (2.0) | 68.7 (4.5) | 78.8 (2.5) | 56.1 (6.0) | 74.6 (3.1) | 50.8 (8.5) |
| Higher SES (Q+ 4,5) | 85.4 (3.3) | 78.5 (7.0) | 80.4 (4.0) | 75.2 (8.8) | 80.4 (4.0) | 72.0 (12.1) |
| Hispanic | ||||||
| Lower SES (Q+ 1-3) | 88.9 (1.0) | 56.0 (2.4) | 82.2 (1.3) | 48.5 (3.2) | 78.1 (1.8) | 41.9 (4.8) |
| Higher SES (Q+ 4,5) | 91.9 (1.5) | 64.4 (4.1) | 84.7 (2.3) | 58.1 (5.3) | 79.7 (3.0) | 53.9 (7.3) |
| Asian/ Pacific Islander | ||||||
| Lower SES (Q+ 1-3) | 90.6 (2.5) | 50.4 (6.0) | 87.5 (3.1) | 35.6 (7.8) | 81.4 (4.6) | 27.8 (13.6) |
| Higher SES (Q+ 4,5) | 92.6 (1.9) | 71.9 (5.3) | 91.0 (2.3) | 62.1 (6.8) | 89.0 (3.2) | 56.0 (9.5) |
| Male | ||||||
| Lower SES (Q+ 1-3) | 86.9 (0.8) | 60.1 (1.7) | 82.1 (1.0) | 51.6 (2.2) | 77.6 (1.2) | 43.7 (3.2) |
| Higher SES (Q+ 4,5) | 91.8 (0.7) | 70.9 (1.7) | 88.3 (0.9) | 60.5 (2.2) | 84.7 (1.1) | 52.0 (3.1) |
| Female | ||||||
| Lower SES (Q+ 1-3) | 91.7 (0.7) | 60.0 (1.9) | 87.1 (0.9) | 50.5 (2.5) | 83.3 (1.2) | 43.8 (3.7) |
| Higher SES (Q+ 4,5) | 94.3 (0.6) | 67.8 (2.0) | 91.4 (0.8) | 60.2 (2.6) | 89.6 (0.9) | 57.7 (3.8) |
| Stages I | ||||||
| Lower SES (Q+ 1-3) | 93.7 (1.1) | 78.1 (2.8) | 91.5 (1.4) | 65.5 (4.0) | 86.4 (2.1) | 54.1 (6.1) |
| Higher SES (Q+ 4,5) | 96.5 (0.9) | 80.1 (2.6) | 95.4 (1.1) | 73.0 (3.5) | 93.3 (1.4) | 65.7 (5.4) |
| Stage II | ||||||
| Lower SES (Q+ 1-3) | 91.8 (0.7) | 70.2 (2.4) | 87.3 (0.9) | 59.3 (3.4) | 84.1 (1.2) | 51.8 (5.4) |
| Higher SES (Q+ 4,5) | 95.2 (0.5) | 79.6 (2.1) | 93.1 (0.7) | 68.6 (3.0) | 90.3 (1.0) | 64.1 (4.3) |
| Stage III/IV | ||||||
| Lower SES (Q+ 1-3) | 83.9 (1.0) | 46.7 (1.8) | 78.6 (1.2) | 39.3 (2.3) | 73.9 (1.6) | 33.3 (3.1) |
| Higher SES (Q+ 4,5) | 87.7 (1.0) | 56.6 (2.1) | 83.0 (1.3) | 47.3 (2.6) | 79.9 (1.5) | 42.2 (3.6) |
Quintile
We calculated relative survival estimates, which adjust for competing causes of death by comparing the observed survival of study patients with their expected survival if they did not have HL, by using NCI’s SEER*Stat software version 6.2.4 (http://seer.cancer.gov/seerstat) and proprietary SES-(16), age-, sex-, and race/ethnicity-specific life tables based on the 1990 US census estimates for California.
There was minimal spatial clustering of HL cases in census block groups, as the majority of block groups had only one (55%) or two (28%) cases diagnosed during the 19-year study period. Nevertheless, we performed secondary analyses to adjust for clustering by computing a robust sandwich covariance matrix estimate that accounts for intracluster dependence (22). Accounting for clustering did not change our findings (data not shown); therefore, we present results unadjusted for spatial clustering.
RESULTS
The 12,492 HL patients were followed for a median of 79.3 months (25.8 months for deceased patients; 104.2 months for living patients), with 14.9% followed for 180 months (15 years) or more. In both age groups, many patient and tumor characteristics varied according to neighborhood SES (Table 1), although most differences were not large. Patient and tumor characteristics also varied by race/ethnicity (Table 2). More than 75% of blacks and Hispanics were in the lower-SES group, compared to fewer than 50% of whites and Asians/Pacific Islanders.
Table 2.
Characteristics of Hodgkin lymphoma (HL) patients (N = 12,492) by age at diagnosis and race/ethnicity, California, 1988-2006.
| Age 15-44 |
p- value |
Age ≥45 |
p- value |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicity |
Race/ethnicity |
|||||||||||||||||
| Characteristic | Non-Hispanic White |
Black | Hispanic | Asian/ Pacific Islander |
Non-Hispanic White |
Black | Hispanic | Asian/ Pacific Islander |
||||||||||
| n=5584 | % | n=535 | % | n=1691 | % | N=418 | % | n=3004 | % | n=225 | % | n=822 | % | N=213 | % | |||
| Sex | ||||||||||||||||||
| Male | 2925 | 52.4 | 272 | 50.8 | 875 | 51.7 | 211 | 50.5 | 1683 | 56.0 | 117 | 52.0 | 492 | 59.9 | 138 | 64.8 | ||
| Female | 2659 | 47.6 | 263 | 49.2 | 816 | 48.3 | 207 | 49.5 | 0.79 | 1321 | 44.0 | 108 | 48.0 | 330 | 40.2 | 75 | 35.2 | 0.01 |
| Year of diagnosis | ||||||||||||||||||
| 1988-1992 | 1726 | 30.9 | 152 | 28.4 | 354 | 20.9 | 71 | 17.0 | 746 | 24.8 | 52 | 23.1 | 157 | 19.1 | 42 | 19.7 | ||
| 1993-1997 | 1467 | 26.3 | 146 | 27.3 | 405 | 24.0 | 82 | 19.6 | 757 | 25.2 | 60 | 26.7 | 188 | 22.9 | 52 | 24.4 | ||
| 1998-2002 | 1331 | 23.8 | 115 | 21.5 | 484 | 28.6 | 126 | 30.1 | 831 | 27.7 | 53 | 23.6 | 236 | 28.7 | 63 | 29.6 | ||
| 2003-2006 | 1060 | 19.0 | 122 | 22.8 | 448 | 26.5 | 139 | 33.3 | <0.001 | 670 | 22.3 | 60 | 26.7 | 241 | 29.3 | 56 | 26.3 | <0.001 |
| Stage at diagnosis | ||||||||||||||||||
| I | 807 | 14.5 | 79 | 14.8 | 227 | 13.4 | 61 | 14.6 | 593 | 19.7 | 41 | 18.2 | 149 | 18.1 | 49 | 23.0 | ||
| II | 2688 | 48.1 | 216 | 40.4 | 744 | 44.0 | 214 | 51.2 | 868 | 28.9 | 57 | 25.3 | 198 | 24.1 | 50 | 23.5 | ||
| III/IV | 1753 | 31.4 | 206 | 38.5 | 611 | 36.1 | 128 | 30.6 | 1301 | 43.3 | 107 | 47.6 | 415 | 50.5 | 97 | 45.5 | ||
| Missing/unknown | 336 | 6.0 | 34 | 6.4 | 109 | 6.5 | 15 | 3.6 | <0.001 | 242 | 8.1 | 20 | 8.9 | 60 | 7.3 | 17 | 8.0 | 0.03 |
| B-Symptoms | ||||||||||||||||||
| No | 2003 | 35.9 | 177 | 33.1 | 563 | 33.3 | 185 | 44.3 | 981 | 32.7 | 73 | 32.4 | 232 | 28.2 | 75 | 35.2 | ||
| Yes | 1756 | 31.5 | 178 | 33.3 | 673 | 39.8 | 140 | 33.5 | 970 | 32.3 | 90 | 40.0 | 339 | 41.2 | 72 | 33.8 | ||
| Missing/unknown | 1825 | 32.7 | 180 | 33.6 | 455 | 26.9 | 93 | 22.3 | <0.001 | 1053 | 35.1 | 62 | 27.6 | 251 | 30.5 | 66 | 31.0 | <0.001 |
| Histologic subtype | ||||||||||||||||||
| Nodular sclerosis | 4377 | 78.4 | 375 | 70.1 | 1197 | 70.8 | 321 | 76.8 | 1441 | 48.0 | 90 | 40.0 | 305 | 37.1 | 93 | 43.7 | ||
| Mixed cellularity | 550 | 9.9 | 74 | 13.8 | 242 | 14.3 | 41 | 9.8 | 749 | 24.9 | 60 | 26.7 | 278 | 33.8 | 70 | 32.9 | ||
| Lymphocyte depletion |
53 | 1.0 | 4 | 0.8 | 28 | 1.7 | 3 | 0.7 | 115 | 3.8 | 12 | 5.3 | 36 | 4.4 | 7 | 3.3 | ||
| Lymphocyte rich | 109 | 2.0 | 29 | 5.4 | 55 | 3.3 | 11 | 2.6 | 142 | 4.7 | 15 | 6.7 | 30 | 3.7 | 10 | 4.7 | ||
| Not otherwise specified | 495 | 8.9 | 53 | 9.9 | 169 | 10.0 | 42 | 10.1 | <0.001 | 557 | 18.5 | 48 | 21.3 | 173 | 21.1 | 33 | 15.5 | <0.001 |
| First course of treatment |
||||||||||||||||||
| Combined modality |
2081 | 37.3 | 132 | 24.7 | 551 | 32.6 | 180 | 43.1 | 528 | 17.6 | 28 | 12.4 | 106 | 12.9 | 36 | 16.9 | ||
| Radiation only | 834 | 14.9 | 54 | 10.1 | 154 | 9.1 | 42 | 10.1 | 408 | 13.6 | 23 | 10.2 | 71 | 8.6 | 28 | 13.2 | ||
| Chemotherapy only |
2128 | 38.1 | 279 | 52.2 | 802 | 47.4 | 168 | 40.2 | 1531 | 51.0 | 137 | 60.9 | 451 | 54.9 | 100 | 47.0 | ||
| None/unknown | 541 | 9.7 | 70 | 13.1 | 184 | 10.9 | 28 | 6.7 | <0.001 | 537 | 17.9 | 37 | 16.4 | 194 | 23.6 | 49 | 23.0 | <0.001 |
| Neighborhood socioeconomic status (quintiles) |
||||||||||||||||||
| 1 (Lowest) | 381 | 6.8 | 156 | 29.2 | 526 | 31.1 | 28 | 6.7 | 272 | 9.1 | 78 | 34.7 | 268 | 32.6 | 27 | 12.7 | ||
| 2 | 906 | 16.2 | 144 | 26.9 | 456 | 27.0 | 60 | 14.4 | 515 | 17.1 | 56 | 24.9 | 204 | 24.8 | 36 | 16.9 | ||
| 3 | 1264 | 22.6 | 107 | 20.0 | 302 | 17.9 | 78 | 18.7 | 639 | 21.3 | 38 | 16.9 | 152 | 18.5 | 40 | 18.8 | ||
| 4 | 1510 | 27.0 | 86 | 16.1 | 253 | 15.0 | 119 | 28.5 | 738 | 24.6 | 27 | 12.0 | 110 | 13.4 | 47 | 22.1 | ||
| 5 (Highest) | 1523 | 27.3 | 42 | 7.9 | 154 | 9.1 | 133 | 31.8 | 840 | 28.0 | 26 | 11.6 | 88 | 10.7 | 63 | 29.6 | ||
| Vital status | ||||||||||||||||||
| Alive | 4852 | 86.9 | 419 | 78.3 | 1431 | 84.6 | 377 | 90.2 | 1327 | 44.2 | 116 | 51.6 | 366 | 44.5 | 107 | 50.2 | ||
| Death from HL | 382 | 6.8 | 64 | 12.0 | 156 | 9.2 | 26 | 6.2 | 697 | 23.2 | 58 | 25.8 | 207 | 25.2 | 48 | 22.5 | ||
| Death from Non- Hodgkin lymphoma or Leukemia |
56 | 1.0 | 14 | 2.6 | 18 | 1.1 | 2 | 0.5 | 214 | 7.1 | 7 | 3.1 | 66 | 8.0 | 14 | 6.6 | ||
| Death from other cancer |
50 | 0.9 | 4 | 0.8 | 6 | 0.4 | 1 | 0.2 | 152 | 5.1 | 11 | 4.9 | 26 | 3.2 | 12 | 5.6 | ||
| Death from circulatory disease |
42 | 0.8 | 6 | 1.1 | 14 | 0.8 | 4 | 1.0 | 225 | 7.5 | 11 | 4.9 | 48 | 5.8 | 9 | 4.2 | ||
| Death from other cause |
125 | 2.2 | 18 | 3.4 | 39 | 2.3 | 4 | 1.0 | 288 | 9.6 | 15 | 6.7 | 77 | 9.3 | 16 | 7.5 | ||
| Death from unknown cause |
77 | 1.4 | 10 | 1.9 | 27 | 1.6 | 4 | 1.0 | <0.001 | 101 | 3.4 | 7 | 3.1 | 32 | 3.9 | 7 | 3.3 | 0.07 |
Table 3 shows that, among young adults, worse HL-specific survival was associated with earlier year of diagnosis, older age, male sex, later stage of disease, presence of B symptoms, and lymphocyte depletion histologic subtype. In addition, worse HL-specific survival was associated significantly and independently both with lower neighborhood SES, with a 23% to 64% greater risk of HL-death for the lower SES quintiles (versus the highest SES quintile), and with black or Hispanic race/ethnicity, with the risk of HL-death 74% greater in blacks and 43% greater in Hispanics than in whites. Worse HL-specific survival in older adults was associated with many of the same factors as in young adults. With the exception that the risk of death from all causes was similar for blacks and whites, worse overall survival (Table 3) was associated with many of the same factors as HL-specific survival. Adding neighborhood SES to the multivariate models in Table 3 attenuated the hazard ratios for race/ethnicity but by less than 10%.
Table 3.
Multivariate adjusted* hazard ratios and 95% confidence interval (95% CI) estimates for death from all causes (overall survival) and death from Hodgkin lymphoma (HL-specific survival) in HL patients diagnosed between 1988 and 2006 in the state of California by age group.
| Characteristic | Age 15 - 44 years | Age ≥ 45 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | HL-Specific Survival | Overall Survival | HL-Specific Survival | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age at diagnosis (per year) |
1.03 | 1.03-1.04 | 1.02 | 1.01-1.03 | 1.07 | 1.06-1.07 | 1.07 | 1.06-1.07 |
| Year of diagnosis | ||||||||
| 1988-1992 | 1.79 | 1.39-2.31 | 2.05 | 1.47-2.87 | 1.24 | 1.07-1.44 | 1.55 | 1.25-1.92 |
| 1993-1997 | 1.36 | 1.06-1.75 | 1.54 | 1.11-2.15 | 1.20 | 1.05-1.38 | 1.46 | 1.20-1.78 |
| 1998-2002 | 1.08 | 0.83-1.41 | 1.35 | 0.96-1.89 | 0.95 | 0.83-1.09 | 1.08 | 0.88-1.32 |
| 2003-2006 | Reference | Reference | Reference | Reference | ||||
| Sex | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 1.35 | 1.19-1.53 | 1.16 | 0.98-1.36 | 1.15 | 1.06-1.25 | 1.05 | 0.92-1.19 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | ||||
| Black | 1.63 | 1.33-2.00 | 1.74 | 1.32-2.29 | 1.05 | 0.86-1.29 | 1.40 | 1.06-1.85 |
| Hispanic | 1.30 | 1.11-1.51 | 1.43 | 1.17-1.75 | 1.11 | 0.99-1.24 | 1.17 | 1.00-1.38 |
| Asian/Pacific Islander |
1.02 | 0.74-1.40 | 1.22 | 0.82-1.82 | 1.00 | 0.82-1.21 | 1.11 | 0.83-1.50 |
| Stage at diagnosis | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 1.35 | 1.09-1.67 | 1.48 | 1.08-2.02 | 1.22 | 1.06-1.39 | 1.75 | 1.38-2.23 |
| III/IV | 2.15 | 1.74-2.65 | 2.59 | 1.90-3.52 | 1.92 | 1.70-2.16 | 3.31 | 2.67-4.11 |
| Missing/unknown | 1.99 | 1.49-2.65 | 1.84 | 1.20-2.84 | 1.25 | 1.05-1.50 | 1.52 | 1.10-2.09 |
| B Symptoms | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.72 | 1.46-2.04 | 2.15 | 1.71-2.70 | 1.31 | 1.17-1.46 | 1.77 | 1.49-2.11 |
| Missing/unknown | 1.41 | 1.18-1.68 | 1.67 | 1.31-2.14 | 1.15 | 1.02-1.29 | 1.41 | 1.16-1.70 |
| Histologic subtype | ||||||||
| Nodular sclerosis | Reference | Reference | Reference | Reference | ||||
| Mixed cellularity | 1.07 | 0.90-1.27 | 0.90 | 0.70-1.15 | 0.98 | 0.89-1.08 | 1.00 | 0.86-1.16 |
| Lymphocyte depletion |
1.69 | 1.13-2.53 | 1.78 | 1.08-2.95 | 1.59 | 1.32-1.90 | 1.68 | 1.30-2.17 |
| Lymphocyte rich | 0.94 | 0.64-1.40 | 0.54 | 0.27-1.10 | 0.82 | 0.66-1.02 | 0.73 | 0.50-1.07 |
| Not otherwise specified |
1.40 | 1.15-1.69 | 1.04 | 0.78-1.38 | 1.19 | 1.06-1.33 | 1.18 | 1.00-1.40 |
| Neighborhood socioeconomic status (quintiles) |
||||||||
| 1 (Lowest) | 1.81 | 1.46-2.24 | 1.64 | 1.23-2.20 | 1.44 | 1.26-1.66 | 1.36 | 1.10-1.68 |
| 2 | 1.57 | 1.29-1.92 | 1.48 | 1.13-1.94 | 1.26 | 1.01-1.44 | 1.27 | 1.04-1.54 |
| 3 | 1.58 | 1.30-1.91 | 1.63 | 1.26-2.10 | 1.31 | 1.15-1.48 | 1.19 | 0.98-1.44 |
| 4 | 1.28 | 1.06-1.56 | 1.23 | 0.95-1.61 | 1.06 | 0.93-1.20 | 1.00 | 0.82-1.21 |
| 5 (Highest) | Reference | Reference | Reference | Reference | ||||
Adjusted for all variables in the table
Neighborhood SES differences in survival varied by stage at diagnosis, so analyses of SES associations were stratified by stage (Table 4). Although power is limited when stratified by age group and stage, neighborhood SES was associated with overall survival among patients with all stages of disease; in young adults, patients in the lower-SES group had risks of death 103% (stage I), 56% (stage II) and 28% (stages III/IV) greater than patients in the higher-SES group. In older adults, patients in the lower-SES group had risks of death 27% to 35% higher than patients in the higher-SES group. Lower-SES patients also appeared to be at a greater risk of HL-specific death than higher-SES patients. Among young adults, risks of death were 96% (stage II) and 21% (stages III/IV) greater in lower-than higher-SES patients; among older adults, risks were 39% (stage II) and 19% (stages III/IV) greater in lower-than in higher-SES patients. Neighborhood SES was not significantly associated with HL-specific survival in patients with stage I disease, possibly because of the relatively small number of deaths from HL (n=49 for patients 15-44 years; n=101 for patients ≥ 45 years).
When we considered death from cancers other than HL and death from circulatory diseases separately, low-SES was associated with worse survival in young (multivariate adjusted HR for death from other cancers = 1.88, 95% confidence intervals (CI): 1.33-2.67; HR for death from circulatory disease = 1.60, 95% CI: 0.95-2.70) and older (HR for death from other cancers = 1.25, 95% CI: 1.04-1.50; HR for death from circulatory disease = 1.75, 95% CI: 1.37-2.23) adults. Racial/ethnic disparities in survival were apparent only in young adult blacks (HR for death from other cancers = 1.66, 95% CI: 0.99-2.77; HR for death from circulatory disease = 1.73, 95% CI: 0.72-4.13) and Hispanics (HR for death from other cancers = 0.91, 95% CI: 0.58-1.44; HR for death from circulatory disease = 1.58, 95% CI: 0.84-2.97).
Young-adult patients of black race/ethnicity had worse survival in all stages of disease: in this group, risk of death from all causes was 52% to over two-fold greater and risk of death from HL 65% to over four-fold greater than that in white patients (Table 4). Older-adult black patients had a suggestively higher risk of death from HL, with the risk 46% greater (stages III/IV) than that in white patients. Hispanic patients with later-stage disease similarly had worse survival than white patients. Among Hispanic young adults, the risk of death from all causes was 30% (stage II) to over 140% (unknown stage) greater, and the risk of death from HL was 64% (stage III/IV) to 158% (unknown stage) greater, than in white patients (Table 4). Older-adult Hispanic patients with stage III/IV disease also had a 25% greater risk of death from all causes than whites. Asians/Pacific Islanders had similar survival to white patients.
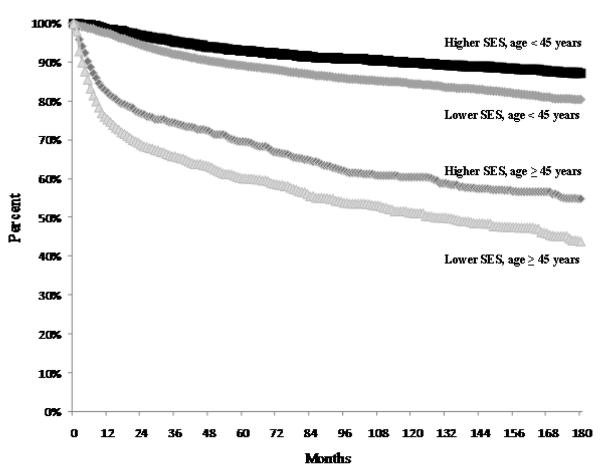
Relative survival estimates varied by age and SES group. Older adults had worse survival than young adults over the entire study period (Figure 1), and within categories defined by race/ethnicity, gender and stage at diagnosis (Table 5). The higher-SES group had higher relative survival than the lower-SES group for all categories of race/ethnicity, gender and stage at diagnosis (Table 5). Although absolute differences in relative survival by neighborhood SES varied over time within patient subgroups, the disparities between the higher- and lower-SES groups generally persisted for the 15-year study period in both age groups.
Figure 1.
Relative survival after Hodgkin lymphoma by age (years) and neighborhood socioeconomic status (SES) group (Lower SES = quintiles 1, 2, 3; Higher SES = quintiles 4, 5), California, 1988-2006.
DISCUSSION
In this large population-based series of classical HL patients in California, we found that survival was poorer for patients living in lower-SES neighborhoods at diagnosis, and for black or Hispanic patients even after adjustment for neighborhood SES. These findings of social class-associated survival disparities for this highly curable cancer underscore the importance of determining and ameliorating the underlying causes of such disparities so that all patients can benefit from the well-established and successful treatments.
Our findings are supported by previous studies that considered SES and racial/ethnic differences in survival separately (9-12) and by our prior study (based on 922 subjects also included in these analyses) that considered neighborhood SES and race/ethnicity simultaneously (23). HL patients diagnosed in the US Surveillance, Epidemiology and End Results (SEER) regions in 1987-1992 had a 5% increased risk of cancer-specific death with each quintile decrease in area-level median household income (9); in a Brazilian HL clinical series (2001-2005), higher SES was associated with better 2-year overall survival (93% vs. 79%) (10). By contrast, in an Austrian clinical series of HL patients (1969-2002), those of higher SES had worse failure-free survival (13), and in a population-based Danish HL cohort (1994-2003), there was no association between socioeconomic or demographic measures and 5-year relative survival (24). In our prior study, lower neighborhood SES and non-white race/ethnicity were associated with poorer survival in patients 15-44, but not 45-96, years of age (23). Some of these differences in findings may be attributable to different health care systems. For example, Austria and Denmark have well-established universal health insurance systems, whereas the US provides government-supported health insurance only to those aged 65 and over, and Brazil’s universal health care system was implemented relatively recently.
At least two studies have considered HL survival in non-white racial/ethnic groups. A US survey of HL patients (diagnosed in 1970-1981), 74% of whom were under 55 years of age at diagnosis, found that blacks had a 44% higher risk of death than whites five to 10 years after diagnosis (12). Our study of SEER data for HL diagnoses in 1983 through 1995 found that non-white young and older adult HL patients had a 40-50% increased risk of HL-specific death, depending on age and symptoms, compared to white patients (11). However, no previous US studies have considered HL survival in Hispanics, and HL survival studies of APIs have been limited to small series (n < 50) of Taiwanese patients (25, 26).
Social disparities in survival may occur because patients with lower-SES or non-white race/ethnicity are diagnosed at a later stage of disease (27), as found in one HL study (28), or receive poorer cancer treatment (27). In our study, the percentages of HL patients with lower neighborhood SES and diagnosed with stage III/IV disease were higher in blacks and Hispanics than in Asians/Pacific Islanders and whites. However, we found neighborhood SES and racial/ethnic differences in survival in patients with all stages of disease, suggesting that factors over and above stage at diagnosis influence the disparities we observed. Improvements in standards for HL treatment likely contributed to the better survival observed in more recent diagnostic years (29). However, we did not have information to evaluate the quality of staging, which largely determines treatment (20), or the completeness of treatment received by study patients. Thus, we could not assess how these factors may have varied by neighborhood SES or race/ethnicity and thereby contributed to our findings. Young-adult black and Hispanic patients with unknown stage of disease had markedly worse survival, suggesting that lower quality of staging in these groups may be related to poorer treatment and, consequently, worse survival. Furthermore, SES is related to having adequate health insurance, a variable not available in this study, and being uninsured or Medicaid-insured has been found to be associated with poorer survival after cancer (30).
Other explanations for our observations of HL outcome disparities by neighborhood SES and race/ethnicity include differences in medical follow-up or integrated care after diagnosis, comorbidities, health behaviors, and other host factors (27). If poor health behaviors and comorbid conditions are more prevalent in lower-SES and/or non-white HL patients, as found for patients with other cancers (27, 31-33), then these factors could increase post-treatment complications and reduce survival. Furthermore, inadequate long-term follow-up in patients could result in a delay in diagnosing and treating complications (10). Finally, host genetic factors also may contribute to the observed survival differences by race/ethnicity. For example, racial/ethnic variation in certain immune-function genes may impact survival after HL (34-38).
In our study, relative survival was worse in the lower neighborhood SES group than the higher-SES group in all racial/ethnic categories. With the exception of older cases at 5 and 10 years after diagnosis, we found better relative survival in females than in males, consistent with previously reported five-year relative survival estimates (1). We also noted better overall survival for young-adult females than males, as found previously, including prior to the era of successful HL treatments (11, 39). While there was some variation in the magnitude of neighborhood SES disparities in relative survival over the duration of follow-up, we found that the gaps persisted for at least 15 years after diagnosis in groups defined by age, gender, and stage of disease.
Our study had a number of strengths. It used a large, population-based series of patients, including all HL patients diagnosed in California over an 19-year time period; thus, our findings are likely more generalizable than those from some prior studies of disparities in HL, as survival has been shown to be different in patients in population-based cancer registries than in patients in clinical trials (40) or treated in comprehensive cancer centers (41). Another strength is that survival time for our study was uniformly collected, which minimizes bias due to differential follow-up. We also used a measure of neighborhood SES shown to have adequate sensitivity for demonstrating SES gradients in HL incidence (17) and survival (23). Our study has the advantage of using customized SES- and race/ethnicity-specific life tables, which likely estimate the relative survival after HL more accurately than unadjusted/general life tables. Because HL incidence varies by neighborhood SES at diagnosis (17) and higher-SES populations have longer survival, the use of general population mortality tables to determine the expected mortality for calculations of relative survival would likely overestimate the relative survival rates of these patients.
Our study also was subject to some limitations. We did not have individual-level measures of SES to consider along with our measure of neighborhood SES. While individual and neighborhood SES are associated, neighborhood SES has been shown to underestimate associations observed with individual-level SES (42). Furthermore, area-based SES measures may reveal differences in health outcomes and risk factors not captured by individual-level SES alone, as 23 of 25 studies found a significant association between area SES measures and health outcomes after adjusting for individual-level SES (43). Because cancer registry data lack information on potentially relevant clinical data such as prognostic serum measures (44) or tumor characteristics (e.g., presence of Epstein-Barr virus in tumor cells (23)), our analyses could not examine the impact of these factors on survival. Finally, our study is also subject to the effects of some potential misclassification in cancer registry data, including for race/ethnicity, although we have detected excellent overall agreement with self-reported race/ethnicity for whites and blacks, and intermediate agreement for Hispanics and Asians (45, 46); and for the underlying cause of death code, although this previously has been found to be between 84% and 90% accurate (47-49).
While over 75% of all HL patients are now considered cured of their disease (2), our data show that this remarkable clinical accomplishment has not been distributed evenly across socioeconomic and racial/ethnic groups. Rather, lower neighborhood SES and non-white patients are less likely to benefit from optimal treatment and clinical care, even among young adults, an age group for which a cancer diagnosis might be expected to provoke particularly close medical attention and follow-up care. Our findings of similar results for overall and HL-specific survival, as well as the persistence of differences in relative survival by neighborhood SES over time, suggest that neighborhood SES and racial/ethnic disparities in HL survival stem more from differences in the initial treatment and management than in the response to late complications resulting from HL. Therefore, targeted efforts to expand access to high-quality staging and treatment for lower-SES and black and Hispanic HL patients should help to bring survival in these groups up to the standard enjoyed by more privileged patients. In the meantime, efforts are needed to identify additional reasons for these marked survival differences.
ACKNOWLEDGEMENTS
The authors thank Laura A. McClure and Sandra J. Horning for their contributions to this work.
Acknowledgement of Financial Support: This work was supported in part by National Cancer Institute funds from R03 CA117454 (T. H. K.). The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975-2004. 2007 [cited based on November 2006 SEER data submission, posted to the SEER web site, 2007; Available from: http://seer.cancer.gov/csr/1975_2004/
- 2.Ng AK, Mauch PM. Late complications of therapy of Hodgkin’s disease: prevention and management. Curr Hematol Rep. 2004;3(1):27–33. [PubMed] [Google Scholar]
- 3.Hoppe RT. The John Ultmann Lecture--the role of radiation therapy in the treatment of Hodgkin’s disease: past, present, and future. Eur J Haematol Suppl. 2005;(66):14–20. doi: 10.1111/j.1600-0609.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 4.Klimm B, Diehl V, Pfistner B, Engert A. Current treatment strategies of the German Hodgkin Study Group (GHSG) Eur J Haematol Suppl. 2005;(66):125–34. doi: 10.1111/j.1600-0609.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 5.Elston L, Cole J, Ben Manachem T, Morlock R. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39:361–372. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 6.VanEenwyk J, Campo J, Ossiander E. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95:39–46. doi: 10.1002/cncr.10645. [DOI] [PubMed] [Google Scholar]
- 7.Yabroff K, Gordis L. Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case-fatality, and mortality? Soc Sci Med. 2003;57:2265–2279. doi: 10.1016/s0277-9536(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 8.Cella DF, Orav EJ, Kornblith AB, Holland JC, Silberfarb PM, Lee KW, et al. Socioeconomic status and cancer survival. J Clin Oncol. 1991;9(8):1500–9. doi: 10.1200/JCO.1991.9.8.1500. [DOI] [PubMed] [Google Scholar]
- 9.Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999;17(7):2244–55. doi: 10.1200/JCO.1999.17.7.2244. [DOI] [PubMed] [Google Scholar]
- 10.Soares A, Biasoli I, Scheliga A, Luiz RR, Costa MA, Land M, et al. Socioeconomic inequality and short-term outcome in Hodgkin’s lymphoma. Int J Cancer. 2007;120(4):875–9. doi: 10.1002/ijc.22417. [DOI] [PubMed] [Google Scholar]
- 11.Clarke CA, Glaser SL, Prehn AW. Age-specific survival after Hodgkin’s disease in a population-based cohort (United States) Cancer Causes Control. 2001;12(9):803–12. doi: 10.1023/a:1012240222032. [DOI] [PubMed] [Google Scholar]
- 12.Zaki A, Natarajan N, Mettlin CJ. Early and late survival in Hodgkin disease among whites and blacks living in the United States. Cancer. 1993;72(2):602–6. doi: 10.1002/1097-0142(19930715)72:2<602::aid-cncr2820720244>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Holzner B, Fischhofer M, Kemmler G, Kopp M, Sperner-Unterweger B, Krugmann J, et al. Is higher income and educational status associated with poorer outcome in patients with Hodgkin’s disease? Eur J Haematol. 2004;73(5):318–24. doi: 10.1111/j.1600-0609.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 14.Hadden WC, Brooke MB. [Accessed 7/9/08];The limits of health surveys for contextual or multi-level analysis.: Federal Committee on Statistical Methodology. National Center for Health Statistics. 1999 www.fcsm.gov/99papers/hadden.pdf.
- 15.Fritz F, Percy C, Jack A, Shanmugaratnan K, Sobin L, Parkin DM, et al., editors. International Classification onf Diseases for Oncology. Third ed World Health Organization; Geneva: 2000. [Google Scholar]
- 16.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 17.Clarke CA, Glaser SL, Keegan TH, Stroup A. Neighborhood socioeconomic status and Hodgkin’s lymphoma incidence in California. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1441–7. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- 18.Vaccher E, Spina M, Tirelli U. Clinical aspects and management of Hodgkin’s disease and other tumours in HIV-infected individuals. Eur J Cancer. 2001;37:1306–15. doi: 10.1016/s0959-8049(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 19.Glaser SL, Clarke CA, Gulley ML, Craig FD, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988 1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 20.DeVita VT, Jr., Hubbard SM. Hodgkin’s disease. N Engl J Med. 1993;328(8):560–5. doi: 10.1056/NEJM199302253280808. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 22.Lee E, Wei LJ, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Kluwer Academic; Netherlands: 1992. [Google Scholar]
- 23.Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, et al. Epstein-Barr virus as a marker of survival after Hodgkin’s lymphoma: a population-based study. J Clin Oncol. 2005;23(30):7604–13. doi: 10.1200/JCO.2005.02.6310. Epub 2005 Sep 26. [DOI] [PubMed] [Google Scholar]
- 24.Roswall N, Olsen A, Christensen J, Rugbjerg K, Mellemkjaer L. Social inequality and incidence of and survival from Hodgkin lymphoma, non-Hodgkin lymphoma and leukaemia in a population-based study in Denmark, 1994 2003. Eur J Cancer. 2008;24:24. doi: 10.1016/j.ejca.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Tan TD, Feng AC. Clinico-pathological study of Hodgkin’s lymphoma in a cancer center in Taiwan. Clin Lab Haematol. 2005;27(6):379–83. doi: 10.1111/j.1365-2257.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 26.Hong RL, Su IJ, Chen YC, Hsieh HC, Wang CH, Liu CH, et al. Hodgkin’s disease and non-Hodgkin’s lymphoma containing Reed-Sternberg-like giant cells in Taiwan. A clinicopathologic analysis of 50 cases. Cancer. 1992;69(5):1254–8. doi: 10.1002/cncr.2820690530. [DOI] [PubMed] [Google Scholar]
- 27.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. Epub 2005 Sep 2. [DOI] [PubMed] [Google Scholar]
- 28.Hu E, Hufford S, Lukes R, Bernstein-Singer M, Sobel G, Gill P, et al. Third-World Hodgkin’s disease at Los Angeles County-University of Southern California Medical Center. J Clin Oncol. 1988;6(8):1285–92. doi: 10.1200/JCO.1988.6.8.1285. [DOI] [PubMed] [Google Scholar]
- 29.Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–84. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 30.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 31.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250–6. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia IB, Mack KA, Murphy W, Mokdad AH, Bales VS. State-specific prevalence of selected chronic disease-related characteristics--Behavioral Risk Factor Surveillance System, 2001. MMWR Surveill Summ. 2003;52(8):1–80. [PubMed] [Google Scholar]
- 33.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294(14):1765–72. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 34.Diepstra A, Niens M, Vellenga E, van Imhoff GW, Nolte IM, Schaapveld M, et al. Association with HLA class I in Epstein-Barr-virus-positive and with HLA class III in Epstein-Barr-virus-negative Hodgkin’s lymphoma. Lancet. 2005;365(9478):2216–24. doi: 10.1016/S0140-6736(05)66780-3. [DOI] [PubMed] [Google Scholar]
- 35.Begovich AB, Moonsamy PV, Mack SJ, Barcellos LF, Steiner LL, Grams S, et al. Genetic variability and linkage disequilibrium within the HLA-DP region: analysis of 15 different populations. Tissue Antigens. 2001;57(5):424–39. doi: 10.1034/j.1399-0039.2001.057005424.x. [DOI] [PubMed] [Google Scholar]
- 36.Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI Minority Workshops: Part IV. Hum Immunol. 2004;65(12):1413–9. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- 37.Herbst H, Samol J, Foss HD, Raff T, Niedobitek G. Modulation of interleukin-6 expression in Hodgkin and Reed-Sternberg cells by Epstein-Barr virus. J Pathol. 1997;182(3):299–306. doi: 10.1002/(SICI)1096-9896(199707)182:3<299::AID-PATH856>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Herbst H, Foss HD, Samol J, Araujo I, Klotzbach H, Krause H, et al. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin’s disease. Blood. 1996;87(7):2918–29. [PubMed] [Google Scholar]
- 39.MacMahon B. Epidemiology of Hodgkin’s disease. Cancer Res. 1966;26(6):1189–201. [PubMed] [Google Scholar]
- 40.Roy P, Hudson G Vaughan, Hudson B Vaughan, Esteve J, Swerdlow AJ. Long-term survival in Hodgkin’s disease patients. A comparison of relative survival in patients in trials and those recorded in population-based cancer registries. Eur J Cancer. 2000;36(3):384–9. doi: 10.1016/s0959-8049(99)00267-1. [DOI] [PubMed] [Google Scholar]
- 41.Davis S, Dahlberg S, Myers MH, Chen A, Steinhorn SC. Hodgkin’s disease in the United States: a comparison of patient characteristics and survival in the Centralized Cancer Patient Data System and the Surveillance, Epidemiology, and End Results Program. J Natl Cancer Inst. 1987;78(3):471–8. [PubMed] [Google Scholar]
- 42.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 45.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–87. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 46.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17(6):771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129(12):1020–6. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 48.Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–50. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Percy C, Ries LG, Van Holten VD. The accuracy of liver cancer as the underlying cause of death on death certificates. Public Health Rep. 1990;105(4):361–7. [PMC free article] [PubMed] [Google Scholar]